Abstract
Aim
This study investigated the effects of St John's wort extract (SJW) on the pharmacokinetics and metabolism of the immunosuppressant cyclosporin A (CSA).
Methods
In an open-label study, 11 renal transplant patients received 600 mg SJW extract daily for 14 days in addition to their regular regimen of CSA. Blood concentrations of CSA and its metabolites AM1, AM1C, AM9, AM19, and AM4N were measured by HPLC.
Results
After 2 weeks of SJW coadministration, dose-corrected AUC0–12, Cmax and Ctrough values for CSA decreased significantly by 46%[geometric mean ratio baseline/SJW (95% CI): 1.83 (1.63–2.05)], 42%[1.72 (1.42–2.08)], and 41%[1.70 (1.17–2.47)], respectively. CSA doses were increased from a median of 2.7 mg day−1 kg−1 at baseline to 4.2 mg day−1 kg−1 at day 15, with the first dose adjustment required only 3 days after initiation of SJW treatment. Additionally, the metabolite pattern of CSA was substantially altered during SJW treatment. Whereas dose-corrected AUC values for AM1, AM1c and AM4N significantly decreased by 59%, 61%, and 23% compared with baseline, AUC values for AM9 and AM19 were unchanged. Following the increase in CSA dose, observed AUC and Cmax values for AM9, AM19, and AM4N increased by 20–51% and 43–90%, respectively.
Conclusion
Administration of SJW extract to patients receiving CSA treatment resulted in a rapid and significant reduction of plasma CSA concentrations. Additionally, the substantial alterations in CSA metabolite kinetics observed may affect the toxicity profile of the drug.
Keywords: cyclosporin A, drug interaction, metabolism, patients, pharmacokinetics, St John's wort
Introduction
St John's wort (SJW, Hypericum perforatum) extracts are frequently used for the treatment of mild to moderate depression. Chronic use of SJW reduces the bioavailability of a number of drugs, including the cardiac glycoside digoxin [1], the HIV protease inhibitor indinavir [2], the antidepressant amitriptyline [3], and the immunosuppressant cyclosporin A (CSA). Several cases of acute heart, liver, and kidney transplant rejections due to decreased CSA blood levels during coadministration of SJW have been reported [4–9].
CSA is the standard immunosuppressant in the prevention of allograft rejection after kidney, liver, heart, and bone marrow transplantations and is also used in the treatment of various autoimmune diseases. Frequently, CSA is administered in combination with steroids, azathioprin, or mycophenolic acid [10]. The drug undergoes extensive biotransformation by cytochrome P450 enzymes (CYP) to more than 30 metabolites that differ in their therapeutic activity and toxicity [11–13]. Metabolic alteration of single functional groups yields the primary metabolites AM1 (hydroxylated at amino acid 1), AM9 (hydroxylated at amino acid 9), and AM4N (N-demethylated at amino acid 4). These are subject to further biotransformation yielding AM1c (cyclized AM1) and AM19 (hydroxylated at amino acids 1 and 9) as the quantitatively most important secondary metabolites [12]. The main enzyme in CSA metabolism is CYP3A4 [14], but other isoforms may be involved [15–17]. CSA is also a substrate for the MDR1-transporter P-glycoprotein (P-gp) [18–20].
Induction of intestinal P-gp can decrease drug absorption by stimulating the active efflux of the drug back into the intestinal lumen [21, 22], an effect that was first proposed to explain the SJW–digoxin interaction [1]. Decreased systemic availability and plasma drug concentrations can also be the result of intestinal and hepatic induction of CYP enzymes. SJW extracts contain a variety of compounds [23], with hypericin, pseudohypericin, and hyperforin among those presumed responsible for its antidepressive activity [24, 25]. Hyperforin has been shown to strongly activate the PXR receptor, which is involved in the regulation of CYP3A and P-gp expression [26, 27].
The present study investigated the effects of 14 days of concomitant treatment with 600 mg hypericum extract per day on the pharmacokinetics of CSA and its primary (AM1, AM9, AM4N) and major secondary (AM1c, AM19) metabolites in renal transplant patients.
Methods
Patients
Eleven renal transplant patients (9M, 2F) at least 2 years after surgery were enrolled and all patients completed the study. Inclusion criteria were: stable CSA dose for 3 months prior to enrolment, trough blood concentrations in the range 100–150 µg l−1 verified by two consecutive measurements, and stable allograft function (creatinine clearance> 30 ml min−1). Patients underwent general blood and urine analysis, ECG, and physical examination prior to enrolment. Detailed patient characteristics are reported in Table 1. Dietary restrictions included caffeine, alcohol and grapefruit juice. Co-medications remained unchanged during the course of the study, which was approved by the ethics committee of the University Medical Centre Charité, Humboldt University of Berlin. All patients gave their written informed consent.
Table 1.
Characteristics of renal transplant patients participating in the study.
| Creatinine (mg dl-1) | Urea (mg dl−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Sex | Age | Weight (kg) | Years post-transplant | Baseline | SJW* | Baseline | SJW* | Baseline CSA dose (mg kg−1 day−1) | Co-medication |
| 1 | M | 34 | 74 | 11 | 1.1 | 1.1 | 55 | 44 | 3.4 | Methylprednisolon, nisoldipin, atenolol |
| 2 | M | 46 | 86 | 10 | 1.3 | 1.4 | 70 | 70 | 2.5 | Methylprednisolon, furosemide, cerivastatin, azathioprine |
| 3 | M | 42 | 81 | 13 | 1.4 | 1.5 | 75 | 69 | 2.7 | Methylprednisolon, metoprolol, benazepril, benzbromaron |
| 4 | F | 48 | 77 | 10 | 0.7 | 0.8 | 44 | 32 | 2.0 | Methylprednisolon, felodipin, losartan |
| 5 | F | 58 | 64 | 10 | 1.4 | 1.3 | 68 | 74 | 2.8 | Methylprednisolon, nitrendipin, benzbromaron |
| 6 | M | 50 | 73 | 16 | 1.5 | 1.7 | 54 | 61 | 2.3 | Methylprednisolon, amlodipin, allopurinol |
| 7 | M | 35 | 63 | 11 | 2.1 | 2.4 | 75 | 74 | 3.2 | Methylprednisolon, nitrendipin |
| 8 | M | 54 | 80 | 6 | 1.5 | 1.3 | 58 | 53 | 3.7 | Methylprednisolon, nitrendipin, celiprolol, cerivastatin, ranitidin, benzbromaron |
| 9 | M | 59 | 83 | 9 | 1.4 | 1.4 | 59 | 53 | 4.2 | Methylprednisolon, amlodipin, doxazosin, benzbromaron, mycophenolate-mofetil, furosemide |
| 10 | M | 52 | 85 | 6 | 2.0 | 2.2 | 77 | 69 | 1.9 | Methylprednisolon, nitrendipin, celiprolol |
| 11 | M | 39 | 95 | 12 | 2.1 | 2.0 | 77 | 61 | 2.1 | Methylprednisolon, amlodipin, cerivastatin, doxazosin, piretanid |
| Mean | 10.4 | 1.5 | 1.6 | 65 | 60 | 2.8 | ||||
| s.d. | 2.9 | 0.4 | 0.5 | 11 | 13 | 0.7 | ||||
Values after 14 days of St John's wort treatment.
Study design
In an open-label design, patients received 600 mg SJW extract (two coated tablets Jarsin300™; Lichtwer Pharma, Berlin, Germany) once daily, together with the CSA morning dose for 14 days in addition to their normal regimen of CSA (Sandimmun® Optoral; Novartis Pharma, Nürnberg, Germany, which contained all-rac-alpha-tocopherol as an antioxidant). One tablet of Jarsin300 consisted of 300 mg of a dried methanolic extract of SJW containing hypericin, pseudohypericin, and hyperforin [3]. Owing to safety considerations in response to case reports on serious CSA and SJW drug interactions [4–9], the selected dose of SJW extract (600 mg day−1) was below the recommended therapeutic dose for this preparation (900 mg day−1). The pharmacokinetics of CSA and its metabolites AM1, AM9, AM4N, AM1c and AM19 were measured on the day before initiation of SJW treatment (study day 1) and after 14 days of SJW treatment (study day 15). At sampling times of 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 12 h post administration of CSA, venous blood samples of 5 ml were drawn into EDTA-coated tubes. Trough concentrations of CSA were also determined on days 4, 8, 11, 18, 22 and 29, and doses were adjusted as required based on trough concentrations to assure CSA blood concentrations in the therapeutic range of 70–150 µg l−1. Doses were kept stable at least 2 days prior to the second CSA kinetic measurements to ensure steady-state conditions.
Sample analysis
CSA and its metabolites AM1, AM9, AM4N, AM1c and AM19 were quantified using a modification of the HPLC method by Christians et al. [28]. Briefly, 0.5 ml blood was spiked with the internal standard (cyclosporin D) and loaded onto a solid-phase extraction cartridge (Baker-Bond C8; J. T. Baker, Phillipsburg, NJ, USA). The samples were washed with 30% acetonitrile in water and n-hexane and eluted with dichloromethane. The solvent was evaporated and samples were reconstituted in 50% acetonitrile in water. Analysis was performed using a Shimadzu HPLC system (Duisburg, Germany) consisting of two pumps LC-10AS, an automatic sampler SIL-10A, and a dual-wavelength u.v. detector SPD-10A set at 210 nm. The compounds were separated by gradient elution at 60°C on a Hypersil ODS column (5 µm, 250 × 4.6 mm i.d.; Optilab, Berlin, Germany) with a guard column (5 µm, 10 × 4.6 mm i.d.). Mobile phase A consisted of 10% acetonitrile and 0.01% phosphoric acid in water and mobile phase B consisted of 90% acetonitrile and 0.01% phosphoric acid in water. Gradient elution started at 50% of B for 10 min, was increased to 75% at 35 min, kept constant until 40 min, was increased to 100% at 50 min and reduced to 50% at 52 min. Total run time was 60 min at a flow rate of 1.0 ml min−1. The compounds were quantified using their peak height ratio to an internal standard based on the calibration curve of CSA. Metabolites were identified by comparison of their retention times with reference standards (Dade Behring, Deerfield, IL, USA) and bile extract. The assay was linear up to 2000 µg l−1. Intra- and inter-assay coefficients of variation ranged from 3.2% to 14.1% and from 2.4% to 12.8%, respectively. The lower limit of quantification was 20 µg l−1. CSA trough concentrations were determined by homogeneous enzyme immunoassay (EmitTM; Dade Behring, Deerfield, IL, USA) using a COBAS MIRA S analyser (Hoffmann-La Roche AG, Basel, Switzerland).
Pharmacokinetics
Steady-state pharmacokinetics of CSA were characterized by the area under the plasma concentration–time curve within one dosing interval (AUC0–12), the drug concentration at the end of one dosing interval (Ctrough), peak concentration in plasma (Cmax) and time to reach Cmax (tmax). AUC0–12 was calculated using the linear trapezoidal rule (WinNonLin Pro 1.5; Pharsight Corp., Mountain View, CA, USA). In order to account for the dose adjustments, values of AUC, Cmax, and Ctrough were corrected by multiplication with the ratio of baseline dose/dose on SJW.
Statistical analysis
The primary aim of the study was to evaluate the influence of SJW co-medication on the steady-state AUC0–12 of CSA. Considering a 30% difference in CSA AUC0–12 to be clinically relevant, a necessary sample size of 11 subjects was calculated with a paired two-sided t-test with a type-I error of 0.05 and a type-II error of 0.20. Calculations were based on the known variability in the AUC0–12 of CSA in kidney transplant patients of 5069 ± 1480 µg h−1 l−1 (mean ± s.d.) [29]. Values were log-transformed and baseline and SJW data were compared using a two-sided t-test (SPSS 10.0; SPSS Inc., Chicago, IL, USA).
Results
CSA pharmacokinetics
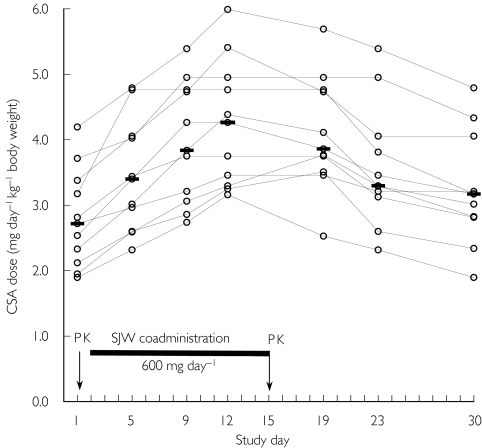
In order to maintain CSA blood concentrations in the therapeutic range (70–150 µg l−1), median daily CSA doses were increased from 2.7 mg day−1 kg−1 at baseline to 4.2 mg day−1 kg−1 after 10 days of SJW treatment on study day 12 (Figure 1). All 11 patients required a first CSA dose adjustment 3 days after the initiation of SJW co-medication. Despite dose adjustments, the CSA pharmacokinetics resulting on day 15 did not quite reach pretreatment values (Table 2, Figure 2). After discontinuation of SJW treatment, CSA doses had to be decreased. However, the median dose was still 3.2 mg day−1 kg−1 on study day 30, and baseline doses were reached only in three patients (Figure 1).
Figure 1.
Individual CSA doses required to maintain sufficient immunosuppression during SJW co-medication. CSA trough concentrations were measured on study days 4, 8, 11, 18, 22 and 29, with dose adjustments effective the following day (ie study days 5, 9, 12, 19, 23 and 30). SJW extract was coadministered between day 2 and day 15 (black bar). CSA pharmacokinetics were measured on study days 1 and 15 (arrows). Horizontal bars indicate the median dose.
Table 2.
Pharmacokinetic parameters for CSA at baseline and after 14 days of SJW co-medication (600 mg day−1).
| Baseline | SJW†observed data | SJWc‡dosecorrected data | Ratio§(95% CI) | |
|---|---|---|---|---|
| AUC0–12 (µg h−1 l−1)¶ | 3319 (3034–3606) | 2832 (2456–3824)* | 1818 (1447–2274)** | 1.83 (1.63–2.05) |
| Cmax (µg l−1) | 1077 (955–1275) | 976 (697–1292) | 627 (503–831)** | 1.72 (1.42–2.08) |
| Ctrough (µg l−1) | 93 (74–121) | 70 (53–102) | 55 (44–70)* | 1.70 (1.17–2.47) |
| tmax (h)†† | 1.0 (1.0–1.5) | 1.0 (1.0–1.5) |
During SJW treatment, CSA doses were successively increased to compensate for decreasing CSA blood concentrations.
Parameters after 14 days of SJW treatment derived from observed data (not corrected for CSA dose).
Parameters after 14 days of SJW treatment derived from data corrected for CSA dose.
Geometric mean ratios between baseline values and dose corrected data, with 95% confidence intervals in parentheses.
Geometric means with 25th and 75th percentiles in parentheses.
Median with 25th and 75th percentiles in parentheses.
P < 0.05;
P < 0.005 (two-way t-test compared with baseline).
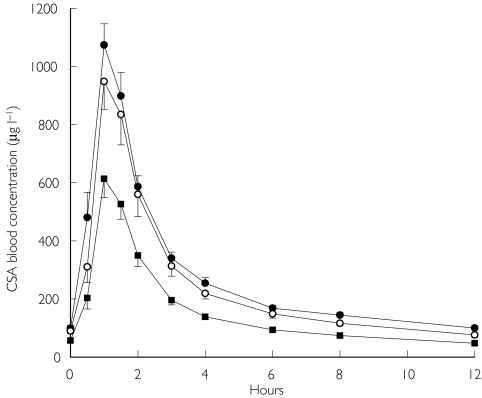
Figure 2.
Blood concentrations of CSA in 11 renal transplant patients at baseline (•), after 14 days of SJW treatment with a compensatory increase in CSA dose (○), and after 14 days of SJW treatment with data corrected for CSA dose ( ). Values are means ± s.e.m.
). Values are means ± s.e.m.
The median dose-corrected values for CSA after 2 weeks of SJW treatment showed a significant decrease in AUC0–12, Cmax, and Ctrough by 41% to 45% (Table 2, Figure 2). Despite subtherapeutic SJW doses, the effect was evident in all 11 patients, with the reduction in CSA AUC0–12 ranging between 30% and 60%.
CSA metabolite pharmacokinetics
Data corrected for CSA dose Dose-corrected: AUC0–12, Cmax, and Ctrough values for AM1 and AM1c were significantly reduced by approximately 60%, an effect larger than the one observed for the parent compound. In contrast, the metabolites AM9 and AM19 remained unaffected by SJW treatment when blood concentrations were corrected for CSA dose. AM4N showed decreased dose-corrected AUC and Ctrough values (−23% and −42%, respectively), whereas Cmax was unchanged (Table 3, Figure 3a).
Table 3.
Pharmacokinetic parameters for CSA metabolites at baseline and after 14 days of SJW co-medication (600 mg day−1)
| AUC0–12(µg h−1l−1) | Cmax(µg l−1) | Ctrough(µg l−1) | |
|---|---|---|---|
| AM1 | |||
| Baseline | 11 901 (10 608–14 230) | 1 534 (1284–1959) | 777 (677–820) |
| SJW† | 7 558 (6950–8596)** | 1 105 (863–1329)* | 400 (339–503)** |
| SJWc‡ | 4 852 (3909–5989)** | 709 (599–816)** | 322 (258–391)** |
| Ratio (95% CI)§ | 2.45 (2.17–2.77) | 2.16 (1.56–3.00) | 2.41 (2.10–2.77) |
| AM1c | |||
| Baseline | 1 355 (1122–1598) | 188 (165–210) | 87 (72–120) |
| SJW | 823 (627–1191)** | 119 (93–167)* | 38 (25–83)* |
| SJWc | 529 (392–761)** | 76 (52–106)** | 31 (22–57)** |
| Ratio (95% CI) | 2.56 (1.92–3.42) | 2.47 (1.76–3.45) | 2.78 (1.69–4.58) |
| AM9 | |||
| Baseline | 3 716 (2635–5295) | 806 (580–1200) | 114 (106–160) |
| SJW | 5 460 (5071–6672)** | 1 269 (925–1560)** | 149 (129–156) |
| SJWc | 3 506 (2988–4448) | 815 (587–1054) | 114 (87–151) |
| Ratio (95% CI) | 1.06 (0.94–1.20) | 0.99 (0.75–1.30) | 1.00 (0.79–1.28) |
| AM19 | |||
| Baseline | 781 (604–1072) | 153 (119–239) | 38 (20–62)¶ |
| SJW | 1 180 (1052–1552)** | 291 (177–387)** | 35 (18–55) |
| SJWc | 758 (592–899) | 187 (102–258) | 24 (11–36)** |
| Ratio (95% CI) | 1.03 (0.92–1.15) | 0.82 (0.65–1.04) | 1.59 (1.32–1.90) |
| AM4N | |||
| Baseline | 1 146 (940–1603) | 312 (211–458) | 46 (28–65)¶ |
| SJW | 1 374 (1237–1681)* | 447 (289–607)** | 41 (24–61) |
| SJWc | 882 (737–1177)** | 287 (173–415) | 27 (17–41)** |
| Ratio (95% CI) | 1.30 (1.16–1.46) | 0.92 (0.85–1.39) | 1.72 (1.39–2.12) |
Geometric means with 25th and 75th percentiles in parentheses. During SJW treatment, CSA doses were successively increased to compensate for decreasing CSA blood concentrations.
Parameters after 14 days of SJW treatment derived from observed data (not corrected for CSA dose).
Parameters after 14 days of SJW treatment derived from data corrected for CSA dose.
Geometric mean ratios between baseline values and dose corrected data, with 95% confidence intervals in parentheses.
n = 10, one patient did not show measurable AM19 and AM4N blood concentrations.
P < 0.05;
P < 0.005 (two-way t-test compared with baseline).
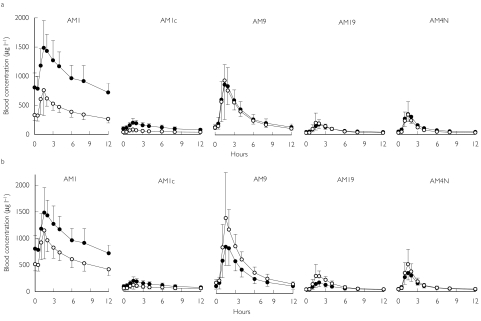
Figure 3.
Blood concentrations of CSA metabolites in 11 renal transplant patients at baseline (•) and after 14 days of SJW treatment (○). Values are means ± s.d.
Data corrected for CSA dose.
Observed uncorrected data.
Observed uncorrected data: At increased CSA doses, the AUC0–12 and Cmax values for AM1 and AM1c remained 35% lower than those at baseline, and Ctrough values were decreased by more than 50% (Table 3, Figure 3b). The AUC values for AM9, AM19, and AM4N increased significantly by 47%, 51%, and 20%, respectively. Corresponding Cmax values increased significantly by 57%, 90%, and 43%, respectively (Table 3, Figure 3b).
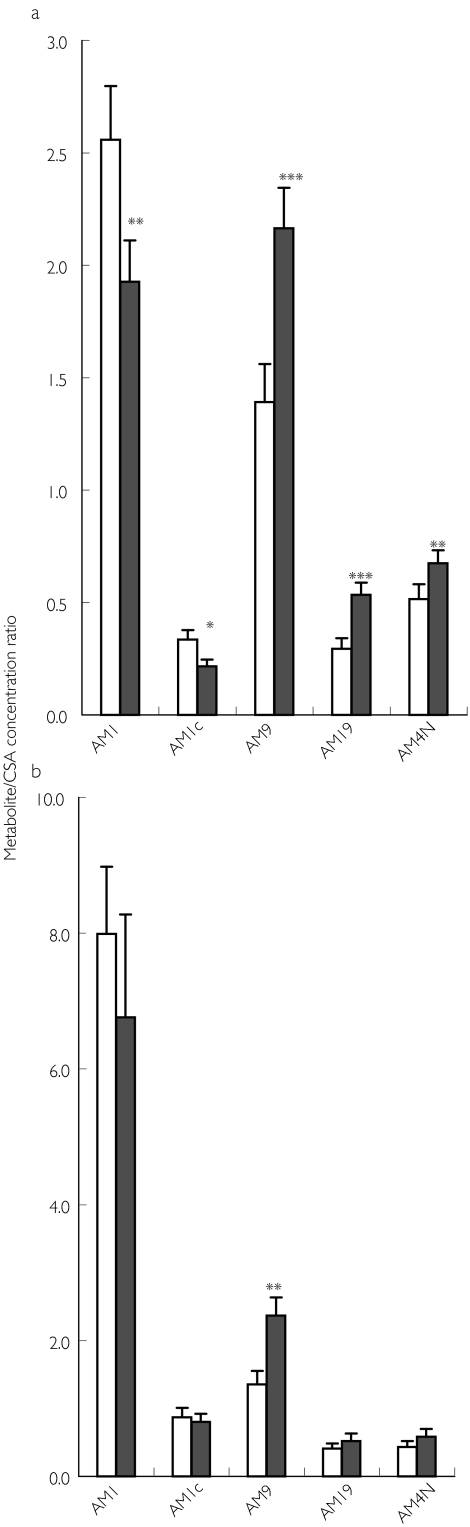
As a consequence of those differential effects on individual metabolic pathways, the pattern of CSA metabolism was considerably altered during SJW treatment, particularly around tmax (1–2 h). Whereas AM1 followed by AM9 showed the highest Cmax values and metabolic ratios at baseline, this order was reversed after 2 weeks of SJW treatment (Table 3, Figures 3b and 4a). Additionally, exposure to AM19 and AM4N increased significantly, whereas the AM1c metabolic ratio was decreased (Figure 4a). Trough concentrations at 12 h post dose showed less pronounced effects. However, AM1 metabolic ratios still tended to be lower and AM9/CSA ratios significantly higher than the corresponding baseline values (Figure 4b).
Figure 4.
Observed metabolite/CSA concentration ratios in 11 renal transplant patients at baseline (□) and after 14 days of SJW treatment (▪). Values are means + s.e.m.
At Cmax (2 h after CSA dose).
At Ctrough (12 h after CSA dose). *P < 0.05; **P < 0.01; ***P < 0.005 (two-way t-test compared with baseline).
Allograft function
Renal function remained stable during SJW treatment as indicated by creatinine and urea concentrations (Table 1).
Discussion
Although several case reports have described serious interactions between CSA and SJW [4–8], detailed pharmacokinetic data have not been available until now. The present study has demonstrated that co-medication with SJW extract rapidly (3 days after initiation of treatment) and substantially (more than a 40% decrease in AUC0–12) altered the pharmacokinetics of CSA in all 11 patients, requiring repeated CSA dose adjustments to ensure continued therapeutic activity of the immunosuppressant (Figure 1). Although the SJW dose used in this study (600 mg day−1) was below that recommended for this formulation (900 mg day−1), the CSA dose had to be increased by 60%. The reversal of the effect after discontinuation of SJW co-medication took longer than 2 weeks in 8/11 patients.
The reduced plasma concentrations of several orally administered drugs that have been observed after co-medication with SJW [1–3] can be explained by induction of drug-metabolizing enzymes (CYP3A) and/or drug transporters (P-gp) via the PXR receptor [21, 22, 26]. CSA undergoes extensive hepatic metabolism by cytochrome P-450 enzymes, mainly CYP3A4 [11, 12, 14] and is also a substrate for P-gp [18–20]. Although more than 80% of CSA metabolism is attributed to CYP3A4 [14], CYP3A5 has also been shown to metabolize CSA [16], and there is some evidence that the formation of cyclic metabolites (AM1c, AM1c9) may be mediated by 3-methylcholanthrene-inducible CYP isoforms such as CYP1A1 or CYP1A2 [15, 17]. Furthermore, the effects observed with different preparations of SJW may vary greatly, depending on the composition of the individual plant extract.
SJW treatment caused differential effects on the pharmacokinetics of CSA metabolites. The profiles of the five metabolites quantified in this study fell into two distinct groups. Thus, AM1 and AM1c showed markedly reduced blood concentrations in all 11 patients even after CSA dose adjustment, whereas the Cmax values of AM9, AM19, and AM4N were unchanged after correction for CSA dose. A similar pattern, although in reverse direction, was previously seen with the calcium antagonist diltiazem, which caused an increase in CSA and AM1 blood concentrations, whereas AM9 levels remained unchanged [30]. CSA and its metabolites are mainly eliminated via bile [31], with 96% of an oral dose recovered in faeces and less than 0.1% of the parent drug eliminated unchanged [32]. It has been shown that the biliary excretion of CSA in P-gp knockout mice is reduced to approximately 30% of that in wild-type animals [33]. It can be hypothesized that the induction of P-gp by SJW results in enhanced biliary excretion of those CSA metabolites with higher affinities for P-gp, and they would also be less likely to be reabsorbed from the intestine. As a result, the enterohepatic cycle would be interrupted and blood concentrations of P-gp substrates would decrease. Poor P-gp substrates, on the other hand, would not be affected by P-gp induction. The observed increase in blood concentrations of AM9, AM19, and AM4N may be the result of CYP3A induction, an effect that could be masked for AM1 and AM1c by their enhanced elimination. In vitro studies characterizing the CSA interaction metabolites with P-gp would be valuable to explain the differential effects of SJW on CSA metabolite kinetics. However, the lack of availability of authentic standards of CSA metabolites limits such studies. Additionally, acute inhibition of CYP3A4, CYP2D6, CYP1A2, and CYP2C9 activity by SJW constituents has been shown in vitro[34]. Considering the long half-lives of the SJW constituents hypericin, pseudohypericin and hyperforin (42 h, 23 h, and 16 h, respectively) [3, 35], such inhibitory effects on hepatic or intestinal CYP activity may also contribute to the interactions of SJW with several drugs.
Although therapeutic CSA concentrations could be maintained under SJW co-medication by appropriate dose adjustments, the resulting pattern of CSA metabolites was substantially altered. Whereas baseline metabolite patterns were consistent with previously reported findings [36], SJW treatment resulted in increased exposure to AM9, AM19, and AM4N, particularly around tmax. High blood concentrations of total CSA metabolites have been repeatedly associated with CSA nephrotoxicity [37–39], and elevated AM1 concentrations have been reported to coincide with severe CNS toxicity symptoms [40]. In the same study, rejection episodes after kidney transplants were associated with low AM1 and AM9 blood concentrations, suggesting that CSA metabolites may contribute to the immunosuppressant activity of the drug [40]. These findings indicate that the influence of chronic SJW coadministration may go beyond a decrease in CSA parent drug bioavailability, but could potentially lead to alterations in treatment efficacy and toxicity. In humans, CSA toxicity affects kidney, liver, pancreas, and CNS, with nephrotoxicity presenting the major limitation in clinical use [41]. The molecular basis of CSA toxicity remains unclear, but proposed mechanisms include a modulation of CYP patterns in liver and kidney, covalent binding to macromolecules, alterations in endotheline production, as well as the occurrence of alternative CSA metabolic pathways [42, 43]. In rat or cell culture models, CSA metabolites show less toxicity than the parent compound [44, 45]. However, synergistic effects between CSA and its metabolites or between different metabolites have been proposed [46].
In summary, administration of SJW extract to renal transplant patients being treated with a stable CSA regimen resulted in a rapid and significant decrease in CSA blood concentrations associated with the risk of inadequate immunosuppression. In order to compensate for the decrease in AUC, CSA doses had to be increased by 60%. Additionally, the metabolite pattern of CSA was substantially altered during SJW co-medication. In light of the fact that the molecular mechanism of CSA toxicity and the role of individual CSA metabolites in this process are as yet unknown, an effect of chronic SJW treatment on the toxicity profile of CSA cannot be excluded. Coadministration of SJW extract to patients during CSA therapy appears to be associated with a substantial risk of therapy failure and a considerable increase in treatment costs and therefore should be avoided.
Acknowledgments
The authors thank Dr A. G. Hildebrandt for the critical reading of the manuscript, as well as Ms C. Schulze for performing the HPLC analysis of CSA metabolites.
References
- 1.Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John's wort (Hypericum perforatum) Clin Pharmacol Ther. 1999;66:338–345. doi: 10.1053/cp.1999.v66.a101944. [DOI] [PubMed] [Google Scholar]
- 2.Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St John's wort. Lancet. 2000;355:547–548. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 3.Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St. John's wort (Hypericum perforatum) J Clin Psychopharmacol. 2002;22:46–54. doi: 10.1097/00004714-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Ruschitzka F, Meier PJ, Turina M, Luscher TF, Noll G. Acute heart transplant rejection due to Saint John's wort. Lancet. 2000;355:548–549. doi: 10.1016/S0140-6736(99)05467-7. [DOI] [PubMed] [Google Scholar]
- 5.Moschella C, Jaber BL. Interaction between cyclosporine and Hypericum perforatum (St. John's wort) after organ transplantation. Am J Kidney Dis. 2001;38:1105–1107. doi: 10.1053/ajkd.2001.28617. [DOI] [PubMed] [Google Scholar]
- 6.Karliova M, Treichel U, Malago M, et al. Interaction of Hypericum perforatum (St. John's wort) with cyclosporin A metabolism in a patient after liver transplantation. J Hepatol. 2000;33:853–855. doi: 10.1016/s0168-8278(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 7.Breidenbach T, Hoffmann MW, Becker T, Schlitt H, Klempnauer J. Drug interaction of St John's wort with cyclosporin. Lancet. 2000;355:1912. doi: 10.1016/s0140-6736(05)73359-6. [DOI] [PubMed] [Google Scholar]
- 8.Mai I, Krüger H, Budde K, et al. Hazardous pharmacokinetic interaction of Saint John's wort (Hypericum perforatum) with the immunosuppressant cyclosporin. Int J Clin Pharmacol Ther. 2000;38:500–502. doi: 10.5414/cpp38500. [DOI] [PubMed] [Google Scholar]
- 9.Turton-Weeks SM, Barone GW, Gurley BJ, et al. St John's wort: a hidden risk for transplant patients. Prog Transplant. 2001;11:116–120. doi: 10.1177/152692480101100207. [DOI] [PubMed] [Google Scholar]
- 10.Gaston RS. Maintenance immunosuppression in the renal transplant recipient: an overview. Am J Kidney Dis. 2001;38:S25–S35. doi: 10.1053/ajkd.2001.28923. [DOI] [PubMed] [Google Scholar]
- 11.Christians U, Strohmeyer S, Kownatzki R, et al. Investigations on the metabolic pathways of cyclosporine. II. Elucidation of the metabolic pathways in vitro by human liver microsomes. Xenobiotica. 1991;21:1199–1210. doi: 10.3109/00498259109039560. [DOI] [PubMed] [Google Scholar]
- 12.Christians U, Sewing KF. Cyclosporin metabolism in transplant patients. Pharmacol Ther. 1993;57:291–345. doi: 10.1016/0163-7258(93)90059-m. [DOI] [PubMed] [Google Scholar]
- 13.Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet. 1993;24:472–495. doi: 10.2165/00003088-199324060-00004. [DOI] [PubMed] [Google Scholar]
- 14.Combalbert J, Fabre I, Fabre G, et al. Metabolism of cyclosporin A. IV. Purification and identification of the rifampicin-inducible human liver cytochrome P-450 (cyclosporin A oxidase) as a product of P450IIIA gene subfamily. Drug Metab Dispos. 1989;17:197–207. [PubMed] [Google Scholar]
- 15.Sewing KF, Christians U, Kohlhaw K, et al. Biologic activity of cyclosporine metabolites. Transplant Proc. 1990;22:1129–1134. [PubMed] [Google Scholar]
- 16.Aoyama T, Yamano S, Waxman DJ, et al. Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J Biol Chem. 1989;264:10388–10395. [PubMed] [Google Scholar]
- 17.Bouis P, Brouillard JF, Fischer V, Donatsch P, Boelsterli UA. Effect of enzyme induction on Sandimmun (cyclosporin A) biotransformation and hepatotoxicity in cultured rat hepatocytes and in vivo. Biochem Pharmacol. 1990;39:257–266. doi: 10.1016/0006-2952(90)90024-f. [DOI] [PubMed] [Google Scholar]
- 18.Hebert MF. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv Drug Deliv Rev. 1997;27:201–214. doi: 10.1016/s0169-409x(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 19.Lown KS, Mayo RR, Leichtman AB, et al. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther. 1997;62:248–260. doi: 10.1016/S0009-9236(97)90027-8. [DOI] [PubMed] [Google Scholar]
- 20.Fricker G, Drewe J, Huwyler J, Gutmann H, Beglinger C. Relevance of p-glycoprotein for the enteral absorption of cyclosporin A: in vitro–in vivo correlation. Br J Pharmacol. 1996;118:1841–1847. doi: 10.1111/j.1476-5381.1996.tb15612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dürr D, Stieger B, Kullak-Ublick GA, et al. St John's Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. doi: 10.1067/mcp.2000.112240. [DOI] [PubMed] [Google Scholar]
- 22.Perloff MD, von Moltke LL, Störmer E, Shader RI, Greenblatt DJ. Saint John's wort. An in vitro analysis of P-glycoprotein induction due to extended exposure. Br J Pharmacol. 2001;134:1601–1608. doi: 10.1038/sj.bjp.0704399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahrstedt A, Butterweck V. Biologically active and other chemical constituents of the herb of Hypericum perforatum L. Pharmacopsychiatry. 1997;30(Suppl. 2):129–134. doi: 10.1055/s-2007-979533. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee SS, Bhattacharya SK, Wonnemann M, Singer A, Muller WE. Hyperforin as a possible antidepressant component of hypericum extracts. Life Sci. 1998;63:499–510. doi: 10.1016/s0024-3205(98)00299-9. [DOI] [PubMed] [Google Scholar]
- 25.Butterweck V, Petereit F, Winterhoff H, Nahrstedt A. Solubilized hypericin and pseudohypericin from Hypericum perforatum exert antidepressant activity in the forced swimming test. Planta Med. 1998;64:291–294. doi: 10.1055/s-2006-957437. [DOI] [PubMed] [Google Scholar]
- 26.Moore LB, Goodwin B, Jones SA, et al. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wentworth JM, Agostini M, Love J, Schwabe JW, Chatterjee VK. St John's wort, a herbal antidepressant, activates the steroid X receptor. J Endocrinol. 2000;166:R11–R16. doi: 10.1677/joe.0.166r011. [DOI] [PubMed] [Google Scholar]
- 28.Christians U, Zimmer KO, Wonigeit K, Maurer G, Sewing KF. Liquid-chromatographic measurement of cyclosporin A and its metabolites in blood, bile, and urine. Clin Chem. 1988;34:34–39. [PubMed] [Google Scholar]
- 29.Mück W, Mai I, Fritsche L, et al. Increase in cerivastatin systemic exposure after single and multiple dosing in cyclosporine-treated kidney transplant recipients. Clin Pharmacol Ther. 1999;65:251–261. doi: 10.1016/S0009-9236(99)70104-9. [DOI] [PubMed] [Google Scholar]
- 30.Brockmöller J, Neumayer HH, Wagner K, et al. Pharmacokinetic interaction between cyclosporin and diltiazem. Eur J Clin Pharmacol. 1990;38:237–242. doi: 10.1007/BF00315023. [DOI] [PubMed] [Google Scholar]
- 31.Christians U, Strohmeyer S, Kownatzki R, et al. Investigations on the metabolic pathways of cyclosporine. I. Excretion of cyclosporine and its metabolites in human bile—isolation of 12 new cyclosporine metabolites. Xenobiotica. 1991;21:1185–1198. doi: 10.3109/00498259109039559. [DOI] [PubMed] [Google Scholar]
- 32.Maurer G, Lemaire M. Biotransformation and distribution in blood of cyclosporine and its metabolites. Transplant Proc. 1986;18:25–34. [PubMed] [Google Scholar]
- 33.Kwei GY, Alvaro RF, Chen Q, et al. Disposition of ivermectin and cyclosporin A in CF-1 mice deficient in mdr1a P-glycoprotein. Drug Metab Dispos. 1999;27:581–587. [PubMed] [Google Scholar]
- 34.Obach RS. Inhibition of human cytochrome P450 enzymes by constituents of St. John's Wort, an herbal preparation used in the treatment of depression. J Pharmacol Exp Ther. 2000;294:88–95. [PubMed] [Google Scholar]
- 35.Kerb R, Brockmöller J, Staffeldt B, Ploch M, Roots I. Single-dose and steady-state pharmacokinetics of hypericin and pseudohypericin. Antimicrob Agents Chemother. 1996;40:2087–2093. doi: 10.1128/aac.40.9.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bleck JS, Schlitt HJ, Christians U, et al. Urinary excretion of cyclosporin and 17 of its metabolites in renal allograft recipients. Pharmacology. 1989;39:160–164. doi: 10.1159/000138593. [DOI] [PubMed] [Google Scholar]
- 37.Wonigeit K, Kohlhaw K, Winkler M, Schaefer O, Pichlmayr R. Cyclosporine monitoring in liver allograft recipients: two distinct patterns of blood level derangement associated with nephrotoxicity. Transplant Proc. 1990;22:1305–1311. [PubMed] [Google Scholar]
- 38.Kohlhaw K, Wonigeit K, Schafer O, et al. Association of very high blood levels of cyclosporin metabolites with clinical complications after liver transplantation. Transplant Proc. 1989;21:2232–2233. [PubMed] [Google Scholar]
- 39.Leunissen KM, Beuman GH, Bosman FT, van Hooff JP. The nephrotoxic effects of cyclosporine metabolites. Transplant Proc. 1988;20:738–739. [PubMed] [Google Scholar]
- 40.Kunzendorf U, Brockmöller J, Jochimsen F, Roots I, Offermann G. Activity of cyclosporin metabolites M17 and M1. Transplant Proc. 1990;22:1697–1699. [PubMed] [Google Scholar]
- 41.Rush DN. Cyclosporine toxicity to organs other than the kidney. Clin Biochem. 1991;24:101–105. doi: 10.1016/0009-9120(91)90399-y. [DOI] [PubMed] [Google Scholar]
- 42.Christians U, Sewing KF. Alternative cyclosporine metabolic pathways and toxicity. Clin Biochem. 1995;28:547–559. doi: 10.1016/0009-9120(95)00037-3. [DOI] [PubMed] [Google Scholar]
- 43.Mayer RD, Berman S, Cockett AT, Maines MD. Differential effects of cyclosporin on hepatic and renal heme, cytochrome P-450 and drug metabolism. Possible role in nephrotoxicity of the drug. Biochem Pharmacol. 1989;38:1001–1007. doi: 10.1016/0006-2952(89)90291-8. [DOI] [PubMed] [Google Scholar]
- 44.Copeland KR, Thliveris JA, Yatscoff RW. Toxicity of cyclosporine metabolites. Ther Drug Monit. 1990;12:525–532. doi: 10.1097/00007691-199011000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Donatsch P, Rickenbacher U, Ryffel B, Brouillard JF. Sandimmun metabolites: their potential to cause adverse reactions in the rat. Transplant Proc. 1990;22:1137–1140. [PubMed] [Google Scholar]
- 46.Radeke HH, Christians U, Bleck JS, Sewing KF, Resch K. Additive and synergistic effects of cyclosporine metabolites on glomerular mesangial cells. Kidney Int. 1991;39:1255–1266. doi: 10.1038/ki.1991.159. [DOI] [PubMed] [Google Scholar]