Abstract
Aims
Evidence of long-term beneficial effects of β-blockers on mortality and morbidity in patients with heart failure has been demonstrated in recent randomized trials. However, not all β-blockers are identical. Carvedilol, a nonselective β- and α-adrenergic blocker, can potentially blunt the release of noradrenaline by blocking presynaptic β2-adrenergic receptors. To test this hypothesis, we have compared the effects of carvedilol and atenolol on plasma noradrenaline during exercise in healthy young volunteers.
Methods
This study investigated the differential effects of 2 weeks pretreatment with carvedilol 25 mg day−1 and atenolol 50 mg day−1 on plasma noradrenaline at rest and during exercise on a treadmill in a double-blind randomized crossover study, involving 12 healthy male volunteers (mean age 21.6 ± 0.3 years).
Results
Haemodynamic parameters at rest and during exercise were not significantly different in either carvedilol or atenolol pretreatment groups. However, carvedilol pretreatment significantly blunted the increase in plasma noradrenaline during exercise [393.8 ± 51.7 pg ml−1 (pretreatment) to 259.7 ± 21.2 pg ml−1 (post-treatment)], when compared with atenolol [340.4 ± 54.6 pg ml−1 (pretreatment) to 396.2 ± 32.0 pg ml−1 (post-treatment)]. The difference between carvedilol and atenolol (95% confidence interval) was −145.2, −351.0, P < 0.05.
Conclusions
We have demonstrated that carvedilol but not atenolol significantly blunted the increase in plasma noradrenaline during exercise. These findings may suggest a sympathoinhibitory effect of carvedilol that may enhance its ability to attenuate the cardiotoxicity associated with adrenergic stimulation in patients with heart failure.
Keywords: β-adrenoceptor antagonist, chronic heart failure, noradrenaline
Introduction
Recent randomized trials have demonstrated that β-blockers have long-term beneficial effects on mortality and morbidity in patients with heart failure [1]. However, β-blockers differ substantially in their pharmacological properties in ways that may impact their relative efficacy and tolerability. Carvedilol, a third-generation β-blocking agent, is reported to have vasodilator activity and antioxidant properties [2, 3]. The basic pharmacology of carvedilol differs considerably from second-generation β-blocking agents [4, 5]. Significantly, carvedilol has ‘atypical’ effects on β-receptors in that it has a high affinity for agonist-binding receptors, a process mediated by an interaction with G protein that, in turn, leads to downregulation of receptors. This property is more prominent for the human β2- than for the β1- adrenoceptor [6]. Finally, carvedilol may have a sympathoinhibitory effect attributable to blockade of peripheral presynaptic β2-adrenoreceptors, which potentially may have additional beneficial effects [7].
In patients with heart failure, activation of the sympathetic nervous system is frequently excessive at rest, as evidenced by increased plasma levels of noradrenaline [8] and increased peroneal nerve activity [9]. Exercise triggers even more excessive activation, with plasma levels of noradrenaline markedly exceeding those noted in normal subjects at comparable workloads [10, 11].
The present study was undertaken to compare the effects of atenolol, a selective β-blocker, and carvedilol on plasma noradrenaline during exercise in healthy volunteers.
Methods
Subjects
Twelve healthy male volunteers (mean age 21.6 ± 0.3 years) were studied. The absence of significant medical problems was verified by history and physical examination. Subjects did not take any medication for at least 2 weeks before and throughout the study. All subjects gave informed consent for this trial, which was approved by the local ethics committee.
Study design
The study was a double-blind randomized crossover comparison of 2 weeks treatment with carvedilol against atenolol, with a 2-week washout between both treatments. Exercise testing was performed before administration of carvedilol or atenolol and after 2 weeks washout period, and also after 2 weeks of administration of either drug. Thus, there were four assessment points. Measurements of interest included: (i) haemodynamic parameters of blood pressure at rest and heart rate at rest and during exercise; and (ii) plasma noradrenaline concentration at rest and during exercise.
Medication
Treatment consisted of either oral carvedilol or oral atenolol in a crossover design with a 2-week washout in between. Carvedilol was started at a dose of 12.5 mg daily for 3 days, followed by a dose of 25 mg daily. Similarly, atenolol was started at a dose of 25 mg daily for the first 3 days and then increased to a daily dose of 50 mg. These doses were selected in order to achieve comparable β-adrenoceptor blockade [12]. Compliance with medication was assessed by tablet counting.
Exercise testing
At each visit, following an overnight fast, the subjects were required to perform a treadmill exercise using the Bruce protocol [13] to stage 4 (total exercise time of 9 min, equivalent to 15 metabolic equivalents (METS). Blood pressure was measured using a semiautomatic sphygmomanometer at rest before the test. Twelve-lead electrocardiograph (ECG) was recorded using Max-1 (Sensor Medics Corporation, Yorba Linda, CA, USA) at rest, at the end of each stage of the Bruce protocol and at the recovery stage. Heart rate was determined from the ECG.
Sample collection and analysis
Blood samples for noradrenaline assessment were collected into a Vacutainer tube containing lithium heparin via an indwelling venous cannula at rest and at the end of stage 4 of the Bruce protocol. Blood samples were placed immediately in an ice-cold box and centrifuged at 1050 g at 4°C for 5 min. Plasma was separated and stored at −70°C until the analysis. Plasma concentration of noradrenaline was assayed using a gas chromatograph mass spectrometer as previously described (14). The intra- and inter-assay coefficients of variation for this assay were 4.76% and 4.76%, respectively. The lower limit of detection was 100 pg ml−1.
Statistical evaluation
Data was analysed using the SPSS program (SPSS Inc, 2335 Wacker Drive, Chicago, IL, USA). All values were presented as mean ± s.e.m. The statistical significance of the differences was evaluated using two-tailed Student's t-test for paired and unpaired values; P < 0.05 was considered statistically significant.
Results
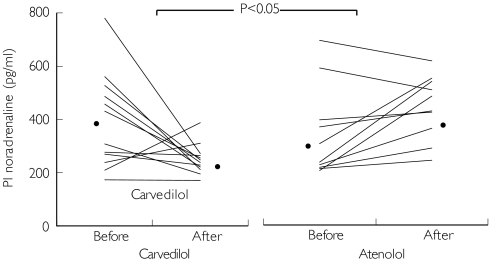
Both carvedilol and atenolol decreased heart rate and blood pressure equally at rest. The increase in heart rate during exercise was also equally blunted by carvedilol and atenolol (Table 1). Plasma noradrenaline concentration at rest also did not significantly differ after 2 weeks of treatment in both groups, from 229.4 ± 12.5 pg ml−1 (pretreatment) to 212.7 ± 14.7 pg ml−1 (post-treatment) in the carvedilol pretreatment group, and from 211.9 ± 7.9 pg ml−1 (pretreatment) to 194.2 ± 13.5 pg ml−1 (post-treatment) in the atenolol pretreatment group. However, carvedilol significantly (P < 0.05) blunted the increase in plasma noradrenaline at peak exercise from 393.8 ± 51.7 pg ml−1 (pretreatment) to 259.7 ± 21.2 pg ml−1 (post-treatment), when compared with atenolol, from 340.4 ± 54.6 pg ml−1 (pretreatment) to 396.2 ± 32.0 pg ml−1 (post-treatment). The difference between carvedilol and atenolol was 248.1 pg ml−1 (95% CI −145.2, −351.0, P < 0.05, Figure 1).
Table 1.
Haemodynamic effects in both carvedilol and atenolol.
| Carvedilol (n = 12) | Atenolol (n = 12) | |||
|---|---|---|---|---|
| Pretreatment | Post-treatment | Pretreatment | Post-treatment | |
| Heart rate at rest (bpm) | 73.6 ± 2.2 | 66.8 ± 3.0 | 77.3 ± 3.1 | 62.5 ± 1.7 |
| Heart rate at peak exercise (bpm) | 137.6 ± 5.31 | 17.5 ± 5.5* | 136.1 ± 5.21 | 19.3 ± 4.1* |
| Systolic pressure at rest (mmHg) | 126.8 ± 3.01 | 19.4 ± 2.4* | 125.4 ± 2.61 | 16.3 ± 2.5* |
| Diastolic pressure at rest (mmHg) | 2.0 ± 2.5 | 69.8 ± 2.5 | 72.8 ± 2.7 | 67.5 ± 2.4 |
Results are expressed as mean ± s.e.m.
P < 0.05 pretreatment vs post-treatment. Haemodynamic parameters did not differ between atenolol and carvedilol pretreatment groups. bpm, Beats per minute.
Figure 1.
Effect of carvedilol or atenolol on plasma noradrenaline at peak exercise. Results are individual pairs of plasma noradrenaline levels obtained from each patient at peak exercise (Stage 4 Bruce exercise protocol, equivalent to 15 METS) prior to and after treatment with carvedilol (left panel) and atenolol (right panel). *Mean plasma noradrenaline level. P < 0.05, 95% confidence interval for the difference carvedilol vs atenolol was −145.2, −351.0.
Discussion
This study has two main findings. Firstly, we showed that both carvedilol and atenolol had similar blunting effects on heart rate and blood pressure at rest and during exercise. Secondly, this study showed that despite similar effects on haemodynamic responses, carvedilol but not atenolol blunted the increase in plasma noradrenaline during exercise.
Plasma noradrenaline levels are commonly elevated during exercise [15]. This level is much higher in congestive heart failure. The mechanisms responsible for this maladaptive process remain to be precisely defined. An impairment of baroreflex sensory mechanism may play an important role underlying the sympatho-excitation [16–18]. A recent report suggests that a rapidly responsive and sensitive arterial baroreflex, and activation of a cardiac sympatho-excitatory reflex related to increased cardiopulmonary filling pressures, could be responsible [19]. The high levels of noradrenaline may lead to systemic vasoconstriction with a further consequent excessive reduction in tissue perfusion (20). Impaired skeletal muscle perfusion during exercise appears to be involved in reduced exercise capacity in patients with chronic heart failure [21),
Previous studies have compared the effects of carvedilol and atenolol on haemodynamic parameters in patients of mild to moderate essential hypertension. Like our study, there were no consistently significant differences between the effects of carvedilol and atenolol on either heart rate or blood pressure during treatment [22, 23].
The key finding of our present study was that carvedilol statistically significantly lowered plasma noradrenaline levels at peak exercise in our subjects. In contrast, atenolol tended to increase the concentration of plasma noradrenaline at peak exercise. These findings suggest that carvedilol may possess a sympathoinhibitory effect, although it should be noted that responses of plasma noradrenaline to exercise are a net effect of a number of processes. Radioisotope noradrenaline kinetic studies would have been useful to differentiate an effect on noradrenaline spillover and clearance. Nevertheless, our results support the findings of two previous studies demonstrating a sympathoinhibitory effect of carvedilol. Gilbert et al. [7] investigated the differential effects of carvedilol and metoprolol on coronary sinus and central venous noradrenaline levels in patients with heart failure at rest. They reported there were no significant differences in haemodynamic effects between the carvedilol and metoprolol active-treatment groups. However, they found carvedilol selectively lowered coronary sinus noradrenaline levels, an index of cardiac adrenergic activity, whereas metoprolol did not lower coronary sinus noradrenaline levels and actually increased central venous noradrenaline levels. In addition, they showed that metoprolol was associated with an increase in cardiac β-receptor density, whereas carvedilol did not change cardiac β-receptor expression. More recently, Azevedo et al. [24] reported that carvedilol, when compared with metoprolol, decreased both resting total body and cardiac noradrenaline spillover. Importantly, microneurographic measures of sympathetic nerve traffic to skeletal muscle did not change in either group. These findings suggest that carvedilol caused a sympathoinhibitory effect by blocking peripheral β-adrenergic receptors. It should be noted that both these studies were conducted on heart failure patients at rest. Our study would suggest such an effect of carvedilol during exercise.
There are some potential advantages of carvedilol over selective β1-blockers. β1-selective blocking agents may re-couple uncoupled cardiac β2-adrenergic receptors [25] through a crossregulatory effect, further predisposing β-adrenergic signal transduction pathways to withdrawal phenomena. Because selective β1-blockers such as metoprolol have been shown to increase systemic noradrenaline levels through effects on noradrenaline clearance [26], they may increase cardiac noradrenaline spillover in chronic heart failure in the short term [27]. Thus, there is obvious potential for drug-related increases in adrenergic activity to produce adverse events in the failing heart. On the other hand, because carvedilol lowers cardiac adrenergic activity, blocks β1-, β2-, and α1-adrenergic receptors at high doses, and does not upregulate or downregulate β1-receptors, its antiadrenergic properties greatly exceed those of metoprolol. This sympathoinhibitory effect of carvedilol as shown in this study could also result in a decrease in the release of other potentially harmful neurotransmitters such as neuropeptide Y (NPY) which coexist with noradrenaline in perivascular sympathetic fibres [28]. NPY has been reported to have a direct vasoconstrictor effect on blood vessels and may potentiate the noradrenaline-evoked response [28]. This sympathoinhibitory effect may enhance the ability of carvedilol to attenuate the cardiotoxicity associated with adrenergic stimulation in patients with heart failure.
Several randomized controlled trials have compared metoprolol and carvedilol in heart failure and the results have been inconsistent. In general, no significant differences have been found, although carvedilol may lower blood pressure and peripheral resistance more than metoprolol due to its α-adrenergic blocking properties [29–31]. It should be noted that the recent BEST trial [32] with bucindolol, which is a nonselective β-blocker, failed to reduce heart failure mortality. The reasons for this have not been defined, although bucindolol, unlike carvedilol, does display intrinsic sympathomimetic activity which may be harmful in heart failure [33]. Whether the pharmacological differences between carvedilol and a selective β1-blocker will be translated into differences in survival is not known, but is being evaluated in the ongoing Carvedilol or Metoprolol European Trial (COMET) [34].
Acknowledgments
We wish to express our gratitude to Mr V. T. Johgalingam, Mr K. S. Chua, Ms G. Y. Christina and Mr Y. P. Voo for their valuable technical assistance, and to Mr M. Ragavan for assistance in statistical evaluation. This study was funded by an IRPA grant from The Ministry of Science, Technology and The Environment, Malaysia. Professor Lang is the recipient of a Tun Razak Award.
References
- 1.Tendera M, Ochala A. Overview of the results of recent beta blocker trials. Curr Opinion Cardiol. 2001;16:180–185. doi: 10.1097/00001573-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hattori Y, Nakaya H, Endou M, et al. Vascular effects of carvedilol, a new β-adrenoceptor antagonist with vasodilating properties, in isolated canine coronary artery. J Cardiovasc Pharmacol. 1989;13:572–579. [PubMed] [Google Scholar]
- 3.Asbrink S, Zickert A, Bratl J, et al. No effect of carvedilol on nitric oxide generation in phagocytes but modulation of production of superoxide ions. Biochem Pharmacol. 2000;59:1007–1013. doi: 10.1016/s0006-2952(99)00393-7. [DOI] [PubMed] [Google Scholar]
- 4.Bristow MR, Larrabee P, Minobe W, et al. Receptor pharmacology of carvedilol in the human heart. J Cardiovasc Pharmacol. 1992;19(Suppl. I):S68–S80. doi: 10.1097/00005344-199219001-00014. [DOI] [PubMed] [Google Scholar]
- 5.Sponer G, Bartsch W, Strein K, et al. Pharmacological profile of carvedilol as a β-blocking agent with vasodilating and hypotensive properties. J Cardiovasc Pharmacol. 1987;9:317–327. doi: 10.1097/00005344-198703000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Yoshikawa T, Port JD, Asano K, et al. Cardiac adrenergic receptor effects of carvedilol. Eur Heart J. 1996;17(Suppl. B):8–16. doi: 10.1093/eurheartj/17.suppl_b.8. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert EM, Abraham WT, Olsen S, et al. Comparative hemodynamic, left ventricular functional, and antiadrenergic effects of chronic treatment with metoprolol versus carvedilol in the failing heart. Circulation. 1996;94:2817–2825. doi: 10.1161/01.cir.94.11.2817. [DOI] [PubMed] [Google Scholar]
- 8.Levine TB, Francis GS, Goldsmith SR, et al. Activity of the sympathetic nervous system and renin angiotensin system assessed by plasma hormone levels and their relationship to hemodynamic abnormalities in congestive heart failure. Am J Cardiol. 1982;49:1659–1666. doi: 10.1016/0002-9149(82)90243-0. [DOI] [PubMed] [Google Scholar]
- 9.Leimbach WN, Wallin G, Victor RG, et al. Direct evidence from intraneuronal recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–919. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- 10.Chidsey CA, Harrison DC, Braunwald E. Augmentation of the plasma norepinephrine response to exercise in patients with congestive heart failure. N Engl J Med. 1962;267:650–654. doi: 10.1056/NEJM196209272671305. [DOI] [PubMed] [Google Scholar]
- 11.Francis GS, Goldsmith SR, Ziesche SM, et al. Response of plasma norepinephrine and epinephrine to dynamic exercise in patients with congestive heart failure. Am J Cardiol. 1982;49:1152–1156. doi: 10.1016/0002-9149(82)90039-x. [DOI] [PubMed] [Google Scholar]
- 12.Klemsdal TO, Mundal HH, Gjesdal K. Effects of carvedilol and atenolol on arterial pulse curves (plethysmography) and finger temperature after hand cooling. Eur J Clin Pharmacol. 1996;50:483–489. doi: 10.1007/s002280050145. [DOI] [PubMed] [Google Scholar]
- 13.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:545–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 14.Herman RB, Mustafa AM, Husain R, et al. Penang, Malaysia: 1999. Assessment of picogram concentration of plasma norepinephrine using gas chromatograph-mass spectrometer (GCMS) Abstracts of Joint 14th Scientific Meeting of the Malaysian Society of Pharmacology and Physiology. Cardiac Rehabilitation Conference and Cardiovascular Counselling Workshop. May. [Google Scholar]
- 15.Smith EE, Guyton AC, Manning RD, et al. Integrated mechanisms of cardiovascular response and control during exercise in the normal human. Prog Cardiovasc Dis. 1976;18:421–443. doi: 10.1016/0033-0620(76)90010-4. [DOI] [PubMed] [Google Scholar]
- 16.Thames MD, Kinugawa T, Smith ML, et al. Abnormalities of baroreflex control in heart failure. J Am Coll Cardiol. 1993;4(Suppl. A):56A–60A. doi: 10.1016/0735-1097(93)90464-c. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson DW, Berg WJ, Roach PJ, et al. Effects of heart failure on baroreflex control of sympathetic neural activity. Am J Cardiol. 1992;69:523–531. doi: 10.1016/0002-9149(92)90998-e. [DOI] [PubMed] [Google Scholar]
- 18.Zucker IH, Wang W, Brandle M, et al. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis. 1995;37:397–414. doi: 10.1016/s0033-0620(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 19.Floras JS. Arterial baroreceptor and cardiopulmonary reflex control of sympathetic outflow in human heart failure. Ann NY Acad Sci. 2001;940:500–513. doi: 10.1111/j.1749-6632.2001.tb03701.x. [DOI] [PubMed] [Google Scholar]
- 20.Lang CC, Chomsky DB, Rayos G, et al. Effects of sympathoinhibition on exercise performance in heart failure. Circulation. 1997;96:238–245. doi: 10.1161/01.cir.96.1.238. [DOI] [PubMed] [Google Scholar]
- 21.Drexler H. Reduced exercise tolerance in chronic heart failure and its relationship to neurohumoral factor. Eur Heart J. 1991;12(Suppl. C):21–28. doi: 10.1093/eurheartj/12.suppl_c.21. [DOI] [PubMed] [Google Scholar]
- 22.Young PH. A comparison of carvedilol with atenolol in the treatment of mild-to-moderate essential hypertension.INT-CAR-07 (U.K.) Study Group. J Cardiovasc Pharmacol. 1992;19(Suppl. 1):S82–S85. doi: 10.1097/00005344-199219001-00016. [DOI] [PubMed] [Google Scholar]
- 23.Ruilope LM. Comparison of a new vasodilating beta-blocker, carvedilol, with atenolol in the treatment of mild to moderate essential hypertension. Am J Hypertens. 1994;7:129–136. doi: 10.1093/ajh/7.2.129. [DOI] [PubMed] [Google Scholar]
- 24.Azevedo ER, Kubo T, Mak S, et al. Non-selective versus selective beta-adrenergic receptor blockade in congestive heart failure: differential effects on sympathetic activity. Circulation. 2001;104:2149–2149. doi: 10.1161/hc4301.098282. [DOI] [PubMed] [Google Scholar]
- 25.Hall JA, Kaumann AJ, Brown MJ. Selective β1-adrenoceptor blockade enhances positive inotropic responses to endogenous catecholamines mediated through β2-adrenoceptor in human atrial myocardium. Circ Res. 1990;66:1610–1623. doi: 10.1161/01.res.66.6.1610. [DOI] [PubMed] [Google Scholar]
- 26.Olsson G, Daleskog M, Hjemdahl P, Rehnqvist N. Unchanged peripheral sympathetic activity following withdrawal of chronic metoprolol treatment: a study of noradrenaline concentrations and kinetics in plasma. Br J Clin Pharmacol. 1984;18:573–579. doi: 10.1111/j.1365-2125.1984.tb02506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newton GE, Parker JD. β1 vs nonselective β-blockade in human congestive heart failure: acute effects on cardiac sympathetic activity. Circulation. 1995;92(Suppl I):I–395. doi: 10.1161/01.cir.94.3.353. Abstr. [DOI] [PubMed] [Google Scholar]
- 28.Grundemar L, Hakanson R. Multiple neuropeptide Y receptors are involved in cardiovascular regulation. Peripheral and central mechanisms. General Pharmacol. 1993;24:785–796. doi: 10.1016/0306-3623(93)90151-m. [DOI] [PubMed] [Google Scholar]
- 29.Sanderson JE, Chan SK, Yip G, et al. Beta-blockade in heart failure: a comparison of carvedilol with metoprolol. J Am Coll Cardiol. 1999;34:1522–1528. doi: 10.1016/s0735-1097(99)00367-8. [DOI] [PubMed] [Google Scholar]
- 30.Kukin ML, Kalman J, Charney RH, et al. Prospective, randomized comparison of effect of long term treatment with metoprolol or carvedilol on symptoms, exercise, ejection fraction and oxidative stress in heart failure. Circulation. 1999;99:2645–2651. doi: 10.1161/01.cir.99.20.2645. [DOI] [PubMed] [Google Scholar]
- 31.Metra M, Giubbini R, Dodari S, et al. Differential effects of beta-blockers in patients with heart failure: a prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation. 2000;102:546–551. doi: 10.1161/01.cir.102.5.546. [DOI] [PubMed] [Google Scholar]
- 32.The Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 33.Andreka P, Aiyar N, Olson LC, et al. Bucindolol displays intrinsic sympathomimetic activity in human myocardium. Circulation. 2002;105:2429–2434. doi: 10.1161/01.cir.0000016050.79810.18. [DOI] [PubMed] [Google Scholar]
- 34.Poole-Wilson PH, Remme WJ. COMET: a multicenter randomized double blind study to compare the effect of carvedilol and metoprolol on morbidity and mortality in patients with moderate or severe congestive heart failure (NYHA II–IV) Cardiovasc Drug Ther. 1999;13:24. [Google Scholar]