Abstract
Aims
To investigate whether the drug–drug interaction between fexofenadine and ketoconazole is localized to efflux transport proteins of the small intestine, and to determine and classify the effective jejunal permeability (Peff) of fexofenadine according to the Biopharmaceutics Classification System (BCS).
Methods
Two separate jejunal perfusion experiments were performed using the Loc-I-Gut® technique in eight healthy volunteers. During treatment 1 (T1), we investigated the acute effect of ketoconazole on the Peff and plasma pharmacokinetics of fexofenadine. In treatment 2 (T2) we examined the effect of oral pretreatment with ketoconazole (200 mg daily for 5 days) on the same absorption parameters. Each experiment was divided into two periods of 100 min and the jejunal segment was perfused with 93 µm fexofenadine during both periods. In period 2 of each treatment, fexofenadine was coadministered with 94 µm ketoconazole. The concentrations of fexofenadine in intestinal perfusate and plasma were measured by liquid chromatography with mass detection.
Results
During T1, the mean (± s.d.) Peff of fexofenadine was low according to the BCS (0.11 ± 0.11 and 0.04 ± 0.13·10−4 cm s−1 in periods 1 and 2, respectively), and the coadministration of ketoconazole in period 2 had no significant acute effect on Peff (95% confidence interval (CI) on the difference −0.37, 0.51). After pretreatment with ketoconazole (T2), the jejunal Peff of fexofenadine increased to 0.29 ± 0.47 and 0.22 ± 0.31·10−4 cm s−1 in both periods 1 and 2, respectively, but the change was not statistically significant when compared with T1 (95% CI on the difference −0.62, 0.27 for T1 0–100 min vs T2 0–100 min; −0.54, 0.34 for T1 0–100 min vs T2 100–200 min). Fexofenadine plasma AUC from 0–100 mg showed no significant difference after pretreatment with ketoconazole (55 ± 101 and 51 ± 33 µg ml−1 min−1 respectively; 95% CI on the difference −108, 115). Total plasma AUC (0–720 min) was 318 ± 426 and 426 ± 232 ng ml−1 min in T1 and T2, respectively (95% CI on the difference −622, 405).
Conclusions
No significant effect of acute coadministration or pretreatment with ketoconazole on the in vivo intestinal absorption of fexofenadine was detected in this study.
Keywords: bioavailability, Biopharmaceutics Classification System, drug absorption, drug–drug interaction, fexofenadine, intestinal permeability, ketoconazole, P-glycoprotein
Introduction
Fexofenadine (Telfast®, Allegra®), the active metabolite of terfenadine, is a selective nonsedating histamine H1 receptor antagonist that is prescribed for oral treatment of allergic rhinitis and chronic idiopathic urticaria, and is administered orally over a range of doses from 60 mg BID to 120–180 mg OD [1]. The pharmacokinetics of fexofenadine are linear over the dose range 20–120 mg, with a small disproportionate increase in the area under the concentration–time curve (AUC) after a 240-mg dose [2]. After oral administration of 14C-fexofenadine (60 mg), 92% of the total dose was recovered, 12% in the urine and 80% in the faeces, and the majority of the dose (> 85%) was recovered as unchanged fexofenadine [3]. This indicates that metabolism is an insignificant elimination route and that fexofenadine is either poorly absorbed and/or is secreted back into the gastrointestinal tract.
The clinical relevance of interactions between antihistamines and other common drugs has been the subject of much research following reports of fatal interactions of antihistamines such as terfenadine when coprescribed with antimicrobials such as ketoconazole [4]. The coadministration of ketoconazole with many commonly prescribed antihistamines, including loratadine and fexofenadine, has been shown to increase the plasma concentrations of the antihistamine in question [1, 5, 6]. Because fexofenadine is not metabolized by cytochrome P450 enzymes to any major extent [3, 7], increases in plasma concentration have been attributed to interactions involving transport proteins. Several of these are expressed in numerous cell types and are located throughout the body, including the intestine, liver, kidneys and blood–brain barrier. P-glycoprotein (P-gp, ABCB1) is the most extensively studied of the ATP-binding cassette (ABC) transport proteins. It is a membrane protein that acts as an efflux pump and may affect the absorption and disposition of a drug by excreting the agent from the cells where the transporter is expressed [8]. It also seems to be more readily expressed in the small intestine than the colon [9]. Therefore, inhibition of P-gp-mediated intestinal transport may result in an increased Cmax and AUC of its substrates, which would be consistent with the 164% and 135% increases in AUC and Cmax, respectively, for fexofenadine reported after coadministration of ketoconazole [1, 5]. This hypothesis is supported by previous studies where fexofenadine was shown to be a substrate for P-gp in Caco-2 and L-MDR1 cells, and its disposition was altered in knockout mice lacking the gene for mdr1a [10, 11].
Understanding of the expression of transporters and their functional activity in different human tissues is at an early stage, and there is a need for a greater appreciation of the clinical significance of interactions mediated by transporters [12]. Traditional pharmacokinetic studies have some limitations with regard to direct investigations of in vivo intestinal transport. For instance, the effect of gastrointestinal transit and luminal pH may influence the interpretation of the pharmacokinetic data. The major advantage of the intestinal perfusion technique (Loc-I-Gut®) used in this study, is that it enables the direct in vivo determination of intestinal permeability with simultaneous measurements of the plasma pharmacokinetic parameters of fexofenadine [13–17]. The absorption conditions, such as pH and the concentrations of substrate and inhibitor, can also be closely controlled. Intestinal permeability, which represents the membrane transport coefficient of the intestinal mucosa for a drug, is a major determinant of absorption and bioavailability [18]. This technique has also been used to establish a human permeability database for the new FDA guideline Biopharmaceutics Classification System (BCS) for oral immediate release products [13–19].
The aims of the present study were to investigate whether the in vivo drug–drug interaction between ketoconazole and fexofenadine is localized to efflux pumps, such as P-gp, in the small intestine, and to classify fexofenadine as a low or high-permeability drug according to the BCS.
Materials and methods
Subjects
Eight healthy volunteers (four male and four female), aged 24–38 years and weighing 57–93 kg, gave informed consent to participate in this study. All subjects underwent a full clinical examination prior to the study and all had normal clinical and laboratory values. None of the subjects received any medication other than the study drugs prior to and during the study.
The inclusion of eight volunteers in each group provides an 80% power to detect an increase of 100% in the mean Peff between the groups, assuming that the common standard deviation is 70% of the respective means.
Study design
This open, two-way, crossover, single-dose study was performed at the Clinical Research Department, University Hospital, Uppsala, Sweden and was approved by the Ethics Committee of the Medical Faculty, Uppsala University. The study was composed of two single-pass jejunal perfusion experiments, treatment 1 (T1) and treatment 2 (T2), separated by a washout period of at least 4 weeks. The same subjects participated in both treatments. Each intestinal perfusion experiment lasted for 200 min and was divided into two periods of 100 min. During T1 only the acute inhibitory effect of ketoconazole on the jejunal efflux transport of fexofenadine was investigated. During T2 the same absorption parameters for fexofenadine were investigated in the same way as in T1, but the study took place after 5 days of oral pretreatment with 200 mg ketoconazole.
Study medication
The study drugs were racemic fexofenadine HCl (Aventis Pharmaceuticals, Kansas City, MO, USA), and micronized ketoconazole (Apoteket AB, Uppsala, Sweden). A low concentration of antipyrine (Astra Läkemedel AB, Södertälje, Sweden) was used as a marker for passive transcellular diffusion in all perfusion experiments. The non-absorbable volume marker 14C-labelled polyethylene glycol 4000 (14C-PEG 4000) (2.5 µCi l−1) was purchased from Amersham Pharmacia Biotech (Little Chalfont, UK).
The perfusion solution consisted of antipyrine 10 mg l−1 (53 µm), potassium chloride 5.4 mm, sodium chloride 30 mm, mannitol 35 mm, D-glucose 10 mm and PEG 4000 1.0 g l−1, all dissolved in a 70-mm phosphate buffer (pH 6.5) with an osmolality of 290 mOsm kg−1. In both periods, the jejunal segment was perfused with this solution containing 50 mg l−1 (93 µm) fexofenadine. In period 2 of both T1 and T2, fexofenadine was administered together with 50 mg l−1 (94 µm) of ketoconazole. The only difference between T1 and T2 was that the subjects had been pretreated with ketoconazole (200 mg daily) for 5 days prior to T2.
In vivojejunal perfusion experiments
Treatment 1 (T1)
After an overnight fast of 10 h, a regional single-pass perfusion of the proximal jejunum was performed using a Loc-I-Gut® perfusion tube (Synectics Medical, Stockholm, Sweden). Having applied local anaesthesia to the throat with a lidocaine spray, the tube was introduced through the mouth. The position of the tube was checked by fluoroscopy and the perfused segment was located in the proximal part of the jejunum. Along with the Loc-I-Gut® instrument, another tube was positioned in the stomach to drain gastric juice during the experiment (Salem sump tube; Sherwood Medical, UK). Once the perfusion tube was in place, the two balloons were inflated with approximately 26–30 ml of air, creating a 10-cm long jejunal segment. A vacuum pump was connected to the proximal drainage channel of the perfusion tube to drain any intestinal fluid above the perfused segment (Ameda suction pump type 23; Ameda AG, Zug, Switzerland). The jejunal segment was then rinsed with isotonic saline (37°C) for at least 20 min. When stable perfusion conditions were achieved, the perfusion solution (37°C) was pumped into the jejunal segment at a flow rate of 2.0 ml min−1 using a calibrated syringe pump (model 355; Sage Instruments, Orion Research Inc., Cambridge, MA, USA). A more extensive description of this intestinal perfusion technique is published elsewhere [13, 20]. The perfusate leaving the jejunal segment under the single-pass perfusion was quantitatively collected on ice at 10-min intervals and immediately frozen at −20°C pending analysis. After completion of the perfusion experiment, the jejunal segment was rinsed with 120 ml isotonic saline before the Loc-I-Gut® instrument was removed. Venous blood samples were collected immediately before the perfusion experiment and again after 20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 240, 300, 360, 480 and 720 min. The blood samples were centrifuged at 3000 g for 10 min and the plasma was frozen (−20°C) awaiting analysis.
Treatment 2 (T2)
Following a washout period of at least 4 weeks, each subject was pretreated orally for 5 days with ketoconazole (200 mg daily) before undergoing the same procedure as described above. None of the subjects took the 200-mg dose of ketoconazole on the day of the jejunal perfusion experiment.
Adsorption and stability tests
The adsorption of fexofenadine and ketoconazole to the catheter was investigated by an in vitro perfusion test in a glass tube for 100 min. A stability test for fexofenadine in the perfusion solution at 37°C for 180 min was performed. Fexofenadine was also incubated at 37°C for 60 min in human jejunal fluid collected from three individuals. There was no adsorption of fexofenadine to the perfusion catheter. Incubation of fexofenadine in the perfusion solution showed no degradation, and fexofenadine was also stable when incubated with the jejunal luminal content. Approximately 50% of ketoconazole was adsorbed to the catheter tube. However, the resulting concentration of ketoconazole in the perfusate was considered to be clinically relevant [17].
Analytical methods
Perfusate
Fexofenadine in the jejunal perfusate and perfusion solution was analysed by a gradient HPLC system with mass spectrometric detection (Thermo Finnigan TSQ-700/SIM, San Jose, CA, USA) at Quintiles, Kansas City, MO, USA. Briefly, 100 µl of perfusate samples were diluted with 2% glacial acetic acid in water and the internal standard (MDL 100 907) was added before injection. The analytical column was a 2 × 30-mm 3-µm Luna C18 column (Phenomenex, Torrance, CA, USA) heated to 40°C. Mobile phase A was composed of 95% water and 5% acetonitrile with 10 mm ammonium hydroxide and 87 mm glacial acetic acid with a pH of 3.5. Mobile phase B was composed of 95% acetonitrile and 5% water with 10 mm ammonium hydroxide and 87 mm glacial acetic acid. The flow rate was 0.4 ml min−1 and the injection volume was 15 µl. The limit of quantification (LOQ) was set to 10 mg l−1 and a double standard curve (10–60 mg l−1) was prepared and assayed with each run and the intraday coefficient of variation was 12–14%.
Ketoconazole concentrations were determined by HPLC with u.v. detection (231 nm). Briefly, 20 µl of perfusate sample were injected directly onto a Nucleosil 100 C18 column (150 × 4.6 mm 5 µm; Chrompack, Sweden) at ambient temperature. The mobile phase was composed of methanol–acetonitrile–phosphate buffer (pH 6.8) (35:40:25) at a flow rate of 1 ml min−1. The LOQ was set to 0.5 mg l−1 (coefficient of variation (CV) 3.4%) and the standard curves were linear in the range of 0.5–10 mg l−1. The CV of the intra-assay variability (n = 6; quality controls containing 1.5, 3 and 7 mg l−1) ranged between 2.7% and 4.0%. The CV of the interassay variability was below 20%.
The concentrations of antipyrine in the perfusate and perfusion solution were analysed by HPLC with u.v. detection using a previously validated method at the Department of Pharmacy, Uppsala University, Uppsala, Sweden [16, 17]. The total radioactivity of 14C-PEG 4000 in the perfusion solution and the perfusate samples was determined by liquid scintillation counting (Mark III; Searle Analytic Inc., Des Plaines, IL, USA). The osmolality and pH of the perfusion solution and perfusate samples were determined using the vapour pressure method (5500 vapour pressure osmometer; Wescor Inc., Logan, UT, USA) and a pH meter (632 pH-Meter; Metrohm AG, Herisau, Switzerland), respectively.
Plasma
Fexofenadine was analysed by HPLC (pump model G1310A; Cohesive Technologies) with mass spectrometric detection (Micromass, VG Quattro 2 mass spectrometer) at Quintiles, Kansas City, USA. Multiple reaction monitoring (MRM) was used to monitor the transition ions 502.1–464.1 and 532.1–494.1 for fexofenadine and the internal standard MDL 26042, respectively. After conditioning with methanol, water and 0.1 m sodium phosphate buffer (pH 6.0), 0.5 ml plasma containing the internal standard was added to solid-phase extraction cartridges (C8 and benzenesulphonic acid copolymer; United Chemical Technologies). The analytes were eluted with 80 : 20 : 2 v/v/v dichloromethane : isopropyl alcohol : ammonium hydroxide after washing with water, 0.1 N HCl and methanol. Samples (10 µl) were then injected onto a Hypersil BDS C8 column (1 × 50 mm, 3 µm packing; Keystone Scientific) held at ambient temperature. The mobile phase consisted of 60 : 40 v/v acetonitrile : 12 mm ammonium acetate (pH 5.0) at a flow rate of 0.05 ml min−1. The LOQ was 1 ng ml−1 (CV 10.7%) and the standard curves were linear over the range 1–150 ng ml−1. The intra-assay coefficients of variation (n = 21–24; quality controls containing 2.5 and 75 ng ml−1) ranged between 2.4% and 13.0%. The interassay coefficients of variation were 6.0–10.7%.
Data analysis
Perfusate data
All calculations from the two single-pass perfusion experiments were made from steady-state concentrations in the outlet jejunal perfusate in period 1 and period 2, respectively. Each data point represents the mean concentration of the aliquots collected for each 10-min interval (0–200 min). The net water flux (NWF, ml h−1 cm−1) in the isolated jejunal segment was calculated according to equation 1:
where PEGin and PEGout are the concentrations of 14C-PEG 4000 (dpm ml−1) entering and leaving the segment, respectively, Qin (ml min−1) is the flow rate of the perfusion solution entering the segment, and L is the length of the perfused segment (10 cm). The concentration of each compound in the perfusate leaving the intestine was corrected for water flux before the fraction of drug being absorbed in the segment (fabs) and the permeability were calculated.
As the study drugs did not bind to the perfusion tube and were stable in the perfusion solution, the amount that disappeared during the single passage through the jejunal segment was assumed to have been absorbed. The fraction of drug being absorbed in the segment during the perfusion (fabs) was calculated from equation 2:
where Cin and Cout are the concentrations entering and leaving the jejunal segment, respectively.
The effective jejunal permeability (Peff) of each drug was calculated according to a well-mixed tank model as shown in equation 3 [21]:
where the cylinder area (2πrL) of the jejunal segment was calculated using the intestinal radius (r = 1.75 cm) and length (L = 10 cm) of the segment. We have measured the human jejunal radius to be 1.75 cm by perfusing the jejunal segment with barium followed by X-ray analysis (Knutson et al. personal communication). Peff, which is calculated from the disappearance of drug from the jejunal segment, is a local absorption rate coefficient that is valid regardless of the mechanisms involved in drug transport across the human epithelium [18].
Pharmacokinetic data
The area under the plasma concentration–time curve from 0 to 100 (AUC100) or 720 min (AUC720) for each subject was calculated using the linear and the logarithmic trapezoidal rules for ascending and descending plasma concentrations, respectively. There was no estimation of any residual area, because the plasma concentration of fexofenadine was below LOQ at the last sampling time (720 min).
Statistical analysis
The effect of ketoconazole on Peff and fabs of fexofenadine and antipyrine between the four perfusion periods (0–100 min and 100–200 min in both T1 and T2) was evaluated using repeated-measures anova with Bonferroni post-test (GraphPad Prism version 3.0; GraphPad Software, San Diego, CA, USA). The effect of ketoconazole on AUC of fexofenadine between T1 and T2 was evaluated using Student's t-test for paired data. Differences between mean values were considered significant at P < 0.05. Throughout the paper all data are expressed as mean ± s.d.). Ninety-five percent confidence intervals on differences (95% CI) are presented where appropriate.
Results
Intestinal perfusion
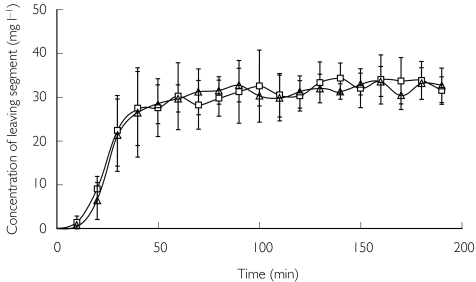
The absorption parameters Peff and fabs were determined during steady-state perfusion conditions, which were attained after 60 and 160 min in periods 1 and 2, respectively, in both T1 and T2 (Figure 1).
Figure 1.
The mean (± s.d.) concentration of fexofenadine in perfusate leaving the jejunal segment during a 200-min perfusion in T1 (□) and T2 (▵), showing that steady-state conditions were attained after 60 and 160 min, respectively, in each period.
Treatment 1 (T1)
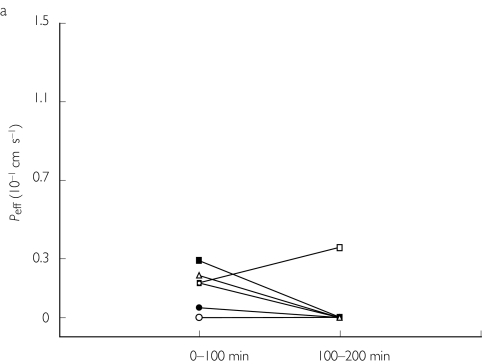
The jejunal Peff of fexofenadine was low and variable (0.11 ± 0.11 and 0.04 ± 0.13·10−4 cm s−1 in periods 1 and 2, respectively) (Table 1 and Figure 2a). The high intersubject variability in this absorption parameter is commonly seen for drugs with incomplete absorption and low bioavailability [22]. The mean fabs for fexofenadine was 3 ± 3% and 1 ± 3% in periods 1 and 2, respectively (Table 1). There was no significant change in Peff (95% CI −0.37, 0.51) or fabs (95% CI −8, 12) for fexofenadine when it was perfused together with ketoconazole in period 2 (Tables 1, 2 and Figure 2a).
Table 1.
Absorption and other pharmacokinetic parameters of fexofenadine (mean ± s.d.) administered alone (0–100 min) or with ketoconazole (100–200 min) before (treatment 1) and after 5 days pretreatment (treatment 2) with ketoconazole.
| Treatment 1 | Treatment 2 | |||
|---|---|---|---|---|
| 0–100 min | 100–200 min | 0–100 min | 100–200 min | |
| Peff (10−4 cm s−1) | 0.11 ± 0.11 | 0.04 ± 0.13 | 0.29 ± 0.47 | 0.22 ± 0.31 |
| fabs (%) | 3 ± 3 | 1 ± 3 | 7 ± 10 | 6 ± 8 |
| AUC100 (ng ml−1 min) | 55 ± 101 | 51 ± 33 | ||
| AUC720 (ng ml−1 min) | 318 ± 426 | 426 ± 232 |
No significant differences were observed. Peff, Effective jejunal permeability; fabs, the fraction of drug being absorbed in the segment; AUC100, AUC720, area under the plasma concentration–time curve after 100 and 720 min, respectively.
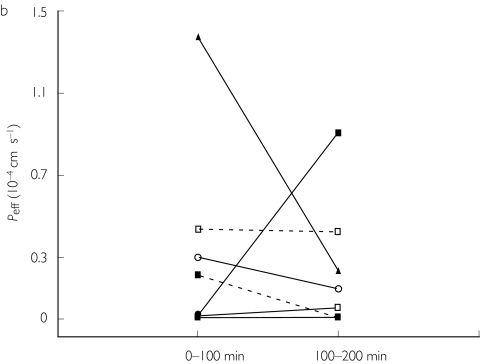
Figure 2.
Individual values of the human jejunal effective permeability (Peff) of fexofenadine at a luminal concentration of 50 mg l−1 administered alone (0–100 min), or with 50 mg l−1 ketoconazole (100–200 min) before (a) and after 5 days pretreatment with ketoconazole (b).
Table 2.
95% confidence intervals (95% CI) on differences for the absorption and other pharmacokinetic parameters of fexofenadine.
| T1 0–100 | T1 0–100 | T1 0–100 | T1 100–200 | T1 100–200 | T2 0–100 | |
|---|---|---|---|---|---|---|
| vs | vs | vs | vs | vs | vs | |
| T1 100–200 | T2 0–100 | T2 100–200 | T2 0–100 | T2 100–200 | T2 100–200 | |
| Peff (10−4 cm s−1) | (−0.37, 0.51) | (−0.62, 0.27) | (−0.54, 0.34) | (−0.69, 0.20) | (−0.61, 0.27) | (−0.37, 0.51) |
| fabs (%) | (−8, 12) | (−14, 7) | (−13, 8) | (−16, 5) | (−15, 6] | (−9, 11) |
| AUC100 (ng ml−1 min) | (−108, 115) | |||||
| AUC720 (ng ml−1 min) | (−622, 405) |
No significant differences were observed. Peff, Effective jejunal permeability; fabs, the fraction of drug being absorbed in the segment; AUC100, AUC720, area under the plasma concentration–time curve after 100 and 720 min, respectively.
The Peff and fabs of antipyrine were similar in both periods (Tables 3, 4). The mean steady-state recovery of the non-absorbable volume marker 14C-PEG 4000 was 99 ± 12% and 87 ± 10% in periods 1 and 2, respectively (Table 3), which agreed with our earlier studies [13–17]. The concentration of ketoconazole in the perfusion solution entering the segment was between 47 and 106 µm, but there was no correlation between individual values of ketoconazole perfusate concentration and Peff for fexofenadine (data not shown) in either T1 or T2. Mean values of the net water flux (NWF) in the isolated jejunal segment, flow rate leaving the segment (Qout), pH and osmolality in the outlet jejunal perfusate for each period are reported in Table 3.
Table 3.
Mean (± s.d.) values of the absorption markers and the other perfusion parameters after treatments 1 and 2.
| Treatment 1 | Treatment 2 | |||
|---|---|---|---|---|
| 0–100 min | 100–200 min | 0–100 min | 100–200 min | |
| Peff antipyrine (10−4 cm s−1) | 3.28 ± 2.44 | 2.24 ± 1.12 | 6.37 ± 3.53* | 5.61 ± 4.29† |
| fabs antipyrine (%) | 46 ± 16 | 36 ± 12 | 59 ± 12* | 56 ± 21† |
| PEG 4000rec (%) | 99 ± 12 | 87 ± 10 | 93 ± 19 | 110 ± 18 |
| NWF (ml h−1 cm−1) | 1.77 ± 0.58 | 2.21 ± 0.51 | 2.27 ± 0.97 | 1.44 ± 1.16 |
| Qout (ml min−1) | 2.35 ± 0.31 | 2.17 ± 0.23 | 2.29 ± 0.36 | 2.48 ± 0.29 |
| pH | 6.57 ± 0.11 | 6.69 ± 0.17 | 6.58 ± 0.08 | 6.66 ± 0.25 |
| Osmolality (mOsm kg−1) | 280 ± 8 | 260 ± 14 | 266 ± 17 | 277 ± 11 |
Peff, Effective jejunal permeability; fabs, the fraction of drug being absorbed in the segment; PEG 4000rec, recovery of 14C-labelled PEG 4000; NWF, net water flux; Qout, flow rate out of the segment.
Significantly different from treatment 1 100–200 min (P < 0.01).
Significantly different from treatment 1 100–200 min (P < 0.05).
Table 4.
95% confidence intervals (95% CI) on differences for the absorption parameters of antipyrine.
| T1 0–100 | T1 0–100 | T1 0–100 | T1 100–200 | T1 100–200 | T2 0–100 | |
|---|---|---|---|---|---|---|
| vs | vs | vs | vs | vs | vs | |
| T1 100–200 | T2 0–100 | T2 100–200 | T2 0–100 | T2 100–200 | T2 100–200 | |
| Peff (10−4 cm s−1) | (−2.17, 4.26) | (−6.31, 0.13) | (−5.55, 0.88) | (−7.35, −0.92) | (−6.59, −0.16) | (−2.46, 3.97) |
| fabs (%) | (−7, 26) | (−31, 3) | (−27, 6) | (−40, −7) | (−37, −3) | (−13, 20) |
Treatment 2 (T2)
During T2, the inhibitory effect of repeated oral administration of ketoconazole was investigated in the same eight individuals. The mean Peff was 0.29 ± 0.47 and 0.22 ± 0.31·10−4 cm s−1 in periods 1 and 2, respectively (Table 1). The mean fabs was 7 ± 10% and 6 ± 8% in periods 1 and 2, respectively (Table 1). The Peff of fexofenadine from each subject is shown in Figure 2b. The increase in Peff and fabs for fexofenadine after 5 days pretreatment with ketoconazole was not statistically significant (Tables 1, 2).
The Peff and fabs of antipyrine increased significantly in T2 compared with T1 (Tables 3, 4). The mean steady-state recovery of 14C-PEG 4000 was 93 ± 19% and 110 ± 18% in periods 1 and 2, respectively (Table 3). The values of all other absorption variables are also shown in Table 3.
Plasma pharmacokinetic data
There was no difference in AUC for fexofenadine in seven subjects between T1 and T2, (as shown in Tables 1, 2) (subject 8 was excluded due to plasma concentrations below LOQ). Mean AUC100 was 55 ± 101 and 51 ± 33 ng ml−1 min (95% CI −108, 115) in T1 and T2, respectively. Mean AUC720 was 318 ± 426 and 426 ± 232 ng ml−1 min in T1 and T2, respectively, but the observed trend towards an increase in AUC was not significant (95% CI −622, 405).
Discussion
The main purpose of this human in vivo jejunal perfusion study was to investigate whether ketoconazole increases the in vivo intestinal absorption of fexofenadine via an interaction at an intestinal efflux pump, such as P-gp. This would provide a mechanistic explanation for the 164% increased plasma AUC of fexofenadine when coadministered with ketoconazole [1, 5]. We have previously shown that ketoconazole is a potent inhibitor of intestinal CYP3A4 at this dose in the Loc-I-Gut perfusion model [17]. However, ketoconazole has also been suggested to inhibit P-gp [23–27]. Hence, an expected acute effect of ketoconazole when added to the jejunal perfusate would have been an increase in jejunal Peff and plasma AUC of fexofenadine. The lack of effect of ketoconazole in this study was somewhat surprising, as previous reports, ranging from in vitro experiments to human in vivo studies, reported that fexofenadine is readily transported by P-gp [7, 10, 11, 28]. However, none of the data in these reports has been validated against a mechanistic in vivo human model such as this jejunal perfusion technique [12]. There are several possible explanations for the absence of effects after acute and/or repeated administration of ketoconazole on fexofenadine absorption found in this perfusion study: (i) fexofenadine is not efficiently transported by intestinal P-gp in vivo; (ii) the intestinal absorption of fexofenadine is complex and involves both absorptive and efflux proteins; (iii) ketoconazole is not very potent as a P-gp inhibitor; and/or (iv) ketoconazole and fexofenadine may act on different binding sites of P-gp.
Assuming that ketoconazole inhibits intestinal P-gp at the perfusate concentration used in this study, our results suggest that P-gp does not limit the intestinal transport of fexofenadine in vivo. This hypothesis is supported by Drescher et al., who recently showed that polymorphism of the MDR1 gene did not explain the high variability in the plasma concentrations of fexofenadine [29]. They also concluded that mechanisms other than P-gp involvement are likely to affect the pharmacokinetics of fexofenadine. For instance, intestinal transport of fexofenadine may be mediated by several transport proteins that, along with passive diffusion, work in the absorptive and secretory direction simultaneously. It has been shown in vitro that fexofenadine is a substrate for members of the organic anion transporting polypeptide family (OATP) [10]. The human OATP family consists of transport proteins that work in the uptake direction in several organs such as the liver and the kidneys. It was recently shown that some forms of OATP were expressed in the small and large intestine [30]. Inhibition of any OATP has also been suggested as the mechanism underlying the decreased absorption of fexofenadine in humans seen after concomitant intake of grapefruit, orange or apple juice [31]. Ketoconazole has been shown to be an inhibitor of OATP in vitro[10], which suggests that absorptive transporters may contribute to the total mucosal transport of fexofenadine and therefore emphasizes the need for more studies to better understand their role in drug absorption.
The lack of effect on the Peff for fexofenadine in this study may also be a consequence of ketoconazole being only a weak inhibitor of P-gp in vivo. The reported in vitro IC50 values for ketoconazole inhibition of P-gp-mediated transport range from 2 to 120 µm[26, 27]. Moreover, ketoconazole seems to be a more potent inhibitor of cytochrome P450 enzymes than of P-gp-mediated transport [32, 33]. This difference in potency has also been observed for verapamil using the present in vivo perfusion technique. Thus, the Peff of verapamil, which is a P-gp substrate, was unaffected by the addition of ketoconazole, whereas its CYP3A4 metabolism to norverapamil was inhibited [17]. Another plausible hypothesis to explain the results is that ketoconazole and fexofenadine act at two different sites on the intestinal P-gp, as it has been reported that multiple binding sites exist [34].
The jejunal transport of fexofenadine was not significantly affected by pretreatment with ketoconazole for 5 days. However, the permeability of antipyrine was increased in both periods during T2. Because antipyrine is a marker for passive transcellular diffusion [35], the results indicate that membrane fluidity, which is important for passive diffusion of drugs, was affected. This is supported by earlier studies that demonstrated that ketoconazole inhibits ergosterol synthesis, resulting in lower cholesterol content within the membrane with the consequent increased fluidity [36].
Finally, our results show that the intestinal Peff of fexofenadine in humans was low when administered alone. This is consistent with the low transport rate reported from in vitro experiments [10]. In addition, the physicochemical properties of fexofenadine (polar surface area (PSA) = 0.124 Å2) predict low passive diffusion [37, 38]. Taken together, these data suggest that the high recovery of fexofenadine in faeces following oral administration is probably due to incomplete intestinal absorption, which is in accordance with a bioavailability estimated at 30%[3, 10]. Therefore, fexofenadine can be classified as a low-permeability drug according to the BCS [18].
In conclusion, we did not find a significant acute effect of ketoconazole on the intestinal Peff of fexofenadine using an in vivo jejunal perfusion technique. The results also indicated that pretreatment with ketoconazole did not influence Peff. Therefore, our data do not support earlier reports that P-gp may play an important role in the intestinal absorption of fexofenadine in vivo. However, further studies are needed to understand fully the mechanism underlying the interaction between fexofenadine and ketoconazole. Finally, according to the Biopharmaceutical Classification System (BCS), fexofenadine was classified as a low-permeability drug.
Acknowledgments
We thank Aventis Pharmaceuticals, Kansas City, USA for their financial support of this study.
References
- 1.Simpson K, Jarvis B. Fexofenadine: a review of its use in the management of seasonal allergic rhinitis and chronic idiopathic urticaria. Drugs. 2000;59:301–321. doi: 10.2165/00003495-200059020-00020. [DOI] [PubMed] [Google Scholar]
- 2.Robbins DK, Castles MA, Pack DJ, Bhargava VO, Weir S. Dose proportionality and comparison of single and multiple dose pharmacokinetics of fexofenadine (MDL 16455) and its enantiomers in healthy male volunteers. Biopharm Drug Dispos. 1998;19:455–463. doi: 10.1002/(sici)1099-081x(199810)19:7<455::aid-bdd130>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Lippert C, Ling J, Brown P, et al. Mass balance and pharmacokinetics of MDL 16,455A in healthy male volunteers. Pharm Res. 1996;12:S390. [Google Scholar]
- 4.Honig PK, Wortham DC, Zamani K, Conner DP, Mullin JC, Cantilena LR. Terfenadine–ketoconazole interaction: pharmacokinetic and electrocardiographic consequenses. JAMA. 1993;269:1513–1518. [PubMed] [Google Scholar]
- 5.Davit B, Reynolds K, Yuan R, et al. FDA evaluations using in vitro metabolism to predict and interpret in vivo metabolic drug–drug interactions: impact on labeling. J Clin Pharmacol. 1999;39:899–910. doi: 10.1177/00912709922008515. [DOI] [PubMed] [Google Scholar]
- 6.Kosoglou T, Salfi M, Lim JM, Batra VK, Cayen MN, Affrime MB. Evaluation of the pharmacokinetics and electrocardiographic pharmacodynamics of loratadine with concomitant administration of ketoconazole or cimetidine. Br J Clin Pharmacol. 2000;50:581–589. doi: 10.1046/j.1365-2125.2000.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamman MA, Bruce MA, Haehner-Daniels BD, Hall SD. The effect of rifampin administration on the disposition of fexofenadine. Clin Pharmacol Ther. 2001;69:114–121. doi: 10.1067/mcp.2001.113697. [DOI] [PubMed] [Google Scholar]
- 8.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Sakeda T, Ohmoto N, et al. Real-time quantitative polymerase chain reaction for MDR1, MRP1, MRP2, and CYP3A-mRNA levels in Caco-2 cell lines, human duodenal enterocytes, normal colorectal tissues, and colorectal adenocarcinomas. Drug Metab Dispos. 2002;30:4–6. doi: 10.1124/dmd.30.1.4. [DOI] [PubMed] [Google Scholar]
- 10.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–871. [PubMed] [Google Scholar]
- 11.Soldner A, Christians U, Susanto M, Wacher VJ, Silverman JA, Benet LZ. Grapefruit juice activates P-glycoprotein-mediated drug transport. Pharm Res. 1999;16:478–485. doi: 10.1023/a:1011902625609. [DOI] [PubMed] [Google Scholar]
- 12.Tucker GT, Houston JB, Huang SM. Optimizing drug development: strategies to assess drug metabolism/transporter interaction potential—towards a consensus. Br J Clin Pharmacol. 2001;52:107–117. doi: 10.1046/j.0306-5251.2001.temp.1441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lennernäs H, Ahrenstedt Ö, Hällgren R, Knutsson L, Ryde M, Paalzow L. Regional jejunal perfusion, a new in vivo approach to study oral drug absorption in man. Pharm Res. 1992;9:1243–1251. doi: 10.1023/a:1015888813741. [DOI] [PubMed] [Google Scholar]
- 14.Lennernäs H. Human intestinal permeability. J Pharm Sci. 1998;87:403–410. doi: 10.1021/js970332a. [DOI] [PubMed] [Google Scholar]
- 15.Lennernäs H. Human jejunal effective permeability and its correlation with preclinical drug absorption models. J Pharm Pharmacol. 1997;49:627–638. doi: 10.1111/j.2042-7158.1997.tb06084.x. [DOI] [PubMed] [Google Scholar]
- 16.Lindahl A, Sandström R, Ungell A-L, et al. Jejunal permeability and hepatic extraction of fluvastatin in humans. Clin Pharmacol Ther. 1996;60:493–503. doi: 10.1016/S0009-9236(96)90145-9. [DOI] [PubMed] [Google Scholar]
- 17.Sandström R, Knutson TW, Knutson L, Jansson B, Lennernäs H. The effect of ketoconazole on the jejunal permeability and CYP3A metabolism of (R/S)-verapamil in humans. Br J Clin Pharmacol. 1999;48:180–189. doi: 10.1046/j.1365-2125.1999.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amidon GL, Lennernäs HL, Shah VP, Crison J. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 19.Food and Drug Administration. 2000 Guidance for Industry: Waiver of in Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System: http://www.fda.gov/cder/guidance/3618fnl.htm.
- 20.Knutson L, Odlind B, Hallgren R. A new technique for segmental jejunal perfusion in man. Am J Gastroenterol. 1989;84:1278–1284. [PubMed] [Google Scholar]
- 21.Lennernäs H, Lee I-D, Fagerholm U, Amidon GL. A residence-time distribution analysis of the hydrodynamics within the intestine in man during a regional single-pass perfusion with Loc-I-Gut: in vivo permeability estimation. J Pharm Pharmacol. 1997;49:682–686. doi: 10.1111/j.2042-7158.1997.tb06092.x. [DOI] [PubMed] [Google Scholar]
- 22.Hellriegel ET, Bjornsson TD, Hauck WW. Interpatient variability in bioavailability is related to the extent of absorption: implications for bioavailability and bioequivalence studies. Clin Pharmacol Ther. 1996;60:601–607. doi: 10.1016/S0009-9236(96)90208-8. [DOI] [PubMed] [Google Scholar]
- 23.Siegsmund MJ, Cardarelli C, Aksentijevich I, Sugimoto Y, Pastan I, Gottesman MM. Ketoconazole effectively reverses multidrug resistance in highly resistant KB cells. J Urol. 1994;151:485–491. doi: 10.1016/s0022-5347(17)34999-6. [DOI] [PubMed] [Google Scholar]
- 24.Kim RB, Wandel C, Leake B, et al. Interrelationship between substrates and inhibitors of human CYP3A and P-glycoprotein. Pharm Res. 1999;16:408–414. doi: 10.1023/a:1018877803319. [DOI] [PubMed] [Google Scholar]
- 25.Takano M, Hasegawa R, Fukuda T, Yumoto R, Nagai J, Murakami T. Interaction with P-glycoprotein and transport of erythromycin, midazolam and ketoconazole in Caco-2 cells. Eur J Pharmacol. 1998;358:289–294. doi: 10.1016/s0014-2999(98)00607-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Hsieh Y, Izumi T, Lin ET, Benet LZ. Effects of ketoconazole on the intestinal metabolism, transport and oral bioavailability of K02, a novel vinylsulfone peptidomimetic cysteine protease inhibitor and a P450 3A, P-glycoprotein dual substrate, in male Sprague–Dawley rats. J Pharmacol Exp Ther. 1998;287:246–252. [PubMed] [Google Scholar]
- 27.Woodland C, Ito S, Koren G. A model for the prediction of digoxin drug interactions at the renal tubular cell level. Ther Drug Monit. 1998;20:134–138. doi: 10.1097/00007691-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Kim RB, Leake BF, Choo EF, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–199. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 29.Drescher S, Schaeffeler E, Hitzl M, et al. MDR1 gene polymorphisms and disposition of the P-glycoprotein substrate fexofenadine. Br J Clin Pharmacol. 2002;53:526–534. doi: 10.1046/j.1365-2125.2002.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamai I, Nezu J-I, Uchino H, et al. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–260. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- 31.Dresser GK, Bailey DG, Leake BF. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 32.Gibbs MA, Thummel KE, Shen DD, Kunze KL. Inhibition of cytochrome P-450 (CYP3A) in human intestinal and liver microsomes. Comparison of Ki values and impact of CYP3A5 expression. Drug Metab Dispos. 1999;27:180–187. [PubMed] [Google Scholar]
- 33.von Moltke LL, Greenblatt DJ, Duan SX, Harmatz JS, Shader RI. In vitro prediction of the terfenadine–ketoconazole pharmacokinetic interaction. J Clin Pharmacol. 1994;34:1222–1227. doi: 10.1002/j.1552-4604.1994.tb04735.x. [DOI] [PubMed] [Google Scholar]
- 34.Martin C, Berridge G, Higgins CF, Mistry P, Charlton P, Callaghan R. Communication between multiple drug binding sites on P-glycoprotein. Mol Pharmacol. 2000;58:624–632. doi: 10.1124/mol.58.3.624. [DOI] [PubMed] [Google Scholar]
- 35.Hilgendorf C, Spahn-Langguth H, Regardh CG, Lipka E, Amidon GL, Langguth P. Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J Pharm Sci. 2000;89:63–75. doi: 10.1002/(SICI)1520-6017(200001)89:1<63::AID-JPS7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Van den Bossche H, Willemsens G, Cools W, Cornelissen F, Lauwers WF, Van Cutsem JM. In vitro and in vivo effects of the antimycotic drug ketoconazole on sterol synthesis. Antimicrob Agents Chemother. 1980;17:922–928. doi: 10.1128/aac.17.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winiwarter S, Bonham NM, Ax F, Hallberg A, Lennernäs H, Karlén A. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J Med Chem. 1998;41:4939–4949. doi: 10.1021/jm9810102. [DOI] [PubMed] [Google Scholar]
- 38.Palm K, Stenberg P, Luthman K, Artursson P. Polar molecular surface properties predict the intestinal absorption of drugs in humans. Pharm Res. 1997;14:568–571. doi: 10.1023/a:1012188625088. [DOI] [PubMed] [Google Scholar]