Abstract
Aims
To study the relationship between the pharmacokinetics (PK) of gliclazide and its long-term pharmacodynamic (PD) effect in a large population of Type 2 diabetic patients and to identify factors predicting intersubject variability.
Methods
A PKPD database of 634 Type 2 diabetic patients with a total of 5258 fasting plasma glucose (FPG) samples was built up from the data collected during the clinical development of a modified release formulation of gliclazide (gliclazide MR). The PKPD analysis used a nonlinear mixed effect modelling approach. A mixture model was used to identify patients with a FPG response to treatment. In patients identified as responders, the decrease in FPG was related to gliclazide exposure (AUC) by an Emax relationship. An effect compartment was used to describe the link between PK and PD. A linear disease-progression model was used to assess the glycaemic deterioration observable over several months of treatment. Simulations were performed to evaluate the predictive performance of the PKPD model and to illustrate the time course of the antidiabetic effect of gliclazide MR.
Results
Disease state was found to be the main explanatory factor for intersubject variability in response to gliclazide. The percentage of responders to gliclazide, used as monotherapy, increased inversely to the number of classes of antidiabetic agents received prior to entry in the studies. In responders, the initial dose (30 mg) of the gliclazide MR dosing regimen induced half of the maximum hypoglycaemic effect. The equilibration half-life between the PK and PD steady states was 3 weeks (intersubject variability of 84%). The rate of disease progression was 0.84 mmol l−1 year−1 (intersubject variability 143%). The PKPD model adequately predicted the FPG profiles of 234 patients who received the current formulation of gliclazide. Simulation of a 1-year parallel dose ranging clinical trial illustrated the influence of dose, time and type of previous antidiabetic treatment on the percentage of patients with clinically significant improvement of blood glucose control.
Conclusions
This population PKPD analysis has characterized the relationship between the exposure to gliclazide and its long-term hypoglycaemic effect, and has established that the intersubject variability in response is mostly related to disease state. These results underline the clinical interest of quickly increasing the dose of gliclazide MR according to the response to treatment in order to achieve effective blood glucose control.
Keywords: disease progression, gliclazide, gliclazide MR, hypoglycaemic effect, mixture model, NONMEM, population PKPD modelling
Introduction
Gliclazide is a second-generation sulphonylurea that is used in the treatment of Type 2 diabetes. Its effectiveness and safety are well known [1, 2]. Gliclazide improves defective insulin secretion by interacting with specific receptors on pancreatic β-cells. This stimulation of insulin secretion leads to a gradual improvement of glycaemic control [3].
The clinical development of a modified release formulation of gliclazide (gliclazide MR) provided the opportunity to study the relationship between the pharmacokinetics (PK) of gliclazide and its long-term pharmacodynamic (PD) effect. This formulation has modified release characteristics in order to allow a once-daily dosing and to better match release of active principle to the known circadian variations in glycaemia seen in Type 2 diabetes. The clinical development programme included a phase II parallel dose ranging study, a phase II dose-escalation study and two phase III confirmatory therapeutic equivalence studies. PK and PD data were collected during these phase II/III trials and a population PKPD analysis was performed. The results of the population PK analysis will be fully published elsewhere [4].
Methods
Clinical studies
Two phase II clinical studies were performed to select the best dose-range regimen of gliclazide MR: a parallel dose-ranging study and a dose-escalation study. The effectiveness of the selected dose range was then compared in two phase III trials with the currently marketed formulation of gliclazide in 19 countries [5]. Only patients who had Type 2 diabetes defined by WHO criteria [6] were included in these studies. The study protocols were approved by the ethics committees of the centres in which the studies were carried out and written informed consent to participate was obtained from each patient prior to enrolment.
Study designs
Phase II studies
The two phase II studies were 10-week clinical trials composed of a 2-week washout period and a 8-week double-blind treatment period. In the parallel dose ranging study, 224 patients were randomly divided into six parallel groups. Each group received placebo or one of the following doses of gliclazide MR: 15, 30, 60, 90 and 135 mg once daily. In the dose-escalation study, the washout period was followed by two successive treatment periods of 4 weeks. Fifty patients were randomly divided into three parallel groups and each group initially received placebo or gliclazide MR (15 or 30 mg) once daily. At the end of the first treatment period, if fasting plasma glucose (FPG) level was < 7.8 mmol l−1, the patients continued on the same dose for the last 4 weeks. If the mean of the two FPG levels was ≥ 7.8 mmol l−1, the dose was doubled for the last 4 weeks. The patients of the placebo group continued to receive placebo.
Phase III studies
The two phase III studies (study A and study B) were comparative trials of gliclazide MR with the currently marketed formulation of gliclazide in different countries. At the end of a washout period of 2 weeks, 1464 patients (800 in study A and 664 in study B) were randomly assigned to receive gliclazide MR or the current formulation. Both groups were treated for 1 year, including three periods: a titration period of 4 months, a maintenance period of 6 months and a follow-up period of 2 months. At the beginning of the titration period, the patients started with one tablet of 80 mg of the current formulation or one tablet of 30 mg of gliclazide MR. The dosage was then increased every 4 weeks by steps of one tablet until the patients were well controlled (i.e. FPG between 4.4 mmol l−1 and 6.6 mmol l−1 for patients less than 65 years old or between 5.5 mmol l−1 and 7.7 mmol l−1 for patients 65 years or older) or had reached the maximum currently prescribed dose (320 mg for the current formulation or 120 mg for gliclazide MR). Doses of gliclazide MR were administered once daily and doses of the current formulation> 80 mg were administered twice a day. At the end of the titration period, the patients entered the maintenance period for 6 months with the lowest dose having led to the best glycaemic control. Finally, all patients entered into a single-blind follow-up period and received gliclazide MR for 2 months. The patients who received 80, 160, 240 or 320 mg of the current formulation were switched to 30, 60, 90 or 120 mg of gliclazide MR, respectively (the difference in dose between the current and the new formulations are mainly due to a difference in bioavailability).
Pharmacokinetic assessment
A sparse sampling design was applied on the last day of treatment in the phase II parallel dose ranging and in the phase III studies. Three blood samples were drawn per patient: just before the last dose (Cmin), 2 h after dosing, and at any convenient time between 4 h and 12 h after dosing. In order to provide enough information for discriminating among pharmacokinetic models, a full PK sampling design was applied in the phase II dose-escalation study. Five blood samples were drawn per patient on three occasions: on the first day, at the end of the first 4-week period, and at the end of the study. Gliclazide plasma concentrations were measured using high-performance liquid chromatography with ultraviolet detection. The lowest concentration giving accuracy and precision within a limit of 20% was 50 ng ml−1. This value was taken as the limit of quantification.
Pharmacodynamic assessment
The primary efficacy endpoints in the phase II/III studies were glycohaemoglobin (HbA1c) and FPG values at the end of the study. Although HbA1c is the most widely accepted measure of overall long-term blood glucose control, FPG was selected to reflect the antidiabetic effectiveness in this PKPD analysis because, unlike HbA1c, FPG changes occur over short periods of time. In the phase II parallel dose-ranging study, FPG was measured before the inclusion visit, and after 1, 4 and 8 weeks of treatment. In the phase II dose-escalation study, FPG was measured before the inclusion visit and every week until the end of the 8-week treatment period. In phase III, FPG was measured before the inclusion visit, every month during the 4-month titration period, every 2 months during the 6-month maintenance period, and every month during the 2-month follow-up period. Plasma glucose measurements were centrally analysed using a glucose oxidase method (Kodak Zektachem Clinical Chemistry Slide). The intra-assay coefficient of variation varied between 1.4% and 1.8%.
Patients
A total of 1007 patients were included in the gliclazide MR PK database. In this database, 373 patients received gliclazide MR only during the last 2 months of the phase III studies (follow-up period). These patients were not kept for the PKPD analysis and then a total of 634 patients were included in the gliclazide MR PKPD database. At the end of the phase III studies, the patient distribution per group of dose of gliclazide MR was well balanced. The main demographic and biochemical characteristics of the PKPD database are given in Table 1.
Table 1.
Demographics and biochemical characteristics of patients of the PKPD database and the simulation database.
| PKPD database | Simulation database | ||||||
|---|---|---|---|---|---|---|---|
| Phase II studies | Phase III studies Glicazide MR vs. glicazide | Phase III studies Glicazide MR vs. glicazide | |||||
| Dose ranging | Dose increase | Study A | Study B | Study A | Total on PKPD database | Study A | |
| Number of patients | 176 | 50 | 242 | 166 | 634 | 232 | |
| Age (years) | 61 ± 10 | 60 ± 8 | 61 ± 10 | 61 ± 10 | 61 ± 10 | 62 ± 10 | |
| Weight (kg) | 77 ± 11 | 85 ± 9 | 79 ± 12 | 81 ± 14 | 80 ± 12 | 79 ± 12 | |
| BMI (kg/m2) | 28 ± 3 | 28 ± 2 | 28 ± 4 | 28 ± 4 | 28 ± 3 | 29 ± 3 | |
| Creatinine clearance(ml min−1) | 92 ± 25 | 122 ± 27 | 102 ± 28 | 103 ± 33 | 102 ± 30 | 101 ± 29 | |
| Gender | |||||||
| Male | 94 | 35 | 134 | 102 | 365 | 132 | |
| Female | 82 | 15 | 108 | 64 | 269 | 100 | |
| Race | |||||||
| Caucasian | 176 | 473 | 240 | 147 | 610 | 230 | |
| Others | 0 | 3 | 3 | 19 | 24 | 2 | |
| HbA1c (%) | 8.1 ± 1.8 | 7.6 ± 1.0 | 8.1 ± 1.0 | 8.2 ± 1.0 | 8.1 ± 1.3 | 8.3 ± 1.0 | |
| FPG (mmol l−1) | 11.4 ± 2.5 | 11.8 ± 2.4 | 10.6 ± 2.1 | 10.8 ± 2.1 | 11.0 ± 2.3 | 10.8 ± 2.0 | |
| Diabetes duration (years) | 6.9 ± 6.6 | 8.6 ± 9.0 | 6.3 ± 5.6 | 5.2 ± 5.0 | 6.3 ± 6.2 | 6.8 ± 5.9 | |
| Previous treatment | |||||||
| Diet alone | 30 | 1 | 56 | 38 | 125 | 51 | |
| 1 OHA class | 122 | 38 | 152 | 108 | 420 | 158 | |
| 2 OHA classes | 24 | 11 | 34 | 20 | 89 | 23 | |
OHA, oral hypoglycaemic agent; BMI, body mass index; FPG, fasting plasma glucose.
Population analyses
The population PK and PKPD analyses were performed using NONMEM [7]. Model selection between hierarchical models is performed by the likelihood ratio test. A significance level of 0.05 was used to distinguish between models. Individual PK and PKPD parameters were obtained from the population parameters with the Bayesian post hoc option. The procedure used to identify explanatory factors (covariates) for intersubject variability in the parameters was conducted as follows [8]. In a first step, the basic population model, composed of the structural model and the random effects models without any covariates, was built. In a second step, the empirical Bayes’ estimates of individual parameters were regressed on the potential covariates using a generalized additive model (GAM). In a third step the relationships found in the GAM were tested in NONMEM and were finally added to the model if they improved the fit as judged by the likelihood ratio criterion. The tested covariates were age, sex, weight, body mass index (BMI), creatinine clearance, type of previous antidiabetic treatment, known diabetes duration, diabetes history and diabetes complications. Weight, BMI and creatinine clearance for a given patient were the mean of his/her covariates collected during the course of the study.
PK modelling
Compartment models were fitted to the gliclazide concentration time data. The parameters of distribution and elimination processes were measured in terms of clearances and volumes of distribution. The parameters for the absorption process were measured in terms of lag-time and rate constant of absorption. Details of this PK analysis will be published elsewhere.
PKPD modelling
A population PKPD model was used to quantify the time course of effect of gliclazide based on repeated FPG determinations. To investigate the PKPD relationship with an effect measured only once a day, it is common to use a secondary pharmacokinetic parameter such as area under the concentration–time curve (AUC) or average concentration [9, 10]. In this analysis, AUC was chosen and individual AUCs were calculated by dividing the daily dose by the total apparent clearances estimated in the PK analysis.
Drug effect model
The relationship between AUC and FPG decrease was assessed using an Emax PKPD model coupled with an effect compartment model according to the following equation:
where Et is the predicted treatment effect at time t, Emax is the maximum effect, AUC is the area under the concentration–time curve, AUC50 is the AUC which induces 50% of maximal effect and Keq is the rate constant of equilibration.
The effect compartment was used to describe the dissociation between the PK steady state of gliclazide, which is reached in approximately 3 days, and the FPG steady state, which is reached after 1 month. The delay in response was estimated by an equilibration half-life equal to ln(2)/Keq.
Disease-progression model
Type 2 diabetes mellitus is a progressive disease [11]. This feature can be observed over long-term therapy by a continuous glycaemic deterioration, especially if there is no dose adjustment during maintenance therapy [12], which was the case in the two phase III trials. Therefore, in addition to the modelling of the magnitude of the drug effect, it was possible to model disease progression. As there was no placebo group in the phase III trials, a simple disease-progression model with a constant rate of change was used [13] according to the following equation: St=α × t, where St is the predicted disease progression at time t and α is the slope of the disease progression.
This hypothesis of linearity was reinforced by the UK Prospective Diabetes Study (UKPDS), which has shown linear glycaemic deterioration over 6 years of therapy in Type 2 diabetes. It was assumed that this process was unchanging over the 1-year therapy.
Mixture model
Type 2 diabetes mellitus is a heterogeneous disease [14]. Some patients can be less susceptible to a given treatment than others and some patients may not even be affected by the treatment. This is illustrated in clinical practice by the use of a titration period in order to adjust the dosage for each patient according to their response. If such variability is not handled in a PKPD analysis this can lead to inaccurate estimates of PKPD parameters or incorrect estimates of the effect size [15]. A mixture model was used to deal with this variability in response. Such a model can test the hypothesis of no treatment effect against the alternative that a subset of the treated patients shows an improvement [16]. The treated population was hypothesized to be composed of two types of individuals, those who are affected by the treatment (i.e. responders) and those who are not (i.e. nonresponders). The probability (P) for a given patient to belong to one of the two subpopulations was estimated along with the parameters of the model. This probability can be interpreted as the proportion (P) of patients in one type of the population. In case of no treatment effect the time-course of FPG was described by the disease-progression model alone, otherwise the time-course of FPG was described by the combination of the PKPD model and the disease-progression model according to the following equations:
where FPGt is the predicted fasting plasma glucose at time t, Base is the predicted baseline fasting plasma glucose, St the predicted disease progression at time t and Et the predicted treatment effect at time t.
Random effects models
Models for intersubject variability
The differences between individuals in each PKPD parameter were regarded as random quantities and were assumed to have a mean equal to zero and variance estimated using a proportional error model. The magnitude of each intersubject variability is expressed as a coefficient of variation of the population PKPD parameter.
Models for residual variability
The differences between the observed FPG and the predicted FPG were regarded as random quantities and were assumed to have a mean equal to zero and a variance estimated by an additive error model. The residual variability is expressed in mmol l−1. This value represents FPG measurement error, mis-specification of the model and background noise of the data.
Simulations
Predictive performance of the PKPD model
Simulations of existing FPG data were performed in order to evaluate the predictive performance of the PKPD model [17]. The PKPD model was developed on the FPG profiles of patients treated with gliclazide MR only. The predictability of this model was then evaluated by predicting the FPG profiles of patients who received the current formulation during the 10-month phase III studies. This evaluation was performed on the PKPD data collected in the largest phase III study: study A.
To simulate the FPG profiles of study A patients, the overall design properties of this phase III study including dose adaptation rules were used. The patients were characterized by their baseline FPG, their apparent gliclazide clearance and their previous antidiabetic treatment. The individual PKPD parameters were independently generated according to the population distributions (mean and variance) and were randomly attributed to the patients. Attempts to estimate the covariance between the parameters did not improve the fit significantly, so simulation was performed without covariance. The residual variability was used to simulate the individual FPG values. The standard error on the PKPD parameters was not taken into account. The simulation was replicated 100 times in order to provide a distribution of the predicted FPG profiles. Simulations were performed using Pharsight Trial Simulator version 2.0.
The predictability of the population PKPD model was assessed using graphical display. In order to evaluate the ability of this model to predict the mean response, the minimum and maximum mean predicted FPG time courses of the 100 simulations were compared with the mean observed FPG time course. In order also to evaluate the ability of this model to predict the intersubject variability in response, the minimum and maximum 5th and 95th predicted percentile lines of the 100 simulations were compared with the 5th and 95th observed percentile lines.
Illustration of the time course effect of gliclazide MR
In order to illustrate the drug effect of gliclazide MR and the repercussion of the disease progression on this effect, a 1-year clinical trial without dose adaptation was simulated. This study was designed as a parallel dose ranging study with administration of the five doses of gliclazide MR: 15, 30, 60, 90 and 120 mg once daily. The 634 patients included in the PKPD database were used to perform the simulation. They were characterized by their baseline FPG, their apparent gliclazide clearance, their PKPD parameters and their previous antidiabetic treatment. In order to have patients with the same characteristics, the simulation was performed in 634 patients for each dose group. FPG values were simulated each month and the percentage of patients with clinically significant improvement of blood glucose control was calculated. Clinically significant improvement of blood glucose control was defined as a reduction in FPG> 10% from baseline. This criterion of effectiveness corresponds to the mean intrasubject variability of a FPG measurement at 24-h intervals. This simulation allowed what proportion of patients who may expect an improvement in blood glucose control as a function of dose and as a function of time to be estimated.
Programs
All PKPD evaluations were performed on an AlphaServer 2100 4/200 (Digital) with NONMEM version 5.1 software using the first order estimation method and a convergence criterion of four significant digits. The Fortran compiler was Digital Fortran for Open VMS, version 7.0. The GAM was implemented into the program Xpose [18], version 2.0, using S-PLUS 2000 software (Statistical Sciences, Seattle, WA, USA: StatSci, a division of MathSoft, Inc., 1995). Graphics and splines were performed using SAS (version 6.12 software; 1990 SAS Institute Inc., Cary, NC, USA) and Excel 97 software. PKPD simulations were performed with Pharsight Trial Simulator version 2.0 and SAS version 6.12 software (SAS Institute Inc., 1990).
Results
Pharmacokinetic results
A one-compartment open model with first-order absorption and elimination was found to describe adequately the pharmacokinetic profile of gliclazide MR. The pharmacokinetics are characterized, for a patient of 80 kg by an apparent clearance of 15 ml min−1 (0.90 l h−1) and an apparent volume of distribution of 19 l. The absorption phase gives a peak between 6 h and 9 h after drug administration. The decline in the plasma concentration is described by a single exponential term with an elimination half-life of about 16 h. The pharmacokinetics of gliclazide is linear after single and repeated administration in the range of doses 15–120 mg. The gliclazide MR apparent clearance was characterized by a moderate intersubject variability of 41% and a low intrasubject variability (i.e. interoccasion variability) of 16%.
Pharmacokinetic/pharmacodynamic results
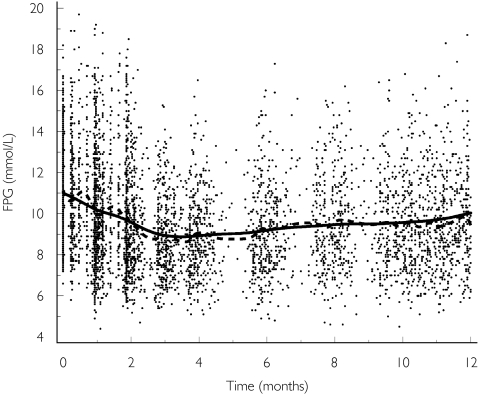
The PKPD database was composed of 634 Type 2 diabetic patients and included a total of 5258 fasting plasma glucose samples. The time course of FPG was fitted using a combination of a drug-effect model, a disease-progression model and a mixture-effect model (Figure 1). The final population PKPD parameters from the NONMEM model and their precision estimates are given in Table 2.
Figure 1.
Mean observed and predicted fasting plasma glucose (FPG) vs time after repeated administration of the modified release formulation of gliclazide. Points represent the observations. The dotted line represents a cubic spline through the observations. The solid line represents a cubic spline through the population predictions.
Table 2.
Results from the final population PKPD model.
| Parameters | Intersubject variability | Residual variability | ||||
|---|---|---|---|---|---|---|
| Estimate | Standard error of estimate | Estimate(%) | Standard error of estimate | Estimate | Standard error of estimate | |
| Baseline FPG (mmol l−1) | 17 | 1 | ||||
| Diet alone | 9.6 | 0.2 | ||||
| 1 OHA class | 10.9 | 0.1 | ||||
| 2 OHA classes | 12.2 | 0.2 | ||||
| Emax (% of baseline FPG) | 29 | 1 | 30 | 7 | ||
| Gliclazide AUC50 (µg.h ml−1) | 20 | 3 | 60 | 47 | ||
| Rate constant of equilibration keq (day−1) | 0.033 | 0.005 | 84 | 41 | ||
| Equilibration half-life (day) | 21 | |||||
| Constant rate of disease progression in FPG (mmol l−1 year−1) | 0.84 | 0.12 | 143 | 18 | ||
| Percentage of nonresponders (%) | ||||||
| Diet alone | 12 | 3 | ||||
| 1 OHA class | 24 | 4 | ||||
| 2 OHA classes | 50 | 7 | ||||
| Additive residual error (mmol l−1) | ||||||
| Responders | 0.8 | 0.03 | ||||
| Nonresponders | 1.7 | 0.06 | ||||
OHA, oral hypoglycaemic agent.
Drug effect model
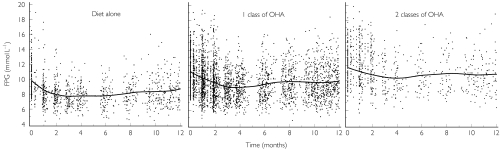
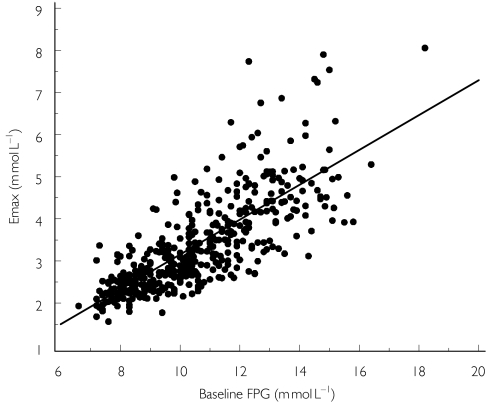
The baseline FPG was found to be related to the type of previous antidiabetic treatment (Figure 2): the baseline value was 9.6 mmol l−1 in patients previously treated with diet alone, 10.9 mmol l−1 in patients previously treated by a single class of oral hypoglycaemic agent (OHA), and 12.2 mmol l−1 in patients previously treated with two classes of OHA. Intersubject variability in baseline FPG was 17%. This variability was 19% before inclusion of the previous treatment in the model. The population Emax was found to be related to the baseline FPG (Figure 3) and was expressed as a percentage of this value (29%). Intersubject variability for Emax was 30%. The final population AUC50 (AUC that induces 50% of maximal effect) was 20 µg h ml−1. Intersubject variability for AUC50 was 60%. The final rate constant of equilibration (Keq) was 0.033 day−1, which corresponds to an equilibration half-life of 21 days. Intersubject variability for Keq was 84%.
Figure 2.
Mean observed fasting plasma glucose (FPG) vs time curve after repeated administration of the modified release formulation of gliclazide. Function of type of previous antidiabetic treatment. Points represent the observations. The solid lines represent a cubic spline through the observations. OHA, Oral hypoglycaemic agent.
Figure 3.
Relationship between the post hoc parameter estimates for maximum hypoglycaemic effect (Emax) of gliclazide and baseline fasting plasma glucose (FPG). Points represent the observations. The solid line represents a linear regression model through the observations.
Disease-progression model
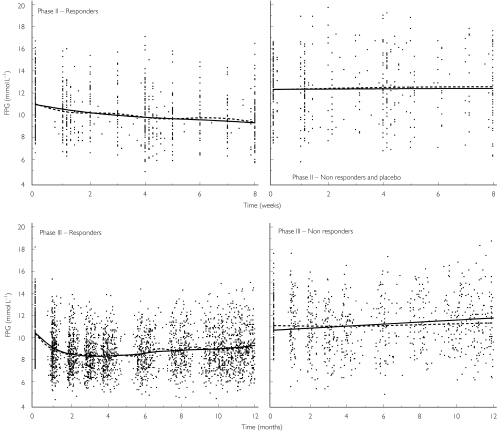
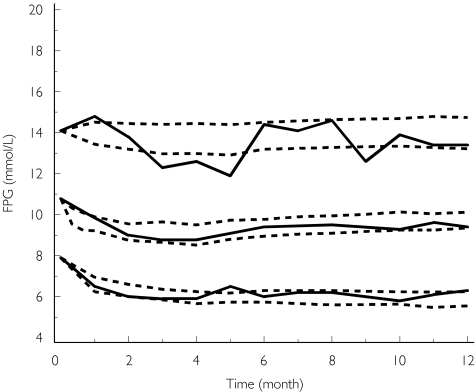
The constant rate of the disease progression (Figure 4) was 0.84 mmol l−1 per year. Intersubject variability for the slope was 143%.
Figure 4.
Mean observed and predicted fasting plasma glucose (FPG) vs time after repeated administration of the modified release formulation of gliclazide. Function of the study phase and function of the response to treatment. Points represent the observations. The dotted line represents a cubic spline through the observations. The solid line represents a cubic spline through the individual predictions.
Mixture model
The percentage of nonresponders was found to be related to the number of classes of OHA prescribed before the patients stopped their treatment and started gliclazide MR as monotherapy. The percentage of nonresponders was 12% in patients previously treated with diet alone, 24% in patients previously treated by a single class of OHA, and 50% in patients previously treated by two classes of OHA. A different residual variability was used for the two subpopulations of patients. The nonresponders had a higher residual variability in their FPG time course (Figure 5). The residual variability was 1.7 mmol l−1 in case of no treatment effect and 0.8 mmol l−1 in case of treatment effect.
Figure 5.
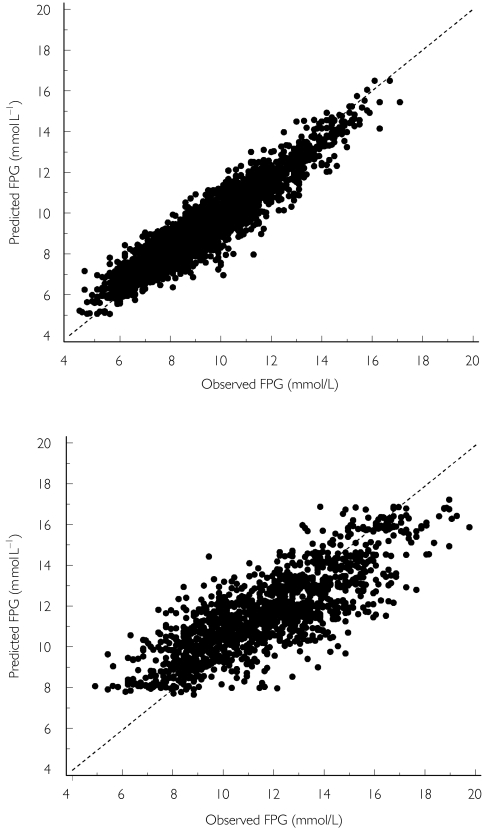
Individual predicted fasting plasma glucose (FPG) vs observed FPG after repeated administration of the modified release formulation of gliclazide. Points represent the observations. The dotted line represents the identity line. Top, responder patients; bottom, non-responder patients.
Covariate analysis
The number of classes of OHA received prior to entry in the studies was the only covariate found to have a significant influence in NONMEM, on baseline FPG and on the percentage of nonresponders as described above. The GAM analysis also suggested that AUC50 increased with the number of classes of OHA and inversely for Emax, that the constant rate of the disease progression increased with BMI, and that the baseline FPG increased with the duration of diabetes. In NONMEM, the inclusion of diabetes duration alone on the baseline FPG was significant, but this influence disappeared after inclusion of the number of previous antidiabetic agents. The reason for this may be that these two covariates are correlated, with an increase in the number of OHA classes from 0 to 2 when the diabetes duration increases from 4 to 9 years.
Simulations
Predictive performance of the PKPD model
A total of 232 Type 2 diabetic patients were included in the simulation database. Main demographic and biochemical characteristics of these patients were similar to those of the patients included in the PKPD database (Table 1). The comparison of the observed FPG time courses and the predicted FPG time courses is shown in Figure 6. The mean observed FPG profile lay within the minimum and maximum mean predicted profiles of the 100 simulations. The 5th and 95th observed percentile lines mostly lay within the minimum and maximum 5th and 95th predicted percentile lines predicted profiles of the 100 simulations. This figure illustrates that the population PKPD model predicts accurately the mean response but also the intersubject variability in response.
Figure 6.
Comparison of observed (solid lines) vs simulated (dotted lines) fasting plasma glucose (FPG) time courses after repeated administration of the current gliclazide formulation in a phase III trial (study A). From the bottom up, the solid lines represent the 5th percentile line, the mean and 95th percentile line of the observed FPG time course, respectively. For each of these observed profiles, the dotted lines represent the minimum and maximum predicted profiles obtained from 100 simulations of the phase III trial.
Illustration of the time-course effect of gliclazide MR
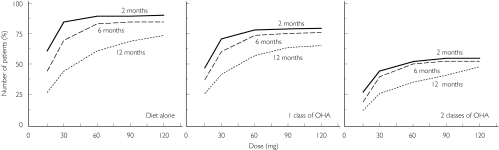
Figure 7 shows the percentage of patients with clinically significant improvement of blood glucose control (i.e. decrease in FPG of at least 10% of baseline) as a function of dose, time and type of previous antidiabetic treatment. Percentages are displayed after 2, 6 and 12 months of treatment. The percentage increases with the dose of gliclazide MR and decreases with the number of OHA classes used prior to entry in the study. Higher percentages were obtained in OHA-naive patients. After 2 months of treatment in patients who previously received a single class of OHA, the first dose inducing improvement of blood glucose control in more than 50% of patients was the 30-mg dose, and more than 75% of these patients had an improvement on the 120-mg dose. The disease progression induced a slight decrease in the percentage after 6 months of treatment which was more pronounced after 12 months. In the patients who previously received a single class of OHA, the first dose inducing improvement of blood glucose control in more than 50% of patients after 12 months of treatment was the 60-mg dose.
Figure 7.
Percentage of patients with clinically significant improvement of blood glucose control (i.e. reduction in fasting plasma glucose (FPG)> 10% from baseline) as a function of dose, time and type of previous antidiabetic treatment after simulation of repeated administration of the modified release formulation of gliclazide. OHA, Oral hypoglycaemic agent.
Discussion
The main purpose of this paper is to report the results of the population PKPD combined analysis of the long-term antidiabetic effect of the modified release formulation of gliclazide (gliclazide MR). This PKPD analysis leads to a better understanding of the kinetics of the hypoglycaemic effect of gliclazide and of its intersubject variability.
The FPG profile over 1 year with gliclazide MR was best described by the combination of three models: a mixture model, a drug-effect model and a disease-progression model. The mixture model was used to estimate the percentage of patients unaffected by the treatment. The probability of response to gliclazide was found to decrease with the number of OHA classes received prior to entry in the studies. Type 2 diabetes mellitus is a progressive disease, which requires increased intensity of therapy and often adding new agents with time [19]. This was illustrated in this analysis by the observed correlation between the diabetes duration and the number of antidiabetic agents prescribed prior to entry in the studies. Therefore, it is consistent to observe a lower percentage of responders to gliclazide, used as monotherapy, in patients in whom glycaemic control was previously achieved with combined therapy only. The gradual deterioration of blood glucose control in Type 2 diabetes was also reflected in the baseline FPG, which was found to increase with the number of previous antidiabetic agents.
In responders, a significant Emax relationship was established between the area under the concentration–time curve of gliclazide (AUC) and the decrease in FPG. The AUC producing half maximum effect (AUC50) was just below the mean AUC of the 30-mg dose, which is the initial dose of gliclazide MR dosing regimen. The highest tested dose of 120 mg would be predicted to produce 87% of the maximum hypoglycaemic effect. The hypoglycaemic efficacy (Emax) of gliclazide was found to be directly related to the baseline FPG level; the higher the FPG level, the higher its decrease from baseline. This correlation agrees with previous findings [20].
The use of a compartment-effect model allowed the delay in response to be described by an equilibration half-life. When a patient had a response at any given dose, the mean equilibration half-life was 21 days, meaning that 50% of the effect was obtained in 3 weeks of treatment and that most of the activity was obtained after 3 months of treatment. This result indicates that a lack of a significant decrease in FPG after 2 weeks of treatment could be a sufficient indication to increase the dose. This is in agreement with recent recommendations to quickly increase the dose of oral hypoglycaemic agent until adequate glycaemic control is achieved or response is not observed. This result also indicates that the washout period of 2 weeks was perhaps too short to remove completely the influence of OHA received prior to entry in the studies. This probably resulted in an underestimation of the baseline value and also of the size of the antihyperglycaemic effect of gliclazide in this analysis.
The decrease in FPG induced by gliclazide is progressively altered by the natural deterioration of glycaemic control in Type 2 diabetes. The progression of the disease was estimated by using a linear model. The slope which characterizes this increasing hyperglycaemia was 0.84 mmol l−1 per year. A large variability in this parameter was found between individuals (143%) and no explanatory factor was identified in this study. Many other studies, such as the UKPDS [21], have illustrated that Type 2 diabetes is a progressive disease [22, 23] with a similar linear increase in fasting plasma glucose. In these studies, the decline in metabolic control is mostly related to the deterioration of the pancreas β-cell function.
The aim of this population PKPD combined analysis was not only to describe a process that develops over time, but also to build a PKPD model which can be used for simulation. Before performing any simulation, it should be established that the PKPD model is predictive. For this purpose, an acceptable way is to predict the FPG profiles of patients different from those used to develop the PKPD model. The FPG profiles of patients who received the current formulation of gliclazide in the phase III clinical trial (study A) were simulated. The mean response and the intersubject variability in response were satisfactorily predicted by the simulations. This ability of the PKPD model to predict both the mean and the range of FPG time courses can be interpreted as a qualification of the model.
When this predictability was established, a parallel dose-ranging study was simulated in order to illustrate the impact of disease progression on the improvement of glucose control induced by gliclazide over 1 year at different dose levels. This simulation shows the need to adjust therapy regularly in Type 2 diabetes mellitus in order to act against the deterioration of glucose control and to take into account the previous treatment when changing therapy.
In conclusion, this population PKPD analysis has characterized the relationship between the exposure to gliclazide and its long-term hypoglycaemic effect, and has established that the intersubject variability in response is mostly related to disease state. The results underline the clinical interest of quickly increasing the dose of gliclazide MR according to the response to treatment in order to achieve effective blood glucose control.
References
- 1.Campbell DB, Lavielle R, Nathan C. The mode of action and clinical pharmacology of gliclazide: a review. Diabetes Res Clin Prac. 1991;14:S21–S36. doi: 10.1016/0168-8227(91)90005-x. [DOI] [PubMed] [Google Scholar]
- 2.Palmer KJ, Brogden RN. Gliclazide an update of its pharmacological properties and therapeutic efficacy in non-insulin-dependent diabetes mellitus. Drugs. 1993;46:92–125. doi: 10.2165/00003495-199346010-00007. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Simonson DC. Oral sulfonylurea agents suppress hepatic glucose production in non-insulin-dependent diabetic individuals. Diabetes Care. 1984;7:72–80. [PubMed] [Google Scholar]
- 4.Francillard M, Frey N, Paraire M, Laveille C, Jochemsen R. Pharmacokinetics of Diamicron modified release (MR) in 1007 type 2 diabetic patients. J Nutr Health Aging. 2001;5:F14. special issue 31 [Abstr.] [Google Scholar]
- 5.Diamicron MR Study group and Drouin P. Diamicron MR once daily is effective and well tolerated in type 2 diabetes: a double-blind, randomised, multinational study. J Diabetes Complications. 2000;14:185–191. doi: 10.1016/s1056-8727(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Study Group on Diabetes Mellitus. Diabetes Mellitus: Report of a WHO Study Group. Geneva: World Health Organization; 1985. pp. 1–113. Technical Report Series no. 727. [PubMed] [Google Scholar]
- 7.Beal SL, Sheiner LB. NONMEM Users Guides. San Francisco: NONMEM Project Group–University of California; 1992. [Google Scholar]
- 8.Mandema JW, Verotta D, Sheiner LB. Building population pharmacokinetic–pharmacodynamic models. I. Models for covariate effects. J Pharmacokin Biopharm. 1992;20:511–528. doi: 10.1007/BF01061469. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson MO. Population pharmacodynamic models in cancer chemotherapy. In: COST B1 Scientific Committee, editor. The Population Approach: Measuring and Managing Variability in Response, Concentration and Dose. Brussels: European Commission; 1997. pp. 115–124. [Google Scholar]
- 10.Holford NHG. Parametric models for the time course of drug action: the population approach. In: Rowland M, Aarons L, editors. New Strategies in Drug Development and Clinical Evaluation: the Population Approach. Brussels: Commission of the European Communities; 1992. pp. 193–206. [Google Scholar]
- 11.UK Prospective Diabetes Study Group. UK Prospective Diabetes Study 16: overview of 6 years’ therapy of type 2 diabetes: a progressive disease. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- 12.Marbury T, Huang WC, Strange P, et al. Repaglinide versus glyburide: a one-year comparison trial. Diabetes Res Clin Prac. 1999;43:155–166. doi: 10.1016/s0168-8227(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 13.Chan PLS, Holford NHG. Drug treatment effects on disease progression. Annu Rev Pharmacol Toxicol. 2001;41:625–659. doi: 10.1146/annurev.pharmtox.41.1.625. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 15.Razzaghi M, Kodell RL. Risk assessment for quantitative responses using a mixture model. Biometrics. 2000;56:519–527. doi: 10.1111/j.0006-341x.2000.00519.x. [DOI] [PubMed] [Google Scholar]
- 16.Boos DD, Brownie C. Mixture models for continuous data in dose–response studies when some animals are unaffected by treatment. Biometrics. 1991;47:1489–1504. [PubMed] [Google Scholar]
- 17.Mandema JW, Kaiko RF, Oshlack B, et al. Characterization and validation of a pharmacokinetic model for controlled-release oxycodone. Br J Clin Pharmacol. 1996;42:747–756. doi: 10.1046/j.1365-2125.1996.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic-pharmacodynamic model building aid for NONMEM. Comput Meth Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 19.European Diabetes Policy Group. A desktop guide to type 2 diabetes mellitus. Diabet Med. 1999;16:716–730. [PubMed] [Google Scholar]
- 20.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 21.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 22.Simonson DC, Kourides IA, Feinglos M, et al. Efficacy, safety, and dose–response characteristics of glipizide gastrointestinal therapeutic system on glycaemic control and insulin secretion in NIDDM. Diabetes Care. 1997;20:597–606. doi: 10.2337/diacare.20.4.597. [DOI] [PubMed] [Google Scholar]
- 23.Wolffenbuttel BHR, Landgraf R. A 1-year multicenter randomized double-blind comparison of repaglinide and gliburide for the treatment of type 2 diabetes. Diabetes Care. 1999;22:463–467. doi: 10.2337/diacare.22.3.463. [DOI] [PubMed] [Google Scholar]