Abstract
Aim
To examine the suspected inhibitory potential of the over-the-counter (OTC) drug ibuprofen on N-acetyltransferase 2 (NAT2) in vitro and in vivo and the possible implications for phenotyping procedures using caffeine as probe drug.
Methods
We first studied the inhibitory effect of ibuprofen on NAT2 in vitro, using human liver cytosol and sulfamethazine as substrate. In vivo 15 fast and 15 slow acetylating healthy volunteers were treated with a single dose of ibuprofen (800 mg) orally and phenotyped for NAT2, CYP1A2, and xanthine oxidase (XO) with caffeine as probe drug before and during drug treatment. Because of unexpected in vivo results with ibuprofen this study was repeated in 20 healthy volunteers with probenecid, a model substrate of renal organic anion transport (OAT). For phenotyping tests a urine sample was collected 6 h after caffeine (200 mg) intake. The caffeine metabolites acetyl-6-formylamino-3-methyluracil (AFMU), 1-methylxanthine (1MX), 1-methyluric acid (1MU), and 1,7-dimethyluric acid (17MU) were quantified by HPLC, and the corresponding metabolic ratios for CYP1A2, NAT2, and XO were then calculated. Genotyping for NAT2 was performed with standard PCR-RFLP methods.
Results
In vitro, with human liver cytosol an inhibition by ibuprofen of the acetylation of sulfamethazine with Ki values between 2.2 and 3.1 mm was observed. Surprisingly, in vivo a significant (P < 0.001) increase of the acetyl-6-formylamino-3-methyluracil/1-methylxanthine (AFMU/1MX) urinary ratio from 0.97 ± 0.16 to 1.08 ± 0.18 (95% CI on the difference 0.049, 0.170) was found, indicating an apparent elevation of NAT2 activity. In contrast, no change was observed for the ratios used for XO and CYP1A2. Because an induction of NAT2 could be excluded, an interaction of ibuprofen with the tubular secretion of some of the caffeine metabolites was assumed. To prove this assumption, the in vivo study was repeated with probenecid, a model substrate of the renal OAT system. Again, a prominent elevation of the AFMU/1MX ratio from 0.97 ± 0.21 to 1.53 ± 0.35 was found (P < 0.002; 95% CI on the difference 0.237, 0.876), but also the XO ratio 1MU/1MX was significantly (P < 0.0001) increased from 1.34 ± 0.09 to 2.24 ± 0.14 (95% CI on difference 0.735, 1.059) due to a reduction of 1MX excretion.
Conclusions
Substrates of OAT interact with renal excretion of caffeine metabolites and may falsify NAT2 and XO phenotyping results. Other phenotyping procedures, which are based on urinary metabolic ratios, should also be validated in this respect, especially in patients under polymedication.
Keywords: caffeine metabolites, ibuprofen, N-acetyltransferase 2, organic anion transport, phenotyping
Introduction
Genetic polymorphism of N-acetyltransferase 2 (NAT2) results in the occurrence of slow and rapid acetylator phenotypes, who differ in the risk of side-effects of certain drugs and in the development of malignancies [1–4]. Phenotyping of this polymorphism is routinely performed by ingesting a single oral dose of caffeine as probe drug and the subsequent determination of the ratio of caffeine metabolites in urine [5, 6]. A good agreement between phenotype and genotype has been observed in most studies, especially with healthy volunteers [7]. However, in some studies with severely ill patients being treated with many drugs, discrepancies have been observed between NAT2 phenotype and genotype [8, 9], for example by the unexplained predominance of slow acetylators in patients with AIDS [10]. We assumed that drug interactions might account for these discrepant observations [11], especially in patients taking over-the-counter (OTC) drugs, which are often used in an uncontrolled fashion. Indeed, recently we found a significant inhibition of NAT2 activity by the OTC drug acetaminophen [12]. The nonsteroidal anti-inflammatory agent ibuprofen is another widely used OTC drug. Although ibuprofen is not a substrate of NAT2, the former may cause a noncompetitive enzyme inhibition at high drug concentrations, as observed in vitro[13, 14]. Thus, clinically significant inhibition of NAT2 by ibuprofen is possible.
Our primary aim was to investigate the interaction potential of ibuprofen with NAT2 in vitro and in vivo, using sulfamethazine and caffeine, respectively. A discrepancy between our in vitro and in vivo results led to an additional in vivo experiment replacing ibuprofen by probenecid. We suspected that ibuprofen, a known substrate of the renal organic anion transport (OAT) [15], might interfere with the renal excretion of the caffeine metabolites.
Subjects and methods
Materials
Ibuprofen tablets were obtained from Knoll (Liestal, Switzerland) and, probenecid tablets were from Merck, Sharp & Dome-Chibret (Glattbrugg, Switzerland). Acetyl-6-formylamino-3-methyluracil (AFMU) was generously supplied by Nestec (Vevey, Switzerland), and methylxanthines and uric acids were from Fluka (Buchs, Switzerland). Ibuprofen for analytical purposes, flurbiprofen, and sulfamethazine were from Sigma (Buchs, Switzerland) and N-acetylsulfamethazine from INC (Costa Mesa, CA, USA). All solvents and buffer substances were purchased from Merck (Darmstadt, Germany) and were of analytical grade. Caffeine capsules for caffeine tests were prepared by the Institute of Hospital Pharmacy, University Hospital (Basel, Switzerland). Double distilled water was used for the preparation of all solutions.
Protocols
In vitro N-acetyltransferase 2 assay
Inhibition of NAT2 in liver cytosol by ibuprofen was investigated using genotyped human liver tissue from one fast and one slow acetylator donor prepared as described previously [11]. NAT2 activity was measured by monitoring the formation of N-acetyl-sulfamethazine from sulfamethazine by HPLC [16]. Substrate concentrations were 10, 30, and 90 µm, covering the previously determined Km values for the slow (23 µm) and fast (73 µm) acetylator liver specimens. Ibuprofen was added to the reaction solution to give final concentrations of 0, 0.27, 1.35, and 2.7 mm, corresponding to the range of therapeutic to toxic concentrations [24]. The reaction was stopped with 10 µl 15% HClO4, and the precipitated proteins were removed by centrifugation for 3 min at 30 000 g. An aliquot of 50 µl of the supernatant was directly injected onto the HPLC column. All incubations were carried out in triplicate.
In vivo ibuprofen study
Subjects
After approval had been obtained from the Ethics Committee of the University Hospital, Basel, Switzerland, 15 previously genotyped fast and 15 slow acetylating healthy adult volunteers (mean age 34 years, 18 females, 12 males, seven smokers) were enrolled in the study with ibuprofen (part 1 of the study) after giving written informed consent. The subjects were not taking any medication from the week before until the end of the study, with the exception of four females who were taking oral contraceptives. All participating volunteers were healthy and had normal liver and kidney function. Individuals with known adverse drug reactions to ibuprofen or probenecid, or known caffeine intolerance were excluded from the study.
In this open study all 30 subjects abstained from methylxanthine-containing food and beverages from 12 h before until the end of the study. Phenotyping for NAT2 was performed with caffeine as the probe drug at the first (baseline) and the second day (treatment with ibuprofen). The subjects received a capsule of 200 mg caffeine p.o. and a spot urine sample was collected after 6 h in a 10-ml tube containing 200 mg of ascorbic acid and frozen at -20°C until analysis. On the following day (day 2) the subjects received 800 mg of ibuprofen (Brufen®) orally 2 h prior to caffeine administration, and again a 6-h spot urine sample was collected for phenotyping. On day 2 a venous blood sample was drawn 2 h after intake of ibuprofen for analysis of the latter used as a measure of compliance.
In vivo probenecid study
The protocol was identical to that for the study with ibuprofen, except that only 20 out of the 30 volunteers took part (mean age 32 years, 14 females and six males, 10 genotyped fast and 10 slow acetylators). The interval between the two studies was 5 months. On the day after phenotyping with caffeine for the baseline enzyme activities (day 2) the subjects received probenecid (Benemid®) tablets (2 × 500 mg) p.o. 3 h prior to caffeine intake (200 mg p.o.).
Analytical procedures
Determination of caffeine metabolites in urine
The pH of thawed urine was adjusted to less than 3.5 with acetic acid and an aliquot of 200 µl was vortexed for 5 s with 25 µl of internal standard solution containing 1,9-dimethyluric acid (1.2 mg l−1) and 135 mg ammonium sulphate. After extraction with 6 ml chloroform:isopropanol (95:5, v/v) the organic phase was evaporated to dryness at 30°C under N2. The residue was dissolved in 750 µl of 10% methanol in 0.05% acetic acid and the following caffeine metabolites were analysed by HPLC: acetyl-6-formylamino-3-methyluracil (AFMU), 1-methylxanthine (1MX), 1-methyluric acid (1MU), and 1,7-dimethyluric acid (17MU). Caffeine metabolites were separated by HPLC on a Superspher® 100 RP-18 column (LiChroCART® 250-4; Merck, Dietikon, Switzerland). The solvent used consisted of a ternary gradient of phase A (6% methanol), phase B (6% acetonitrile), and phase C (25% acetonitrile), all in 0.05% acetic acid. The settings for the gradient were as follows: 0–10 min 100% A; 10–14 min 50% A and 50% B; 14–20 min 35% A, 35% B, and 30% C; 20–30 min 100% C; re-equilibration with A from 30 to 50 min. The flow was 0.86 ml min−1 at a temperature of 32°C, the injection volume was 15 µl, and the diode array detector was set at 280 nm. The within-assay coefficients of variation (CV) for AFMU, 1MU, 1MX, 17MU, and 17MX in the concentration range between 60 and 170 µmol l−1 were 1.4, 1.0, 1.5, 2.0 and 2.2%, respectively. The corresponding between-assay CVs were 2.5, 3.8, 9.0, 4.3, and 8.9%, respectively. The chromatogram did not show any interfering peaks following analysis of samples from subjects who had taken ibuprofen or probenecid.
Quantification of ibuprofen in serum
Ibuprofen was measured in serum after extraction using the HPLC method described by Geisslinger et al.[19]. Briefly, a 500-µl serum aliquot was acidified by adding 100 µl of 2 m hydrochloric acid. The compounds of interest were extracted into 5 ml of ice-cold hexane-diethyl ether (8:2, v/v) containing 20.5 µm flurbiprofen as internal standard. After centrifugation for 5 min at 1500 g, 4.5 ml of the organic layer were removed and evaporated to dryness under a gentle stream of dry nitrogen. The residue was dissolved in 500 µl of mobile phase and analysed by HPLC with u.v. detection at 220 nm. Ibuprofen was separated on a Superspher® RP-18e column (LiChroCART® 250-4; Merck, Dietikon, Switzerland). The mobile phase consisted of methanol–water (65:35, v/v), containing 1 ml l−1 of concentrated phosphoric acid. The flow was maintained at 0.75 ml min−1 at a temperature of 40°C. A five-point standard curve was constructed for ibuprofen concentrations between 7.3 and 730 µm. The retention times were 13.7 min for ibuprofen and 10.2 min for flurbiprofen. The detection limit was 0.45 µm for ibuprofen. Within- and between-assay coefficients of variation for ibuprofen at a concentration of 368 µmol l−1 were 2.9%, and 3.6%, respectively.
Determination of creatinine in urine
Creatinine in all urine samples was measured by an enzymatic F DAOS method (Wako Chemicals, Neuss, Germany) on a Hitachi 917 clinical chemistry analyser.
Genotyping for NAT2
A 5-ml EDTA blood sample was drawn from each subject for genomic DNA isolation by column extraction with the QIAamp® DNA blood kit (Qiagen, Basel, Switzerland). NAT2 was analysed by PCR-RFLP for the wild-type allele NAT2*4 and the most common alleles NAT2*5, NAT2*6, NAT2*7 and NAT2*14, which account for almost all of the slow acetylator phenotypes in Caucasians [20, 21].
Data analysis
For the determination of the kinetic constants (Km, Vmax) data were fitted by nonlinear regression to the Michaelis–Menten equation using the GraphPad Prism® 3.0 program (GraphPad Software, Inc. San Diego, CA, USA).
The inhibition constant (Ki) of ibuprofen in liver cytosol was calculated on the basis of a Dixon Plot with a kinetic software package (EKI 1.0; Institute of Physiological Chemistry, University of Tübingen, Germany) using means from triplicate determinations. The estimate of the apparent Ki values and the nature of inhibition were obtained from Dixon plots, where the apparent Ki was given by the intersection point of the linear regression lines for data sets of 1/v against the concentration of inhibitor.
Statistical analysis was performed using the SPSS software package (SPSS, Chicago, IL, USA). A nonparametric Wilcoxon test for paired data was used to analyse changes in metabolic ratios before and after drug ingestion and for the creatinine-corrected urinary metabolite concentrations. A two-sided P-value < 0.05 was considered to be significant. Data are presented as mean ± standard error of the mean (s.e.m.).
The molar ratios AFMU/1MX and AFMU/(AFMU + 1MX + 1MU) were used for NAT2 phenotyping. For the AFMU/1MX ratio an antimode of 0.55 was used to discriminate slow from fast acetylators [7].
Results
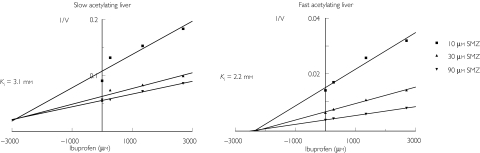
As shown in Figure 1, using ibuprofen inhibited the acetylation of the NAT2 substrate sulfamethazine in an apparent noncompetitive fashion in the liver cytosol from the slow acetylator (Ki 3.1 mm) and competitively in that from the fast acetylator (Ki 2.2 mm).
Figure 1.
Dixon plot showing inhibition of sulfamethazine (SMZ) acetylation by ibuprofen human liver homogenates (for one fast (left) and one slow (right) acetylator). V is expressed as pmol min−1 mg−1 protein.
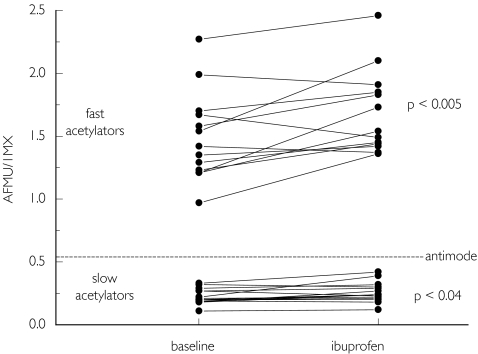
The effect of ibuprofen on markers of NAT2 activity in vivo in 30 previously genotyped healthy volunteers is shown in Figure 2 and Table 1. The serum concentration of ibuprofen 2 h after drug intake was 209 ± 9 µmol l−1. In contrast to the in vitro ibuprofen, administration increased the AFMU/1MX ratio significantly in both slow and fast acetylators, suggesting a higher activity of NAT2. A similar trend was found for the ratio AFMU/(AFMU + 1MX + 1MU), another in vivo marker of NAT2 activity [18], which reached statistical significance in fast acetylators. Fast and slow acetylators were well separated by both phenotyping ratios, and a 100% correlation between phenotype and genotype was found before and during ibuprofen treatment. In contrast to the metabolic ratios reflecting NAT2 activity, the ratios 1MU/1MX (a marker for xanthine oxidase activity) and (AFMU + 1MX + 1MU)/17MU (a marker for CYP1A2 activity) were not significantly affected by treatment with ibuprofen (Table 1).
Figure 2.
AFMU/1MX ratios in urine in 15 fast and 15 slow acetylators before (baseline) and during treatment with ibruprofen. An antimode of 0.55 separates fast from slow acetylators.
Table 1.
Effect of ibuprofen on different metabolic caffeine ratios commonly used for NAT2 phenotyping (a, b), and as indices of CYP1A2 (c), and XO activity (d) in 15 healthy fast and 15 slow acetylators
| AFMU/1MX(a) | P-value | AFMU/(AFMU +1MX + 1MU) (b) | P-value | (AFMU + 1MX +1MU)/17MU (c) | P-value | 1MU/1MX(d) | P-value | |
|---|---|---|---|---|---|---|---|---|
| All volunteers (n = 30) | ||||||||
| Baseline value | 0.97 ± 0.16 | 0.25 ± 0.03 | 3.83 ± 0.27 | 1.11 ± 0.05 | ||||
| During ibuprofen | 1.08 ± 0.18 | < 0.001 | 0.27 ± 0.03 | < 0.04 | 4.00 ± 0.34 | 0.52 | 1.21 ± 0.05 | 0.26 |
| 95% CI | 0.049, 0.170 | 0.0019, 0.0248 | −0.550, 0.471 | −0.028, 0.222 | ||||
| Fast acetylators (n = 15) | ||||||||
| Baseline value | 1.72 ± 0.18 | 0.40 ± 0.02 | 4.01 ± 1.19 | 1.28 ± 0.32 | ||||
| During ibuprofen | 1.91 ± 0.17 | < 0.005 | 0.43 ± 0.02 | 0.13 | 4.34 ± 2.07 | 0.80 | 1.34 ± 0.25 | 0.56 |
| 95% CI | 0.083, 0.291 | −0.0012, 0.0378 | −0.454, 0.984 | −0.102, 0.295 | ||||
| Slow acetylators (n = 15) | ||||||||
| Baseline value | 0.22 ± 0.02 | 0.10 ± 0.01 | 3.66 ± 1.73 | 0.95 ± 0.15 | ||||
| During ibuprofen | 0.26 ± 0.02 | < 0.03 | 0.12 ± 0.01 | 0.27 | 3.51 ± 1.65 | 0.45 | 1.09 ± 0.25 | 0.36 |
| 95% CI | 0.0053, 0.0593 | −0.0044, 0.0213 | −1.06, 0.37 | −0.062, 0.257 |
Data are given as mean ±s.e.m. with 95% CI on differences. AFMU, Acetyl-amino-6-formylamino-3-methyluracil; 1MX, 1-methylxanthine; 1MU, 1-methyluric acid.
Because 1MX and 1MU are at least partially eliminated by renal tubular secretion [22] and because ibuprofen and its metabolites can interfere with renal secretion of drugs [23], the effect of ibuprofen on the renal elimination of IMX and IMU was investigated after normalization with creatinine concentrations to correct for urine dilution (Table 2). Ibuprofen had a tendency to increase the renal excretion of AFMU and to decrease the renal excretion of 1MX and 17MU.
Table 2.
Effect of ibuprofen and probenecid on urinary concentrations of acetyl-amino-6-formylamino-3-methyluracil (AFMU), 1-methylxanthine (1MX), 1-methyluric acid (1MU), and 1,7-dimethyluric acid (17MU) normalized to creatinine concentration
| AFMU | P-value | 1MX | P-value | 1MU | P-value | 17MU | P-value | |
|---|---|---|---|---|---|---|---|---|
| Ibuprofen (n = 30) | ||||||||
| Baseline value | 10.8 ± 1.6 | 15.2 ± 1.9 | 16.7 ± 2.0 | 10.2 ± 0.9 | ||||
| During ibuprofen | 11.9 ± 2.1 | 0.40 | 14.6 ± 2.0 | 0.49 | 16.8 ± 2.1 | 0.98 | 8.9 ± 1.3 | 0.09 |
| 95% CI | −0.69, 3.03 | −2.64, 1.42 | −2.68, 2.89 | −3.18, 0.51 | ||||
| Probenecid (n = 20) | ||||||||
| Baseline value | 10.9 ± 2.1 | 14.9 ± 1.9 | 19.6 ± 2.8 | 11.5 ± 1.3 | ||||
| During probenecid | 9.1 ± 1.6 | 0.08 | 9.6 ± 1.6 | < 0.0005 | 21.0 ± 3.9 | 0.69 | 10.5 ± 1.2 | 0.27 |
| 95% CI | −3.51, −0.08 | −7.35, −3.31 | −3.25, 6.07 | −3.61, 1.69 |
Data are expressed as µmol l−1 of caffeine metabolite per mmol l−1 creatinine (mean ±s.e.m.) with 95% CI on differences.
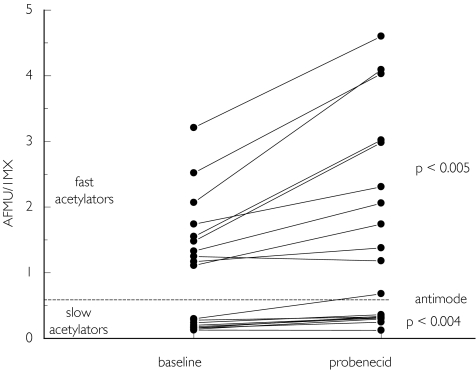
To evaluate the role of the OAT system in the renal elimination of AFMU, 1MU, and 1MX in vivo, we performed a study with probenecid, a substrate and inhibitor of the OAT system. As shown in Table 2, probenecid caused a large and significant (P < 0.0001) reduction of the excretion of 1MX. Correspondingly, the AFMU/1MX ratio also increased significantly, in both slow and fast metabolizers (Figure 3 and Table 3). The alternative ratio AFMU/(AFMU + 1MU + 1MX) increased slightly in subjects treated with probenecid, but this reached statistical significance only in slow acetylators (P = 0.048). The ratio (AFMU + 1MX + 1MU)/17MU was not affected by probenicid (Table 3). No subject reported an adverse reaction during any part of the study.
Figure 3.
AFMU/1MX ratios in urine in 10 fast and 10 slow acetylators before (baseline) and during treatment with probenecid. An antimode of 0.55 separates fast from slow acetylators.
Table 3.
Effect of probenecid on the different metabolic caffeine ratios commonly used for NAT2 phenotyping (a, b), and as indices of CYP1A2 (c), and XO (d) in 10 healthy fast and 10 slow acetylators
| AFMU/1MX(a) | P-value | AFMU/(AFMU +1MX + 1MU) (b) | P-value | (AFMU + 1MX +1MU)/17MU (c) | P-value | 1 MU/1MX(d) | P-value | |
|---|---|---|---|---|---|---|---|---|
| All volunteers (n = 30) | ||||||||
| Baseline value | 0.97 ± 0.21 | 0.24 ± 0.04 | 4.82 ± 0.33 | 1.34 ± 0.09 | ||||
| During probenecid | 1.53 ± 0.35 | < 0.002 | 0.26 ± 0.04 | 0.054 | 3.83 ± 0.37 | 0.25 | 2.24 ± 0.14 | < 0.0001 |
| 95% CI | 0.237, 0.876 | −0.0012, 0.0303 | −0.801, 0.309 | 0.74, 1.06 | ||||
| Fast acetylators (n = 15) | ||||||||
| Baseline value | 1.74 ± 0.21 | 0.40 ± 0.02 | 3.88 ± 1.33 | 1.52 ± 0.44 | ||||
| During probenecid | 2.73 ± 0.42 | < 0.004 | 0.42 ± 0.03 | 0.33 | 3.95 ± 1.58 | 0.77 | 2.52 ± 0.60 | < 0.002 |
| 95% CI | 0.47, 1.51 | −0.014, 0.046 | −0.644, 0.775 | 0.75, 1.26 | ||||
| Slow acetylators (n = 15) | ||||||||
| Baseline value | 0.21 ± 0.18 | 0.09 ± 0.08 | 4.28 ± 1.68 | 1.17 ± 0.31 | ||||
| During probenecid | 0.33 ± 0.04 | < 0.004 | 0.10 ± 0.01 | < 0.05 | 3.70 ± 1.83 | 0.19 | 1.96 ± 0.48 | < 0.002 |
| 95% CI | 0.063, 0.189 | 0.0007, 0.0250 | −1.40, 0.29 | 0.604, 0.189 |
Data are given as mean ±>s.e.m. with 95% CI on differences. AFMU, Acetyl-amino-6-formylamino-3-methyluracil; 1MX, 1-methylxanthine; 1MU, 1-methyuric acid.
Discussion
Our study shows that ibuprofen inhibits NAT2 in vitro but leads to an apparent increase of NAT2 activity in vivo. The in vitro inhibition of NAT2 activity in the liver of a slow and a fast acetylator is in agreement with studies by Chung et al.[13, 14] of the in vitro inhibition of other forms of NAT. The Ki values obtained (2.2 and 3.1 mm in the livers from the slow and fast acetylators, respectively) are clearly above the maximum serum concentrations Cmax of 0.1–0.4 mm, after therapeutic doses of ibuprofen in humans [24], suggesting that after normal ibuprofen doses no change in human NAT2 activity occurs, but not excluding the possibility [25]. In contrast, we observed in vivo an apparent elevation in NAT2 activity, if we applied the urinary ratio of the caffeine metabolites using the AFMU/1MX ratio, a common index for this enzyme. The alternative NAT2 ratio, AFMU/(AFMU + 1MU + 1MX), was affected only in fast acetylators, and the ratios for XO (1MU/1MX) and CYP1A2 ((AFMU + 1MU + 1MX)/17MU) showed no change during treatment with ibuprofen. Because NAT2 is thought to be not inducible and induction would be unlikely to occur within hours after treatment, we assumed an effect of ibuprofen on the renal excretion of some of the caffeine metabolites. Ibuprofen and other NSAIDs are known to inhibit the OAT system [15]. Although the knowledge on the mechanisms of the renal excretion of caffeine metabolites is limited, related compounds such as xanthine and uric acid are known substrates of OAT [26]. In addition, it has been shown that the renal clearance of 1MX and 1MU is higher than the glomerular filtration rate, suggesting that renal secretory mechanisms are involved in their elimination [22], whereas AFMU is thought to be reabsorbed in the kidney [27]. Therefore, we hypothesized that a drug interaction between ibuprofen and caffeine metabolites at the level of the renal OAT system might best explain the results of our in vivo study. Such an interaction might have masked any inhibition of NAT2 activity by ibuprofen.
In order to test this hypothesis, we studied the effect of probenecid, a model substrate and inhibitor of the renal OAT system, on caffeine metabolic ratios. A significant elevation of the AFMU/1MX ratio was found, which led to the misclassification of one slow metabolizer. Apparent drug-induced conversion of slow to fast acetylators has not been reported previously [12, 28, 29]. In contrast to the study with ibuprofen, the xanthine oxidase marker ratio 1MU/1MX was almost doubled during probenecid administration. Because caffeine phenotyping was performed using an established protocol [5, 6], only spot urine samples were collected. This precludes precise determination of the cumulative amount of excreted metabolites. To estimate indirectly which of the caffeine metabolites were most affected by probenicid, the urinary concentrations were normalized to urinary creatinine concentrations. Whereas probenecid and ibuprofen significantly decreased the renal excretion of 1MX, the latter increased renal excretion of AFMU. However, the changes did not reach statistical significance. The interpretation of these data is difficult, because creatinine also undergoes tubular secretion, which may be impaired by probenecid and ibuprofen or its metabolites.
The OAT system is a complex family of related transporters with different substrate specificities [31–33]. Thus, it is possible that different OAT subtypes (OAT1–OAT4) are involved in the transport of probenecid, ibuprofen, and caffeine metabolites, respectively, which may explain some of the findings of the present study. In addition, a large number of other anionic drug transporting proteins are present in the kidney, including sodium/phosphate cotransporter type I (NPT1), multidrug resistance proteins (MRP1–MRP6), a subfamily within the ATP-binding cassette (ABC) transporter superfamily, and organic anion transporting polypeptides (OATPs) [33]. However, the molecular identity and exact localization in renal tissue of some of these transporters is still elusive. Probenecid and to a lesser extent ibuprofen are potential inhibitors of most of these transporters [33, 35, 36], although ibuprofen is not efficiently transported by OAT1 [35] or OAT3 [36]. Considering the very large number of known endogenous and exogenous substrates of the OAT family [26, 31, 33], the potential for interactions with probe drugs used for enzyme phenotyping is considerable, especially in patients receiving polymedication. However, it is not yet clear to what extent these interactions are responsible for the reported discrepancies between phenotype and genotype [8–10].
As demonstrated previously [17], the present study indicates that the alternative ratio for NAT2 phenotyping AFMU/(AFMU + 1MU + 1MX) is less affected by drug interactions than the more commonly used ratio AFMU/1MX.
Genotyping could circumvent problems associated with phenotyping. However, the latter remains the method of choice for defining functional enzyme activity in patients. However, the majority of probe drugs for phenotyping have been evaluated in healthy volunteers or in well-defined patient populations and the number of safe and validated test procedures is still limited. This is especially true for phenotyping cocktails [39] because the potential for drug interactions increases with the addition of each probe drug.
In conclusion, this study indicates that the NAT2 phenotyping procedure using caffeine as a probe drug can be influenced by concomitant intake of probenecid or the widely used OTC drug ibuprofen and, therefore, probably by other substrates of the renal OAT and/or other transport systems. This drug interaction might also affect the determination of xanthine oxidase activity, whereas the urinary ratio used as a marker of CYP1A2 activity was not significantly affected by either drug. It remains to be determined whether other phenotyping procedures employing urinary metabolic ratios are affected by interactions involving renal excretion. Phenotyping procedures based on urinary ratios should be interpreted very carefully, especially in patients receiving multiple drug therapy.
Acknowledgments
These studies were supported by grant 32-49666.96 of the Swiss National Research Foundation. W.E.H. was supported by grant 01EC9902 from the German Ministry for Education and Research (BMBF).
References
- 1.Meyer UA. Pharmacogenetics and adverse drug reactions. Lancet. 2000;356:1667–1671. doi: 10.1016/S0140-6736(00)03167-6. [DOI] [PubMed] [Google Scholar]
- 2.Cascorbi I, Brockmöller J, Mrozikiewicz PM, Muller A, Roots I. Arylamine N-acetyltransferase activity in man. Drug Metab Rev. 1999;31:489–502. doi: 10.1081/dmr-100101932. [DOI] [PubMed] [Google Scholar]
- 3.Hein DW, Doll MA, Fretland AJ, et al. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol Biomarkers Prev. 2000;9:29–42. [PubMed] [Google Scholar]
- 4.Grant DM, Goodfellow GH, Sugamori K, Durette K. Pharmacogenetics of the human arylamine N-acetyltransferases. Pharmacology. 2000;61:204–211. doi: 10.1159/000028402. [DOI] [PubMed] [Google Scholar]
- 5.Grant DM, Tang BK, Kalow W. A simple test for acetylator phenotype using caffeine. Br J Clin Pharmacol. 1984;17:459–464. doi: 10.1111/j.1365-2125.1984.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang BK, Kadar D, Qian L, Iriah J, Yip J, Kalow W. Caffeine as a metabolic probe: validation of its use for acetylator phenotyping. Clin Pharmacol Ther. 1991;49:648–657. doi: 10.1038/clpt.1991.82. [DOI] [PubMed] [Google Scholar]
- 7.Hickman D, Sim E. N-acetyltransferase polymorphism. Comparison of phenotype and genotype in humans. Biochem Pharmacol. 1991;42:1007–1014. doi: 10.1016/0006-2952(91)90282-a. [DOI] [PubMed] [Google Scholar]
- 8.O'Neil WM, Gilfix BM, DiGirolamo A, Tsoukas CM, Wainer IW. N-acetylation among HIV-positive patients and patients with AIDS: when is fast, fast and slow, slow? Clin Pharmacol Ther. 1997;62:261–271. doi: 10.1016/S0009-9236(97)90028-X. [DOI] [PubMed] [Google Scholar]
- 9.O'Neil WM, Drobitch RK, MacArthur RD, Farrough MJ, Doll MA, Fretland AJ, et al. Acetylator phenotype and genotype in patients infected with HIV: discordance between methods for phenotype determination and genotype. Pharmacogenetics. 2000;10:171–182. doi: 10.1097/00008571-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Lee BL, Wong D, Benowitz NL, Sullam PM. Altered patterns of drug metabolism in patients with acquired immunodeficiency syndrome. Clin Pharmacol Ther. 1993;53:529–535. doi: 10.1038/clpt.1993.66. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann GR, Wenk M, Taeschner W, et al. N-acetyltransferase 2 polymorphism in patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 1996;60:62–67. doi: 10.1016/S0009-9236(96)90168-X. [DOI] [PubMed] [Google Scholar]
- 12.Rothen JP, Haefeli WE, Meyer UA, Todesco L, Wenk M. Acetaminophen is an inhibitor of hepatic N-acetyltransferase 2 in vitro and in vivo. Pharmacogenetics. 1998;8:553–559. doi: 10.1097/00008571-199812000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Chung JG, Lo HH, Hsieh SE, Yen YS. Ibuprofen inhibits arylamine N-acetyltransferase activity in the bacteria Klebsiella pneumoniae. Curr Microbiol. 1997;35:195–200. doi: 10.1007/s002849900238. [DOI] [PubMed] [Google Scholar]
- 14.Chung JG, Chang HL, Lin WC, Yeh FT, Hung CF. Effects of ibuprofen on arylamine N-acetyltransferase activity in human colon tumor cells. J Appl Toxicol. 1999;19:1–6. doi: 10.1002/(sici)1099-1263(199901/02)19:1<1::aid-jat527>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Apiwattanakul N, Sekine T, Chairoungdua A, et al. Transport properties of nonsteroidal anti-inflammatory drugs by organic anion transporter 1 expressed in Xenopus laevis oocytes. Mol Pharmacol. 1999;55:847–854. [PubMed] [Google Scholar]
- 16.Grant DM, Morike K, Eichelbaum M, Meyer UA. Acetylation pharmacogenetics. The slow acetylator phenotype is caused by decreased or absent arylamine N-acetyltransferase in human liver. J Clin Invest. 1990;85:968–972. doi: 10.1172/JCI114527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs P, Haefeli WE, Ledermann HR, Wenk M. Xanthine oxidase inhibition by allopurinol affects the reliability of urinary caffeine metabolic ratios as markers for N-acetyltransferase 2 and CYP1A2 activities. Eur J Clin Pharmacol. 1999;54:869–876. doi: 10.1007/s002280050569. [DOI] [PubMed] [Google Scholar]
- 18.Kalow W, Tang BK. The use of caffeine for enzyme assays: a critical appraisal. Clin Pharmacol Ther. 1993;53:503–514. doi: 10.1038/clpt.1993.63. [DOI] [PubMed] [Google Scholar]
- 19.Geisslinger G, Dietzel K, Loew D, et al. High-performance liquid chromatographic determination of ibuprofen, its metabolites and enantiomers in biological fluids. J Chromatogr. 1989;491:139–149. doi: 10.1016/s0378-4347(00)82827-3. [DOI] [PubMed] [Google Scholar]
- 20.Blum M, Demierre A, Grant DM, Heim M, Meyer UA. Molecular mechanism of slow acetylation of drugs and carcinogens in humans. Proc Natl Acad Sci USA. 1991;88:5237–5241. doi: 10.1073/pnas.88.12.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin HJ, Han CY, Lin BK, Hardy S. Ethnic distribution of slow acetylator mutations in the polymorphic N-acetyltransferase (NAT2) gene. Pharmacogenetics. 1994;4:125–134. doi: 10.1097/00008571-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Tang-Liu D, Williams RL, Riegelman S. Disposition of caffeine and its metabolites in man. J Pharmacol Exp Ther. 1983;224:180–185. [PubMed] [Google Scholar]
- 23.Mulato AS, Ho ES, Cihlar T. Nonsteroidal anti-inflammatory drugs efficiently reduce the transport and cytotoxicity of adenovir mediated by the human renal organic anion transporter 1. J Pharmacol Exp Ther. 2000;295:10–15. [PubMed] [Google Scholar]
- 24.Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet. 1998;34:101–154. doi: 10.2165/00003088-199834020-00002. [DOI] [PubMed] [Google Scholar]
- 25.Bertz RJ, Granneman GR. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32:210–258. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 26.Møller JV, Sheikh MI. Renal organic anion transport system: pharmacological, physiological, and biochemical aspects. Pharmacol Rev. 1982;34:315–358. [PubMed] [Google Scholar]
- 27.Yesair DW, Branfman AR, Callahan MM. Human disposition and some biochemical aspects of methylxanthines. Prog Clin Biol Res. 1984;158:215–233. [PubMed] [Google Scholar]
- 28.Ahmad RA, Rogers HJ, Vandenburg M, Wright P. Effects of concurrent administration of other substrates of N-acetyltransferase on dapsone acetylation. Br J Clin Pharmacol. 1981;12:83–86. doi: 10.1111/j.1365-2125.1981.tb01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zysset T, Peretti E. Effect of concomitant isoniazid administration on determination of acetylator phenotype by sulfadimidine. Eur J Clin Pharmacol. 1986;30:463–466. doi: 10.1007/BF00607961. [DOI] [PubMed] [Google Scholar]
- 30.Brooks CD, Ulrich JE. Effect of ibuprofen or aspirin on probenecid-induced uricosuria. J Int Med Res. 1980;8:283–285. doi: 10.1177/030006058000800407. [DOI] [PubMed] [Google Scholar]
- 31.Sekine T, Cha SH, Endou H. The multispecific organic anion transporter (OAT) family. Eur J Physiol. 2000;440:337–350. doi: 10.1007/s004240000297. [DOI] [PubMed] [Google Scholar]
- 32.Sun W, Wu RR, van Poelje PD, Erion MD. Isolation of a family of organic anion transporters from human liver and kidney. Biochem Biophys Res Commun. 2001;283:417–422. doi: 10.1006/bbrc.2001.4774. [DOI] [PubMed] [Google Scholar]
- 33.Russel FGM, Masereeuw R, van Aubel RAMH. Molecular aspects of renal anionic drug transport. Annu Rev Physiol. 2002;64:563–594. doi: 10.1146/annurev.physiol.64.081501.155913. [DOI] [PubMed] [Google Scholar]
- 34.Inui KI, Masuda S, Saito H. Cellular and molecular aspects of drug transport in the kidney. Kidney Int. 2000;58:944–958. doi: 10.1046/j.1523-1755.2000.00251.x. [DOI] [PubMed] [Google Scholar]
- 35.Mulato AS, Ho ES, Cihlar T. Nonsteroidal anti-inflammatory drugs efficiently reduce the transport and cytotoxicity of adefovir mediated by the human renal organic anion transporter 1. J Pharmacol Exp Ther. 2000;295:10–15. [PubMed] [Google Scholar]
- 36.Cha SH, Sekine T, Fukushima J, et al. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59:1277–1286. doi: 10.1124/mol.59.5.1277. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez FJ, Idle JR. Pharmacogenetic phenotyping and genotyping. Present status and future potential. Clin Pharmacokinet. 1994;26:59–70. doi: 10.2165/00003088-199426010-00005. [DOI] [PubMed] [Google Scholar]
- 38.Meisel C, Roots I, Cascorbi I, Brinkmann U, Brockmöller J. How to manage individualized drug therapy: application of pharmacogenetics knowledge of drug metabolism and transport. Clin Chem Lab Med. 2000;38:869–876. doi: 10.1515/CCLM.2000.126. [DOI] [PubMed] [Google Scholar]
- 39.Streetman DS, Bleakley JF, Kim JS, et al. Combined phenotypic assessment of CYP1A2, CYP2C19, CYP2D6, CYP3A, N-acetyltransferase-2, and xanthine oxidase with the ‘Cooperstown cocktail’. Clin Pharmacol Ther. 2000;68:375–383. doi: 10.1067/mcp.2000.109519. [DOI] [PubMed] [Google Scholar]