Abstract
The loss of dopamine neurons combined or not with the subsequent administration of levodopa (l-DOPA) in patients with Parkinson’s disease or in experimental models of the disease results in altered GABAergic signaling throughout the basal ganglia, including the striatum and the substantia nigra, pars reticulata. However, the molecular mechanisms involved in altered GABA neurotransmission remain poorly understood. In order to be released from synaptic vesicles, newly synthesized GABA is transported from the cytosol into synaptic vesicles by a vesicular GABA transporter. The objective of this study was to examine the hypothesis that expression of the vesicular GABA transporter vGAT is altered in the unilateral 6-hydroxydopamine model of Parkinson’s disease. Our results provide evidence that a unilateral 6-hydroxydopamine lesion results in increased and decreased vGAT mRNA levels in striatopallidal and striatonigral neurons, respectively. These two subsets of neurons were identified by the co-expression or lack of co-expression of preproenkephalin, a marker of striatopallidal neurons, using double-labeling in situ hybridization histochemistry. Such changes occurred in the striatum ipsilateral to the 6-hydroxydopamine lesion and were paralleled by decreased vGAT protein levels in the SNr. On the other hand, the subchronic systemic administration of l-DOPA increased vGAT mRNA levels in preproenkephalin-negative neurons on the side ipsilateral and, to a lesser extent, the side contralateral to the 6-hydroxydopamine lesion. Systemic l-DOPA also increased vGAT protein levels in the ipsi- and contralateral SNr. As a whole, the results provide original evidence that vGAT expression is altered in the 6-hydroxydopamine model of Parkinson’s disease. They also suggest that the behavioral effects induced by a subchronic administration of l-DOPA to 6-hydroxydopamine-lesioned rats involve an increase in the vesicular release of GABA by striatonigral neurons.
Dopamine (DA) produced by neurons of the substantia nigra, pars compacta (SNc), plays a key role in the regulation of GABA (γ-aminobutyric acid) neurotransmission in the basal ganglia, including the striatum and substantia nigra, pars reticulata (SNr). As documented in experimental models of Parkinson’s disease, loss of DA neurons results in increased gene expression of the GABA-synthesizing enzyme, glutamic acid decarboxylase (GAD), in striatopallidal neurons (Lindefors et al., 1989; Segovia et al., 1990; Soghomonian et al., 1992; Soghomonian and Laprade, 1997) while the chronic administration of the dopamine precursor levodopa (l-DOPA) increases GAD expression in striatonigral neurons (Carta et al., 2001; 2003; Nielsen and Soghomonian, 2004; Zeng et al., 1995). These opposite effects on GAD gene expression in different subsets of striatal efferent neurons are paralleled by opposite changes in the expression of GABAA receptors in the globus pallidus and the SNr/entopeduncular nucleus complex (Pan et al., 1985; Gnanalingham and Robertson, 1993; Chadha et al., 2000; Nielsen and Soghomonian, 2004; Katz et al., 2005). In agreement with these studies, it is documented that systemic l-DOPA administration increases GABA release in the SNr in the dopamine-depleted side of adult rats with a unilateral 6-OHDA lesion of dopamine neurons (Ochi et al., 2004; Yamamoto et al., 2006). Altogether, these findings support the hypothesis that loss of dopamine neurons and subsequent administration of l-DOPA induces an increase in GABA-mediated signaling in striatopallidal and striatonigral neurons, respectively.
Before being released from GABAergic neurons in a vesicular-dependent manner, newly synthesized GABA is first transported from the cytosol into synaptic vesicles. This transport involves a vesicular transporter, whose activity is driven by the proton gradient between the cytosol and the lumen of the synaptic vesicle (Gasnier et al., 2000). A gene encoding for a vesicular transporter selective for inhibitory amino acids has been identified in mammals (McIntire et al., 1997; Sagné et al., 1997; Chaudhry et al., 1998). This vesicular transporter, known as vGAT (vGAT=vesicular GABA transporter), is widely expressed in synaptic vesicles of GABA neurons in the rodent brain (Sagné et al., 1997; Chaudhry et al., 1998). Various experimental manipulations have been found to alter the expression of vGAT protein and/or its gene, resulting in an alteration of vesicular GABA release (Kang et al., 2003; Vemuganti, 2005; Zink et al., 2004; 2005; Erickson et al., 2006). Although there is evidence that the expression of markers of GABAergic activity is altered in experimental models of Parkinson’s disease, the possibility that vGAT and vesicular GABA release is altered in these models remains unclear. The objective of this study was to 1-examine the effects of a unilateral 6-OHDA lesion of dopamine neurons in adult rats followed or not by a subchronic administration of l-DOPA on vGAT mRNA levels in subsets of striatal neurons co-expressing or not preproenkephalin (PPE), a marker of striatopallidal neurons and 2-examine the effects of the treatments on vGAT protein distribution and levels in the SNr, a major target of striatal efferent neurons. Our results provide original evidence that the 6-OHDA lesion of dopamine neurons or the subsequent systemic administration of l-DOPA alters vGAT expression in different subsets of striatal neurons and in the SNr.
EXPERIMENTAL PROCEDURES
Animals and drug treatments
Adult male Sprague-Dawley rats (Charles River, Wilmington, MA) were maintained under a 12-hour light/dark cycle with constant temperature and humidity. Food and water was available ad libitum. All experimental procedures were performed in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines at Boston University School of Medicine. Surgical procedures were performed under anesthesia with a mixture of ketamine (80mg/kg) and xylazine (10mg/kg) injected intraperitoneally (i.p.).
Rats were unilaterally depleted of dopamine following stereotaxic injections of 8μg of freebase 6-hydroxydopamine (6-OHDA hydrobromide; Sigma Chemical Co., St. Louis, MO) into the left rostral substantia nigra, pars compacta (SNc) (H=2.8 mm, AP=3.4 mm, L=2.0 mm), and the left median forebrain bundle (H=1.9 mm, AP=4.2 mm, L=1.2 mm) with the incisor bar at 0 mm. A group of sham-operated rats was injected at the same stereotaxic coordinates with vehicle (saline with 0.1% ascorbic acid). Vehicle or 6-OHDA was injected with a Hamilton syringe over 2 minutes, and the syringe was kept in place for another 5 minutes after the injection. Three to four weeks following the surgery, a subset of 6-OHDA-lesioned rats received s.c. injections of levodopa (l-DOPA methyl-ester; Sigma Chemical Co., St. Louis, MO) at a dose of 50 mg/kg/day twice-daily (saline with 0.1% ascorbic acid including the peripheral decarboxylase inhibitor benserazide at a dose of 15 mg/kg; Sigma Chemical Co., St. Louis, MO). The remaining 6-OHDA-lesioned or sham-operated rats received twice-daily vehicle injections for 7 days. The dose of l-DOPA was chosen to allow comparisons with earlier studies of GAD or GABAA receptor expression (i.e. Nielsen and Soghomonian, 2004; Katz et al., 2005). All rats were injected with l-DOPA or with vehicle between 9:00 and 11:00 AM. On alternate days, rats were observed for three hours after the injection of l-DOPA in order to assess the rotational response to l-DOPA. The mean number of rotation for each testing day was calculated and the differences in rotational scores between days were calculated with a non-parametric Mann-Whitney test, significance at p<0.05. Three hours after the last injection of l-DOPA or vehicle, rats were rendered unconscious by CO2 inhalation and sacrificed by decapitation and the brains quickly frozen on dry ice. Ten μm-thick brain sections were produced on a cryostat at the level of the striatum (interaural=10.00) or the substantia nigra (interaural=3.80) according to Paxinos and Watson, 1986, thaw-mounted on chromalum gelatin-coated glass slides and stored at −80°C until further processing.
3H-mazindol binding radioautography
3H-mazindol binding was used to assess the extent of loss of dopamine re-uptake sites in the striatum. Fresh-frozen tissue sections were dried under a flow of air. Two sections per rat were rinsed for 5 minutes at 4° C in 50 mM Tris buffer with 120 mM NaCl and 5 mM KCl to wash off endogenous ligand. Sections were then incubated for 40 minutes at 4° C in 15 nM 3H-mazindol (PerkinElmer Life Sciences, Boston, MA, specific activity 21.0 Ci/mmol) in 50 mM Tris buffer with 300 mM NaCl and 5 mM KCl. Desipramine (0.3mM; Sigma Chemical Co., St. Louis, MO) was added to block binding to norepinephrine transporters. Nonspecific binding was determined in the presence of 30μM unlabeled benztropine (Sigma Chemical Co., St Louis, MO). Sections were then quickly rinsed in ice-cold buffer, distilled water and air-dried. All sections were apposed to Kodak Biomax MR X-ray films at room temperature for 35–45 days. The films were developed in Kodak D-19 for 3.5 minutes at 14° C.
In situ hybridization histochemistry
Coronal brain sections at the level of the striatum were quickly dried under a constant flow of air at room temperature and immediately fixed for 5 min in 3% paraformaldehyde in a phosphate buffer (pH 7.2). Sections were then sequentially rinsed in 2×SSC, phosphate buffer saline (0.4M), 0.25% acetic anhydride with triethanolamine for 10 min, Tris-glycine for 30 min and dehydrated in ethanol. Sections were hybridized with a 35S-labeled oligonucleotide for vGAT (bp 1192–1246; McIntire et al., 1997) or with a 35S-labeled cRNA probe. The oligonucleotide was labeled by the 3′-tailing addition of 35S-ATP (PerkinElmer Life Sciences, Boston). The cRNA was synthesized by in vitro transcription in the presence of 35S-UTP (PerkinElmer Life Sciences, Boston) using a 2814 pb mouse cDNA encoding for vGAT (Open Biosystems, Huntsville, AL; accession number BC052020) inserted into pYX-A. The synthesis was carried out with an excess of unlabeled ATP, CTP and GTP using a ribroprobe transcription system (Promega, Madison, WI). Each section was covered with 3×105 cpm in 50μl of radiolabeled oligonucleotide or 3.5ng in 20μl of radiolabeled riboprobe in a hybridization solution containing 40% formamide, 10% dextran sulfate, 4×SSC, 10mM dithiothreitol, 1.0% sheared salmon sperm DNA, 1.0% yeast tRNA and 1X Denhardt’s solution. Hybridization was overnight at 42°C (oligonucleotide probe) or for 4 hours at 52°C (cRNA probe). Post-hybridization washes were in 1×SSC for 1 hr and 0.5×SSC for 30 min for the oligonucleotide probe or in 50% formamide in 2×SSC for 30 min and RNAseA for 30 min for the cRNA probe as previously described (Nielsen and Soghomonian, 2003). Following dehydration, the slides were apposed to Kodak BioMax MR films for 10–15 days.
Double-labeling experiments were conducted using a combination of the 35S-labeled vGAT riboprobe and a digoxigenin-labeled (DIG-labeled) preproenkephalin (PPE) ribroprobe. Synthesis of the DIG–labeled PPE probe was performed by in vitro transcription from a cDNA encoding for PPE (Yoshikawa et al., 1984; inserted into pSP64) in the presence of digoxigenin-labeled UTP (Roche Applied Science, Indianapolis, IN). Sections were covered with 200–400ng of the DIG-labeled PPE probe and 3.5ng of the radioactive vGAT probe. Pre-hybridization and post-hybridization washes were as described above. At the end of the post-hybridization washes, sections were covered with 80μl of an anti-digoxigenin Fab fragment conjugated with alkaline phosphatase (Roche Applied Science, Indianapolis, IN) and incubated overnight at 4°C. Sections were then incubated in the dark for 1.5–3 hours in a solution containing 75mg/ml nitroblue tetrazolium chloride and 50mg/ml X-phosphate (Roche Applied Science, Indianapolis, IN) and 0.24mg/ml levamisol (Sigma, St. Louis, MO). The reaction was stopped in 10 mM Tris buffer with 1mM EDTA (pH 8.0) for 15 minutes. Following a quick dehydration in 70% ethanol, sections were dipped into Amersham LM-1 nuclear emulsion, air dried and stored at room temperature in light-tight boxes with desiccant for 7–12 days. Sections were developed in Kodak D-19 developer for 3.5 min. at 14° C and mounted with crystal mount™ water-based medium (Biomedia, Foster City, CA).
Quantification of film and emulsion radioautographs
vGAT mRNA levels in the dorsal striatum were first quantified on X-ray films as previously described (Nielsen and Soghomonian, 2004) in an area illustrated on Figure 2A. Briefly, films were viewed on a light-box and digitized using a Sony CCD video-camera connected to a Macintosh computer. Relative optical density (OD) measurements were obtained by computerized densitometry utilizing NIH Image 1.61 software after standardization against Kodak gelatin filters. A minimum of two sections per animal was quantified. Differences between ipsi- and contralateral sides and between groups were analyzed with a Mann-Whitney test (p<0.05 considered significant).
Figure 2.

Negative images of x-ray films from sections at the level of the striatum processed by in situ hybridization histochemistry with a 35S-labeled vGAT cRNA probe. Sections are from a sham-operated rat injected with vehicle (A), a rat lesioned with 6-OHDA and injected with vehicle (B) and a rat lesioned with 6-OHDA and injected with l-DOPA (C). Left side is ipsilateral to the 6-OHDA lesion. The striatal area selected for quantitative analysis is illustrated with a black line on the contralateral striatum. Scale bar=1.7mm.
Levels of vGAT mRNA in neuronal profiles labeled or not with PPE in the striatum were quantified at the single cell level on sections processed for in situ hybridization and emulsion radioautography as previously described (Nielsen and Soghomonian, 2004). The area covered by silver grains in individual neurons was visualized at 60x with a Nikon microscope under bright-field illumination (for the quantification of vGAT in PPE-negative neurons) or under a combination of bright- and dark-field illumination using a Dark-lite™ stage (MicroVideo Instruments, Avon, MA) (for the quantification of vGAT in PPE-positive neurons). For each section analyzed, the microscope stage was moved in the x and the y axis in order to systematically scan a selected striatal area. The first 40–50 labeled neuronal profiles encountered during the scanning of the striatum were included in the analysis. A comparable sector in the dorsal half of the dorsal striatum was selected across experimental groups for the analysis. This sector was considered representative of the dorsal striatum based on the relatively homogenous vGAT mRNA labeling seen on x-ray films in different aspects, ventral to medial and dorsal to ventral, of the structure. The microscopic fields were captured using a Sony CCD camera connected to a MacIntosh computer. The area covered by silver grains in each neuron was measured by computerized image analysis using the density slice function of NIH image (www.zippy.nimh.nih.gov). Values were expressed as a number of pixels per neuron. vGAT mRNA levels were quantified on an average of 40–50 PPE-negative and 40–50 PPE-positive neuronal profiles per section and per side and 2 sections per rat were analyzed. For the analysis of emulsion radioautographs, the investigator was blind to the experimental groups. The average number of pixels per neuron for each rat and each brain side was the mean number of 80–100 neuronal profiles. Statistical comparisons between the mean numbers of pixels per neuronal profile in the ipsi- and contralateral sides and in the different experimental groups were carried out with a Mann-Whitney test (p<0.05 considered significant).
Western-blots
Fresh-frozen brains were sliced as 500μm-thick sections and punches taken from the SNr. The tissue was sonicated in ice-cold Tris buffer (10 mM; pH 7.4) containing 1% SDS and a proteinase inhibitor cocktail. The samples were denatured at 95°C for 5 minutes and run on an electrophoresis Bio-Rad precast Tris-gel (10% polyacrylamide). Proteins were then transferred to a PVDF membrane (Millipore Co, Billerica, MA). The membrane was blocked with 5% non-fat milk for 1 hour at room temperature to minimize non-specific binding and then probed with a vGAT or tubulin antibody (Chemicon, Temula, CA). The bands were visualized on x-ray films by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). The bands were digitized using a light-box and a Sony CCD video camera connected to a Macintosh computer. The intensity of the bands was measured by densitometry with NIH image. The values for vGAT were normalized against the intensity of the tubulin bands. Statistical differences between sides and groups were analyzed with a Mann-Whitney test with p<0.05 considered significant.
Immunohistochemistry
Immunohistochemistry was performed using a peroxidase-based method (Vectastain Elite ABC Kit; Vector Laboratories) on coronal 10μm thick fresh-frozen sections. Sections were fixed in 4% paraformaldehyde for 5 min and washed with 0.2M potassium-phosphate buffer saline (KPBS) plus Triton X-100 and preincubated in the same buffer containing 5% normal goat serum (NGS). Subsequently, sections were incubated overnight at 4°C using a polyclonal primary antibody against vGAT (1:1000; Chemicon, Temecula, CA) in the presence of 1% NGS and 0.2% Triton X-100. The next day, sections were washed in 0.2M KPBS plus Triton X-100 and incubated in an affinity-purified biotinylated goat anti-rabbit IgG (1:500; Chemicon, Temecula, CA) for 1 hour at room temperature. Sections were then washed in 0.2M KPBS plus Triton X-100 and incubated in an avidin-biotin complex (ABC, Vector Laboratories, Burlingame, CA). Immunostaining was revealed following incubation of the sections in a 1x DAB/Metal concentrate in presence of 1x peroxide buffer (Pierce Labs, Rockford, IL). Sections were then dehydrated and mounted in Eukitt mounting medium (Electron Microscopy Sciences, Hatfield, PA).
RESULTS
Effects of 6-OHDA and l-DOPA on vGAT mRNA levels in the striatum
In agreement with previous observations (Nielsen and Soghomonian, 2003), the administration of l-DOPA induced a rotational behavior contralateral to the 6-OHDA lesion. The number of rotations increased progressively from day 1 to day 7 following a twice-daily administration of l-DOPA (701±111 vs. 1989±279.3 for day 1 and day 7, respectively; p=0.0012 with a Mann-Whitney test; n=7). The rotational behavior and its sensitization were indicative of a significant loss of dopamine neurons. The extent of loss of dopamine neurons induced by 6-OHDA in rats injected with vehicle or l-DOPA was further assessed by post-mortem detection of 3H-mazindol binding on film radioautographs. The observation of radioautographs confirmed that 6-OHDA-injected rats had a marked loss of striatal dopamine re-uptake sites in the striatum on the side ipsilateral to 6-OHDA (Figure 1).
Figure 1.

Photomicrograph of a cryostat-cut section processed for 3H-Mazindol binding to dopamine transporters at the level of the striatum. Left side is ipsilateral to a 6-OHDA injection in the SNc/MFB and illustrates the marked decrease in 3H-Mazindol binding in the striatum. Scale bar=2.5mm.
The examination and quantification of film or emulsion radioautographs labeled with a vGAT 35S-labeled oligonucleotide or cRNA probe yielded similar results. In the following paragraphs, we present results gathered with the vGAT cRNA probe, which gave a more sensitive signal. On x-ray film radioautographs, intense vGAT mRNA labeling was seen in the striatum and cerebral cortex (Figure 2). vGAT mRNA levels were first quantified on film radioautographs by computerized densitometry over the striatum as shown on Figure 2A. In 6-OHDA-lesioned rats injected with vehicle, vGAT mRNA levels were significantly higher in the ipsi- compared to contralateral striatum (Figure 2B and 3). Labeling in the contralateral striatum of 6-OHDA-lesioned rats injected with vehicle was not significantly different from labeling in the ipsi- or contralateral striatum of sham-operated rats (Figure 2A and B). In 6-OHDA-lesioned rats injected with l-DOPA, vGAT mRNA levels were higher in the ipsi- when compared to contralateral striatum (Figure 2C and 3). Further comparisons between groups indicated that vGAT mRNA levels in the ipsilateral striatum were higher in 6-OHDA-lesioned rats injected with l-DOPA compared to rats injected with vehicle (Figure 2B, 2C and 3). vGAT mRNA levels in the contralateral striatum were slightly higher in 6-OHDA-lesioned rats injected with l-DOPA compared to rats injected with vehicle (Figure 2B, 2C and 3).
Figure 3.

vGAT mRNA levels were quantified on x-ray films over the striatum on the side ipsi-(I) or contralateral (C) to the 6-OHDA injection in rats injected with vehicle (Veh) or l-DOPA (LD). Values are mean ± SEM (n=7) and are expressed as a percent of the contralateral side of vehicle-injected rats. *p<0.005 when compared to the contralateral side. # when compared to vehicle-injected rats for the respective contralateral (p<0.05) or ipsilateral (p<0.005) side (Mann-Whitney non-parametric test).
In a second experiment, vGAT mRNA labeling was observed and quantified on emulsion radioautographs at the single-cell level on sections double-labeled with a combination of a digoxigenin-labeled PPE and a 35S-labeled vGAT cRNA probe. vGAT mRNA labeling was distributed throughout the striatum in neuronal profiles labeled or unlabeled with PPE (Figure 4). Quantitative analysis of vGAT mRNA levels in PPE-negative neurons was carried out by bright-field microscopy as described in the methods section. In 6-OHDA-lesioned rats injected with vehicle, vGAT mRNA levels in PPE-negative neurons were significantly lower in the ipsi-compared to contralateral striatum (Figure 4A, 4B and 6A). In contrast, in 6-OHDA-lesioned rats injected with l-DOPA, vGAT mRNA levels in PPE-negative neurons were significantly higher in the ipsi- compared to contralateral striatum (Figure 4C, 4D and 6A). Further comparisons between groups indicated that vGAT mRNA levels in PPE-negative neurons in the ipsi- or contralateral striatum of rats injected with l-DOPA were significantly higher than in the respective side of rats injected with vehicle (Figure 4A, 4C and 6A).
Figure 4.
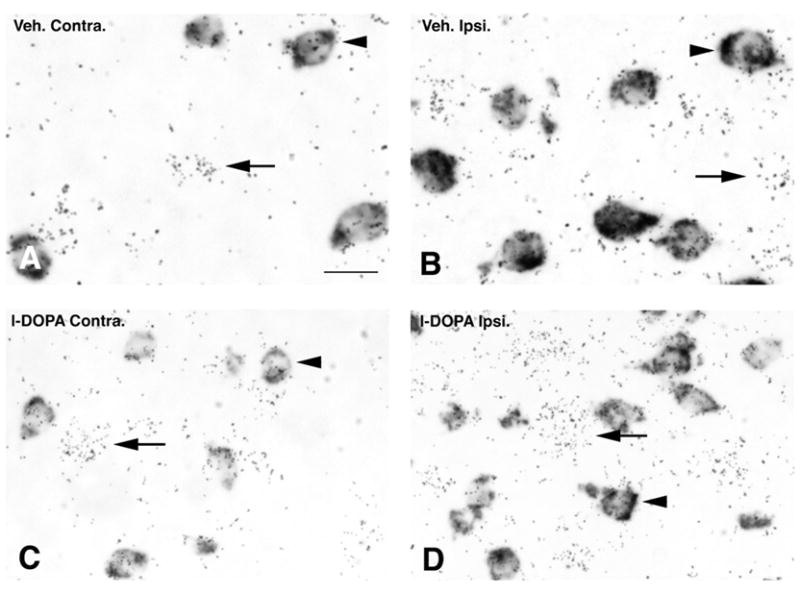
Bright-field photomicrographs of emulsion radioautographs from sections processed for in situ hybridization histochemistry with a radioactive probe for vGAT and a non-radioactive probe for preproenkephalin. Radioactive labeling appears as clusters of silver grains (arrows) whereas the non-radioactive label appears as a dark precipitate over striatal cell bodies (arrowheads). Labeling is illustrated in a 6-OHDA-lesioned rat injected with vehicle (A and B) or l-DOPA (C and D) in the striatum on the side contralateral (A and C) or ipsilateral (B and D) to the 6-OHDA lesion. Scale bar=15μm.
Figure 6.

Quantification of vGAT mRNA levels at the single cell level in the contralateral (C) or ipsilateral (I) striatum of rats unilaterally lesioned with 6-OHDA and subsequently injected with vehicle (Veh) or l-DOPA (50mg/kg; twice-daily for 7 days) (LD). Values are mean ± SEM (n=5) and were gathered in neuronal profiles co-labeled (PPE-positive) (B) or not (PPE-negative) (A) with a non-radioactive oligonucleotide probe for prepropenkephalin. (A) *p<0.05 when compared to the contralateral side. # when compared to vehicle-treated rats for the contralateral (p<0.05) or ipsilateral (p<0.001) side. (B) *p<0.05 when compared to the respective contralateral side. (Mann-Whitney non-parametric test).
Quantitative analysis of vGAT mRNA levels in PPE-positive neuronal profiles was carried out on emulsion radioautographs using a combination of bright- and dark-field microscopy (Figure 5A–D). In 6-OHDA-lesioned rats injected with vehicle, vGAT mRNA levels in PPE-positive neurons were significantly higher in the ipsi- compared to contralateral striatum (Figure 5A, 5B and 6B). In 6-OHDA-lesioned rats injected with l-DOPA, vGAT mRNA levels in PPE-positive neurons were also significantly higher in the ipsi- compared to contralateral striatum (Figure 5C, 5D and 6B). Further comparisons between groups indicated that vGAT mRNA levels in PPE-positive neurons in the striatum ipsi- or contralateral to 6-OHDA were not significantly different between vehicle- and l-DOPA-injected rats (Figure 6B).
Figure 5.
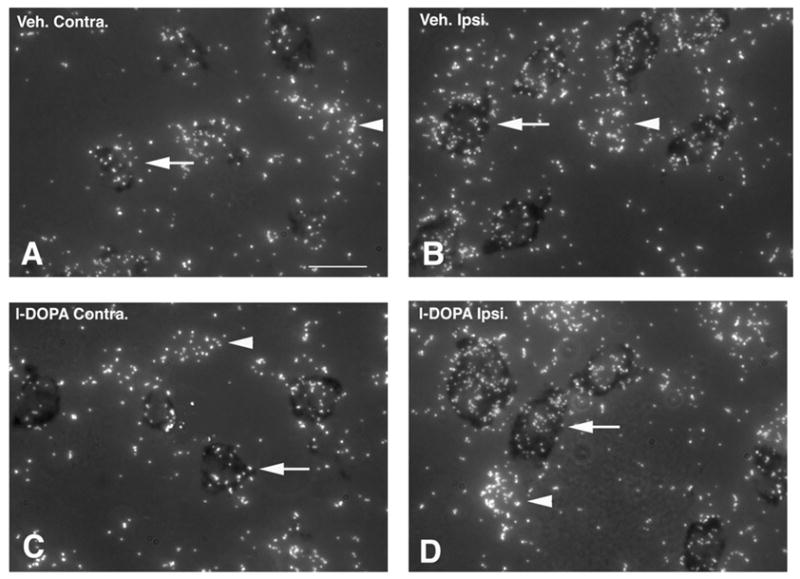
Combined bright- and dark-field photomicrographs of emulsion radioautographs from sections processed for in situ hybridization histochemistry with a radioactive probe for vGAT and a non-radioactive probe for preproenkephalin. Radioactive labeling appears as clusters of white silver grains whereas the non-radioactive label appears as a dark precipitate over striatal cell bodies (arrows). Labeling is illustrated in a 6-OHDA-lesioned rat injected with vehicle (A and B) or 50mg/kg l-DOPA for 7 days (C and D) in the striatum on the side contralateral (A and C) or ipsilateral (B and D) to the 6-OHDA lesion. Scale bar=15μm.
Effects of 6-OHDA and l-DOPA on vGAT protein levels in the SNr
First, vGAT in the SNr was detected by immunohistochemistry on tissue sections. Bright-field examination of sections revealed intense immunostaining in the SNr compared to adjacent brain regions (Figure 7A). At high magnification, vGAT immunoperoxidase staining was primarily detected in small profiles but was absent from cell bodies (Figure 7B). Observation of sections at low magnification revealed differences in immunostaining intensity between the SNr ipsi- and contralateral to the 6-OHDA lesion. Most noticeably, in 6-OHDA-lesioned rats injected with vehicle, vGAT immunostaining was more intense in the contra- compared to ipsilateral SNr (Figure 7C). In contrast, in 6-OHDA-lesioned rats injected with l-DOPA, vGAT immunostaining was uniformly intense and comparable between the ipsi- and contralateral SNr (Figure 7D).
Figure 7.

vGAT immunolabeling at the level of the substantia nigra. (A) illustrates vGAT immunostaining on a coronal brain section in the SNr and surrounding brain regions. (B) higher magnification of vGAT immunostaining in the SNr. (C) vGAT immunostaining in the ipsi- (i) or contralateral (c) SNr of a 6-OHDA-lesioned rat injected with vehicle (D) vGAT immunostaining in the ipsi- (i) or contralateral (c) in the SNr of a 6-OHDA-lesioned rat injected with l-DOPA.
In a second experiment, quantitative analysis of vGAT protein expression in the SNr was carried out on Western-blots (Figure 8). A band at the expected apparent molecular weight of 55–60kD (Chaudhry et al., 1998) was detected with the vGAT antibody in all experimental groups. In 6-OHDA-lesioned rats injected with vehicle, vGAT protein levels were significantly lower in the SNr on the side ipsi- compared to contralateral to 6-OHDA (Figure 8). In 6-OHDA-lesioned rats injected with l-DOPA, vGAT protein levels were not significantly different between the side ipsi- and contralateral to 6-OHDA. Further comparisons between groups showed that vGAT levels in rats injected with l-DOPA were significantly higher in the ipsi- or contralateral SNr compared to the respective SNr of rats injected with vehicle (Figure 8).
Figure 8.

Western-blot analysis of vGAT protein levels in the ipsi- and contralateral SNr. The left upper corner shows a representative immunoblot of the contralateral (C) and ipsilateral (I) SNr of a rat injected with vehicle or l-DOPA. Bars (values are mean ± SEM; n=4) illustrate the quantitative analysis of vGAT protein levels in the SNr of 6-OHDA-lesioned rats injected with vehicle (Veh) or l-DOPA (LD) on the side ipsi- (I) or contralateral (C) to the 6-OHDA lesion. *p<0.05 when compared to the contralateral side. #p<0.05 when compared to the respective side of rats injected with vehicle (Mann-Whitney non-parametric test).
DISCUSSION
Results in this paper provide original evidence that a unilateral 6-OHDA lesion of DA neurons in the adult rat decreases and increases vGAT mRNA levels in PPE-negative and PPE-positive striatal neurons, respectively. In addition, the systemic subchronic administration of l-DOPA to 6-OHDA-lesioned rats increases vGAT mRNA levels in PPE-negative striatal neurons in the ipsi- and, to a lesser extent, the contralateral striatum. The effects of a 6-OHDA lesion or l-DOPA in PPE-negative neurons were paralleled by changes in vGAT protein levels in the SNr. As a whole, these results indicate that vGAT expression is regulated in a different manner by dopamine in different subsets of striatal neurons.
Effects of unilateral 6-OHDA lesions on striatal GABA neurons
It is well documented that striatal efferent neurons use GABA as their neurotransmitter and co-express the two isoforms of the rate-limiting synthesizing enzyme of GABA, glutamic acid decarboxylase, GAD65 and GAD67 (Mercugliano et al., 1992; Laprade and Soghomonian, 1997). Striatal GABA efferent neurons in the adult rat can be subdivided into two subsets based on anatomical and chemical differences. Striatopallidal neurons primarily project to the globus pallidus and co-express the peptide enkephalin whereas striatonigral neurons primarily project to the SNr and the entopeduncular nucleus and co-express the peptides dynorphin and substanceP (Gerfen and Young, 1988). The present results using double-labeling in situ hybridization demonstrate that striatal neurons labeled or unlabeled with PPE, the prepropeptide precursor of enkephalin, also co-express vGAT. We thus conclude that vGAT is expressed in striatonigral as well as striatopallidal efferent neurons, a conclusion consistent with the hypothesis that GABA can be synthesized and released via a vesicular mechanism by axon terminals of striatal efferent neurons.
The finding that a unilateral 6-OHDA lesion increases and decreases vGAT mRNA levels in PPE-positive and PPE-negative neurons, respectively, is consistent with published evidence that 6-OHDA induces opposite effects on peptide gene expression in these two subsets of neurons. For instance, it is well established that loss of dopamine neurons results in increased PPE expression in striatopallidal and decreased preprotachykinin and preprodynorphin mRNA expression in striatonigral neurons (e.g. Gerfen et al., 1990). Double-labeling studies indicate that GAD mRNA levels are increased in striatal PPE-positive neurons in the 6-OHDA-lesioned rat or the MPTP-treated primate (Soghomonian and Laprade, 1997; Carta et al., 2003). On the other hand, previous studies did not report a decrease in GAD mRNA levels in PPE-negative striatal neurons (Soghomonian et al., 1997; Carta et al., 2003). Although it is possible that the expression of the GAD and vGAT genes is differentially altered in PPE-negative neurons following the lesion of dopamine neurons, the lack of parallel could also be explained by technical limitations. Indeed, because GAD mRNA is expressed at very low levels in PPE-negative neurons, the sensitivity of the in situ hybridization technique may not be sufficient to detect small decreases in mRNA levels. In any case, the finding that the 6-OHDA lesion had a dual effect on vGAT expression in presumed striatopallidal and striatonigral neurons supports the idea that GABA neurotransmission is oppositely affected in the two major subsets of striatal efferent neurons. This idea is otherwise supported by ligand binding and in situ hybridization studies showing that the expression of GABAA receptors or their subunits is decreased in the globus pallidus and increased in the SNr or entopeduncular nucleus on the side ipsilateral to a unilateral 6-OHDA lesion in the adult rat (Pan et al., 1985; Gnanalingham and Robertson, 1993; Chada et al., 2000; Nielsen and Soghomonian, 2004; Katz et al., 2005). Our results thus suggest that the altered expression of GABAA receptors in the globus pallidus or SNr induced by a 6-OHDA lesion of dopamine neurons represents a compensatory response to altered vesicular GABA release from striatal afferent inputs.
In our study, the 6-OHDA-induced decrease in vGAT mRNA levels in striatonigral neurons was paralleled by decreased vGAT protein levels in the ipsilateral SNr. Previous studies have shown that although vGAT mRNA is distributed in cell bodies of GABA neurons, the protein accumulates in synaptic vesicles of inhibitory axon terminals (Chaudhry et al., 1998; Dumoulin et al., 1999). Our immunohistochemical observations indicated that vGAT immunostaining in the SNr was distributed throughout the neuropil but was conspicuously absent from cell bodies. Thus, we speculate that vGAT protein in the SNr was primarily distributed in striatal GABAergic afferents to the SNr. Therefore, the decrease in vGAT protein levels induced by 6-OHDA could have been secondary to a decrease in vGAT transcription in cell bodies of striatonigral neurons. Because SNr neurons also receive a GABA input from the globus pallidus (Smith et al., 1998), it cannot be ruled out that some of the vGAT protein levels detected in the SNr could also be localized in pallidal afferents to the SNr. However, experimental evidence indicates that a 6-OHDA lesion of dopamine neurons increases GAD gene expression in neurons of the globus pallidus (Soghomonian and Chesselet, 1992; Nielsen and Soghomonian, 2003), arguing against the possibility that this projection contributed to the decrease in vGAT expression detected in the ipsilateral SNr.
Effects of subchronic l-DOPA administration on striatal GABA neurons
As discussed in the previous paragraphs and based on the finding that the subchronic administration of l-DOPA to 6-OHDA-lesioned rats increases vGAT mRNA levels in PPE-negative neurons and vGAT protein levels in the SNr, we hypothesize that l-DOPA increased vGAT expression in striatonigral neurons. This hypothesis is consistent with previous reports that l-DOPA increases GAD mRNA levels in striatonigral neurons (Carta et al., 2003; Nielsen and Soghomonian, 2004) and GABA release in the SNr (Ochi et al., 2004; Yamamoto et al., 2006). Anatomical studies have shown that the subchronic administration of l-DOPA to rats with a unilateral 6-OHDA lesion decreases flunitrazepam binding to GABAA receptors or the expression of individual subunits of GABAA receptors in the SNr on the side ipsilateral to the lesion (Nielsen and Soghomonian, 2004; Katz et al., 2005), an effect that can be interpreted as a compensatory down-regulation of post-synaptic GABAA receptors in response to increased GABA release in the SNr. Our results thus complement earlier studies and suggest that the effects of l-DOPA on striatonigral GABA neurons involve an alteration in the vesicular release of GABA.
The finding that systemic l-DOPA induced an increase in vGAT expression in PPE-negative striatal neurons not only in the dopamine-depleted but also, though to a lesser extent, in the dopamine-intact contralateral striatum is consistent with earlier studies that assessed the effects of direct agonists of dopamine receptors on GABA neurons in the normal rat. For instance, it is documented that the intrastriatal administration of direct agonists of dopamine receptors releases GABA in the SNr (You et al., 1994) or the entopeduncular nucleus (Ferré et al., 1996) or that the systemic administration of agonists increases GAD65 mRNA levels in PPE-negative striatonigral neurons (Laprade and Soghomonian, 1995; 1997). However, the possibility that the systemic administration of l-DOPA modulates GABA neurotransmission in the dopamine-intact striatum or SNr in the unilateral 6-OHDA rodent model of Parkinson’s disease is not established. In particular, an effect of systemic l-DOPA on GAD mRNA levels in striatonigral neurons or GABAA receptors in the SNr on the side contralateral to a 6-OHDA lesion was not reported in previous studies (Carta et al., 2003; Nielsen and Soghomonian, 2004; Katz et al., 2005). These observations could be explained by a different responsiveness of genes encoding for different markers of GABAergic activity. However, it is also possible that small effects of l-DOPA on the dopamine-intact side were overlooked in previous studies because of the hypersensitivity of dopamine-mediated responses on the dopamine-depleted side. For instance, it is well documented that the systemic administration of direct or indirect agonists of dopamine receptors triggers an enhanced and abnormal pattern of gene expression in striatonigral neurons in the dopamine-depleted striatum (i.e. Cenci et al., 2002; Konradi et al., 2004). Furthermore, systemic l-DOPA administration results in large increases in dopamine levels in the dopamine-depleted striatum but much smaller increases are detected in the dopamine-intact striatum (Abercrombie et al., 1990; Miller and Abercrombie, 1999), increases which could be expected to have more modest effects on striatal neurons. Our results thus provide support for the hypothesis that systemic l-DOPA alters GABA neurotransmission in the dopamine-depleted and dopamine-intact striatum in the unilateral 6-OHDA rodent model of Parkinson’s disease.
If, as we hypothesize, l-DOPA-induced increases in vGAT protein levels in the SNr are linked to increases in vGAT mRNA expression in PPE-negative striatal neurons, l-DOPA-induced asymmetrical vGAT mRNA labeling between the striatum ipsi- and contralateral to 6-OHDA should be matched by asymmetrical vGAT protein levels in the SNr. However, in our study, vGAT protein levels appeared comparably high in the ipsi- and contralateral SNr of l-DOPA-treated rats. This suggests that relatively modest increases in vGAT mRNA levels in the striatum contralateral to 6-OHDA could result in robust increases in vGAT protein levels in the SNr. Alternately, GABA neurons other than the striatonigral projection may have contributed to the increases in vGAT protein levels detected in the SNr in rats injected with l-DOPA. On the other hand, the possibility that the apparent mismatch between relative changes in mRNA and protein levels were due to variations in the sampling of SNr tissue in our immunoblot experiments is unlikely since vGAT protein levels in the ipsi- and contralateral SNr were also comparable on sections processed for immunohistochemistry.
Functional considerations
vGAT is constitutively phosphorylated on serine and threonine residues but phosphorylation does not appear to be a mechanism involved in the regulation of vesicular loading (Bedet et al., 2000). The finding that vGAT mRNA levels are altered following manipulations of the DA system suggests that transcriptional regulation may be a primary mechanism for the modulation of vesicular GABA uptake and release in the basal ganglia in response to long-term changes in dopaminergic activity. There is evidence that modulation of the expression of amino-acid transporters can alter quantal size (Song et al., 1997; Colliver et al., 2000; Gasnier et al., 2000; Pothos et al., 2000; DeGois et al., 2005; Erickson et al., 2006). In this context, it can be speculated that manipulation of the dopaminergic system could alter the frequency of vesicular GABA release and/or the quantal size of individual GABA-mediated events in the basal ganglia, possibilities that should be assessed in future investigations.
Theoretical models of the basal ganglia suggest that long-term loss of dopamine neurons results in increased GABA neurotransmission in the indirect striatopallidal pathway whereas the systemic administration of l-DOPA leads to increased GABA neurotransmission in the striatonigral pathway (Albin et al., 1989). Results in this study are consistent with this model and provide original evidence that such changes may involve altered vesicular GABA release. The long-term chronic administration of l-DOPA to patients with Parkinson’s disease is known to eventually lead to the appearance of abnormal movements such as dyskinesias. There is evidence that abnormal cellular signaling in the direct striatonigral pathway plays a key role in l-DOPA-induced dyskinesias (review in Canales and Graybiel, 2000; Cenci, 2002). Our findings support the concept that enhanced GABA neurotransmission in the striatonigral pathway is involved in l-DOPA-induced dyskinesias and they provide new evidence that enhanced vesicular GABA release may be involved in this disorder.
Acknowledgments
This work was sponsored by the National Institutes of Health NS40783 (JJS).
Abbreviations
- 6-OHDA
6-hydroxydopamine
- ATP
adenosine triphosphate
- CTP
cytosine triphosphate
- EP
entopeduncular nucleus
- GABA
gamma aminobutyric acid
- GAD
glutamic acid decarboxylase
- GTP
guanine triphosphate
- IA
interaural
- i.p
intraperitoneal
- L-DOPA
levodopa
- mRNA
messenger ribonucleic acid
- PPE
preproenkephalin
- s.c
subcutaneous
- SNc
substantia nigra, pars compacta
- SNr
substantia nigra, pars reticulata
- UTP
uracil triphosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of L-dopa on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 1990;525:36–44. doi: 10.1016/0006-8993(90)91318-b. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Bedet C, Isambert MF, Henry JP, Gasnier B. Constitutive phosphorylation of the vesicular inhibitory amino acid transporter in rat central nervous system. J Neurochem. 2000;75:1654–1663. doi: 10.1046/j.1471-4159.2000.0751654.x. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. Patterns of gene expression and behavior induced by chronic dopamine treatments. Ann Neurol. 2000;47:S53–59. [PubMed] [Google Scholar]
- Carta A, Fenu S, Morelli M. Alterations in GAD67, dynorphin and enkephalin mRNA in striatal output neurons following priming in the 6-OHDA model of Parkinson’s disease. Neurol Sci. 2001;22:59–60. doi: 10.1007/s100720170046. [DOI] [PubMed] [Google Scholar]
- Carta AR, Fenu S, Pala P, Tronci E, Morelli M. Selective modifications in GAD67 mRNA levels in striatonigral and striatopallidal pathways correlate to dopamine agonist priming in 6-hydroxydopamine-lesioned rats. Eur J Neurosci. 2003;18:2563–2572. doi: 10.1046/j.1460-9568.2003.02983.x. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- Cenci MA. Transcription factors involved in the pathogenesis of L-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Amino Acids. 2002;23:105–109. doi: 10.1007/s00726-001-0116-4. [DOI] [PubMed] [Google Scholar]
- Chadha A, Dawson LG, Jenner PG, Duty S. Effect of unilateral 6-hydroxydopamine lesions of the nigrostriatal pathway on GABA(A) receptor subunit gene expression in the rodent basal ganglia and thalamus. Neuroscience. 2000;95:119–126. doi: 10.1016/s0306-4522(99)00413-3. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gois S, Schafer MK, Defamie N, Chen C, Ricci A, Weihe E, Varoqui H, Erickson JD. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci. 2005;25:7121–7133. doi: 10.1523/JNEUROSCI.5221-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin A, Rostaing P, Bedet C, Levi S, Isambert MF, Henry JP, Triller A, Gasnier B. Presence of the vesicular inhibitory amino acid transporter in GABAergic and glycinergic synaptic terminal boutons. J Cell Sci. 1999;112(Pt 6):811–823. doi: 10.1242/jcs.112.6.811. [DOI] [PubMed] [Google Scholar]
- Erickson JD, De Gois S, Varoqui H, Schafer MK, Weihe E. Activity-dependent regulation of vesicular glutamate and GABA transporters: A means to scale quantal size. Neurochem Int. 2006;48:643–649. doi: 10.1016/j.neuint.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Ferre S, O’Connor WT, Svenningsson P, Bjorklund L, Lindberg J, Tinner B, Stromberg I, Goldstein M, Ogren SO, Ungerstedt U, Fredholm BB, Fuxe K. Dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission in the rat strioentopenduncular pathway and its modulation by adenosine A1 receptor-mediated mechanisms. Eur J Neurosci. 1996;8:1545–1553. doi: 10.1111/j.1460-9568.1996.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Gasnier B. The loading of neurotransmitters into synaptic vesicles. Biochimie. 2000;82:327–337. doi: 10.1016/s0300-9084(00)00221-2. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Young WS., 3rd Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Robertson RG. Chronic continuous and intermittent L-3,4-dihydroxyphenylalanine treatments differentially affect basal ganglia function in 6-hydroxydopamine lesioned rats-an autoradiographic study using [3H]flunitrazepam. Neuroscience. 1993;57:673–681. doi: 10.1016/0306-4522(93)90014-7. [DOI] [PubMed] [Google Scholar]
- Kang TC, An SJ, Park SK, Hwang IK, Bae JC, Won MH. Changed vesicular GABA transporter immunoreactivity in the gerbil hippocampus following spontaneous seizure and vigabatrin administration. Neurosci Lett. 2003;335:207–211. doi: 10.1016/s0304-3940(02)01166-7. [DOI] [PubMed] [Google Scholar]
- Katz J, Nielsen KM, Soghomonian JJ. Comparative effects of acute or chronic administration of levodopa to 6-hydroxydopamine-lesioned rats on the expression of glutamic acid decarboxylase in the neostriatum and GABAA receptors subunits in the substantia nigra, pars reticulata. Neuroscience. 2005;132:833–842. doi: 10.1016/j.neuroscience.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Konradi C, Westin JE, Carta M, Eaton ME, Kuter K, Dekundy A, Lundblad M, Cenci MA. Transcriptome analysis in a rat model of L-DOPA-induced dyskinesia. Neurobiol Dis. 2004;17:219–236. doi: 10.1016/j.nbd.2004.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprade N, Soghomonian J-J. Differential regulation of mRNA levels encoding for the two isoforms of glutamate decarboxylase (GAD65 and GAD67) by dopamine receptors in the rat striatum. Mol Brain Res. 1995;34:65–74. doi: 10.1016/0169-328x(95)00139-j. [DOI] [PubMed] [Google Scholar]
- Laprade N, Soghomonian J-J. Glutamate decarboxylase (GAD65) gene expression is increased by dopamine receptor agonists in a subpopulation of rat striatal neurons. Mol Brain Res. 1997;48:333–345. doi: 10.1016/s0169-328x(97)00112-5. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Brene S, Herrera-Marschitz M, Persson H. Region specific regulation of glutamic acid decarboxylase mRNA expression by dopamine neurons in rat brain. Exp Brain Res. 1989;77:611–620. doi: 10.1007/BF00249614. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- Mercugliano M, Soghomonian JJ, Qin Y, Nguyen HQ, Feldblum S, Erlander MG, Tobin AJ, Chesselet MF. Comparative distribution of messenger RNAs encoding glutamic acid decarboxylases (Mr 65,000 and Mr 67,000) in the basal ganglia of the rat. J Comp Neurol. 1992;318:245–254. doi: 10.1002/cne.903180302. [DOI] [PubMed] [Google Scholar]
- Miller DW, Abercrombie ED. Role of high-affinity dopamine uptake and impulse activity in the appearance of extracellular dopamine in striatum after administration of exogenous L-DOPA: studies in intact and 6-hydroxydopamine-treated rats. J Neurochem. 1999;72:1516–1522. doi: 10.1046/j.1471-4159.1999.721516.x. [DOI] [PubMed] [Google Scholar]
- Nielsen KM, Soghomonian JJ. Dual effects of intermittent or continuous L-DOPA administration on gene expression in the globus pallidus and subthalamic nucleus of adult rats with a unilateral 6-OHDA lesion. Synapse. 2003;49:246–260. doi: 10.1002/syn.10234. [DOI] [PubMed] [Google Scholar]
- Nielsen KM, Soghomonian JJ. Normalization of glutamate decarboxylase gene expression in the entopeduncular nucleus of rats with a unilateral 6-hydroxydopamine lesion correlates with increased GABAergic input following intermittent but not continuous levodopa. Neuroscience. 2004;123:31–42. doi: 10.1016/j.neuroscience.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Ochi M, Shiozaki S, Kase H. L-DOPA-induced modulation of GABA and glutamate release in substantia nigra pars reticulata in a rodent model of Parkinson’s disease. Synapse. 2004;52:163–165. doi: 10.1002/syn.20006. [DOI] [PubMed] [Google Scholar]
- Pan HS, Penney JB, Young AB. Gamma-aminobutyric acid and benzodiazepine receptor changes induced by unilateral 6-hydroxydopamine lesions of the medial forebrain bundle. J Neurochem. 1985;45:1396–1404. doi: 10.1111/j.1471-4159.1985.tb07205.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Larsen KE, Krantz DE, Liu Y, Haycock JW, Setlik W, Gershon MD, Edwards RH, Sulzer D. Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J Neurosci. 2000;20:7297–7306. doi: 10.1523/JNEUROSCI.20-19-07297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagné C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997;417:177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]
- Segovia J, Tillakaratne NJ, Whelan K, Tobin AJ, Gale K. Parallel increases in striatal glutamic acid decarboxylase activity and mRNA levels in rats with lesions of the nigrostriatal pathway. Brain Res. 1990;529:345–348. doi: 10.1016/0006-8993(90)90849-7. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Gonzales C, Chesselet MF. Messenger RNAs encoding glutamate-decarboxylases are differentially affected by nigrostriatal lesions in subpopulations of striatal neurons. Brain Res. 1992;576:68–79. doi: 10.1016/0006-8993(92)90610-l. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Chesselet MF. Effects of nigrostriatal lesions on the levels of messenger RNAs encoding two isoforms of glutamate decarboxylase in the globus pallidus and entopeduncular nucleus of the rat. Synapse. 1992;11:124–133. doi: 10.1002/syn.890110205. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Laprade N. Glutamate decarboxylase (GAD67 and GAD65) gene expression is increased in a subpopulation of neurons in the putamen of Parkinsonian monkeys. Synapse. 1997;27:122–132. doi: 10.1002/(SICI)1098-2396(199710)27:2<122::AID-SYN3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Vemuganti R. Decreased expression of vesicular GABA transporter, but not vesicular glutamate, acetylcholine and monoamine transporters in rat brain following focal ischemia. Neurochem Int. 2005;47:136–142. doi: 10.1016/j.neuint.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Pierce RC, Soghomonian JJ. Subchronic administration of l-DOPA to adult rats with a unilateral 6-hydroxydopamine lesion of dopamine neurons results in a sensitization of enhanced GABA release in the substantia nigra, pars reticulata. Brain Res. 2006 doi: 10.1016/j.brainres.2006.09.027. In press. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Williams C, Sabol SL. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984;259:14301–14308. [PubMed] [Google Scholar]
- You ZB, Herrera-Marschitz M, Nylander I, Goiny M, O’Connor WT, Ungerstedt U, Terenius L. The striatonigral dynorphin pathway of the rat studied with in vivo microdialysis--II. Effects of dopamine D1 and D2 receptor agonists. Neuroscience. 1994;63:427–434. doi: 10.1016/0306-4522(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Zeng BY, Jolkkonen J, Jenner P, Marsden CD. Chronic L-DOPA treatment differentially regulates gene expression of glutamate decarboxylase, preproenkephalin and preprotachykinin in the striatum of 6-hydroxydopamine-lesioned rat. Neuroscience. 1995;66:19–28. doi: 10.1016/0306-4522(94)00574-o. [DOI] [PubMed] [Google Scholar]
- Zink M, Spanagel R. Ethanol induces GAD67 and VGAT in slice cultures of newborn rat cerebral cortex. Neuroreport. 2005;16:377–380. doi: 10.1097/00001756-200503150-00014. [DOI] [PubMed] [Google Scholar]
- Zink M, Schmitt A, May B, Muller B, Braus DF, Henn FA. Differential effects of long-term treatment with clozapine or haloperidol on GABA transporter expression. Pharmacopsychiatry. 2004;37:171–174. doi: 10.1055/s-2004-827173. [DOI] [PubMed] [Google Scholar]


