Abstract
VegT and β-Catenin are key players in the hierarchy of factors that are required for induction and patterning of mesendoderm in Xenopus embryogenesis. By descending the genetic cascades, cells lose their pluripotent status and are determined to differentiate into distinct tissues. Mammalian Oct-3/4, a POU factor of subclass V (POU-V), is required for the maintenance of pluripotency of embryonic stem cells. However, its molecular function within the early embryo is yet poorly understood. We here show that the two maternal Xenopus POU-V factors, Oct-60 and Oct-25, inhibit transcription of genes activated by VegT and β-Catenin. Maternal POU-V factors and maternal VegT show an opposite distribution along the animal/vegetal axis. Oct-25, VegT and Tcf3 interact with each other and form repression complexes on promoters of VegT and β-Catenin target genes. We suggest that POU-V factors antagonize primary inducers to allow germ layer specification in a temporally and spatially coordinated manner.
Keywords: β-Catenin, germ layers, POU-V factors, VegT, Xenopus laevis
Introduction
Numerous studies have provided profound insights into the molecular mechanisms of the formation of the three germ layers, ectoderm, mesoderm and endoderm, in Xenopus (De Robertis and Kuroda, 2004; Heasman, 2006). During early cleavage stages, unevenly distributed maternal factors drive the initial signaling pathways that induce the mesodermal and endodermal germ layers (combined as mesendoderm hereafter). Of special importance, the T-box transcription factor VegT is maternally expressed and localizes to the vegetal pole in full-grown oocytes and early cleavage stages. Depletion of maternal VegT transcripts results in the defect of primary germ layer induction (Zhang et al, 1998) and in loss of expression of zygotic FGFs and TGF-β/nodal (Kofron et al, 1999). In fact, maternal VegT activates transcription of genes encoding ligands for TGF-β/nodal signaling (Clements et al, 1999; Hyde and Old, 2000; White et al, 2002). Moreover, the endoderm inducing genes Milk, Mix.1, Mixer or Sox17 are either activated by maternal VegT or by zygotic Xenopus nodal-related (Xnr) proteins (Xanthos et al, 2001; Shivdasani, 2002). Therefore, maternal VegT plays a key role in mesendoderm induction.
Another signal, mediated by maternal β-Catenin, specifies patterning of germ layers along the dorsal–ventral axis. Upon fertilization, radial symmetry of the fertilized egg is broken by nuclear accumulation of β-Catenin at the dorsal side. β-Catenin interacts with TCF/LEF transcription factors (Behrens et al, 1996) to activate expression of the target gene Siamois in the Nieuwkoop center (Wodarz and Nusse, 1998). β-Catenin also acts synergistically with VegT to enhance transcription of Xnrs (Agius et al, 2000; Takahashi et al, 2000). Thus, a gradient of inducing signals is established along the dorsal–ventral axis. High level of these signals induces the overlying equatorial region to form dorsal mesoderm, that is, the organizer, whereas low level of signals induces the overlying equatorial region to form ventral mesoderm. Expression of neural inducers, Xnr3, Chordin and Noggin, in the organizer is initiated by the β-Catenin cascade (Wessely et al, 2001). Ectopic activation of β-Catenin signaling causes the formation of a complete secondary axis, while blocking endogenous signaling results in loss of dorsal–anterior structures (Heasman et al, 2000). Hence maternal β-Catenin is responsible for dorsalizing the three germ layers.
After the blastula stage and concomitant with germ layer formation, the cells lose their pluripotent status and begin to differentiate. This loss of pluripotency is accompanied by a switch of genetic programming, which has been extensively studied in mammalian embryonic stem (ES) cells (Ivanova et al, 2006). One of the factors required for self-renewal of ES cells and for the maintenance of pluripotency is the POU factor Oct-3/4, a member of subclass V (Nichols et al, 1998). Up- or downregulation of Oct-3/4 induces divergent differentiation programs in ES cells (Niwa et al, 2000; Hay et al, 2004). These studies demonstrate that the level of Oct-3/4 expression is essential for ES cell differentiation. In Xenopus laevis, three POU-V factors, Oct-60, Oct-25 and Oct-91, are expressed during oogenesis and early embryogenesis (Hinkley et al, 1992; Whitfield et al, 1993, 1995). Recent studies have demonstrated that Xenopus Oct factors are functional homologues to mammalian Oct-3/4 (Cao et al, 2006; Morrison and Brickman, 2006). However, the molecular mechanisms underlying the loss of pluripotency in embryonic development are yet largely unknown.
In this study we demonstrate that Xenopus Oct proteins repress mesendodermal germ layer induction and patterning via inhibition of maternal VegT activity and β-Catenin signaling. Oct-25, VegT and Tcf3 interact with each other and form repressing complexes on the promoters of VegT and β-Catenin target genes. We therefore propose a model in which a decreasing activity of POU-V factors from the animal to the vegetal pole antagonizes the activity of VegT decreasing from the vegetal to the animal pole. These opposite distributions along with the suppression of β-Catenin signaling at the dorsal side ensure the temporally and spatially coordinated induction and patterning of mesendoderm in gastrulating Xenopus embryos.
Results
Maternal Oct factors inhibit expression of genes that are essential for germ layer induction and patterning
To investigate the role of POU-V factors in germ layer induction, we have analyzed the effects of maternal Oct factors on the expression of mesodermal and endodermal inducers by gain- and loss-of-function studies. In Xenopus, Oct-60 is only maternally transcribed, Oct-25 is both maternally and zygotically transcribed, whereas Oct-91 is only zygotically expressed (Hinkley et al, 1992). The major part of Oct-60 transcript is found in the animal half of mature oocytes, with minor part in the vegetal half (Hinkley et al, 1992), which is known as a source of primary germ layer inducers such as VegT. Although at early cleavage stages Oct-25 is less abundant than Oct-60 RNA, we found by immunoblotting that Oct-25 protein is expressed (data not shown). The distribution of Oct-25 RNA was analyzed by RT–PCR in eight-cell (stage 4) and blastula (stage 8.5) embryos. At stage 4, Oct-60 and Oct-25 transcripts were found enriched in animal blastomeres. At stage 8.5, highest amounts of these RNAs were also detected in the animal region, with decreasing amounts in the equatorial and vegetal regions (Figure 1A). In contrast, the major part of VegT transcripts locates in the vegetal region. Although Oct-25 and Oct-60 show an opposite distribution to that of VegT, they overlap with VegT in the vegetal–equatorial region of embryo where mesoderm and endoderm are formed.
Figure 1.
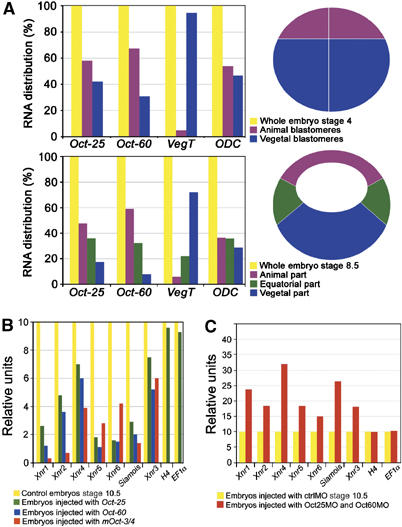
Maternal POU-V factors regulate transcription of Xnrs and Siamois. (A) Distribution of Oct-25, Oct-60 and VegT in eight-cell and blastula embryos. Animal and vegetal blastomeres were dissected from stage 4 embryos. Animal, equatorial and vegetal parts were excised from stage 8.5 embryos and subjected to real-time RT–PCR. Quantification of expression level in each part was normalized to the yield of RNA and to the respective expression level in whole embryos. (B) A total of 400 pg Oct-25, Oct-60 or mOct-3/4 mRNA was injected into all vegetal blastomeres at the eight-cell stage. Controls and injected embryos were grown to stage 10.5 and subjected to RT–PCR. (C) A mixture of 15 ng of Oct25MO and 40 ng of Oct60MO was injected into the equatorial region of four blastomeres at the four-cell stage. Controls and injected embryos were grown to stage 10.5 and subjected to RT–PCR.
We have overexpressed Oct-25, Oct-60, and their corresponding mouse orthologue Oct-3/4 (mOct-3/4) by microinjection of mRNAs into the vegetal part of embryos. At stage 10.5, expression of the nodal-related genes Xnr1–6 and the Siamois gene, known to be responsible for germ layer formation and patterning, was severely repressed (Figure 1B). In contrast, functional knockdown of Oct-25 and Oct-60 by injection of a mixture of characterized antisense morpholino oligos against Oct-25 (Oct25MOs) and Oct-60 (Oct60MOs) (Cao et al, 2006) into the equatorial region of embryos led to elevated expression of Xnrs and Siamois (Figure 1C). In both experiments, we observed no significant alteration in the transcription of H4 and EF1α, suggesting that POU-V proteins do not affect general transcription. Thus, gain- and loss-of-function assays clearly demonstrate that POU-V proteins inhibit the expression of Xnrs and Siamois in gastrulating embryos.
Oct-25 or Oct-60 overexpression inhibits VegT and β-Catenin activated gene transcription in animal caps
Maternal POU-V proteins inhibit expression of the factors that are required for mesendoderm formation. These factors are primarily induced by maternal VegT and β-Catenin. Therefore, we have asked if Oct-25 can indeed inhibit the inducing activity of overexpressed VegT and β-Catenin in animal cap assays. Real-time RT–PCR showed that stimulation of Xnrs, Mixer and XSox17α by overexpression of VegT alone was dramatically diminished when Oct-25 or Oct-60 was co-injected (Figure 2A). We next examined if Oct-25 inhibits gene activation by β-Catenin. Similarly, activation of Xnr3, Siamois, chordin and noggin, triggered by overexpression of β-Catenin alone, was strongly inhibited by co-injected Oct-25 or Oct-60 (Figure 2B). VegT and β-Catenin act synergistically to enhance mesendodermal gene transcription in the blastula-stage dorsal endoderm, the Nieuwkoop center (Takahashi et al, 2000). When each lower amount of VegT and β-Catenin RNAs was co-injected, Xnr5, Xnr6, Mixer and Xsox17α were more strongly induced in animal caps than by a higher amount of VegT alone (Figure 2A and C). When Oct-25 or Oct-60 was co-injected, a severe inhibition was observed (Figure 2C). Therefore, both Oct-25 and Oct-60 inhibit not only the individual but also the synergistic function of VegT and β-Catenin regarding the transcription of target genes. H4 and EF1α are not affected in caps injected with VegT or β-Catenin alone or together with Oct-25 (Supplementary Figure S1A). Moreover, the inhibitory effect of Oct-25 seems to be direct, because transcription of direct target genes of VegT and β-Catenin was still repressed in the presence of cycloheximide (CHX), a protein biosynthesis inhibitor (Supplementary Figure S1B and C).
Figure 2.
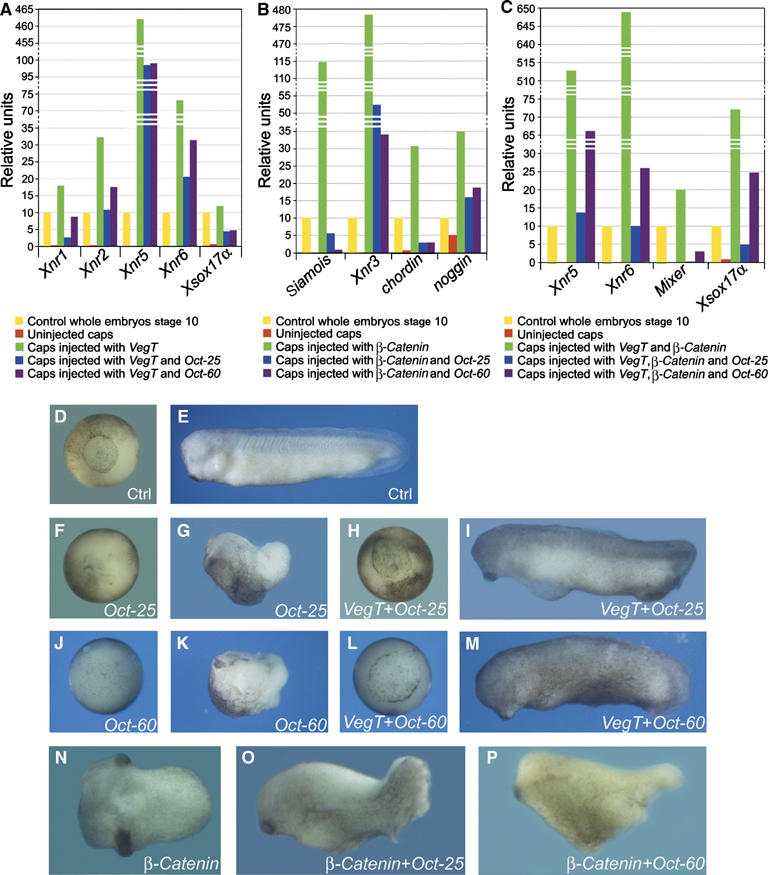
Oct-25 and Oct-60 counteract the activities of VegT and β-Catenin. (A–C) Real-time RT–PCR showed that injection of 600 pg Oct-25 and Oct-60 RNA into the animal pole region of four-cell-stage embryos inhibited gene expression in animal caps activated by 500 pg VegT alone (A), by 500 pg β-Catenin alone (B) and, synergistically, by 200 pg VegT and 200 pg β-Catenin RNA together (C). (D–M) The phenotype of Oct-25 or Oct-60 overexpression was rescued by VegT. (D, E) Uninjected control embryos showed normal development at gastrula (D) and tail-bud stages (E). (F, G) Embryos injected vegetally with 400 pg Oct-25 RNA showed failure of gastrulation (F) and body axis formation (G). (H, I) The phenotypic effect of Oct-25 was rescued by co-injection of 700 pg VegT RNA, as shown by restored blastopore during gastrulation (H) and nearly normal body axis formation during tail-bud stage (I). (J, K) Embryos injected vegetally with 400 pg Oct-60 RNA showed failure of gastrulation (J) and body axis formation (K). (L, M) Co-injection with 700 pg VegT RNA restored gastrulation (L) and body axis formation (M). (N–P) POU-V factors repressed axis formation induced by β-Catenin. (N) Ventral–vegetal overexpression of 400 pg β-Catenin RNA induced axis duplication. The secondary axis was abolished by co-injection of 400 pg Oct-25 (O) or Oct-60 RNA (P).
Since VegT activates genes that are responsible for mesendoderm induction, we have asked whether an increase of VegT in embryos would be able to reverse the previously described gastrulation failure caused by Oct-25 and Oct-60 overexpression (Cao et al, 2006). In contrast to uninjected controls (Figure 2D and E), embryos injected vegetally with Oct-25 (Figure 2F and G) or Oct-60 RNA (Figure 2J and K) showed no blastopore during gastrulation and no clear body axis formation. However, when a suitable dose of VegT RNA was injected together with Oct-25 (Figure 2H and I) or Oct-60 RNA (Figure 2L and M), the major part of embryos showed nearly normal blastopore formation and developed with a clearly visible body axis (Supplementary Table S1). Therefore, addition of an appropriate dose of VegT can prevent the phenotypic effect of Oct-25 or Oct-60.
As β-Catenin itself has no mesendoderm inducing activity, which is required for formation of the blastopore, we did not try to rescue the Oct-25 or Oct-60 overexpression phenotype with β-Catenin. Vice versa, we tested whether Oct proteins can inhibit the secondary axis formation induced by β-Catenin. Injection of β-Catenin RNA into ventral–vegetal blastomeres induces axis duplication (Figure 2N). When Oct-25 or Oct-60 was co-injected, formation of secondary axis was abolished (Figure 2O and P; Supplementary Table S1). Thus, maternal POU-V factors inhibit the activity of β-Catenin to induce a secondary axis.
Interactions between Oct-25, VegT and Tcf3
To examine the mechanism of POU-V-mediated inhibition of VegT- and β-Catenin-induced gene activation, we have analyzed whether Oct-25 can directly interact with VegT and/or with components of the β-Catenin signaling pathway. GST pull-down assays with radiolabeled VegT- and GST-tagged Oct-25 fusion protein, and vice versa, with radiolabeled Oct-25- and GST-tagged VegT fusion protein demonstrated a physical interaction between Oct-25 and VegT (Figure 3A). We did not observe Oct-25 binding to β-Catenin. However, we found an interaction between Oct-25 and Tcf3, the best characterized maternal nuclear transducer of β-Catenin signaling that is responsible for the establishment of the dorsal body axis (Molenaar et al, 1996; Roel et al, 2002). Transcription regulators that do not bind to Oct-25 are, for example, Xenopus HoxD1, ETO and Notch-ICD (Supplementary Figure S2). The interaction between Oct-25 and Tcf3 obviously did not influence the binding between Tcf3 and β-Catenin. Oct-25, Tcf3 and β-Catenin formed a ternary complex, because radiolabeled β-Catenin was precipitated by GST/Oct-25 via unlabeled Tcf3 (Figure 3A, last panel). VegT or Tcf3 bound not only to Oct-25, but also to Oct-60 and Oct-91 (Figure 3B). Interestingly, even the mouse orthologue Oct-3/4 was found as an interaction partner of Xenopus VegT and Tcf3 (Figure 3B). These findings suggest that an evolutionary conserved mechanism for the inhibition of primary germ layer induction by POU-V factors might also exist in mammals.
Figure 3.
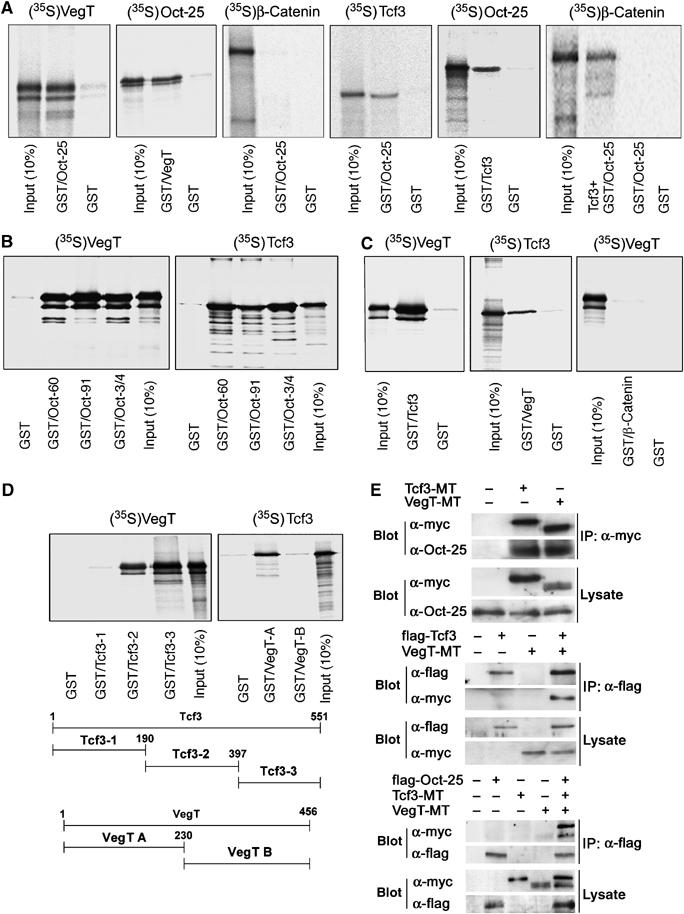
GST pull-down and immunoprecipitation demonstrate interactions between Oct-25, VegT and Tcf3. (A) Radiolabeled VegT bound to GST/Oct-25 fusion protein and vice versa, but β-Catenin did not interact with GST/Oct-25. Radiolabeled Tcf3 interacted with GST/Oct-25 and vice versa. Radiolabeled β-Catenin was precipitated by GST/Oct-25, when in vitro translated unlabeled Tcf3 was simultaneously added. (B) Both VegT and Tcf3 bound to Oct-60, Oct-91 as well as mouse Oct-3/4. (C) Radiolabeled Tcf3 bound to GST/VegT fusion protein and vice versa. In contrast, VegT did not interact with GST/β-Catenin. (D) VegT did not interact with 1–190 aa, but interacted with 191–397 aa and 398–551 aa regions of Tcf3. Vice versa, Tcf3 interacted with the N-terminal region (1–120 aa) but did not interact with the C-terminal region (121–456 aa) of VegT. (E) Co-immunoprecipitation with anti-myc antibody (IP:α-myc) showed that both myc-tagged Tcf3 (Tcf3-MT) and VegT (VegT-MT) precipitated endogenous Oct-25, whereas anti-flag antibody (IP:α-flag) precipitated a complex of flag-tagged Tcf3 (flag-Tcf3) and VegT-MT and a ternary complex of flag-tagged Oct-25 (flag–Oct-25), Tcf3-MT and VegT-MT.
Furthermore, we have examined if VegT can bind to β-Catenin or Tcf3, because a synergism between VegT and β-Catenin enhances VegT target gene expression. We found that VegT did not bind to β-Catenin but to Tcf3 (Figure 3C). We have also determined the regions within VegT and Tcf3 that are required for binding (Figure 3D). VegT did not bind to the N-terminal domain of Tcf3 (1–190 aa), but bound to the remaining region (191–397 aa and 398–551 aa) that includes the Groucho binding domain and the HMG box (Hurlstone and Clevers, 2002). Vice versa, Tcf3 bound to the N-terminal region (1–230 aa) of VegT containing the DNA binding domain, but not to the C-terminal region (231–456 aa) that is responsible for transactivation (Conlon et al, 2001).
Co-immunoprecipitations with fusion proteins demonstrated that VegT and Tcf3 precipitated endogenous Oct-25, and that Tcf3 precipitated VegT. Moreover, Oct-25, VegT and Tcf3 could form a ternary complex (Figure 3E). In summary, the three maternal factors, Oct-25, VegT and Tcf3, which are critical for specification of germ layers, interact with each other in vitro and in vivo.
Oct-25, VegT and Tcf3 form complexes on Xnr1 and Siamois promoters
To further investigate, how Oct-25 interacts with a promoter regulated by VegT, we have isolated the Xnr1 promoter region −907/−5 (Hyde and Old, 2000) and performed electrophoretic mobility shift assays (EMSA). The Xnr1 promoter contains the T-box sites required for the activation by VegT (Hyde and Old, 2000) and, additionally, one putative TCF/LEF and an Oct binding site (Figure 4A). Besides the previously reported binding between VegT and the first half T-box site (Hyde and Old, 2000), EMSAs demonstrated that bacterially expressed Oct-25 as well as Oct-25 in embryonic protein extracts specifically bound to the DNA fragment that contains the Oct binding site (Figure 4B and C). A faint band visible in protein extracts from uninjected embryos was strongly enhanced by Oct-25 RNA injection. This band was no longer visible with extracts from MO-injected embryos and with a mutated Oct binding site (Figure 4C). Tcf3 bound to the DNA fragment that contains the TCF/LEF binding site (Figure 4D). Mutation of these sites in the Xnr1 promoter abolished binding of Oct-25, VegT and Tcf3 proteins (Supplementary Figure S3). Therefore, Oct-25, VegT and Tcf3 possess cognate binding sites within the Xnr1 promoter. Like Oct-25, also Oct-60 and Oct-3/4 were shown to bind to the promoter fragment that contains the Oct binding site (Supplementary Figure S4A), suggesting again that the Oct proteins employ the same mechanism to regulate gene expression.
Figure 4.
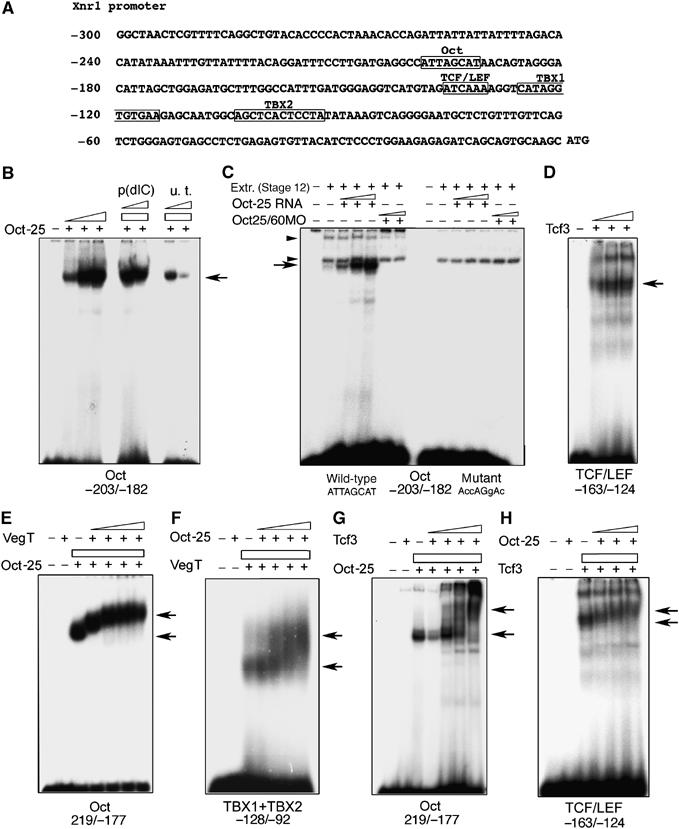
EMSAs with the Xnr1 promoter. (A) Xnr1 promoter sequence. The first nucleotide in front of the start codon ATG is designated as −1. The Oct binding site, TCF/LEF binding site and the two half T-box sites, TBX1 and TBX2, are boxed and labeled. The promoter fragments used for EMSAs are indicated below each panel. (B) Oct-25 bound specifically to the promoter fragment that contains the Oct binding site. Nonspecific competitive probe (p(dIC)) did not interfere with the binding, while the unlabeled target probe (u.t.) competed with the labeled target probe for binding with Oct-25. (C) EMSA with embryonic protein extracts (stage 12) showed that a probe containing the Oct binding site formed a complex with Oct-25. Arrow indicates specific protein/DNA complex, whereas arrowheads indicate unspecific complexes. The specific complex became more intense using extracts from embryos after injecting increasing doses of Oct-25. In contrast, the protein/DNA complex was not formed in extracts from embryos injected with increasing doses of Oct25MO and Oct60MO, or when a probe containing a mutated Oct binding site was used. (D) Tcf3 bound to the promoter fragment containing the TCF/LEF binding site. The labeled probe containing the TCF/LEF binding site was incubated with increasing amounts of Tcf3. (E) Oct-25 and VegT formed a complex on the Oct binding site. Oct-25 but not VegT bound to the promoter fragment containing the Oct binding site. When Oct-25 was incubated with increasing amounts of VegT, the mobility of the Oct-25/DNA complex was reduced. (F) Oct-25 and VegT formed a complex on the T-box site as revealed by the reduced mobility of the VegT/DNA complex. (G, H) Oct-25 and Tcf3 formed a complex on the promoter fragment containing either the Oct binding site (F) or the TCF/LEF binding site (G).
We next explored if these proteins can form complexes on the binding sites on the Xnr1 promoter. First, Oct-25 alone but not VegT or Tcf3 bound to the oligonucleotide containing only the Oct binding site (Figure 4E and G). However, when increasing amounts of VegT or Tcf3 were added, the resulting supershifts indicated that the mobility of the Oct-25/promoter complex was significantly reduced. Therefore, both VegT and Tcf3 form complexes with Oct-25 on the Oct binding site. Vice versa, we found that Oct-25 formed a complex with VegT on the promoter sequence that contains the T-box sites (Figure 4F). While VegT alone but not Oct-25 alone formed a complex with the T-box containing probe, addition of Oct-25 led to a supershift indicating reduced mobility of the VegT/DNA complex. Similarly, we found that Oct-25 and Tcf3 formed a complex on the TCF/LEF binding site (Figure 4H). As expected by the results from GST pull-down assays, β-Catenin did not yield a supershift of the Oct-25/DNA complex (Supplementary Figure S4B).
Subsequently, we examined if Oct-25, Tcf3 and VegT could also form complexes on the Siamois promoter, which is primarily stimulated by β-Catenin signaling via the TCF/LEF binding sites (Brannon et al, 1997; Fan et al, 1998). A Siamois promoter fragment –802/+9 (Brannon et al, 1997) was isolated. In addition to the four possible TCF/LEF binding sites (S1–S4) (Brannon et al, 1997), we detected a putative Oct binding site within the –300/+9 fragment (Figure 5A). EMSA demonstrated that Oct-25 bound to a promoter fragment containing this putative site (Figure 5B). It had been shown that the S1 and S3 sites are more important than S2 and S4 for Wnt stimulation of the Siamois promoter (Brannon et al, 1997). We observed that Tcf3 efficiently bound to both the S1 site and the S3 site (Figure 5C and F). When the Oct binding site or the four TCF/LEF binding sites were separately mutated, binding of Oct-25 or Tcf3 was abolished, showing that the binding sites for Oct-25 and Tcf3 are specific (Supplementary Figure S5). Considering again that Oct-25, Tcf3 and VegT interact with each other, we postulated that these proteins also form complexes on the Siamois promoter. First, Oct-25 did not bind to the S1 site, however, addition of different amounts of Oct-25 protein retarded the mobility of Tcf3–promoter complex (Figure 5D). Second, VegT formed a complex with Tcf3 on either S1 or S3 TCF/LEF binding sites, as shown by retardation of the mobility of the Tcf3/promoter complex (Figure 5E and F). Hence, Oct-25 and VegT may regulate transcription of Siamois by forming protein complexes with Tcf3 on the TCF/LEF binding sites on the Siamois promoter.
Figure 5.
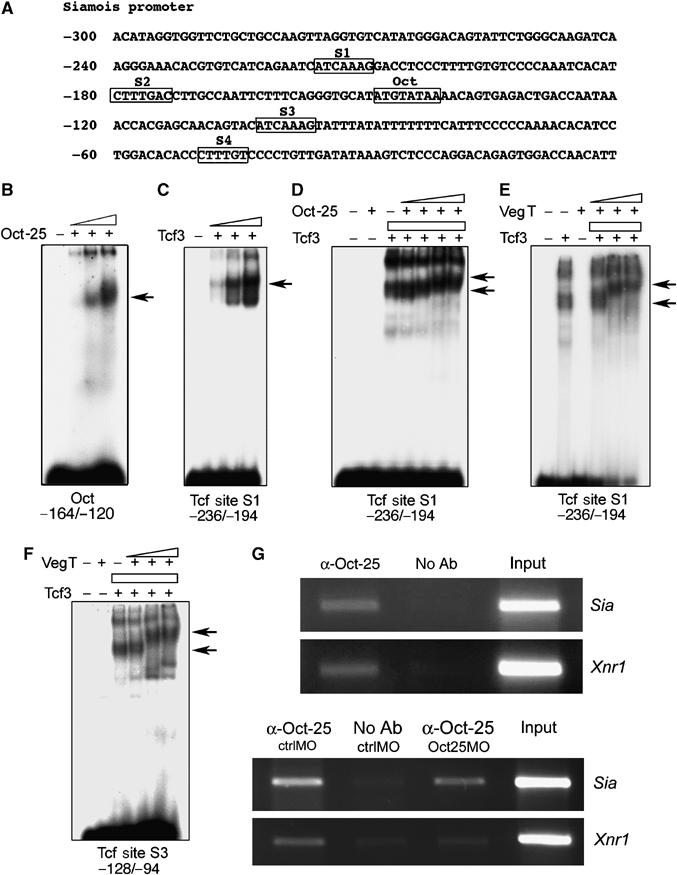
EMSAs with the Siamois promoter and ChIP. (A) Siamois promoter sequence. The first nucleotide in front of the transcription start site is designated as −1. The Oct binding site and the four TCF/LEF sites (S1, S2, S3, S4) are boxed and labeled. The promoter fragments used for EMSAs are indicated below each panel. (B) Oct-25 bound to the promoter fragment that contains the Oct binding site. (C) Tcf3 bound to the promoter fragment that contains the S1 TCF/LEF binding site. (D) Oct-25 and Tcf3 formed a complex on the S1 TCF/LEF binding site. (E, F) VegT and Tcf3 formed a complex on both S1 (E) and S3 (F) TCF/LEF sites. (G) ChIP assays revealed that the Siamois promoter region spanning the Oct and TCF/LEF binding sites, as well as the Xnr1 promoter region including the Oct, TCF/LEF and T-box sites, were amplified from chromatin precipitated by an Oct-25 antibody (α-Oct-25) but not in the absence of antibody (no Ab). When Oct-25 translation was blocked by Oct25MO, PCR products for Siamois and Xnr1 promoter regions were reduced as compared with ctrlMO injections.
In chromatin immunoprecipitation (ChIP) assays (Figure 5G), Oct-25/Xnr1 and Oct-25/Siamois promoter complexes were precipitated by an Oct-25 antibody (α-Oct-25) (Cao et al, 2006). Because Oct25MO can efficiently block Oct-25 protein translation in vivo (Cao et al, 2006), we expected less formation of Oct-25/chromatin complex in embryos injected with Oct25MO than in those injected with the control morpholino (ctrlMO). Indeed, ChIP assays detected weaker bands representing the precipitated Siamois and Xnr1 promoter fragments in Oct25MO injected embryos (Figure 5G). In conclusion, both in vitro and in vivo studies support that Oct-25 interacts with Xnr1 and Siamois promoters.
Oct-25 represses the Xnr1 and Siamois promoters
To investigate the functional role of distinct protein complexes and distinct binding sites for promoter activity, we prepared luciferase reporter constructs for the wild-type Xnr1 promoter fragments −907/−5, −279/−5 and a series of promoter mutants (Figure 6A). Corresponding promoter/reporter constructs were either injected alone or together with VegT, Xwnt8, β-Catenin or Oct-25 mRNA, respectively. The wild-type reporter Xnr1Luc(−907) was not only stimulated by VegT but also by Xwnt8 and the downstream effector β-Catenin (Figure 6B). Co-injection of VegT and β-Catenin resulted in a significant enhancement of luciferase activity. However, luciferase activity was strongly inhibited in all cases when Oct-25 was co-injected (Figure 6B). In contrast, injection of Oct25MOs and Oct60MOs augmented significantly the stimulation of the Xnr1 promoter (Figure 6C), clearly demonstrating that POU-V factors inhibit Xnr1 gene transcription. Since Oct-25, VegT and Tcf3 form protein complexes on the Xnr1 promoter, we examined how these complexes regulate promoter/reporter activity in vivo, when distinct binding sites were destroyed. Mutations were introduced for the Oct binding site (−Oct), TCF/LEF binding site (−TCF) and VegT binding site (−Tbox) (Figure 6A). In line with the observation that Oct-25, VegT or Tcf3 do not directly bind to the promoter when their respective cognate binding sites are lost (Supplementary Figure S3), the mutant promoters lacking the T-box did not respond to VegT injection and those lacking the TCF/LEF binding site did not respond to β-Catenin injection any more (Figure 6D and E). This means that neither VegT nor Tcf3/β-Catenin can activate the Xnr1 promoter by utilizing the binding site of the interaction partner. Therefore, we tested the effect of Oct-25 on VegT stimulation separately by using promoter mutants that contain the T-box (Figures 6F–I) or the effect of Oct-25 on β-Catenin stimulation by using mutants that contain the TCF/LEF binding site (Figures 6J–M). As Tcf3 is required for the repression of zygotic expression of Xnrs (Houston et al, 2002; Hilton et al, 2003), the correlation between Tcf3 and Oct-25 was analyzed by injecting the mRNA for dominant-negative Tcf3 (dnTcf3), the constitutive repression form of Tcf3 (Molenaar et al, 1996). Activities of the wild-type Xnr1/Luc(−279) (Figure 6F), the mutants lacking the Oct binding site Xnr1Luc(−279-Oct) (Figure 6G), lacking the TCF/LEF binding site Xnr1Luc(−279-TCF) (Figure 6H) and lacking both sites (−279-Oct-TCF) (Figure 6I) were all dramatically stimulated by VegT. This stimulation was significantly repressed by co-injecting either dnTcf3 or Oct-25 (Figure 6F–I). Moreover, an enhanced repression effect was observed when Oct-25 and dnTcf3 were co-injected (Figure 6F–I). Therefore, Oct-25 and Tcf3 exert an inhibitory function on Xnr1 transcription, and the inhibitory effect is even stronger when Oct-25 and Tcf3 work together. Intriguingly, inhibition by Oct-25 or Tcf3 does not rely upon their respective binding sites on the promoter, suggesting that Oct-25 and Tcf3 form complexes with VegT, which can repress VegT activated gene transcription.
Figure 6.
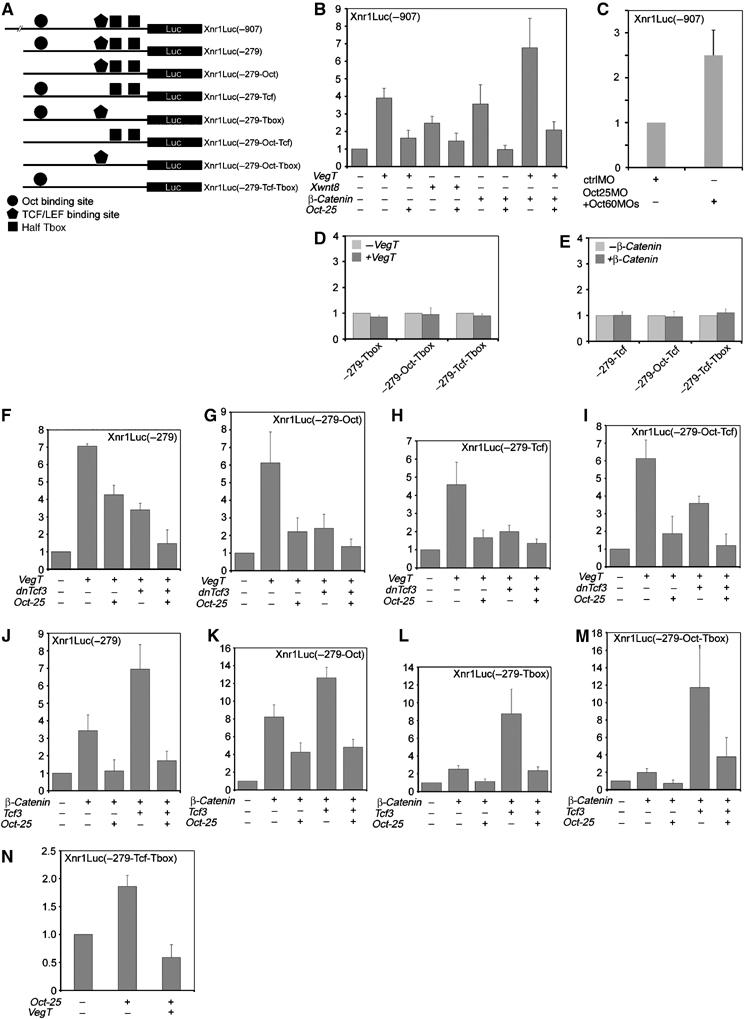
Regulation of Xnr1 promoter/reporter activity by VegT, β-Catenin and Oct-25. (A) Wild-type and mutant Xnr1 promoter/reporter constructs used for luciferase assays. (B) The wild-type −907 promoter was stimulated by overexpression of VegT, Xwnt8 or β-Catenin. Stimulation was enhanced by co-injection of VegT and β-Catenin. In all cases, stimulation was repressed by overexpression of Oct-25. (C) In whole embryos, knockdown of Oct-25/Oct-60 led to an increased stimulation of Xnr1 promoter. (D, E) Promoter/reporter mutants lacking the T-box binding site were not stimulated by overexpression of VegT (D) and those lacking the TCF/LEF binding site were not stimulated by overexpression of β-Catenin (E). (F–I) The wild-type −279 promoter/reporter (F) or the mutants lacking the Oct binding site (G), TCF/LEF binding site (H) or both (I) were stimulated by overexpression of VegT. Stimulation was repressed by overexpression of Oct-25 or dnTcf3, and even more drastically, by co-injection of Oct-25 and dnTcf3. (J–M) The wild-type −279 promoter reporter (J) or the mutants lacking the Oct binding site (K), the T-box binding site (L) or both (M) were stimulated by overexpression of β-Catenin or, much more strongly, by overexpression of both β-Catenin and Tcf3. The stimulation was drastically repressed by overexpression of Oct-25. (N) Oct-25 stimulated slightly the promoter mutant containing only the Oct binding site, which by co-injection of VegT was in turn repressed.
Next we tested the effect of Oct-25 on β-Catenin stimulation of promoter mutants that contain the TCF/LEF binding site (Figure 6J–M). In fact, overexpression of β-Catenin stimulated not only the wild-type promoter reporter Xnr1Luc(−279) but also the mutants lacking the Oct binding site Xnr1Luc (−279-Oct), lacking the T-box binding site Xnr1Luc(−279-Tbox) and lacking both sites Xnr1Luc (−279-Oct-Tbox) (Figure 6J–M). In contrast, stimulation of all these mutants by β-Catenin was inhibited, when Oct-25 was co-injected (Figure 6J–M). If β-Catenin was co-injected with Tcf3, luciferase reporter activity of all mutants was significantly higher than with β-Catenin alone. Nevertheless, when Oct-25 was simultaneously overexpressed, the enhanced activation was also severely repressed (Figure 6J–M). Considering the observation that Oct-25 does not disrupt the β-Catenin/Tcf3 activation complex, but instead forms a ternary complex with these two proteins (Figure 3A), joining of Oct-25 turns the activation complex into a repressive one. As revealed by promoter mutants lacking the Oct binding site, the inhibitory effect of Oct-25 does not depend on its direct binding to DNA. In summary, all these findings suggest that Oct-25 inhibits the activation potential of VegT and β-Catenin on the Xnr1 promoter by forming repression complexes with VegT and Tcf3.
We subsequently performed luciferase reporter assays to investigate how Oct-25 can influence Siamois transcription. A −802/+9 promoter fragment and a series of thereof-derived mutants were fused to the luciferase reporter (Figure 7A). By EMSA we demonstrated that Oct-25 or Tcf3 did not bind to the promoter mutant lacking the Oct binding site or the TCF/LEF binding sites (Supplementary Figure S5). In addition, the corresponding luciferase reporter construct lost response to β-Catenin overexpression, when the TCF/LEF binding sites were missing (Figure 7B). Therefore, we tested the effect of Oct-25 on the Wnt/β-Catenin pathway by using Siamois promoter mutants in which the TCF/LEF binding sites were intact. The wild-type promoter/reporters −802/+9 and −250/+9 were stimulated by overexpression of Xwnt8 and β-Catenin or, even stronger, by overexpression of both β-Catenin and Tcf3. This stimulation was severely repressed when Oct-25 was co-injected (Figure 7C and E), revealing an inhibitory effect of Oct-25 on Siamois transcription. The effect was confirmed by an enhanced stimulation of Siamois reporter when Oct-25 and Oct-60 were knocked down (Figure 7D). We further tested whether Oct-25 represses Siamois promoter in the absence of Oct binding site. Overexpression of β-Catenin alone or β-Catenin and Tcf3 together stimulated the promoter reporter mutant lacking the Oct binding site (Figure 7F). A significant inhibitory effect was still observed in response to Oct-25 injection (Figure 7F), suggesting that the inhibitory activity of Oct-25 is not dependent on its binding to the target site but on its interaction with Tcf3 on the TCF/LEF binding sites.
Figure 7.

Regulation of Siamois promoter/reporter activity by VegT, β-Catenin and Oct-25. (A) Wild-type and mutant Siamois promoter reporter constructs used for luciferase assays. (B) The promoter mutant lacking the TCF/LEF sites was not stimulated by overexpression of β-Catenin. (C, E) Both wild type −802 (C) and −250 (E) promoter/reporters were significantly stimulated by overexpression of either Xwnt8 or β-Catenin, and, much more strongly, by co-injection of β-Catenin and Tcf3. Stimulation was repressed by co-injection of Oct-25. (D) Knockdown of Oct-25/Oct-60 in whole embryos resulted in enhanced stimulation of Siamois promoter. (F) The promoter/reporter mutant lacking the Oct binding site was stimulated by injection of β-Catenin or by injection of both β-Catenin and Tcf3. However, overexpression of Oct-25 repressed this stimulation. (G) Overexpression of VegT activates the −250 promoter/reporter. When VegT and β-Catenin were co-injected, a higher luciferase activity was obtained. This activity was drastically inhibited by overexpression of Oct-25. (H) Overexpression of Oct-25 stimulated the Siamois promoter/reporter mutant that contains only the Oct binding site. Co-injection of VegT led to a repression. (I) Model for mesendoderm specification and patterning. POU-V factors antagonize VegT and β-Catenin, which induce and pattern the mesendodermal germ layer. In the animal region, Oct-25 promotes formation of neuroectoderm (see text for details). Org, organizer.
Although we have no evidence for a VegT binding site on the analyzed Siamois promoter fragment, the results obtained by GST pull-down assays and by EMSA suggest that VegT and Tcf3 might form a complex on the TCF/LEF binding sites of the Siamois promoter. The protein interactions motivated us to examine whether VegT also enhances Siamois transcription, just as β-Catenin enhances Xnr1 transcription. VegT overexpression alone was sufficient to stimulate Siamois promoter/reporter activity, albeit to a lower extent than β-Catenin overexpression (Figure 7G). Intriguingly, co-injection of both VegT and β-Catenin stimulated luciferase activity to an apparently much higher level than it was expected, as a result of an additive effect. These findings reveal that VegT indeed enhances Siamois transcription. Again, stimulation of the Siamois promoter by VegT alone or by VegT and β-Catenin together was repressed by Oct-25 overexpression (Figure 7G).
Since Oct-25 exhibits an inhibitory activity for both, VegT and β-Catenin signaling, on the Xnr1 and the Siamois promoters, we next examined how Oct-25 regulates promoter activity when only an Oct binding site is present. Co-injection of Oct-25 with the Xnr1 promoter lacking both the TCF/LEF and T-box sites (Xnr1Luc(−279-TCF-Tbox)) induced about two-fold luciferase activity (Figure 6N). An even higher stimulation by Oct-25 was observed with the Siamois promoter containing only the Oct binding site (SiaLuc(−250-S1234); Figure 7H). Therefore it seems, that Oct-25 by itself tends to activate gene expression when it directly interacts with its binding site. However, co-injection of VegT inhibited stimulation of promoter/reporters by Oct-25 (Figures 6N and 7H). These results extend our previous conclusion in that Oct-25 forms repression complexes with VegT even in the absence of the cognate binding sites.
Discussion
POU-V factors antagonize primary inducing activities
In Xenopus, mesoderm and endoderm are induced by signaling networks triggered by maternal VegT. These germ layers are dorsalized by a signaling pathway that is induced by another maternal factor β-Catenin. When Oct-25 or Oct-60 is overexpressed in the vegetal region, target genes of VegT and β-Catenin, Xnr1–6 and Siamois, are all inhibited. Vice versa, when Oct factors are functionally depleted, the expression levels of these genes are significantly elevated. These results suggest that Oct factors are essential for controlling the expression levels of Xnrs and Siamois. Previous studies have shown that Oct factors block the FGF and nodal/activin pathways required for mesendoderm formation (Henig et al, 1998; Cao et al, 2006; Snir et al, 2006). The present findings reveal that POU-V factors already suppress the expression of the ligands governing these pathways. A recent analysis of Oct-4-dependent transcriptional networks in human ES cells yielded similar results (Babaie et al, 2007). Affected genes include regulators of various signaling pathways, and Oct-4 target genes are either positively regulated, like Sox2, or negatively regulated, like brachyury. In the animal cap assays, Oct factors inhibit transcription of genes activated by VegT or β-Catenin. In whole embryos, VegT can rescue the failure of gastrulation and body axis formation resulting from Oct-25 or Oct-60 overexpression, and additionally, Oct-25 and Oct-60 inhibit axis duplication induced by ectopic expression of β-Catenin. Therefore, Oct factors antagonize the functions of maternal VegT and β-Catenin. Such a regulatory relationship, in combination with the positional information of these signals, suggests a model for germ layer induction and patterning in blastula-stage embryos. Maternal VegT is primarily localized to the vegetal region of embryos and decreases from the vegetal to the animal pole. High levels of VegT induce endoderm, whereas low levels tend to induce mesoderm (Kavka and Green, 2000). Fertilization results in accumulation of nuclear β-Catenin at the dorsal side. Therefore, VegT and β-Catenin overlap in the dorsal, vegetal part of embryos, where they enhance transcription of factors, which dorsalize mesendodermal germ layers. POU-V factors are primarily localized in the animal half and their concentration decreases toward the vegetal pole. Such a distribution of Oct factors functions to titrate the strength of both VegT activity and β-Catenin signaling, so as to ensure correct induction and patterning of the mesendodermal germ layer. It should also be noted that Oct factors within the animal region promote formation of neuroectoderm but prevent neuronal differentiation (Cao et al, 2006; Snir et al, 2006). A corresponding model is presented in Figure 7I.
Oct-25 functions as a co-repressor to inhibit transcription of VegT and β-Catenin target genes
In an attempt to dissect how Oct-25 can inhibit VegT- or β-Catenin-induced gene activation at the molecular level, we found that Oct-25 directly interacts with VegT and Tcf3. Interestingly, a physical interaction also occurs between VegT and Tcf3, thereby providing a direct link between VegT and maternal β-Catenin signaling. Furthermore, we observed that Oct-25, VegT and Tcf3 form a ternary complex in vivo. These findings suggest a mechanism for Oct-25 to regulate VegT- and β-Catenin-activated gene transcription, by which it serves as a co-repressor. We have then analyzed the regulatory effect of Oct-25 on the Xnr1 and Siamois promoters, which are activated by maternal VegT and β-Catenin. EMSA supershifts demonstrated that Oct-25 and VegT form a complex on the T-box site of the Xnr1 promoter, and that Oct-25 and Tcf3 form complexes on the TCF/LEF site of both the Xnr1 and Siamois promoters. Luciferase reporter assays demonstrated that the activities of Xnr1 or Siamois promoters stimulated by VegT or by components of the β-Catenin pathway are all repressed by Oct-25, which is in agreement with the results of animal cap assays. Moreover, Oct-25 is still able to inhibit the stimulation of Xnr1 and Siamois promoter mutants lacking the Oct binding site, suggesting that direct binding of Oct-25 to the Xnr1 or Siamois promoter might be not necessary for the repression of VegT- or β-Catenin-induced gene transcription. Instead, we suggest that repression is achieved by the formation of a complex between Oct-25 and VegT on the T-box site, or between Oct-25 and Tcf3 on the TCF/LEF site. Therefore, in the absence of its binding site, Oct-25 serves as a co-repressor for VegT or Tcf3 to regulate transcription of target genes.
As Oct-25 represses Xnr1 or Siamois promoter activity even in the absence of a direct binding site, the functional significance of the Oct binding sites may be questioned. Xnr1 and Siamois promoter/reporter mutants containing only the Oct-binding site do not respond to overexpression of VegT or β-Catenin; however, they are stimulated by overexpression of Oct-25. This observation is congruent with a previous finding that Oct-25 stimulates an artificial promoter/reporter containing six reiterated copies of the Oct binding motif (Niwa et al, 2002; Morrison and Brickman, 2006). EMSA revealed that VegT or Tcf3 forms a complex with Oct-25 on the Oct binding site. When VegT is co-injected, stimulation of the promoter/reporter mutants by Oct-25 is inhibited. We did not observe a significant effect of Tcf3 overexpression on the stimulation of these promoter mutants by Oct-25 (data not shown). Nevertheless, the protein complex formation on the Oct binding site provides an additional mode of repression, that is, Oct-25 diverts VegT or Tcf3 from binding to their respective sites, thereby inhibiting VegT- or β-Catenin-induced gene transcription.
It may be asked whether the repression of VegT- and β-Catenin-mediated gene activations is specific for POU-V factors or whether other POU factors, for example, Oct-1 of subclass II, perform a similar function. However, animal or vegetal overexpression of Xenopus Oct-1 yielded a different phenotype (Supplementary Figure S6A–E) (Veenstra et al, 1998). Moreover, Oct-1 overexpression did not lead to a repression of Xnr1 and Siamois promoter/reporter activities (Supplementary Figure S6F–G). Therefore, it seems that the inhibitory effect on VegT and β-Catenin target genes is specific for POU-V factors.
Nucleotide sequence comparisons between the promoter regions of Xnr1, 2, 3, 5, 6 and Siamois reveal the existence of an Oct binding site in all of these genes (Supplementary Figure S7). TCF/LEF sites exist in all of these promoters, except for Xnr6, whereas T-box sites are found in all but Xnr3 and Siamois promoter regions. The conserved localization of these elements might suggest that these genes are regulated by similar mechanisms. They are either activated by β-Catenin alone or by a combination of VegT and β-Catenin. As demonstrated by gain-of-function in whole embryos and animal caps, overexpression of Oct-25 inhibits the activation of all these genes.
Interactions between VegT and Tcf3 may explain the synergistic effect of VegT and β-Catenin on activating Siamois transcription
Although we could not detect a canonical T-box motif within the analyzed part of the Siamois promoter, VegT stimulates Siamois promoter/reporter activity. Indeed, in animal cap assays, a synergistic effect has been observed for VegT and Xwnt8 on the activation of Siamois (Rex et al, 2002). Moreover, depletion of VegT reduced expression of Siamois and Xnr3 in embryos, and a more severe reduction was found when both VegT and β-Catenin were depleted (Xanthos et al, 2002). There are two reasonable explanations for this observation. First, we cannot exclude that VegT activates expression of another factor that synergizes with β-Catenin/Tcf3 in the activation of the Siamois promoter. Second, and highly plausible in light of our results, this synergism could be achieved by forming a ternary activation complex composed of β-Catenin, Tcf3 and VegT.
A mechanism for the maintenance of pluripotency
Xenopus Oct factors behave as functional homologues to mammalian Oct-3/4 (Cao et al, 2006; Morrison and Brickman, 2006), which plays a key role in the maintenance of pluripotency of ES cells. However, the mechanisms to maintain pluripotency at early cleavage stages within the embryo have remained largely mysterious. Our results suggest that Xenopus POU-V factors repress mesendodermal germ layer formation by antagonizing the function of maternal embryonic inducers, especially that of VegT. β-Catenin signaling is well conserved throughout vertebrates to play a role in axis specification. Oct-25 represses β-Catenin signaling via interaction with Tcf3. The TCF/LEF family is composed of multiple members with conserved functional domains. Therefore, Oct-25 probably interacts also with other members of the TCF/LEF family. Moreover, distinct TCF/LEF factors transduce β-Catenin signaling at different stages of embryogenesis or in different types of tissues. Therefore, in addition to its maternal function, Oct-25 might also interfere with β-Catenin signaling in general.
Although a direct functional homologue to Xenopus maternal VegT has not been found in mammals, it cannot be excluded that certain maternally expressed T-box proteins display a function similar as Xenopus VegT. Intriguingly, we found that mouse Oct-3/4 shows physical interactions with Xenopus Tcf3 and VegT, suggesting that this mammalian POU-V factor probably employs a similar mechanism to inhibit mesendoderm formation. The activin/nodal and FGF signaling pathways, which are functionally conserved in vertebrate mesendoderm formation, are shown to be blocked by Oct factors in early embryos (Henig et al, 1998; Cao et al, 2006; Morrison and Brickman, 2006). It has also to be mentioned that Oct factors activate Sox2 both in Xenopus and in mammals (Chew et al, 2005; Cao et al, 2006; Babaie et al, 2007). Sox2 is another important factor for the maintenance of pluripotency and, especially, for keeping the undifferentiated state of neuroectodermal precursor cells (Boiani and Schöler, 2005). These findings suggest a general principle, that is, the maintenance of pluripotency is, on one hand, to disable the signals responsible for induction of differentiation and, on the other hand, to promote the signals that keep cells in an undifferentiated state and inhibit terminal differentiation. The molecular mechanisms depend upon the temporal and spatial distribution of POU-V proteins and their cofactors, which in a coordinated manner determine whether genes are repressed or activated.
Materials and methods
Embryos and explants
Embryos were obtained by in vitro fertilization and cultured in 0.1 × MBSH (1 × MBSH: 88 mM NaCl, 2.4 mM NaHCO3, 1 mM KCl, 0.82 mM MgSO4, 0.41 mM CaCl2, 0.33 mM Ca(NO3)2, 10 mM HEPES, pH 7.4). Animal cap explants used for gene expression analysis, luciferase assays or co-immunoprecipitation were cut from uninjected or injected embryos at stage 8.5 and cultured in 0.5 × MBSH until the desired stages. To analyze the distribution of RNAs, animal and vegetal blastomeres at stage 4 were manually separated. Embryos at stage 8.5 were divided into animal, equatorial and vegetal parts.
In vitro transcription of RNA and morpholino oligos
In vitro transcription of Oct-25, Oct-60 and mOct-3/4 mRNAs was performed as described (Cao et al, 2006). Plasmids pCS2+VegT, pCS2+dnTcf3, pSP64T-Xwnt8, pSP64T-β-Catenin and pTO-NPC(Oct-1) were transcribed using mMessage mMachine SP6 or T7 kit (Ambion). Antisense morpholino oligos used for Oct-25 and Oct-60 functional knockdown were as described (Cao et al, 2006).
Gene expression analysis with real-time RT–PCR
Real-time RT–PCR was performed in the same way as described (Cao et al, 2006). Primers and cycling conditions are listed in (Supplementary Table S2). Results are presented as histograms with relative units.
Preparation of GST-fusion proteins and GST pull-down assays
Preparation of His-tagged fusion proteins used for EMSA assays and GST-tagged fusion proteins used for GST pull-down assays were as described (Cao et al, 2004). 35S-labeled proteins and unlabeled Tcf3 were prepared using the TNT® Coupled Reticulocyte Lysate Systems (Promega).
Luciferase reporter assay
Promoter reporter plasmid DNAs and mRNAs were injected into the animal pole of all blastomeres at the four-cell stage. DNA was injected at 40 pg, mRNAs were injected at 200 pg, except for Oct-25 and Oct-1, which were injected at 400 pg/embryo. Promoter reporter constructs were also injected with ctrlMO or Oct25MO/Oct60MO (45 ng) into the equatorial region of all blastomeres in the four-cell stage. Whole embryos were collected at stage 10.5. Details are described in Supplementary data.
EMSA and ChIP
EMSA and ChIP were performed essentially as described (Cao et al, 2004). Uninjected embryos, embryos injected with 20 ng of ctrlMO or Oct25MO were collected at stage 10.5 and subjected to ChIP. An anti-Oct-25 peptide antibody (Cao et al, 2006) was used to precipitate chromatin–protein complexes. Primers used for amplifying Xnr1 promoter region were as follows 5′-TTAGCATGCAGTTTGCTAGGG-3′ (−419 to −399), reverse 5′-GAGCTGCCATTGCTCTTCAC-3′ (−100 to −119). Primers used for amplifying Siamois promoter region were as follows: forward 5′-GTGTAAGTAAGGGACTTTGAAGTCTTG-3′ (−370 to −344), reverse 5′-GGTGTGTCCAGGATGTGTTTTG-3′ (−51 to −72). For EMSAs with embryonic extracts and for supershift assays, refer to Supplementary data.
Co-immunoprecipitation
Each 200 pg of mRNAs of flag-Oct-25, VegT-MT and Tcf3-MT was injected into the animal pole of embryos at the four-cell stage. Caps were collected at stage 10.5 and subjected to co-immunoprecipitation. For further details, including Western blotting and plasmid construction, refer to Supplementary data.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Figure S7
Supplementary Figure Legends and Tables
Acknowledgments
We thank A Rößner for skilful technical assistance and ML King, HR Schöler, R Moon and GJC Veenstra for the gifts of plasmids. This work was supported by a grant from the Deutsche Forschungsgemeinschaft to WK (SFB 497/A1).
References
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM (2000) Endodermal nodal-related signals and mesoderm induction in Xenopus. Development 127: 1173–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaie Y, Herwig G, Greber B, Brink TC, Wruck W, Groth D, Lehrach H, Burdon T, Adjaye J (2007) Analysis of OCT4 dependent transcriptional networks regulating self renewal and pluripotency in human embryonic stem cells. Stem Cells 25: 500–510 [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W (1996) Functional interaction of beta-Catenin with the transcription factor LEF-1. Nature 382: 638–642 [DOI] [PubMed] [Google Scholar]
- Boiani M, Schöler HR (2005) Regulatory networks in embryo derived pluripotent stem cells. Nat Rev Mol Cell Biol 6: 872–884 [DOI] [PubMed] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D (1997) A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev 11: 2359–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Knöchel S, Donow C, Miethe J, Kaufmann E, Knöchel W (2004) The POU factor Oct-25 regulates the Xvent-2B gene and counteracts terminal differentiation in Xenopus embryos. J Biol Chem 279: 43735–43743 [DOI] [PubMed] [Google Scholar]
- Cao Y, Siegel D, Knöchel W (2006) Xenopus POU factors of subclass V inhibit activin/nodal signaling during gastrulation. Mech Dev 123: 614–625 [DOI] [PubMed] [Google Scholar]
- Clements D, Friday RV, Woodland HR (1999) Mode of action of VegT in mesoderm and endoderm formation. Development 126: 4903–4911 [DOI] [PubMed] [Google Scholar]
- Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, Li P, Ang YS, Lim B, Robson P, Ng HH (2005) Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol 25: 6031–6046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon FL, Fairclough L, Price BM, Casey ES, Smith JC (2001) Determinants of T box protein specificity. Development 128: 3749–3758 [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H (2004) Dorsal–ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol 20: 285–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan MJ, Gruning W, Walz G, Sokol SY (1998) Wnt signaling and transcriptional control of Siamois in Xenopus embryos. Proc Natl Acad Sci USA 95: 5626–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DC, Sutherland L, Clark J, Burdon T (2004) Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells 22: 225–235 [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C (2000) Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol 222: 124–134 [DOI] [PubMed] [Google Scholar]
- Heasman J (2006) Maternal determinants of embryonic cell fate. Semin Cell Dev Biol 17: 93–98 [DOI] [PubMed] [Google Scholar]
- Henig C, Elias S, Frank D (1998) A POU protein regulates mesodermal competence to FGF in Xenopus. Mech Dev 71: 131–142 [DOI] [PubMed] [Google Scholar]
- Hilton E, Rex M, Old R (2003) VegT activation of the early zygotic gene Xnr5 requires lifting of Tcf-mediated repression in the Xenopus blastula. Mech Dev 120: 1127–1138 [DOI] [PubMed] [Google Scholar]
- Hinkley CS, Martin JF, Leibham D, Perry M (1992) Sequential expression of multiple POU proteins during amphibian early development. Mol Cell Biol 12: 638–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DW, Kofron M, Resnik E, Langland R, Destree O, Wylie C, Heasman J (2002) Repression of organizer genes in dorsal and ventral Xenopus cells mediated by maternal XTcf3. Development 129: 4015–4025 [DOI] [PubMed] [Google Scholar]
- Hurlstone A, Clevers H (2002) T-cell factors: turn-ons and turn-offs. EMBO J 21: 2303–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde CE, Old RW (2000) Regulation of the early expression of the Xenopus nodal-related 1 gene, Xnr1. Development 127: 1221–1229 [DOI] [PubMed] [Google Scholar]
- Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR (2006) Dissecting self-renewal in stem cells with RNA interference. Nature 442: 533–538 [DOI] [PubMed] [Google Scholar]
- Kavka AI, Green JBA (2000) Evidence for dual mechanisms of mesoderm establishment in Xenopus embryos. Dev Dyn 219: 77–83 [DOI] [PubMed] [Google Scholar]
- Kofron M, Demel T, Xanthos J, Lohr J, Sun B, Sive H, Osada S, Wright C, Wylie C, Heasman J (1999) Mesoderm induction in Xenopus is a zygotic event regulated by maternal VegT via TGFbeta growth factors. Development 126: 5759–5770 [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H (1996) XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86: 391–399 [DOI] [PubMed] [Google Scholar]
- Morrison GM, Brickman JM (2006) Conserved roles for Oct4 homologues in maintaining multipotency during early vertebrate development. Development 133: 2011–2022 [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95: 379–391 [DOI] [PubMed] [Google Scholar]
- Niwa H, Masui S, Chambers I, Smith AG, Miyazaki J (2002) Phenotypic complementation establishes requirements for specific POU domain and generic transactivation function of Oct-3/4 in embryonic stem cells. Mol Cell Biol 22: 1526–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG (2000) Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 24: 372–376 [DOI] [PubMed] [Google Scholar]
- Rex M, Hilton E, Old R (2002) Multiple interactions between maternally-activated signalling pathways control Xenopus nodal-related genes. Int J Dev Biol 46: 217–226 [PubMed] [Google Scholar]
- Roel G, Hamilton FS, Gent Y, Bain AA, Destree O, Hoppler S (2002) Lef-1 and Tcf-3 transcription factors mediate tissue-specific Wnt signaling during Xenopus development. Curr Biol 12: 1941–1945 [DOI] [PubMed] [Google Scholar]
- Shivdasani RA (2002) Molecular regulation of vertebrate early endoderm development. Dev Biol 249: 191–203 [DOI] [PubMed] [Google Scholar]
- Snir M, Ofir R, Elias S, Frank D (2006) Xenopus laevis POU91 protein, an Oct3/4 homologue, regulates competence transitions from mesoderm to neural cell fates. EMBO J 25: 3664–3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Yokota C, Takano K, Tanegashima K, Onuma Y, Goto J, Asashima M (2000) Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development 127: 5319–5329 [DOI] [PubMed] [Google Scholar]
- Veenstra GJC, Peterson-Maduro J, Mathu MT, van der Vliet PC, Destree OHJ (1998) Non-cell autonomous induction of apoptosis and loss of posterior structures by activation domain-specific interactions of Oct-1 in the Xenopus embryo. Cell Death Differ 5: 774–784 [DOI] [PubMed] [Google Scholar]
- Wessely O, Agius E, Oelgeschlager M, Pera EM, DeRobertis EM (2001) Neural induction in the absence of mesoderm: β-Catenin dependent expression of secreted BMP antagonists at the blastula stage in Xenopus. Dev Biol 234: 161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ, Sun BI, Sive HL, Smith JC (2002) Direct and indirect regulation of derriere, a Xenopus mesoderm-inducing factor, by VegT. Development 129: 4867–4876 [DOI] [PubMed] [Google Scholar]
- Whitfield T, Heasman J, Wylie C (1993) XLPOU-60, a Xenopus POU-domain mRNA, is oocyte-specific from very early stages of oogenesis, and localised to presumptive mesoderm and ectoderm in the blastula. Dev Biol 155: 361–370 [DOI] [PubMed] [Google Scholar]
- Whitfield TT, Heasman J, Wylie CC (1995) Early embryonic expression of XLPOU-60, a Xenopus POU-domain protein. Dev Biol 169: 759–769 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R (1998) Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 14: 59–88 [DOI] [PubMed] [Google Scholar]
- Xanthos JB, Kofron M, Wylie C, Heasman J (2001) Maternal VegT is the initiator of a molecular network specifying endoderm in Xenopus laevis. Development 128: 167–180 [DOI] [PubMed] [Google Scholar]
- Xanthos JB, Kofron M, Tao Q, Schaible K, Wylie C, Heasman J (2002) The roles of three signaling pathways in the formation and function of the Spemann Organizer. Development 129: 4027–4043 [DOI] [PubMed] [Google Scholar]
- Zhang J, Houston DW, King ML, Payne C, Wylie C, Heasman J (1998) The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell 94: 515–524 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Figure S7
Supplementary Figure Legends and Tables


