Abstract
Rationale
Mice lacking the dopamine transporter (DAT−/−) exhibit high extracellular dopamine levels and marked hyperactivity. This hyperlocomotion is paradoxically decreased by acute administration of amphetamine-like psychostimulants, an effect that has been previously related to the activation of serotonergic neurotransmission.
Objectives
The goal of the present study was to investigate the effects of acute and daily administration of d-amphetamine on the locomotor activity of DAT−/− mice and examine the development of behavioral sensitization. In addition, we tested the implication of the serotonin system in the observed effects.
Methods
DAT+/+, DAT+/−, and DAT−/− mice were injected with acute amphetamine (0, 0.3, 1, 3, or 10 mg/kg, s.c.), repeated amphetamine (1 mg/kg for 8 days, s.c.), or with the serotonin reuptake inhibitor fluoxetine (0, 5, 10, or 20 mg/kg, s.c.) and their locomotor activity was evaluated. Moreover, the expression of the serotonin transporter and 5-HT1A receptors in the brain of DAT−/− mice was studied using autoradiography.
Results
Acute and repeated d-amphetamine injection (1 mg/kg) induced an hypolocomotor response in DAT−/− and DAT+/− mice, but only DAT+/− mice developed locomotor sensitization to the drug. Acute treatment with fluoxetine decreased locomotion in DAT−/− mice in a dose-dependent manner. The common hypolocomotor effect induced by d-amphetamine and fluoxetine in DAT−/− mice suggests an action on the serotonin transporter. However, autoradiography of the serotonin transporter and 5-HT1A receptors showed normal density and distribution in the brain, suggesting no compensatory effects due to the deletion of the DAT.
Conclusions
These findings indicate that partial or total DAT gene deletion result in decreased locomotion in response to d-amphetamine and modify behavioral sensitization depending on the proportion of DAT removed, suggesting that inhibition of the DAT is necessary for the development of sensitization to psychostimulant drugs.
Keywords: Animals; Dextroamphetamine; administration & dosage; Dopamine Plasma Membrane Transport Proteins; Dopamine Uptake Inhibitors; administration & dosage; Dose-Response Relationship, Drug; Drug Administration Schedule; Gene Deletion; Membrane Glycoproteins; Membrane Transport Proteins; deficiency; genetics; Mice; Mice, Inbred C57BL; Motor Activity; drug effects; genetics; Nerve Tissue Proteins
Keywords: dopamine transporter, d-amphetamine, locomotor activity, stereotypy, behavioral sensitization, fluoxetine, autoradiography
INTRODUCTION
The dopamine transporter (DAT) is located on the plasma membrane of dopamine (DA) neurons, where it controls the concentrations of extracellular DA by rapidly removing the transmitter back into the cytoplasm (Giros and Caron 1993; Povlock and Amara 1997). Recently, a strain of mice that lacks the DAT has been developed (DAT−/− mice) (Giros et al. 1996). Deletion of the DAT gene results in a five- to ten-fold increase in extracellular DA, measured by microdialysis and voltammetry studies in the striatum and nucleus accumbens of the mutant mice (Jones et al. 1998; Spielewoy et al. 2000a). This phenotype is accompanied by numerous compensatory changes in pre- and postsynaptic neuronal markers, including reduced intracellular stores of DA (Jones et al. 1998), decreased D1 andD2 receptor density (Giros et al. 1996), loss of D2 autoreceptor functions (Jones et al. 1999), and increased D1 receptor sequestration in the cytoplasmic compartment (Dumartin et al. 2000). The most prominent behavioral characteristic of DAT−/− mice is their marked hyperlocomotion (Giros et al. 1996). This hyperactivity is novelty-driven, long lasting, and persists after repeated exposure to the same environment (Giros et al. 1996; Gainetdinov et al. 1999; Spielewoy et al. 2000a, 2000b). Thus, the removal of the DAT induces a spontaneous hyperlocomotion that is virtually indistinguishable from that obtained with amphetamine-like psychostimulants in DAT+/+ mice (Giros et al. 1996).
Although cocaine and d-amphetamine bind with similar affinity to the DAT, the serotonin transporter (5-HTT) and the norepinephrine transporter (Amara and Kuhar 1993), it has been widely assumed that the motor stimulating effects and the reinforcing properties of these drugs result from their action at the DAT (Kuhar et al. 1991; Seiden et al. 1993). Surprisingly, two studies have recently demonstrated that despite the absence of DAT, the mutant mice are still sensitive to the reinforcing properties of cocaine under self-administration (Rocha et al. 1998) and conditioned place preference (Sora et al. 1998) paradigms. Moreover, DAT−/− mice have been shown to exhibit an hypolocomotor response to acute cocaine and d-amphetamine (Gainetdinov et al. 1999). These paradoxical effects of psychostimulants in DAT−/− mice have been explained by an action on the 5-HT system (Rocha et al. 1998; Gainetdinov et al. 1999). Gainetdinov et al. (1999) reported that agents increasing 5-HT neurotransmission (fluoxetine, 5-hydroxytryptophan and L-tryptophan) substantially reduce hyperlocomotion in DAT−/− mice and concluded that the calming effects of psychostimulants in DAT−/− mice depend on an action on the 5-HT system.
The aim of the present work was to investigate the effect of acute and chronic treatment with d-amphetamine on the hyperactivity of DAT−/− mice and to study the possible implication of 5-HT neurotransmission in the hypolocomotor effects of psychostimulants in these mutant mice. We first performed a locomotor dose-response to acute d-amphetamine in DAT+/+, DAT+/−, and DAT−/− mice, to identify the dose range that decreased hyperactivity in DAT−/− mice under our experimental conditions. Because the hypolocomotor effect of d-amphetamine in DAT−/− mice could be due to an increase in stereotyped behavior induced by the drug, we also measured stereotypies after acute administration of d-amphetamine in the three groups of mice. In a second experiment, mice were treated daily with d-amphetamine for eight days, followed by a saline or drug challenge, to examine locomotor sensitization. Additionally, in order to further study the locomotor effects of the 5-HTT inhibitor fluoxetine in DAT mutant mice, we did a dose-response curve. Finally, we performed in vitro autoradiography of 5-HTT binding sites and immunoautoradiography labeling of 5-HT1A receptors in the brain of DAT−/− mice and their control littermates, to examine possible compensatory effects in the 5-HT system during development.
MATERIALS AND METHODS
Animals
Homozygous DAT−/− mice were obtained by genetic manipulation (Giros et al. 1996) and were backcrossed for 12 generations on a C57BL/6 background. DAT−/−, heterozygous DAT+/−, and wild-type DAT+/+ littermates were obtained from the mating of DAT+/− mice and their genotypes were determined by Southern blot analysis as previously described (Giros et al. 1996). Mice were maintained in a temperature-controlled colony room (22°C, humidity 60%), under a 12-h light/dark cycle (lights on from 7:30 to 19:30). All mice used were 8 to 10 weeks old and drug-naive. They were handled daily during the week preceding the experiments. All experiments were conducted in accordance with standard ethical guidelines (European Communities Council Directive 86/609/EEC for the care and use of laboratory animals) and approved by the local ethical committee.
Drugs
D-amphetamine sulfate (Sigma) and fluoxetine (Lilly) were dissolved in saline (0.9% NaCl) and distilled water, respectively. Drugs were injected subcutaneously (s.c.) in a volume of 0.01 ml/g. Control mice received vehicle injections.
Behavioral analysis
Locomotion was evaluated in activity boxes (20 × 15 × 25 cm) located in a sound-attenuated experimental room under moderate illumination (<5 lux). The animal’s displacements were measured by photocell beams located across the long axis, 15 mm (horizontal activity) and 30 mm (vertical activity) above the floor. Each box was connected by an interface to a computer (Imetronic, Bordeaux, France).
The acute locomotor dose-responses to d-amphetamine and fluoxetine were evaluated between 11:00 and 16:00 h. Mice were randomly assigned to a treatment group: d-amphetamine (0, 0.3, 1, 3, and 10 mg/kg, s.c.) or fluoxetine (0, 5, 10, and 20 mg/kg, s.c.). They were weighed, injected and immediately placed in the activity boxes for a 1-h session.
Stereotypies induced by acute amphetamine (1 and 10 mg/kg, s.c.) were evaluated with a rating scale (Creese and Iversen 1973). Each mouse was observed for a 15-sec period at 5-min intervals over the 1-h period following drug administration. Subjects were scored as follows: 0, asleep/inactive; 1, intermittent locomotor activity; 2, continuous locomotor activity with stereotyped sniffing, rearing or grooming; 3, stereotyped behavior maintained over a wide range of the cage; 4, continuous stereotypy in a restricted area of the cage; 5, continuous stereotyped behavior in a restricted location with licking at the walls or floor; 6, continuous stereotyped behavior in a restricted location with biting.
The locomotor response to chronic treatment with d-amphetamine was studied between 11:00 and 15:00 h. The experiment was divided in three phases: preexposure, pairing phase and test phase. During the preexposure session, performed the day before the beginning of the pairing phase, mice received no injection and were placed in the activity boxes for 1 h to measure their basal locomotor activity. During the pairing phase (days 1–8), mice were injected daily with saline or d-amphetamine (1 mg/kg, s.c.) and immediately placed in the activity boxes for a 1-h session. The test phase lasted two days (days 9–10), and examined the environmental and pharmacological conditioning to the drug. On day 9, mice were injected with saline before the introduction in the activity boxes, and on day 10 all mice were given a challenge injection of d-amphetamine (0.5 mg/kg, s.c.).
Autoradiography of 5-HTT binding sites
Mice were killed by decapitation, the brains were rapidly removed, frozen in isopentane chilled at −30°C with dry ice and stored at −20°C. Coronal sections (20 μm) were cut in a cryostat at −20°C, thaw-mounted onto gelatin-coated slides and stored at −20°C until used. Autoradiographic experiments with [3H]citalopram, a selective 5-HTT inhibitor, were performed as described (D’Amato et al. 1987). Briefly, slides were brought to room temperature during 15 min and then preincubated for 15 min in 50 mM Tris-HCl buffer, pH 7.4, containing 5 mM KCl and 120 mM NaCl, at 25°C. The incubation proceeded for 2 h at 25°C in fresh buffer with 0.7 nM [3H]citalopram (85 Ci/mmol, Amersham). Non-specific binding was estimated from adjacent sections incubated in the presence of 10 mM fluoxetine. Sections were then washed four times for 2 min each in Tris buffer at 4°C, and rapidly immersed in ice-cold distilled water. They were dried under a stream of cold air and apposed to 3H-Hyperfilm (Amersham) for 10 days at 4°C. Optical density on the autoradiographic films was measured using a computerized image analysis system (Biocom, Les Ulis, France) and converted to fmol/mg tissue of specifically bound [3H]citalopram according to a [3H] standard scale (Amersham).
Immunoautoradiography of 5-HT1A receptors
Mice were anesthetized with pentobarbital (100 mg/kg, i.p.) and perfused transcardially with 100 ml of 0.9% (w/v) NaCl and 0.1% (w/v) NaNO2. After decapitation, the brain was removed, frozen in isopentane at −30°C and stored at −20°C until used. Polyclonal antibodies that specifically recognize 5-HT1A receptors were purified and used as described (Gérard et al. 1994). Briefly, coronal sections (20 μm) were fixed for 5 min at 4°C with 4% paraformaldehyde in phosphate buffered saline (PBS; 50 mM NaH2PO4/Na2HPO4, 154 mM NaCl, pH 7.4), and preincubated for 1 h in PBS with 3% (w/v) bovine serum albumin and 1% (v/v) donkey serum. They were then incubated overnight at 4°C with purified anti-5-HT1A receptor antibodies (1/750 final dilution). After extensive washings, sections were incubated for 2 h at room temperature in PBS with donkey anti-rabbit [125I]IgG (750–3000 Ci/mmol, 0.2 mCi/ml, Amersham), then washed, dried and apposed to βmax film (Amersham) for 4–5 days. Optical density on the immunoautoradiograms was measured using an image analysis system (Biocom).
Statistical analysis
Analyses of variance (ANOVA) were used to compare the results of the behavioral experiments. Post-hoc comparisons were made using Tukey’s test. The results of the autoradiography and immunoautoradiography studies were analyzed using Student’s t-test. Data are presented as mean ± SEM. Statistical analyses were performed using the CRUNCH statistical package (Crunch Software Corporation, Oakland, CA, USA).
RESULTS
Effect of acute d-amphetamine
Analysis of the acute locomotor response of DAT+/+, DAT+/− and DAT−/− mice to saline and increasing doses of d-amphetamine (0.3, 1, 3, and 10 mg/kg, s.c.) revealed a genotype effect [F(2,210)=37.03, p<0.0001], a dose effect [F(4,210)=7.59, p<0.0001], and a genotype x dose interaction [F(8,210)=13.12, p<0.0001] (Figure 1). DAT+/+ mice showed an increase in locomotion at 3 mg/kg d-amphetamine, while the lower doses (0.3 and 1 mg/kg) were inactive and the higher dose (10 mg/kg) induced stereotypies that interfered with the expression of hyperlocomotion [dose effect: F(4,72)=15.96, p<0.0001; Tukey’s test 3 mg/kg vs. saline: p<0.01]. In DAT+/− mice, the dose-response curve to d-amphetamine showed a decrease in locomotion at 1 mg/kg, and an increase at 10 mg/kg [dose effect: F(4,74)=10.39, p<0.0001; Tukey’s test: p<0.05 for 1 mg/kg, and p<0.01 for 10 mg/kg]. In contrast, d-amphetamine decreased the hyperlocomotion of DAT−/− mice at the doses of 1, 3, and 10 mg/kg [dose effect: F(4,62)=8.77, p<0.0001; Tukey’s test: p<0.05 for 1 and 3 mg/kg, and p<0.01 for 10 mg/kg].
Figure 1.

Locomotor dose-response to acute d-amphetamine in DAT+/+, DAT+/−, and DAT−/− mice. Animals were injected with d-amphetamine (0.3, 1, 3 and 10 mg/kg, s.c.) or saline, and placed in the activity boxes. Locomotion was measured as the number of photocell beam breaks during 1 h. Values represent mean ± SEM; DAT+/+, n = 10–16; DAT+/−, n = 11–16; and DAT−/−, n = 8–15. * p<0.05 and ** p<0.01 vs. saline-treated mice (ANOVA followed by Tukey’s test).
Analysis of stereotypies of DAT+/+, DAT+/−, and DAT−/− mice in response to saline and d-amphetamine (1 and 10 mg/kg, s.c.) revealed no genotype effect [F(2,44)=1.77, p>0.1], a dose effect [F(2,44)=7.55, p<0.002], and a genotype x dose interaction [F(4,44)=4.58, p<0.004] (Figure 2). As expected, DAT+/+ mice showed a large increase in stereotypies after 10 mg/kg of d-amphetamine (Tukey’s test: p<0.01), whereas the dose of 1 mg/kg was inactive. In contrast, stereotypies were not significantly modified by either dose of the drug in DAT+/− and DAT−/− mice.
Figure 2.
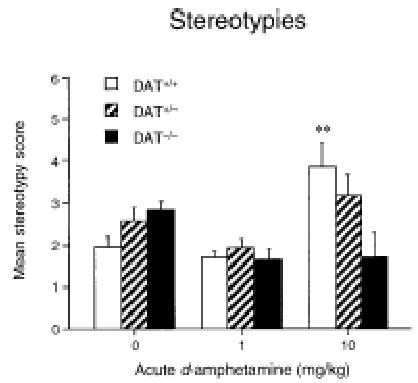
Effect of acute d-amphetamine on stereotypies in DAT+/+, DAT+/−, and DAT−/− mice. Animals (n=5) were injected with d-amphetamine (1 or 10 mg/kg, s.c.) or saline, and placed in the experimental boxes. Stereotypies were evaluated with a rating scale at 5-min intervals over the 1-h period following drug administration. Values represent mean ± SEM; ** p<0.01 vs. saline-treated mice (ANOVA followed by Tukey’s test).
Effect of daily d-amphetamine
For the chronic treatment with d-amphetamine, we selected the dose of 1 mg/kg because it decreased locomotion in DAT+/− and DAT−/− mice. Analysis of the locomotor response to daily administration of d-amphetamine or saline during the pairing phase revealed a genotype effect [F(2,42)=43.03, p<0.0001], a treatment effect [F(1,42)=9.39, p<0.004], a day effect [F(7,301)=3.28, p<0.002], a genotype x treatment interaction [F(2,42)=15.18, p<0.0001], and a genotype x day interaction [F(14,301)=2.23, p<0.007], but no treatment x day or genotype x treatment x day interactions (Figure 3).
Figure 3.

Effect of repeated administration of d-amphetamine on the locomotor response of DAT+/+, DAT+/−, and DAT−/− mice. Animals were first exposed to the testing environment without any injection (preexposure phase, not shown), followed by a daily injection of d-amphetamine (1 mg/kg, s.c.; filled symbols) or saline (open symbols) in the same environment for eight days (pairing phase). During the saline test (day 9), all mice were exposed in a drug-free state to the environment repeatedly associated to the effect of d-amphetamine or saline. During the drug test (day 10), all mice were injected with d-amphetamine (0.5 mg/kg, s.c.) to examine the pharmacological sensitization to the behavioral effects of the drug. Locomotion was evaluated daily during a 1 h session and is expressed as total number of photocell beam breaks. Please note that DAT−/− mice are hyperactive when compared to DAT+/+ and DAT+/− mice and that the vertical scales in the three graphs are different. Values represent mean ± SEM; DAT+/+, n = 6–9; DAT+/−, n = 6–8; and DAT−/−, n = 6–8. ANOVA followed by Tukey’s test: * p<0.05 and ** p<0.01, vs. saline-treated mice of the same genotype at the same time point; # p<0.05, vs. the first pairing day within the same treatment group; § p<0.05 and §§ p<0.001, vs. the last pairing day within the same treatment group.
In DAT+/+ mice, analysis of the locomotor activity during the pairing phase revealed a treatment effect [F(1,14)=12.52, p<0.004], but no day effect or treatment x day interaction (Figure 3). In d-amphetamine-treated mice, locomotion increased during daily injections [day effect: F(7,63)=2.15, p<0.05]. The acute challenge with saline on day 9 induced a higher level of locomotion in drug-pretreated than in saline-pretreated mice, indicating the development of environmental conditioning of the locomotor response (Tukey’s test: p<0.05). The last day, DAT+/+ mice pretreated with the psychostimulant displayed higher locomotor activity after the d-amphetamine challenge (0.5 mg/kg, s.c.) than saline-pretreated mice (p<0.01), indicating the development of behavioral sensitization.
In DAT+/− mice, the results of the pairing phase showed a treatment effect [F(1,13)=10.08, p<0.01], a day effect [F(7,98)=4.06, p<0.001], and a treatment x day interaction [F(7,98)=2.46, p<0.02] (Figure 3). DAT+/− mice injected daily with d-amphetamine displayed a progressive decrease in locomotion [day effect: F(7,56)=5.83, p<0.0001], whereas the locomotor activity of saline-treated DAT+/− mice did not change significantly over time [F(7,42)=1.86, p>0.1]. During the saline test performed on day 9, d-amphetamine-pretreated mice displayed a higher level of locomotion than control animals (p<0.05). Thus, whereas saline-pretreated mice showed the same level of locomotion in response to the saline challenge as the day before, drug-pretreated mice exhibited an increase in locomotor activity compared with the last day of the pairing phase (p<0.01), indicating the absence of conditioned response to the hypolocomotor action of d-amphetamine. The drug test on day 10 revealed no differences between the two groups of DAT+/− mice.
The results of the pairing phase in DAT−/− mice showed a treatment effect [F(1,13)=13.54, p<0.003], a day effect [F(7,98)=2.53, p<0.02], but no treatment x day interaction (Figure 3). DAT−/− mice injected with d-amphetamine displayed an hypolocomotor response which did not increase progressively over daily injections [F(7,56)=2.45, p<0.03], whereas DAT−/− mice given repeated saline remained hyperactive. The injection of saline on day 9 had no effect on the locomotor activity of d-amphetamine and saline-pretreated DAT−/− mice, and both groups were hyperactive, suggesting the absence of environmental conditioning of the hypolocomotor response to the psychostimulant. The acute challenge with d-amphetamine (0.5 mg/kg) on day 10 induced a marked decrease in hyperlocomotion in both saline- and drug-pretreated mice.
Effect of acute fluoxetine
Figure 4 shows the dose-response curve for acute fluoxetine (5, 10, and 20 mg/kg, s.c.) on the locomotor activity of DAT+/+, DAT+/−, and DAT−/− mice. The ANOVA revealed a genotype effect [F(2,59)=165.45, p<0.0001], a dose effect [F(3,59)=9.56, p<0.0001], and a genotype x dose interaction [F(6,59)=7.21, p<0.0001]. DAT+/+ mice showed a decrease in locomotion after 10 mg/kg of fluoxetine, while the lower and the higher doses (5 and 20 mg/kg) had no effect [dose effect: F(3,19)=3.56, p<0.04; Tukey’s test 10 mg/kg vs. vehicle: p<0.05]. Fluoxetine did not affect locomotor activity in DAT+/− mice at any of the doses studied [dose effect: F(3,19)=0.93, p<0.4]. In contrast, fluoxetine 10 and 20 mg/kg decreased the hyperlocomotion of DAT−/− mice, but the effect reached significance only at the higher dose [dose effect: F(3,19)=9.86, p<0.001; Tukey’s test 20 mg/kg vs. vehicle: p<0.01].
Figure 4.

Locomotor dose-response to acute fluoxetine in DAT+/+, DAT+/−, and DAT−/− mice. Animals (n = 5) were injected with fluoxetine (5, 10, and 20 mg/kg, s.c.) or vehicle, and immediately placed in the activity boxes during 1 h. Locomotion was measured as the number of photocell beam breaks (mean ± SEM). * p<0.05 and ** p<0.01 vs. saline-treated mice (ANOVA followed by Tukey’s test).
5-HTT and 5-HT1A binding sites
Quantitative autoradiographic studies with the 5-HTT selective radioligand [3H]citalopram did not reveal any significant differences in the distribution and density of 5-HTT binding sites between DAT+/+ and DAT−/− mice in the substantia nigra, globus pallidus, striatum and hippocampus (Table 1). Similarly, optical density measurements on immunoautoradiograms using 5-HT1A receptor antibodies revealed no significant differences in 5-HT1A immunolabeling between DAT+/+ and DAT−/− mice in the hippocampus and dorsal raphe nucleus (Table 2).
Table 1.
Quantification of the autoradiographic labeling of the 5-HTT by [3H]citalopram in the brain of DAT+/+ and DAT−/− mice.
| Brain region | DAT+/+ | DAT−/− |
|---|---|---|
| Substantia nigra | 16.08 ± 2.45 | 16.36 ± 2.26 |
| Globus pallidus | 15.15 ± 0.66 | 17.46 ± 0.62 |
| Striatum | 6.47 ± 0.54 | 5.51 ± 0.68 |
| Hippocampus | 15.65 ± 1.55 | 15.93 ± 1.68 |
Values represent mean ± SEM fmol/mg tissue of four mice per group.
Table 2.
Quantification of the immunoautoradiographic labeling of 5-HT1A receptors by anti-5-HT1A receptor antibodies in the brain of DAT+/+ and DAT−/− mice.
| Brain region | DAT+/+ | DAT−/− |
|---|---|---|
| Dorsal raphe nucleus | 12.02 ± 2.04 | 14.09 ± 2.23 |
| Hippocampus | 10.38 ± 1.14 | 11.08 ± 0.92 |
Values represent mean ± SEM optical densities (arbitrary units) of four-seven mice per group.
DISCUSSION
In the present study, we further characterized the hypolocomotor effect of d-amphetamine in DAT−/− mice. We first showed that besides the hypolocomotor effect of 2 mg/kg of d-amphetamine reported by Gainetdinov et al. (1999), lower (1 mg/kg) and higher doses (3 and 10 mg/kg) of the drug also decreased the locomotor hyperactivity of DAT−/− mice. Interestingly, we revealed that DAT+/− mice exhibited a biphasic dose-response to d-amphetamine, characterized by hypolocomotion at a low dose (1 mg/kg), as in DAT−/− mice, and increased locomotion at higher doses (3 and 10 mg/kg). Moreover, we demonstrated that the decrease in locomotion induced by d-amphetamine in DAT−/− and DAT+/− mice is not due to an increase in stereotypies. In fact, stereotyped behaviors (sniffing, rearing and grooming) appeared to be slightly decreased in response to d-amphetamine 1 mg/kg in both groups of mice.
Our results also showed that the hypolocomotor response to d-amphetamine (1 mg/kg) in DAT+/− and DAT−/− mice persisted during daily injections of the drug, whereas the same treatment produced a progressive increment in the motor activity of DAT+/+ mice. In DAT+/− mice, the hypolocomotor effect of d-amphetamine increased after repeated drug exposure, indicating that the partial removal of the DAT leads to the development of sensitization to the hypolocomotor action of the psychostimulant drug. In contrast, DAT−/− mice displayed a strong decrease of locomotion after acute d-amphetamine administration that was not enhanced by repeated injections. These findings suggest that neuronal changes in DA transmission resulting from total removal of the DAT disrupted the establishment of sensitization. Psychostimulant-induced sensitization has been commonly associated with disturbances in the synaptic regulation of DA in the nucleus accumbens and striatum, the best documented dysregulation being an increase in the release of DA (Pierce and Kalivas 1997). Deletion of the DAT gene results in a five- to ten-fold increase in extracellular DA levels in the nucleus accumbens (Spielewoy et al. 2000a) and in the striatum (Jones et al. 1998) that mimics the action of d-amphetamine and may have prevented the development of sensitization. Furthermore, impaired DA autoreceptor function and postsynaptic events, such as enhanced responsiveness of D1 receptors in the nucleus accumbens, have also been implicated in the development of behavioral sensitization (Henry and White 1991; White and Kalivas 1998). In DAT−/− mice, D2 receptor mRNA and binding sites are decreased by 50% in both terminal fields and cell body regions (Giros et al. 1996; Jones et al. 1999), and the function of the remaining receptors is decreased by approximately 90% (Jones et al. 1999). DAT−/− mice also exhibit a 55% decrease in D1 receptor mRNA (Giros et al. 1996), together with a striking accumulation of the receptor in the cytoplasmic compartment and decreased delivery to the plasma membrane (Dumartin et al. 2000). These compensatory changes provide additional mechanisms for the disturbed expression of psychostimulant-induced sensitization in DAT−/− mice. By contrast, DAT+/− mice have been described as having an intermediate biochemical phenotype, characterized by a two-fold increase in extracellular DA levels and 25% to 30% reduction in DA receptor sites and autoreceptor functions (Giros et al. 1996; Jones et al. 1998, 1999). This phenotype resulted in the establishment of sensitization to the hypolocomotor effects of d-amphetamine, a phenomenon that is diametrically opposite to what is observed in DAT+/+ mice.
The degree of locomotor sensitization depends not only on the pharmacological sensitization to the psychostimulant action, but also on the environmental context in which repeated drug injections are given (Anagnostaras and Robinson 1996). Environmental conditioning to the stimulant effect of d-amphetamine has been related to the activation of the mesolimbic DA system (Gold et al. 1988; Kalivas et al. 1993). During the saline test performed after the last injection of d-amphetamine, DAT+/+ mice developed the expected conditioned hyperlocomotor response to contextual cues, whereas DAT+/− and DAT−/− mice showed no environmental conditioning to the hypolocomotor action of the drug. One possible explanation could be that, under our experimental conditions, the hypolocomotor effect of d-amphetamine does not condition to the environment, even if DAT were not deleted. Alternatively, this finding could suggest that the synaptic DA dysregulations present in DAT+/− and DAT−/− mice prevented the neural processes underlying conditioned locomotion induced by d-amphetamine. Hence, DAT+/− mice showed an environmental-independent sensitization to the hypolocomotor action of d-amphetamine that might probably be triggered by different mechanisms than the environmental-dependent sensitization to the hyperlocomotor effect of the drug observed in DAT+/+ mice. This absence of environmental conditioning in DAT−/− mice is particularly interesting because it has recently been reported that despite the lack of DAT, these mutant mice display a conditioned response to cocaine, expressed as self-administration and conditioned place preference (Rocha et al. 1998; Sora et al. 1998). These results indicate that conditioning of the locomotor and the rewarding effects of psychostimulant drugs are not equally affected in DAT−/− mice and may thus involve different neural mechanisms.
During the drug challenge performed 48 h after the last daily injection, the three groups of d-amphetamine-pretreated mice responded to the injection of 0.5 mg/kg d-amphetamine, a dose that did not modify locomotion when administered to drug-naive subjects (not shown). This observation supports the establishment of a pharmacological sensitization to the action of the drug, indicated by a hyper- or hypolocomotor response to d-amphetamine in DAT+/+ and DAT+/− mice, respectively. Interestingly, both drug- and saline-pretreated DAT−/− mice showed decreased hyperactivity in response to this dose of d-amphetamine, indicating that the drug effect was independent of the pretreatment, and therefore did not result from pharmacological sensitization in drug-pretreated mice. It is possible that repeated exposure to the testing environment allowed this low dose of d-amphetamine to unmask an habituation effect in both groups of DAT−/− mice, thereby inducing an hypolocomotor response at a dose that was inactive when administered in a novel environment. One could argue that a similar habituation process might have developed during repeated exposure to the testing environment in DAT+/− mice, as saline-pretreated mice showed a tendency towards decreased locomotion. This possibility would confound the interpretation of the sensitization effect observed in the DAT+/− amphetamine-treated group. However, the decrease in locomotion observed in DAT+/− mice treated chronically with saline was not statistically significant, whereas the progressive decrease in locomotor activity in amphetamine-treated DAT+/− mice was significant. Moreover, it has been shown previously that DAT+/− mice fail to develop habituation over consecutive days of testing in the same environment (Spielewoy et al. 2000b).
Recently, 5-HT systems have been implicated in the hypolocomotor action of d-amphetamine in DAT−/− mice, because agents increasing serotonergic neurotransmission also reduced the hyperlocomotion of these mice (Gainetdinov et al. 1999), suggesting that an interaction of psychostimulants with the 5-HTT could mediate the observed effects. In agreement with this hypothesis, in the present study we replicated the result of Gainetdinov et al. (1999), showing that fluoxetine (20 mg/kg) mimics the hypolocomotor effect of d-amphetamine in DAT−/− mice. Furthermore, we report that the action of fluoxetine is dose-dependent since the hypolocomotor effect is not observed after 5 or 10 mg/kg. We also show that fluoxetine is efficient in reducing hyperactivity in DAT−/− mice not only when administered 30 min after placement of the animals in the testing boxes (Gainetdinov et al. 1999), but also when injected immediately before the beginning of the test (present results). In contrast, fluoxetine did not reduce locomotion in DAT+/− mice, at any of the doses examined. However, since the hypolocomotor effect of d-amphetamine in DAT+/− mice was only observed at the dose of 1 mg/kg (but not after 0.3 or 3 mg/kg), the lack of effect of fluoxetine in these mice suggests that we did not find the dose that would reduce locomotor activity and that additional doses need to be tested.
The hypolocomotor effect of d-amphetamine in DAT−/− mice could be explained by adaptive changes in 5-HT neuronal markers that have developed in response to DAT removal and may have facilitated the effect of d-amphetamine on 5-HT release, thus decreasing the locomotor activity of these mice. 5-HTT and 5-HT1A receptors are known to actively regulate extracellular 5-HT levels and 5-HT neuron firing (Baumgarten and Grozdanovic 1994); 5-HT1A receptors also mediate the action of 5-HT on other neurotransmitter neurons (Glennon and Dukat 1995). However, we revealed no compensatory changes in the density and distribution of the 5-HTT and 5-HT1A receptors in the DAT−/− brain. Furthermore, no changes were observed in basal 5-HT levels or 5-HT efflux induced by administration of paroxetine in the ventral hippocampus and the frontal cortex of DAT−/− mice (Smadja et al. 1999). These results suggest either that the 5-HT system is not altered in DAT−/− mice or that compensatory changes affected the functional properties of the receptors and the transporter without modifying their expression levels.
In conclusion, we demonstrated that genetic deletion of the DAT gene in mice results in an hypolocomotor response to d-amphetamine, abolishes environmental conditioning to the locomotor effect of the drug and allows the establishment of pharmacological sensitization depending on the proportion of DAT removed. These data suggest that inhibition of the DAT is mandatory for the establishment of locomotor sensitization, independently of the direction of the locomotor changes induced by the psychostimulant, thus strengthening the implication of DA transmission in these long-lasting changes. Our findings also suggest that in the absence of DAT, d-amphetamine could be acting on alternative targets such as the 5-HTT, as suggested by the hypolocomotor effects induced by fluoxetine in DAT−/− mice. However, considering that d-amphetamine also binds to the norepinephrine transporter, this system also needs to be investigated in DAT−/− mice.
Acknowledgments
We thank Dr. Marie-Pascale Martres and Dr. Véronique Fabre for help in the autoradiography experiments. This work was supported by grants from INSERM to M.H. and B.G. and Mission Interministérielle de Lutte centre les Drogues et la Toxicomanie (convention 96D04) to B.G. C.S. was supported by a fellowship from the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche, G.B. by INSERM and C.R. by Sanofi Research.
Footnotes
Present address: Department of Neuropharmacology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA
References
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Baumgarten HG, Grozdanovic Z. Neuroanatomy and neurophysiology of central serotonergic system. J Seroton Res. 1994;3:171–179. [Google Scholar]
- Creese I, Iversen SD. Blockage of amphetamine induced motor stimulation and stereotypy in the adult rat following neonatal treatment with 6-hydroxydopamine. Brain Res. 1973;55:369–382. doi: 10.1016/0006-8993(73)90302-8. [DOI] [PubMed] [Google Scholar]
- D’Amato RJ, Largent BL, Snowman AM, Snyder SH. Selective labeling of serotonin uptake sites in rat brain by 3H citalopram contrasted to labeling of multiple sites by 3H imipramine. J Pharmacol Exp Ther. 1987;242:364–371. [PubMed] [Google Scholar]
- Dumartin B, Jaber M, Gonon F, Caron MG, Giros B, Bloch B. Dopamine tone regulates D1 receptor trafficking and delivery in striatal neurons in dopamine transporter-deficient mice. Proc Natl Acad Sci USA. 2000;97:1879–1884. doi: 10.1073/pnas.97.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Gerard C, Langlois X, Gringrich J, Doucet E, Vergé D, Kia HK, Raisman R, Gozlan H, el Mestikawy S, Hamon M. Production and characterization of polyclonal antibodies recognizing the intracytoplasmic third loop of the 5-hydroxytryptamine-1A receptor. Neuroscience. 1994;62:721–739. doi: 10.1016/0306-4522(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Dukat M. Serotonin receptor subtypes. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 415–429. [Google Scholar]
- Gold LH, Swerdlow NR, Koob GF. The role of mesolimbic dopamine in conditioned locomotion produced by amphetamine. Behav Neurosci. 1988;102:544–552. doi: 10.1037//0735-7044.102.4.544. [DOI] [PubMed] [Google Scholar]
- Henry D, White F. Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J Pharmacol Exp Ther. 1991;258:882–890. [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu X-T, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in the mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Sorg BA, Hook MS. The pharmacology and neural circuitry of the sensitization to psychostimulants. Behav Pharmacol. 1993;4:315–334. [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Povlock SL, Amara SG. The structure and function of norepinephrine, dopamine, and serotonin transporters. In: Reith MEA, editor. Neurotransmitter transporters: structure, function, and regulation. Humana Press; Totowa, NJ: 1997. pp. 1–28. [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Smadja C, Deschamps A, Spielewoy C, Durieux C, Jacquot C, Giros B, Gardier AM. Effects of paroxetine on serotonin efflux in dopamine transporter knockout mice: an in vivo microdialysis study. Soc Neurosci Abstr. 1999;25:162. [Google Scholar]
- Sora I, Wickems C, Takahashi N, Li X-F, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielewoy C, Gonon F, Roubert C, Fauchey V, Jaber M, Caron MG, Roques BP, Hamon M, Betancur C, Maldonado R, Giros B. Increased rewarding properties of morphine in dopamine-transporter knockout mice. Eur J Neurosci. 2000a;12:1827–1837. doi: 10.1046/j.1460-9568.2000.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielewoy C, Roubert C, Hamon M, Nosten-Bertrand M, Betancur C, Giros B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav Pharmacol. 2000b;11:279–290. doi: 10.1097/00008877-200006000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F, Kalivas P. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]


