Abstract
Background: Concurrent medical comorbidity influences the accurate diagnosis and treatment of major depressive disorder (MDD).
Objective: The objective of this study was to validate previous findings from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study using a confirmation analysis in a previously unanalyzed cohort.
Design: Baseline cross-sectional case-control study of patients enrolling in a prospective randomized multistage treatment study of nonpsychotic MDD.
Setting: Fourteen regional U.S. centers representing 18 primary care and 23 psychiatric practices.
Participants: 2541 outpatients with DSM-IV nonpsychotic MDD.
Measurements: Sociodemographic status, medical illness ratings, psychiatric status, quality of life, and DSM-IV depression symptom ratings.
Results: The prevalence of significant general medical comorbidity in this population was 50.0% (95% CI = 48.1% to 52.0%), consistent with findings reported for the first cohort. Concurrent significant medical comorbidity was associated with older age, lower income, unemployment, limited education, and longer duration of index depressive episode. The group with significant medical comorbidity reported higher rates of somatic symptoms, gastrointestinal symptoms, sympathetic arousal, and leaden paralysis. These results were generally consistent between the 2 cohorts from STAR*D.
Conclusions: Major depressive disorder with concurrent general medical conditions is associated with a specific sociodemographic profile and pattern of depressive symptoms. This association has implications for diagnosis and clinical care.
Major depressive disorder (MDD) is a common psychiatric disorder that is treated in a variety of clinical settings1 including specialty psychiatric care, primary care, nonpsychiatric specialty care, and non-medical mental health settings.2,3 Many general medical conditions (GMCs) are common in MDD4 and can have a significant impact on multiple domains of health and well-being.5
The interaction between medical illness and depression is complex. One challenge for clinicians in treating MDD is to assess and manage MDD in the face of concurrent or comorbid GMCs. Comorbid GMCs may complicate clinical management of MDD in many ways6 as well as assessment, e.g., difficulty in assigning common symptoms of MDD, such as insomnia or fatigue, to a “medical” versus a “psychological” cause.7–9 Pharmacologic treatment for medical conditions may induce a picture of clinical depression, e.g., interferon-induced depression in hepatitis C. Pharmacologic treatment of MDD may induce medical disorder, e.g., bupropion-induced hypertension. Also, specific sociodemographic characteristics in those with MDD and a GMC may influence the affordability of and access to mental health treatment. These complex interactions underscore the need for clinical research in those with both a medical condition and MDD.
We have previously examined the sociodemographic and clinical differences in MDD patients with and without GMCs in an initial cohort of 1500 participants (Cohort I) from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study.10–12 In that analysis, MDD with GMC comorbidity was associated with a specific sociodemographic profile. GMC comorbidity was more common in older participants and those with lower education, lower income, greater unemployment, and lack of private medical insurance. Additionally, GMC comorbidity was associated with longer duration of depressive illness, more severe depressive symptoms, more impairment in function, and higher rates of somatic and gastrointestinal symptoms. General medical condition comorbidity was also associated with lower rates of some symptoms common in MDD, such as impaired mood reactivity and heightened interpersonal sensitivity.
Since the Cohort I analysis, over 2500 additional participants have enrolled in the STAR*D study, which makes STAR*D the largest clinical study of MDD ever completed. We conducted a confirmatory analysis of the second STAR*D cohort (Cohort II) to validate the results found in the initial cohort study, hypothesizing that analysis of Cohort II would confirm previous associations found with GMC comorbidity. Additionally, we hypothesized that this confirmatory analysis would provide support for some trend findings from the original analysis. Finally, we hypothesized that comorbid GMCs influence the rates of common psychiatric comorbidities found in patients with depression, which was not assessed in the analyses of Cohort I.
METHOD
The population and methods of STAR*D are described in more detail elsewhere.11 The key elements of the methods are described below.
Study Overview
The goal of STAR*D was to define prospectively which of several treatments are most effective for participants with nonpsychotic MDD who have an unsatisfactory clinical outcome to an initial and, if necessary, subsequent treatment(s). Eligible and consenting participants were enrolled into the first treatment step (Level 1), which included 12 to 14 weeks of treatment with a selective serotonin reuptake inhibitor (citalopram). Participants with an adequate clinical response could enter a 12-month naturalistic follow-up phase. Those without such an outcome were eligible to enter a series of randomized clinical trials involving antidepressants and psychotherapy.11
Study Organization
The STAR*D infrastructure included the National Coordinating Center in Dallas, Tex.; the Data Coordinating Center in Pittsburgh, Pa.; and 14 Regional Centers (RCs) across the United States, representing 18 primary care and 23 psychiatric practices. Each RC oversaw implementation of the protocol at 2 to 4 clinical sites that were chosen based on multiple factors, including the availability of a large number of potential enrollees and the availability of clinicians, administrative support for the study, and participants from minority populations.
Clinical Research Coordinators (CRCs) at each RC were trained and certified in implementing the treatment protocol and in data collection methods (e.g., screening procedures, inclusion and exclusion criteria, data collection). Research outcome data were collected by telephone interviews with trained Research Outcome Assessors (masked to treatment) and by a telephone-based Interactive Voice Response (IVR) system. Data were collected in English or Spanish depending on participant preference.
Study Population
Recruitment
To achieve the goal of recruiting a broadly representative group of participants with MDD, clinical sites were selected from groups that provide primary and specialty care in either the public or private sector. To further ensure a study sample representative of the “real world,” clinical sites included practice sites that did not typically engage in traditional randomized clinical trials. Further, advertising for participant recruitment was not permitted in STAR*D, as this method may enroll a less representative spectrum of participants.13 Every effort was made to recruit a broad spectrum of participants representing all racial groups and both genders.
The study protocol was developed according to the principles of the Declaration of Helsinki. All risks, benefits, and adverse events associated with each treatment were explained to participants, who provided written informed consent prior to study participation.
Inclusion/exclusion criteria
The inclusion/exclusion criteria were broadly inclusive to acquire a sample representative of persons with MDD who would receive treatment in everyday practice.14 The inclusion and exclusion criteria are summarized below.
Inclusion criteria.
Outpatients with nonpsychotic MDD (based on structured interview using DSM-IV criteria)
A score of > 14 on the 17-item Hamilton Rating Scale for Depression (HAM-D17), administered by the CRC15,16
The treating clinician deemed antidepressant treatment to be appropriate
Age of 18 to 75 years
Participants with suicidal ideation were eligible, as long as outpatient treatment was deemed safe by the clinician (i.e., inpatient care is not called for)
Participants with most GMCs were eligible. Participants whose GMCs could conceivably be physiologically causing their depressive symptoms received treatment as usual for their GMCs as well as protocol treatment for their MDD
Exclusion criteria.
Participants with a well-documented history of nonresponse or clear intolerability in the current major depressive episode to 1 or more treatments required by protocol at Level 1 or 2, delivered at an adequate dose (i.e., > 40 mg/day of citalopram for at least 6 weeks or > 16 sessions of cognitive therapy)
Participants with a lifetime history of bipolar disorder, schizophrenia, schizoaffective disorder, anorexia nervosa, or MDD with psychotic features
Participants who currently suffer from a primary diagnosis of bulimia nervosa or obsessive-compulsive disorder
Participants with severe, unstable concurrent psychiatric conditions likely to require hospitalization within 6 months from study entry (e.g., participants with severe alcohol dependence and a history of recent admissions aimed at detoxification)
Participants with substance dependence disorders who required inpatient detoxification at study entry
Participants with certain concurrent psychiatric or medical conditions that are relative or absolute contraindications to the use of any protocol treatment within Levels 1 and 2
Participants currently receiving a targeted psychotherapy (e.g., cognitive, behavioral, interpersonal therapy) aimed at their depression. Those receiving counseling or therapy for other problems (e.g., marital counseling) could enter the study
Participants who are pregnant or intend to conceive within the subsequent 6 to 9 months
Baseline Assessments
After giving written informed consent, participants were evaluated by the CRC at baseline. Clinical and demographic information was collected, including prior course of illness, current and past substance abuse, prior suicide attempts, family history of mood disorders, current general medical illnesses, and prior history of treatment for the current major depressive episode (both medication and psychotherapy). The CRC completed a baseline HAM-D17 and the 16-item Quick Inventory of Depressive Symptomatology–Clinician Rating (QIDS-C16)17 and reviewed inclusion/exclusion criteria. The participant completed the Self-Report version of the QIDS (QIDS-SR16).18
The Research Outcome Assessor completed the HAM-D17, the 30-item Inventory of Depressive Symptomatology–Clinician-Rated (IDS-C30),19–21 and the 5-item Income and Public Assistance Questionnaire. Finally, the QIDS-C16 was collected by the IVR.22
Function and quality-of-life measures were also collected by IVR. The 12-item Short-Form Health Survey (SF-12),23 an abbreviated version of the SF-36,24 estimated participant perceptions of mental and physical function. The Work and Social Adjustment Scale,25 obtained by IVR, measured impairment in the occupational and interpersonal domains. The 16-item Quality of Life Enjoyment and Satisfaction Questionnaire,26 also collected by IVR, assessed quality of life.
Current GMCs were assessed using the 14-Item Cumulative Illness Rating Scale (CIRS),27,28 which was completed by the CRC or clinician using a manual29 to guide scoring. The CIRS gauges the severity/morbidity of GMCs relevant to different organ systems,30 and it has been applied successfully to clinical populations of depressed individuals.31,32 The CIRS is scored from 0 to 4, with 0 indicating no problem, 1 indicating current mild problem or past significant problem, 2 indicating moderate disability or morbidity requiring therapeutic treatment, 3 indicating severe disability, and 4 indicating extremely severe disability.
Medical illness comorbidity was defined as a score of ≥ 2 on a CIRS item, which indicates moderate or greater impairment of at least 1 medical system evaluated by the CIRS. A threshold score of 2 was chosen based on our assessment that it indicated a clinically relevant level of severity. The 14th CIRS category (Psychiatric Illness) was omitted to identify a group with nonpsychiatric medical comorbidity.
Comorbid Psychiatric Disorders
Participants completed a modified paper-and-pencil version of the Psychiatric Diagnostic Screening Questionnaire,33 consisting of 123 yes/no questions assessing the symptoms of 13 DSM-IV disorders in 5 areas. The items on each diagnostic subscale were grouped together, with subscales clearly demarcated from each other. For the purpose of this article, cutoff scores are used for each category to determine whether threshold levels for corresponding disorders indicate presence or absence of the disorder.33
Statistical Analyses
Group comparisons were made between those with and without co-occurring significant GMCs as defined by the CIRS. This strategy was similar to that completed for Cohort I. For categorical variables, group percentages were calculated and χ2 tests performed. For continuous variables, means and standard deviations were calculated. T tests were performed to statistically compare the 2 groups. Statistical adjustment calculations were made controlling for potential confounding group differences in age and depression severity as measured by the total IDS-C30 score. Due to the multiple statistics tests, a conservative figure of p < .01 was used to indicate a statistically significant difference. p Values from .05 to .01 were considered to indicate a statistical trend.
RESULTS
Individual General Medical Comorbidities
Information for classifying GMC status was available in 2541 participants, with 1271 endorsing at least 1 medical comorbidity (prevalence = 50.0%, 95% CI = 48.1% to 52.0%). This prevalence estimate approximates the estimate from Cohort I of 52.8% (95% CI = 50.3% to 55.3%). The ranking of the rates of comorbidities in specific systems in Cohort II was very similar to Cohort I, with rates for individual systems from the CIRS as follows: 18% musculoskeletal/integument, 16% vascular, 14% endocrine/metabolic and breast, 12% respiratory, 12% ENT/larynx, 12% upper gastrointestinal, 6% heart, 6% neurologic, 5% genitourinary, 4% lower gastrointestinal, 4% liver, 2% hematopoietic, and 1% renal. It is possible that some common medical conditions that often do not produce impairment, e.g., hypertension, may be underrep-resented in the GMC sample.
Sociodemographic Summary
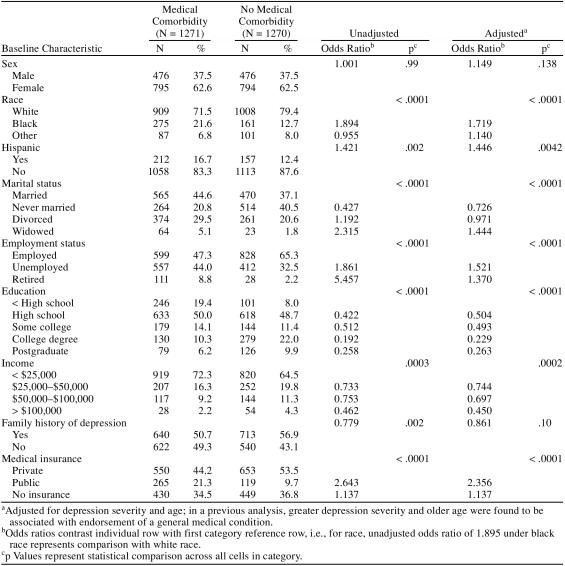
Table 1 summarizes the sociodemographic characteristics of Cohort II. As expected, the GMC group was older (mean age = 45.0 years, SD = 12.9 years) than the non-GMC group (mean age = 36.0 years, SD = 12.2 years). Since endorsement of a GMC was associated with depression severity and older age, an adjusted odds ratio is presented to control for these 2 variables. Women comprised a similar majority of both groups, with a female-to-male ratio of 1.7:1. Similar to Cohort I, black and Hispanic participants were more likely to endorse at least 1 GMC. Marital status and employment status were associated with the presence of GMCs, as were education and income. Endorsement of a GMC was associated with public insurance coverage. The sociodemographic status findings in Cohort II match those found for Cohort I.
Table 1.
Baseline Characteristics of Sample
Clinical Features
Similar to the pattern of Cohort I, Cohort II participants with significant GMCs showed greater depression severity on the HAM-D17 (20.7 vs. 18.6, adjusted p = .0001). Means for the IDS-C30 (37.0 vs. 33.8) and QIDS-SR16 (15.8 vs. 15.2) did not reach statistical significance.
In Cohort II, the SF-12 physical subscale revealed greater impairment in the GMC group (43.8 vs. 53.8, adjusted p value < .0001), while the mental subscale demonstrated less impairment (30.2 vs. 27.0, p < .0001). Lower satisfaction with life was found in those with GMCs (41.2 vs. 45.7, p < .0001). Similar findings were noted for Cohort I. Additionally, similar to Cohort I, Cohort II participants with significant GMCs had a longer duration of the index depressive episode (29.3 months vs. 18.7 months, p < .0001).
Symptom Features
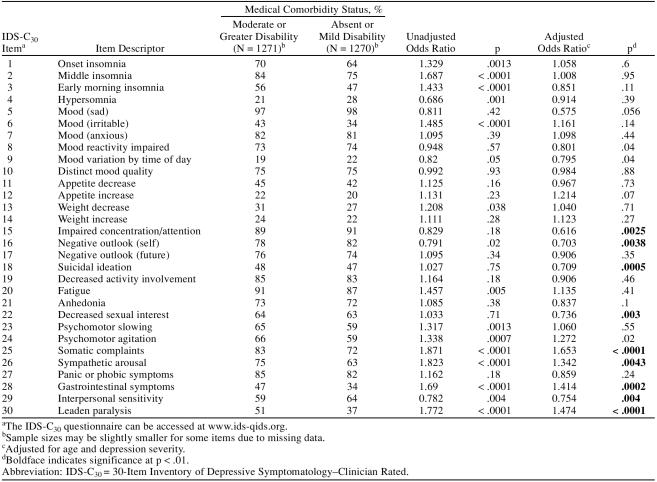
The IDS-C30 measures the presence/severity of 30 depressive symptoms, with each item measuring a different symptom. Table 2 summarizes the rates of endorsement for each item (score of ≥ 1) from this instrument in Cohort II. Nine symptom frequencies differed in those with a GMC compared to those without a GMC based on a p < .01 significance level. GMC subjects were more likely to endorse leaden paralysis, gastrointestinal symptoms, sympathetic arousal, and the somatic complaints item from the IDS-C30. The specific questions related to these items can be found in the IDS-C30 at www.ids-qids.org. Leaden paralysis is assessed in the IDS-C30 by these ratings: 0 = does not experience the physical sensation of feeling weighted down and without physical energy to 3 = feels physically weighted down (without physical energy) most of the time, several hours per day, several days per week. The gastrointestinal item queries for change in bowel habits, diarrhea, or constipations. The sympathetic arousal item queries for palpitations, tremors, blurred vision, tinnitus, sweating, dyspnea, hot or cold flushes, and chest pain. The somatic complaint item queries for headaches, abdominal pain, back pain, joint pain, and limb pain.
Table 2.
Individual IDS-C30 Items for Those With and Without a General Medical Comorbidity
Those with a GMC were less likely to endorse the impaired concentration, negative self-outlook, suicidal ideation, interpersonal sensitivity, and decreased sexual interest items. Interpersonal sensitivity is queried in the IDS-C30 by examining how easily the subject feels rejected, slighted, criticized, or hurt by others.
Because of the large sample sizes, some statistical differences between the groups are relatively small and are probably not clinically significant. However, endorsement rate differences of 5% would generally be considered clinically significant. The clinically significant higher symptom endorsement rates for those with GMCs compared to those without GMCs would include leaden paralysis, gastrointestinal symptoms, sympathetic arousal, and somatic complaints. Those without GMCs endorsed interpersonal sensitivity at a clinically significant (5% absolute difference) level.
Comorbid Psychiatric Disorders
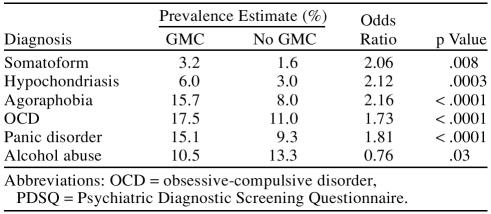
Psychiatric comorbidity rates were not previously reported for Cohort I.10 The findings for Cohort II both for statistically significant differences and for trends are summarized in Table 3. It is noteworthy that the GMC group had higher rates for several psychiatric disorders. The odds ratio estimate for the GMC group was highest for agoraphobia, followed by hypochondriasis, somatoform disorder, panic disorder, and obsessive-compulsive disorder. (Odds ratios depend on the prevalence of the Axis II disorder.) Only alcohol abuse/dependence was more likely to be found in the no-GMC group. The 2 groups did not differ in rates for social phobia, posttraumatic stress disorder, drug abuse, or bulimia nervosa.
Table 3.
Psychiatric Comorbidities From the PDSQ in Those With and Those Without a General Medical Condition (GMC)
DISCUSSION
One of the keys to scientific discovery is the replication of findings. Most reports confirming or refuting a prior finding have confounds, such as the inclusion of different sites or different patient populations due to different recruitment strategies or different inclusion/exclusion criteria. STAR*D provides a unique opportunity to assess relationships and then confirm the relationship in 2 large samples that do not suffer from these confounds. In addition, both the initial and the confirmatory samples are larger than any other previous report of the characteristics of MDD patients with general medical comorbidities.
This confirmatory analysis supports previous findings of a specific sociodemographic pattern for participants with both MDD and a significant GMC.
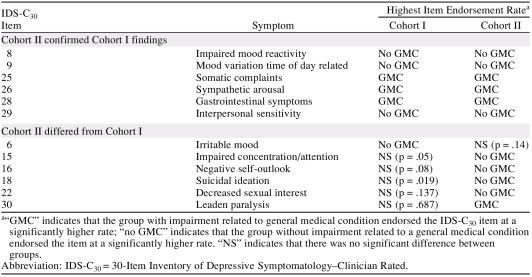
Our primary hypothesis stated that there would be a confirmation of depression symptom endorsement between the 2 cohorts. For the most part, this hypothesis is confirmed. Unique to Cohort II were the increased rates of impaired concentration, negative self-outlook, suicidal ideation, and decreased sexual interest for those without GMCs. The increased rate of irritable mood found for the no-GMC group in Cohort I was not found in Cohort II. Table 4 summarizes the depression symptom findings for the 2 cohorts.
Table 4.
Summary of IDS-C30 Item Endorsement Rates in Cohorts I and II
Consistent with the results from Cohort I, the endorsement rates for most symptoms of depression did not differ between the 2 groups. We were able to confirm that participants with MDD and a GMC had higher rates of somatic symptoms, gastrointestinal symptoms, and symptoms of autonomic hyperactivity. All of these differences reached a clinically significant absolute 5% level.
We also confirmed higher rates for several depression symptoms in those without a GMC. This group endorsed higher rates of impaired mood reactivity, a diurnal pattern to mood variation, and symptoms of interpersonal sensitivity. Increased rates of interpersonal sensitivity reached a clinically significant level. Along with these statistically significant results in both cohorts, some trend findings from Cohort I were supported by statistically significant results in Cohort II. The no-GMC group reported statistically higher rates of impaired concentration/attention and suicidal ideation in Cohort II.
A new analysis in Cohort II suggests that those with MDD and impairment due to a GMC may have higher rates of several psychiatric comorbidities including somatoform disorders, hypochondriasis, agoraphobia, obsessive-compulsive disorder, and panic disorder. Those without a GMC endorsed higher rates of alcohol abuse.
There are several limitations in the study design. The study relied on self-report of impairment due to a GMC. The CIRS instrument has stronger validation when administered by clinicians rather than in the self-report method used in this study. Physician confirmation of the seriousness of the participants' medical condition was not obtained. These limitations need to be considered when assessing the results of the study.
We chose to make a dichotomous selection for the presence of a medical condition. This decision simplified the analysis of medical illness effects but failed to examine medical illness severity along a continuum. Additionally, our initial strategy did not examine the effects of specific systems illness on MDD. In future studies, we plan to examine medical illness in a more continuous way and to examine the effects of specific medical systems on clinical presentation and outcome. These additional studies will provide insight into how dichotomous assignment of medical illness might have affected our results.
Additionally, both cohorts came from a study that enrolled participants in a psychopharmacologic treatment trial of depression. It is not known whether our findings are generalizable to community populations. However, the study participants would appear to be highly representative of a group that is typically encountered in clinical practices.
This study has several implications for the diagnosis and treatment of depression in those with a GMC. The depression symptom endorsement rates in those with a GMC are very similar to the rates in those with no GMC for the majority of symptoms. Patients with GMCs endorse sad mood at high rates when specifically asked. Although somatic symptoms endorsement rates are high in those with GMCs, this is unlikely to affect accurate diagnosis when a complete depression symptoms inventory is completed by interview or by self-report instrument. Clinicians diagnosing depression in those with GMCs need to screen for comorbid anxiety disorders, as several anxiety disorders may complicate clinical management.
In summary, this study showed that patients with comorbid GMCs along with MDD are a relatively disadvantaged group with significant challenges that can impact access to assessment as well as clinical management. They are likely to represent a significant percentage of patients with mental disorders who do not receive adequate treatment in the United States.1 Interventions designed to increase the number of patients treated for depression will need to consider this important group. Primary care physicians will play a key role in identifying MDD in those with a GMC, and in providing access to care. Although most depression symptoms appear similar in the GMC and no-GMC groups studied, clinicians should be aware of the clinical presentation differences. Future treatment programs for MDD in those with a GMC will need to include accurate assessment of somatoform and anxiety disorder comorbidities, which complicate accurate assessment and management. Future studies will provide information on how GMC comorbidity influences response to treatment and prognosis for those with MDD.
Drug names: bupropion (Wellbutrin and others), citalopram (Celexa and others).
Acknowledgments
Financial disclosure: Dr. Yates has received research support from Forest, Eli Lilly, Pfizer, and Pherin Pharmaceuticals and has been on the advisory boards of and/or a consultant for Forest, Eli Lilly, Otsuka, and Wyeth-Ayerst. Dr. Mitchell has received research support from Bristol-Myers Squibb, Jazz Pharmaceuticals, Eli Lilly, and Ortho McNeil; has been on the advisory boards of and/or a consultant for Eli Lilly; has been on the speakers bureaus of Eli Lilly and Forest Laboratories; and has equity holdings (excluding mutual funds/blinded trusts) in Eli Lilly, Forest, Pfizer, and Sanofi-Aventis. Dr. Rush has received research support from the Robert Wood Johnson Foundation, the National Institute of Mental Health, and the Stanley Medical Research Institute; has been on the advisory boards of and/or a consultant for Advanced Neuromodulation Systems, Best Practice Project Management, Bristol-Myers Squibb, Cyberonics, Forest, Gerson Lehman Group, GlaxoSmithKline, Healthcare Technology Systems, Jazz Pharmaceuticals, Eli Lilly, Merck, Neuronetics, Ono Pharmaceutical, Organon USA, Personality Disorder Research Corp., Pfizer, The Urban Institute, and Wyeth-Ayerst; has been on the speakers bureaus of Cyberonics, Forest, GlaxoSmithKline, Eli Lilly, and Merck; has equity holdings (excluding mutual funds/blinded trusts) in Pfizer; and has royalty/patent/other income affiliations with Guilford Publications and Healthcare Technology Systems. Dr. Trivedi has received research support from Bristol-Myers Squibb, Cephalon, Corcept Therapeutics, Eli Lilly, GlaxoSmithKline, Janssen, National Institute of Mental Health, National Alliance for Research on Schizophrenia and Depression, Pfizer, Predix Pharmaceuticals, and Wyeth-Ayerst; has been on the advisory boards of and/or a consultant for Abbott, Akzo (Organon), Bayer, Bristol-Myers Squibb, Cyberonics, Forest, GlaxoSmithKline, Janssen, Johnson & Johnson Product Research and Development, Eli Lilly, Mead Johnson, Parke-Davis, Pfizer, Pharmacia & Upjohn, Sepracor, Solvay, and Wyeth-Ayerst; and has been on the speakers bureaus of Akzo (Organon), Bristol-Myers Squibb, Cyberonics, Forest, Janssen, Eli Lilly, Pharmacia & Upjohn, Solvay, and Wyeth-Ayerst. Dr. Wisniewski has received research support from the National Institute of Mental Health and has been on the advisory board of and/or a consultant for Cyberonics. Dr. Warden has received research support from the National Institute of Mental Health and has equity holdings (excluding mutual funds/blinded trusts) in Bristol-Myers Squibb and Pfizer. Dr. Fava has received research support from Abbott, Alkermes, Aspect Medical Systems, AstraZeneca, Bristol-Myers Squibb, Cephalon, Forest, GlaxoSmithKline, J & J Pharmaceuticals, Lichtwer Pharma GmbH, Eli Lilly, Lorex, Novartis, Organon, PamLab, Pfizer, Pharmavite, Roche, Sanofi/Synthelabo, Solvay, Wyeth-Ayerst; has been on the advisory boards of and/or a consultant for Aspect Medical Systems, AstraZeneca, Bayer AG, Biovail Pharmaceuticals, BrainCells, Inc., Bristol-Myers Squibb, Cephalon, Compellis, Cypress Pharmaceuticals, Dov Pharmaceuticals, EPIX Pharmaceuticals, Fabre-Kramer, Forest, GlaxoSmithKline, Grunenthal GmBH, J & J Pharmaceuticals, Janssen, Jazz Pharmaceuticals, Knoll, Eli Lilly, Lundbeck, MedAvante, Novartis, Nutrition 21, Organon, PamLab, Pfizer, PharmaStar, Pharmavite, Roche, Sanofi/ Synthelabo, Sepracor, Solvay, Somerset, and Wyeth-Ayerst; has been on the speakers bureaus of AstraZeneca, Bristol-Myers Squibb, Cephalon, Forest, GlaxoSmithKline, Eli Lilly, Novartis, Organon, Pfizer, PharmaStar, and Wyeth-Ayerst; and has equity holdings (excluding mutual funds/blinded trusts) in Compellis and MedAvante. Dr. Husain has received research support from the National Institute of Mental Health, Stanley Medical Research Institute, Cyberonics, Pfizer (in process), Neuronetics, Magstim, and Medtronics (potential research sponsor); has been on the advisory boards of and/or a consultant for AstraZeneca, VersusMed, Avinar, Boston Scientific, MEASURE, Bristol-Myers Squibb, and Clinical Advisors (inactive/ terminated); and has been on the speakers bureaus of AstraZeneca, Avinar, Bristol-Myers Squibb, Cerebrio, Inc., Cyberonics, Forest (inactive/terminated), GlaxoSmithKline (inactive/terminated), Janssen (inactive/terminated), and Optima/Forest Pharmaceuticals (inactive/ terminated). Dr. Gaynes has received research support from the National Institute of Mental Health, Agency for Healthcare Research and Quality, Robert Wood Johnson Foundation, Pfizer, and Ovation Pharmaceuticals; has been on the advisory boards of and/or a consultant for Pfizer, Shire, and Wyeth-Ayerst; and has been on the speakers bureau of GlaxoSmithKline. Ms. Bryan reports no additional financial or other relationship relevant to the subject of this article.
Footnotes
This project has been funded with Federal funds from the National Institute of Mental Health, National Institutes of Health, under Contract N01MH90003 to UT Southwestern Medical Center at Dallas (principal investigator: Dr. Rush).
Financial disclosure is listed at the end of the article.
The authors appreciate the editorial support of Jon Kilner, M.S., M.A., and the secretarial support of Fast Word Information Processing Inc., Dallas, Tex.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES CITED
- Kessler RC, Demler O, and Frank RG. et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005 352:2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, and Demler O. et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003 289:3095–3105. [DOI] [PubMed] [Google Scholar]
- Robinson WD, Geske JA, and Prest LA. et al. Depression in primary care. J Am Board Fam Pract. 2005 18:79–86. [DOI] [PubMed] [Google Scholar]
- Patten SB, Beck CA, and Kassam A. et al. Long-term medical conditions and major depression: strength of association for specific conditions in the general population. Can J Psychiatry. 2005 50:195–202. [DOI] [PubMed] [Google Scholar]
- Noel PH, Williams JW Jr, and Unutzer J. et al. Depression and comorbid illness in elderly primary care patients: impact on multiple domains of health status and well-being. Ann Fam Med. 2004 2:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS, and Lewis L. et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005 58:175–189. [DOI] [PubMed] [Google Scholar]
- McDaniel JS, Brown FW, and Cole SA. Assessment of depression and grief reactions in the medically ill. In: Stoudemire A, Fogel BS, Greenberg DB, eds. Psychiatric Care of the Medical Patient, 2nd Edition. Oxford, England: Oxford University Press. 2000 149–164. [Google Scholar]
- Endicott J.. Measurement of depression in patients with cancer. Cancer. 1984;53:2243–2248. doi: 10.1002/cncr.1984.53.s10.2243. [DOI] [PubMed] [Google Scholar]
- Kathol RG, Mutgi A, and Williams J. et al. Diagnosis of major depression in cancer patients according to four sets of criteria. Am J Psychiatry. 1990 147:1021–1024. [DOI] [PubMed] [Google Scholar]
- Yates WR, Mitchell J, and Rush AJ. et al. Clinical features of depressed outpatients with and without co-occurring medical conditions in STAR*D. Gen Hosp Psychiatry. 2004 26:421–429. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Fava M, and Wisniewski SR. et al. Sequenced Treatment Alternatives to Relieve Depression (STAR*D): rationale and design. Control Clin Trials. 2004 25:119–142. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, and Trivedi MH. et al. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatr Clin North Am. 2003 26:457–494. [DOI] [PubMed] [Google Scholar]
- Bielski RJ, Lydiard RB.. Therapeutic trial participants: where do we find them and what does it cost? Psychopharmacol Bull. 1997;33:75–78. [PubMed] [Google Scholar]
- Depression Guideline Panel. Clinical Practice Guideline. Number 5. Depression in Primary Care: Volume 2. Treatment of Major Depression. Rockville, Md: US Dept. Health and Human Services, Public Health Service, Agency for Health Care Policy and Research. AHCPR Publication No. 93-0551. 1993 [Google Scholar]
- Hamilton M.. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M.. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, and Ibrahim HM. et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003 54:806–814. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, and Ibrahim HM. et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004 34:73–82. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Carmody T, Reimitz P-E.. The Inventory of Depressive Symptomatology (IDS): Clinician (IDS-C) and Self-Report (IDS-SR) ratings of depressive symptoms. Int J Methods Psychiatr Res. 2000;9:45–59. [Google Scholar]
- Rush AJ, Giles DE, and Schlesser MA. et al. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986 18:65–87. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, and Basco MR. et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996 26:477–486. [DOI] [PubMed] [Google Scholar]
- Kobak KA, Greist JH, and Jefferson JW. et al. Computerized assessment of depression and anxiety over the telephone using interactive voice response. MD Comput. 1999 16:64–68. [PubMed] [Google Scholar]
- Ware J Jr, Kosinski M, Keller SD.. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE Jr, Raczek AE.. The MOS 36-Item Short-Form Health Survey (SF-36), 2: psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Mundt JC, Marks IM, and Shear MK. et al. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry. 2002 180:461–464. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, and Harrison W. et al. Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q): a new measure. Psychopharmacol Bull. 1993 29:321–326. [PubMed] [Google Scholar]
- Linn BS, Linn MW, Gurel L.. Cumulative Illness Rating Scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, and Houck PR. et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992 41:237–248. [DOI] [PubMed] [Google Scholar]
- Miller MD, Towers A. A Manual of Guidelines for Scoring the Cumulative Illness Rating Scale for Geriatrics (CIRS-G). Pittsburgh, Pa: University of Pittsburgh. 1991 [Google Scholar]
- de Groot V, Beckerman H, and Lankhorst GJ. et al. How to measure comorbidity: a critical review of the literature. J Clin Epidemiol. 2003 56:221–229. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, and Iosifescu DV. et al. Axis III disorders in treatment-resistant major depressive disorder. Psychiatry Res. 2003 118:183–188. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, and Houck PR. et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992 41:237–248. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI.. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60:677–683. doi: 10.4088/jcp.v60n1006. [DOI] [PubMed] [Google Scholar]