Abstract
Objectives: To review the amyloid hypothesis as the predominant mechanistic theory of Alzheimer's disease and update the status of new disease-modifying, anti-amyloid treatments in clinical development.
Data Sources: Governmental Web sites and those of professional Alzheimer's disease associations and drug manufacturers were searched for new drugs in development. An English-language search of PubMed (January 2003–January 2006) was conducted using the search terms Alzheimer's disease and amyloid hypothesis and each of the drugs and immunotherapies from the 4 identified classes of anti-amyloid, disease-modifying therapies.
Study Selection and Data Extraction: Studies and reports were selected on the basis of recent publication, adequate methodology, and completeness of data.
Data Synthesis: Immunotherapy, γ-secretase inhibitors, selective neurotoxic aggregated 42-amino acid peptide subspecies of amyloid β (Aβ42)–lowering agents (tarenflurbil), inhibitors of amyloid aggregation (tramiprosate), and statins show promise in clinical trials. Safety remains an important factor. Disease-modifying drugs that specifically target the amyloid cascade and do not interact with essential biological pathways are expected to possess a lower rate of unintended adverse events.
Agents that selectively target Aβ42 production (e.g., tarenflurbil), block Aβ aggregation (e.g., tramiprosate), or enhance α-secretase activity (statins) offer hope for disease modification and prevention and do not appear to interfere with other biological pathways.
Conclusions: Discovery of safe and effective disease-modifying therapies will usher in a new age of Alzheimer's disease treatment.
In the year 2000, there were an estimated 4.5 million Americans with Alzheimer's disease, approximately 1.8 million of whom were over the age of 85 years. Given the rapid growth of the elderly population, the prevalence of Alzheimer's disease is expected to increase dramatically in the future. If treatments that prevent or significantly slow the onset of the disease are not developed, there could be as many as 13.2 million U.S. adults with Alzheimer's disease by the year 2050.1
Remarkably few treatments are available considering the prevalence of Alzheimer's disease. Moreover, the treatments that are approved for use offer only modest relief of cognitive and behavioral symptoms for some patients.2,3 None of the available treatments prevent the progression from mild cognitive impairment to frank dementia and, ultimately, death.4 The 3 cholinesterase inhibitors (donepezil, rivastigmine, galantamine) in wide clinical use bolster deteriorating cholinergic function in the brain. The findings of 2 studies demonstrated that long-term cholinesterase-inhibitor treatment delayed nursing home placement over 3 years in 1 study5 but not in another,6 and both failed to show a decline in the rate of cognitive or functional disability over the 3-year period.5,6 Some degree of neuroprotection was suggested by neuroimaging studies that showed a slower rate of hippocampal atrophy with donepezil versus placebo after 6 months7 or 1 year.8 These findings are of great interest and raise the possibility of hippocampal atrophy as a surrogate marker of disease progression. The other approved treatment is the n-methyl-d-aspartate receptor antagonist memantine, which protects against excessive activity of the excitatory neurotransmitter glutamate.9 The combination of me-mantine plus donepezil in patients with moderate-to-severe Alzheimer's disease significantly improved measures of cognition, activities of daily living, and behavior compared with placebo over 6 months.10 The durability of clinical improvements associated with memantine treatment is not known.
Unlike treatments that target symptoms of cognitive dysfunction, disease-modifying therapies would slow or arrest disease progression by interrupting underlying pathophysiologic processes.3,4 Disease-modifying and symptomatic treatments represent opposite ends of a continuum of possible therapeutic mechanisms. Disease-modifying treatments would interrupt early pathologic events (e.g., decreased neurotoxic aggregated 42-amino acid peptide subspecies of amyloid β [Aβ42] production, increased plaque clearance, decreased plaque formation), thus preventing later pathologic processes. In contrast, currently available drugs provide transient, symptomatic relief of cognitive impairment for some patients; the natural course of the disease is either not altered or altered very slightly.
The costs associated with Alzheimer's disease are enormous—$100 billion in the year 2000—and consist of direct costs of patient care ($15 billion) and indirect costs of lost earnings by patients and their usually unpaid care-givers ($85 billion).11 Public health modeling has predicted that the availability of disease-modifying treatments would have a significant effect on both prevalence and overall costs. It has been estimated that the prevalence of Alzheimer's disease would decline 38% by 2050 if treatments that delay the onset of Alzheimer's disease by 6.7 years were available by the year 2010.12 Moreover, it is possible that disease-modifying treatments could reduce the annual cost of care by up to $24,000 per patient, thereby reducing the national cost of Alzheimer's disease by trillions of dollars through the year 2050.13 Clearly, treatments that target the underlying causes and alter the natural course of Alzheimer's disease are desperately needed.
DATA SOURCES AND STUDY SELECTION
This review provides an update on the predominant mechanistic theory of Alzheimer's disease—the amyloid hypothesis—and overviews the status of new disease-modifying, anti-amyloid treatments in clinical development. Several different resources were used to identify new treatments and data from ongoing clinical trials. Web sites from the Alzheimer Research Forum (The Drugs in Clinical Trials section; www.alzforum.org), the National Institutes of Health (www.clinicaltrials.gov), the Alzheimer's Association (www.alz.org), and the Alzheimer's Disease Education and Referral Center of the National Institute on Aging (www.alzheimers.org) were searched for anti-amyloid drugs currently being studied in clinical trials. Web sites of scientific organizations and manufacturers of the investigational drugs in question were scanned for additional information about ongoing and completed studies. Subsequently, an English-language search using PubMed (January 2003–January 2006) was conducted using the search terms Alzheimer's disease and amyloid hypothesis and each of the drugs and immuno-therapies from the 4 classes of anti-amyloid disease-modifying therapies identified from the governmental and professional organization Web sites. Review articles, original research reports, and abstracts presented at national/international meetings were chosen on the basis of currency of publication, study methods, peer-reviewed status, and completeness of data. Treatment of Alzheimer's disease is an extremely active area of clinical investigation with many late-breaking reports. The rapidly changing nature of the clinical trials database required the use of Internet sources and abstracts that, for less quickly moving fields, would not normally be considered in a literature review of this sort.
AMYLOID HYPOTHESIS
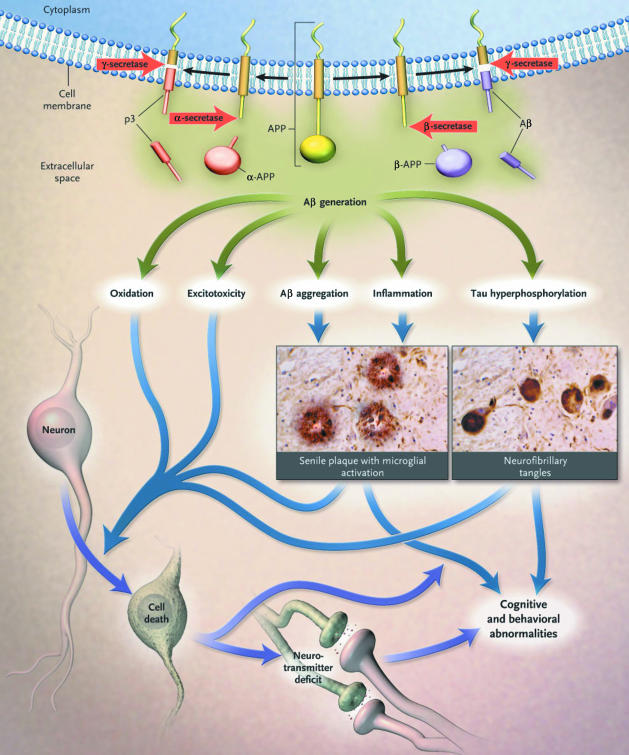
The principal theory of the pathogenesis of Alzheimer's disease is the amyloid hypothesis, which identifies biological targets for disease-modifying treatments. According to the amyloid hypothesis, the increased production or decreased clearance of a small peptide, Aβ, initiates a pathologic process terminating in neurodegeneration, dementia, and death.3,14 The hallmark pathologic lesions of Alzheimer's disease are extracellular cerebral plaques that are composed of highly neurotoxic Aβ42, less neurotoxic Aβ subspecies (i.e., Aβ38–40), and intraneuronal neurofibrillary tangles.14–16
The pathway for Aβ plaque formation begins with the pathologic processing of amyloid precursor protein (APP). Amyloid precursor protein is cleaved first by the protease β-secretase (i.e., BACE-1) and subsequently by γ-secretase to form either the benign peptides Aβ38–40 or the neurotoxic peptide Aβ42. An alternate pathway in the processing of APP involves α-secretase, which cleaves at a site that precludes Aβ42 formation.3,14,15 Under normal circumstances, the vast majority of Aβ (> 95%) consists of Aβ38–40; Aβ42 makes up less than 5% of total Aβ. By largely unknown mechanisms, genetic and environmental factors may shift this balance toward increased production of toxic Aβ42.
Accumulation and oligomerization of Aβ42 results in the formation of amyloid plaques and initiates a cascade of events associated with neuronal and synaptic dysfunction, inflammatory responses, hyperphosphorylation of structural tau proteins (resulting in neurofibrillary tangle formation), synaptic dysfunction, neuronal death, neurotransmitter deficits, and ultimately clinical dementia (Figure 1).3,17 Amyloid plaques are distributed in brain regions that serve memory and cognition: the hippocampus, entorhinal cortex, amygdala, and the frontal, temporal, and parietal lobes.16
Figure 1.
The Amyloid Hypothesis of Alzheimer's Diseasea
Some researchers argue that plaque burden does not correlate with cognitive impairment in Alzheimer's disease, thus fueling a debate about the validity of the amyloid hypothesis. However, the observation that a small minority of patients have genetically transmitted autosomal dominant forms of Alzheimer's disease caused by mutations in genes that express APP, presenilin-1 (PS-1), or PS-2 strongly supports the amyloid hypothesis.17 The majority of patients have a sporadic form of Alzheimer's disease, which may be associated with inheritance of the polymorphic apolipoprotein E ε4 allele as well as other poorly understood genetic and environmental factors. In addition, data from both animal and clinical studies provide compelling evidence for the role of altered amyloid processing in the pathogenesis of Alzheimer's disease. The amyloid hypothesis is strongly supported by data showing that memory deficits accompany increased amyloid plaque burden in transgenic mice18 and also correlate strongly with Aβ42 concentrations in the brains of patients with Alzheimer's disease.19 Moreover, increased brain concentrations of small Aβ oligomers, also referred to as Aβ-derived diffusible ligands,20 have been shown postmortem to correlate with memory loss in patients with Alzheimer's disease.21 Finally, seminal data in APP transgenic mice show that early administration of either an anti-amyloid vaccine or an inhibitor of γ-secretase reduces amyloid plaque formation and intracellular Aβ accumulation and increases clearance of tau proteins.22
DATA EXTRACTION AND SYNTHESIS
Review articles, cited references in review articles, primary research reports, abstracts presented at scientific meetings, and scientific, governmental, and commercial Web sites were assessed and used in the review as determined by recent publication, adequate methodology, and completeness of data.
Anti-Amyloid Drugs in Clinical Trials
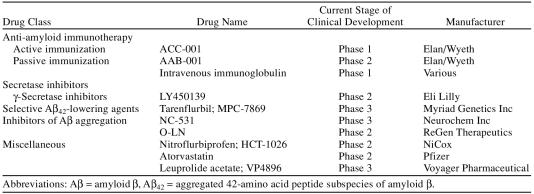
There are 4 classes of potentially disease-modifying treatments that have successfully advanced to later-stage clinical trials: (1) immunotherapies, (2) secretase inhibitors, (3) selective Aβ42-lowering agents (SALAs), and (4) anti-Aβ aggregation agents (Table 1).
Table 1.
Disease-Modifying Therapies for Alzheimer's Disease Currently in Clinical Development
Immunotherapy
A number of approaches to immunotherapy for Aβ have been studied in animal models and found worthy of clinical study.4 Active and passive immunization in Alzheimer's disease theoretically increases amyloid clearance via phagocytosis and/or increased efflux of Aβ from the brain.23 Early studies in APP transgenic mice using active immunization with aggregated Aβ42 (AN1792; Elan Pharmaceuticals/Wyeth Pharmaceuticals) showed reduction in amyloid pathology,24 delayed cognitive deficits, and improved behavioral performance on memory tasks.25,26 Clinical studies of AN1792 were conducted on the basis of these encouraging animal model findings and the results of a phase 1 pilot study in 20 patients.27 Unfortunately, 18 of 300 phase 2 study patients (6%) developed autoimmune meningoencephalitis, which led to the discontinuation of the AN1792 clinical trial program.23,28–30 One-year follow-up of patients who received 1 or more doses of AN1792 showed that patients who successfully mounted an anti-Aβ antibody response exhibited slower rates of cognitive and functional decline31 and reduced cerebral spinal fluid (CSF) concentrations of tau protein compared with nonresponders.28 Interestingly, however, in a subset of patients who underwent baseline and postbaseline CSF sampling, antibody response did not correlate with reduced Aβ42 concentrations in the CSF.28 A potentially troublesome, and as yet unexplained, observation in antibody responders was the reduction in whole-brain volume and increased ventricular volume.32
Taken in the aggregate, the development of immune-based therapy for Alzheimer's disease remains a viable avenue of clinical research despite the cancellation of AN1792 trials. The primary focus of new vaccine development programs is the design of immunotherapies that are effective without the serious adverse events associated with AN1792. Active immunization with the immunoconjugate ACC-001 (Elan Pharmaceuticals/ Wyeth Pharmaceuticals) is being evaluated for its ability to induce a highly specific antibody response to Aβ in a single-dose, placebo-controlled, 12-month, phase 1 study of 70 patients with mild-to-moderate Alzheimer's disease.33 Passive immunotherapy with the humanized monoclonal antibody, AAB-001 (Elan Pharmaceuticals/ Wyeth Pharmaceuticals), is in phase 2 evaluation in 200 patients with mild-to-moderate Alzheimer's disease. This placebo-controlled, 18-month, multiple-dose study is designed to assess safety, tolerability, and clinical endpoints. A corresponding neuroimaging trial also is underway to measure changes in amyloid plaque burden.34 The potential efficacy of another form of passive immunization has recently been demonstrated in a 6-month study in 8 patients with mild Alzheimer's disease. Administration of intravenous immunoglobulin resulted in transient elevations in plasma Aβ levels and reduced CSF Aβ concentrations, suggesting that nonspecific anti-amyloid antibodies may warrant further study.35
Secretase inhibitors
Inhibitors of γ-secretase and β-secretase (i.e., BACE) are potential disease-modifying treatments for Alzheimer's disease. In theory, they would block formation of Aβ42 and its subsequent neuropathology. However, mechanistic and pharmacokinetic problems have hindered progress in drug development for this class of compounds.4
γ-Secretase inhibitors. A number of compounds that inhibit γ-secretase activity in the brain have been identified. However, γ-secretase has many biologically essential substrates.36 One physiologically important γ-secretase substrate is the Notch signaling protein, which is an intermediate in the differentiation and proliferation of embryonic cells, T cells, gastrointestinal goblet cells, and splenic B cells. Experience with transgenic mice has shown that administration of a γ-secretase inhibitor in doses sufficient to reduce Aβ concentrations interferes with lymphocyte differentiation and alters the structure of intestinal goblet cells.37 In addition, hippocampal neuroplasticity,38 neuro-degeneration, and impaired memory39 are evident in adult mice bred to be deficient for the Notch protein. Safety is therefore an important consideration for compounds that nonselectively inhibit γ-secretase.
A nonselective γ-secretase inhibitor, LY450139 (Eli Lilly), has been evaluated in a phase 1 placebo-controlled study in 37 healthy adults (dose range, 5 mg–50 mg).40 Amyloid β concentrations in the CSF were reduced in both active treatment and placebo groups, but between-group differences were not statistically significant. Transient gastrointestinal adverse effects (bleeding, abdominal pain) were reported by 2 patients in the 50-mg group.40 Preliminary findings of a 6-week phase 2 study of 70 patients with mild-to-moderate Alzheimer's disease who were stabilized on cholinesterase inhibitors have been reported.41 Reductions in CSF Aβ concentrations were observed in both the active and placebo groups, with no statistically significant differences between groups. Changes in cognitive function were similar for both the LY450139 and placebo groups, but the study was not designed to detect these differences. No serious adverse events were reported.41
However, to date, compounds that specifically target the γ-secretase isoform involved in APP processing have not reached clinical study.3,4,42 Progress is being made in identifying potential targets43 and highly specific γ-secretase inhibitors44 that may someday translate into the development of efficacious and safe treatments.
β-Secretase inhibitors. β-secretase also is an important biological target for new drug development, but clinical trials have not yet been conducted.45 While inhibition of β-secretase is not expected to incur the same safety risk as γ-secretase inhibitors,46 BACE-1 deficiency in genetically engineered mice is associated with impaired learning.47 In addition, there are significant pharmacokinetic challenges in developing a viable BACE inhibitor. To date, the compounds that effectively inhibit BACE activity are large molecules that do not penetrate the blood-brain barrier.4,48
SALAs
Tarenflurbil. Tarenflurbil is the first agent in a new class of drugs that modulate γ-secretase activity—the SALAs. An important advantage for the SALA class of drugs is lack of interference with Notch or other γ-secretase substrates.49 Tarenflurbil binds to a γ-secretase site other than the active/catalytic center of relevance to production of Aβ42, thereby altering the conformation of γ-secretase and shifting production away from Aβ42, while avoiding interference with other physiologically essential γ-secretase substrates.
Tarenflurbil (MPC-7869; Myriad Pharmaceuticals), which is the pure, R-enantiomer of flurbiprofen, shifts cleavage of APP away from Aβ42, thereby producing shorter, nontoxic fragments (e.g., Aβ38).50,51 In contrast with S-flurbiprofen or other nonsteroidal anti-inflammatory drugs (NSAIDs), tarenflurbil does not inhibit cyclooxygenase (COX) I or COX II and is not associated with gastrointestinal toxicity.52 Administration of tarenflurbil to transgenic mice reduces amyloid plaque burden and prevents learning and behavioral deterioration.53
The findings of a 3-week, placebo-controlled, phase 1 pharmacokinetic study of tarenflurbil (twice-daily doses of 200 mg, 400 mg, or 800 mg) in 36 healthy, older volunteers showed that tarenflurbil was as well tolerated as placebo, with no evidence of gastrointestinal or renal toxicity.54 A phase 2 study was conducted in 207 patients with mild-to-moderate Alzheimer's disease who were randomly assigned to receive twice-daily doses of tarenflurbil 400 mg, tarenflurbil 800 mg, or placebo for 12 months. In mild Alzheimer's disease patients (Mini-Mental State Examination [MMSE] score = 20–26) randomly assigned to the 800-mg twice-daily group, statistically significant benefit was observed at 12 months in activities of daily living (p = .033) as measured by the Alzheimer's Disease Cooperative Study-Activities of Daily Living Scale (ADCS-ADL) and global function (p = .042) as measured by the Clinical Dementia Rating-sum of the boxes (CDR-sb), with a positive trend observed in cognition (Alzheimer's Disease Assessment Scale-cognitive subscale; ADAS-cog). In addition, there was a significant plasma concentration–to-response relationship. For patients with mild Alzheimer's disease who achieved tarenflurbil plasma concentrations higher than 75 µg/mL (i.e., generally patients in the 800-mg twice-daily group), the rate of deterioration in activities of daily living (ADCS-ADL) and global function (CDR-sb) was reduced by 62% (p = .025) and 51% (p = .035), respectively. In this study, no benefit was observed in moderate Alzheimer's disease patients (MMSE score < 20).
Overall, tarenflurbil appeared very well tolerated. Discontinuations due to adverse events were comparable between the 800-mg twice-daily and placebo groups. Adverse events observed at a higher frequency in the treated groups compared with placebo included transient eosinophilia, mild anemia, blood pressure elevation, lower respiratory infection, and rash. Adverse events observed at a lower frequency than placebo included urinary incontinence and psychiatric events. At the 6-month point in an ongoing 12-month follow-on, patients originally treated with tarenflurbil 800-mg twice daily achieved a 33% improvement in cognition on the ADAS-cog, a slowing in the rate of decline in global functioning on the CDR-sb, and maintenance of activities of daily living scores on the ADCS-ADL as compared with their status at the end of the placebo-controlled phase of the study.55 A phase 3 study designed to assess the efficacy of tarenflurbil 800 mg twice daily in patients with mild Alzheimer's disease is ongoing.55
Anti-aggregation agents
Several anti-Aβ aggregation agents are currently in clinical testing. Their mechanisms of action vary and are not completely understood, but are believed to involve prevention of fibril formation and facilitation of soluble Aβ clearance.
Tramiprosate. Tramiprosate (Neurochem, Inc.) is a small-molecule glycosaminoglycan (GAG) mimetic. Glycosaminoglycan binds to soluble Aβ, facilitating fibril formation and deposition of amyloid plaque. GAG mimetics compete for GAG-binding sites, thereby blocking fibril formation56 and reducing soluble Aβ.57,58 Tramiprosate reduces plaque burden and decreases CSF concentrations of Aβ in transgenic mice.58 However, changes in cognitive and behavioral outcomes in this animal model have not been reported. No serious adverse events were reported in a single-dose phase 1 pharmaco-kinetic evaluation in healthy adults, and investigators concluded that tramiprosate was well tolerated.57 A 3-month phase 2 study was subsequently conducted in 58 patients with mild-to-moderate Alzheimer's disease who were randomly assigned to tramiprosate 50 mg, 100 mg, or 150 mg twice daily or placebo. Patients who completed the study were eligible to receive 150 mg twice daily during a 21-month open-label extension. Baseline CSF Aβ42 concentrations declined by up to 70% after 3 months for patients randomly assigned to the 100-mg or 150-mg twice-daily groups, but there were no differences in cognitive function between the tramiprosate and placebo groups. Open-label treatment with tramiprosate for 1 year resulted in a slightly slower rate of decline on the ADAS and the MMSE scores59 than would be expected in historical controls. Phase 3 studies of tramiprosate are ongoing.
Colostrinin. Colostrinin is a proline-rich polypeptide complex derived from sheep colostrum (O-CLN; ReGen Therapeutics). Colostrinin inhibits Aβ aggregation and neurotoxicity in cellular assays60 and improves cognitive performance in laboratory animals.61 Colostrinin was reported to be well tolerated in a 3-week phase 1 study of patients with Alzheimer's disease who received doses of up to 200 µg daily or 100 µg every other day.61 The findings of phase 2 studies show modest improvements in MMSE scores for patients with mild Alzheimer's disease over a treatment period of 12 to 16 months, but this level of response was not sustained during 18 to 28 months of continued treatment. Improvements in MMSE scores were greater for patients with mild versus moderate Alzheimer's disease.61–63
Clioquinol. Clioquinol (PBT-1; Prana Biotechnology) is a quinolone with antibacterial and antifungal properties that was withdrawn from the market decades ago because of subacute myelo-optic neuropathy. Its mechanism relative to Alzheimer's disease is theorized to involve chelation of copper, a metal believed to facilitate plaque formation. Early clinical studies showed a reduction in the rate of cognitive decline,64 but clinical trials were halted because of toxic impurities inherent in the formulation. Second-generation metal chelators are reported to be entering clinical trial development.
Other potential disease-modifying treatments in clinical trials
The NSAIDs, statins, and a gonadotropin-releasing hormone agonist are being investigated for possible disease-modifying effects in patients with mild-to-moderate Alzheimer's disease. Clinical development of phenserine, a “dual action” drug that inhibits cholinesterase and APP production,65 was recently suspended following negative phase 3 study results.66
NSAIDs. A large body of epidemiologic evidence suggests that long-term use of some NSAIDs protects against the development of Alzheimer's disease.67–69 However, prospective studies of rofecoxib, naproxen, or diclofenac failed to slow progression of cognitive decline in patients with mild-to-moderate Alzheimer's disease.70–72 In contrast, indomethacin may delay cognitive decline in this subset of patients, but gastrointestinal toxicity is treatment-limiting.73,74 Because of general concerns about lack of efficacy, gastrointestinal toxicity, and, most recently, myocardial infarction and stroke, the NSAIDs are not considered to be viable treatment options for patients with Alzheimer's disease.75 Indeed, a large primary prevention trial of naproxen, celecoxib, and placebo was recently halted because of concerns about cardiac and cerebrovascular events.76
Nitroflurbiprofen (HCT-1026; NicOx) is a nitric oxide-donating derivative of the NSAID flurbiprofen that improved cognitive function in rats following chronic lipopolysaccharide infusions77 and reduced plaque burden in mice.78 In human studies, nitroflurbiprofen penetrated the blood-brain barrier79 and reduced the rate of gastrointestinal ulcers by 60% to 80% compared with flur-biprofen.80 Nitroflurbiprofen is currently being evaluated in a phase 2 study in patients with Alzheimer's disease.81
Statins. Some epidemiologic studies have suggested that elderly patients treated with long-term statin therapy have lower rates of incident Alzheimer's disease.82 The mechanism whereby the statins exert this putative protective effect is not completely understood, but may be related to reduced serum cholesterol levels and/or anti-inflammatory properties.83 However, the statins (i.e., the HMG-CoA reductase inhibitors) enhance the activity of α-secretase, which cleaves APP into soluble products and precludes the production of Aβ42. The statins are believed to promote α-secretase activity by inhibiting Rho-associated protein kinase 1 (i.e., ROCK1), an enzyme that modulates (i.e., blocks) α-secretase activity.84 Clinical studies of atorvastatin and simvastatin therapy in patients with Alzheimer's disease are ongoing. Results from a phase 2 trial of atorvastatin have been published.83 In this study, 63 evaluable patients with mild-to-moderate Alzheimer's disease were randomized to placebo or 80 mg atorvastatin daily and followed for 1 year. Atorvastatin treatment was associated with a slower rate of decline on the ADAS-cog and MMSE at 1 year compared with patients in the placebo group. The authors concluded that statin therapy may slow the progression of cognitive impairment in patients with mild-to-moderate Alzheimer's disease.83
Gonadotropin-releasing hormone agonist. Leuprolide acetate (VP4896; Voyager Pharmaceutical) is a gonadotropin-releasing hormone agonist that is entering phase 3 testing in patients with mild-to-moderate Alzheimer's disease.85 The theory underlying the clinical trial program of leuprolide (i.e., age-related increases in luteinizing hormone cause Alzheimer's disease) is not consistent with the amyloid hypothesis. However, 1 report describes reductions in Aβ40 and Aβ42 levels in brain tissue from standard adult control mice.86 Mice bred to overproduce amyloid (i.e., APP transgenic mice) were not used in this study, and cognitive and behavioral effects were not assessed. The possible role of this agent in the clinical setting cannot be predicted at this time.
CONCLUSIONS: THE FUTURE OF ALZHEIMER'S TREATMENT
The burgeoning growth of the elderly population and with it the projected epidemic of Alzheimer's disease underscores the urgent need for treatments that safely and effectively slow or arrest cognitive and functional deterioration. Currently available drugs offer symptomatic relief that is temporary at best. New and more durable disease-modifying treatments are needed. The widespread acceptance of the amyloid hypothesis has spurred intense research efforts to identify disease-modifying treatments that interrupt the natural course of Alzheimer's disease by blocking the pathologic processing of APP to Aβ42 or enhancing its clearance or decreasing its toxicity. Molecular milestones along the amyloid pathway, including APP, the enzymes involved in generating Aβ42 (i.e., γ-secretase, β-secretase) or less toxic derivatives (i.e., α-secretase), and Aβ42 itself, are promising targets for therapeutic intervention.
There has been much progress to date. Although initial setbacks associated with unanticipated aseptic meningo-encephalitis have slowed development of immunotherapeutic approaches, active or passive immunization against Aβ42 remains an area of active investigation. Researchers continue to explore the therapeutic potential of γ- or β-secretase inhibitors, but untoward events associated with the nonselective inhibition of biologically essential γ-secretase substrates (e.g., Notch) pose a significant challenge. In contrast, drugs that selectively target Aβ42 production (e.g., tarenflurbil) or block Aβ aggregation (e.g., tramiprosate) have advanced the farthest in the drug development pipeline and, to date, offer the greatest hope for clinical availability of disease-modifying therapy in the near future.
Safety is as important as efficacy, particularly in the elderly population, which is especially susceptible to adverse drug events. Alzheimer's disease treatments that target specific elements of the amyloid cascade and do not interfere with other essential biological pathways are expected to be better tolerated. The long-term efficacy and safety of the disease-modifying drugs currently being studied await the results of ongoing clinical trials.
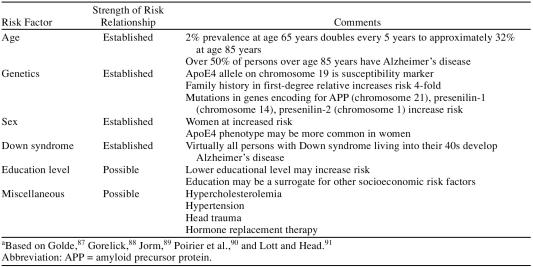
Continued clinical trials are necessary to better characterize candidate populations and optimal doses for the disease-modifying drugs in development. The possibility that mild cognitive impairment is a precursor to Alzheimer's disease and a possible starting point for disease-modifying treatment is the subject of a broad research effort. The relative efficacy and safety of different classes of disease-modifying drugs are not yet known. Only extensive clinical experience and the findings of direct, head-to-head trials will inform treatment decisions about the relative strengths and weaknesses of different classes of drugs. Looking ahead, it is likely that the treatment regimens of tomorrow will begin with mild cognitive impairment and eventually extend to primary prevention in high-risk populations (Table 2). Combination therapy with the currently available symptomatic treatments may prove beneficial for patients whose symptoms have already begun. No drugs in current development offer the hope of complete symptom reversal and “cure.”
Table 2.
Known and Possible Risk Factors for Alzheimer's Diseasea
Prospects for the future of Alzheimer's disease treatment are very encouraging. The diversity of different therapeutic strategies being explored in clinical trials offers hope that some day in the not too distant future, disease-modifying treatments will become the standard of care and serve as the springboard for permanently changing the course of Alzheimer's disease.
Drug names: atorvastatin (Lipitor), celecoxib (Celebrex), diclofenac (Cataflam, Voltaren, and others), donepezil (Aricept), flurbiprofen (Ansaid and others), galantamine (Razadyne), indomethacin (Indocin and others), memantine (Namenda), naproxen (Naprosyn and others), rivastigmine (Exelon), simvastatin (Zocor and others).
Footnotes
Dr. Christensen is a consultant for Bayer Healthcare, Bristol-Myers Squibb, Designer Genes, GlaxoSmithKline, Janssen, Eli Lilly, Myriad Genetics, Novartis, NPS, Pfizer, Ribomed, Solvay, and Wyeth-Ayerst; is a member of the speakers bureau for Abbott, Bayer Healthcare, Bristol-Myers Squibb, Eisai, GlaxoSmithKline, Janssen, Eli Lilly, Novartis, Pfizer, Solvay, Upjohn, and Wyeth-Ayerst; and has received grant/research support from Abbott, Bristol-Myers Squibb, Designer Genes, Eccles Institute of Human Genetics, GlaxoSmithKline, Janssen, Myriad Genetics, Novartis, NPS, Organon, Pfizer, Ribomed, Solvay, and Wyeth-Ayerst.
REFERENCES CITED
- Hebert LE, Scherr PA, and Scherr JL. et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003 60:1119–1122. [DOI] [PubMed] [Google Scholar]
- Kaduszkiewicz H, Zimmermann T, and Beck-Bornholdt HP. et al. Cholines-terase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. Br Med J. 2005 331:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg RN.. Translational research on the way to effective therapy for Alzheimer disease. Arch Gen Psychiatry. 2005;62:1186–1192. doi: 10.1001/archpsyc.62.11.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Ibegbu CC, and Todd CW. et al. Emerging prospects for the disease-modifying treatment of Alzheimer's disease. Biochem Pharmacol. 2005 69:1001–1008. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, and Wisniewski S. et al. Cholinesterase inhibitor treatment alters the natural history of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2002 72:310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney C, Farrell D, and Gray R. et al. Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomised double-blind trial. Lancet. 2004 363:2105–2115. [DOI] [PubMed] [Google Scholar]
- Krishnan KRR, Charles HC, and Doraiswamy PM. et al. Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer's disease. Am J Psychiatry. 2003 160:2003–2011. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Kazui H, and Matsumoto K. et al. Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer's disease? Am J Psychiatry. 2005 162:676–682. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Doody R, and Stöffler A. et al. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003 348:1333–1341. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Farlow MR, and Grossberg GT. et al. Memantine treatment in patients with moderate to severe Alzheimer's disease already receiving donepezil: a randomized controlled trial. JAMA. 2004 291:317–324. [DOI] [PubMed] [Google Scholar]
- Kirschstein R. Disease-specific estimates of direct and indirect costs of illness and NIH support. Fiscal year 2000 update. National Institutes of Health. Available at: http://ospp.od.nih.gov/ecostudies/COIreportweb.htm. Accessed Jan 22, 2006. [Google Scholar]
- Sloane PD, Zimmerman S, and Suchindran C. et al. The public health impact of Alzheimer's disease, 2000–2050: potential implication of treatment advances. Annu Rev Public Health. 2002 23:213–231. [DOI] [PubMed] [Google Scholar]
- Leon J, Cheng CK, Neumann PJ.. Alzheimer's disease care: costs and potential savings. Health Aff. 1998;17:206–216. doi: 10.1377/hlthaff.17.6.206. [DOI] [PubMed] [Google Scholar]
- Gandy S.. The role of cerebral amyloid β accumulation in common forms of Alzheimer disease. J Clin Invest. 2005;115:1121–1129. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy S, Martins RN, Buxbaum J.. Molecular and cellular basis for anti-amyloid therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17:259–266. doi: 10.1097/00002093-200310000-00011. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Schenk D.. Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Ann Rev Pharmacol Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- Cummings JL.. Alzheimer's disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, and Nilsen S. et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996 274:99–102. [DOI] [PubMed] [Google Scholar]
- Näslund J, Haroutunian V, and Mohs R. et al. Correlation between elevated levels of amyloid β peptide in the brain and cognitive decline. JAMA. 2000 283:1571–1577. [DOI] [PubMed] [Google Scholar]
- Gong Y, Chang L, and Viola KL. et al. Alzheimer's disease–affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. PNAS. 2003 100:10417–10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georganopoulou DG, Chang L, and Nam J-M. et al. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer's disease. PNAS. 2005 102:2273–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Billings L, and Kesslak JP. et al. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004 43:321–332. [DOI] [PubMed] [Google Scholar]
- Gelinas DS, DaSilva K, and Fenili D. et al. Immunotherapy for Alzheimer's disease. Proc Natl Acad Sci U S A. 2004 101suppl 2. 14657–14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D, Barbour R, and Dunn W. et al. Immunization with amyloid-βattenuates Alzheimer disease–like pathology in the PDAPP mouse. Nature. 1999 400:173–177. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, and McLaurin J. et al. A beta peptide immunization reduces behavioral impairment and plaques in a model of Alzheimer's disease. Nature. 2000 408:979–982. [DOI] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, and Gottschall PE. et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000 408:982–985. [DOI] [PubMed] [Google Scholar]
- Bayer AJ, Bullock R, and Jones RW. et al. Evaluation of the safety and immunogenicity of synthetic Aβ42 (AN1792) in patients with AD. Neurology. 2005 64:94–101. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koller M, and Black RS. et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005 64:1553–1562. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, and Dartigues JF. et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology. 2003 61:46–54. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Bishop GM, and Lee HG. et al. Lessons from the AN1792 Alzheimer vaccine: lest we forget. Neurobiol Aging. 2004 25:609–615. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, and Streffer JR. et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003 38:547–554. [DOI] [PubMed] [Google Scholar]
- Fox NC, Black RS, and Gilman S. et al. Effects of Abeta immunotherapy (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005 64:1563–1572. [DOI] [PubMed] [Google Scholar]
- Alzforum: Drugs in Clinical Trials. ACC-001. 2005. Available at: http://www.alzforum.org/drg/drc/detail.asp?id=102. Accessed Jan 22, 2006. [Google Scholar]
- Alzforum: Drugs in Clinical Trials. AAB-001. 2005. Available at: http://www.alzforum.org/drg/drc/detail.asp?id=101. Accessed Jan 22, 2006. [Google Scholar]
- Relkin N, Szabo P, and Adamiak B. et al. Intravenous immunoglobulin (IVIg) treatment causes dose-dependent alterations in B-amyloid (AB) levels and anti-AB antibody titers in plasma and cerebrospinal fluid (csf) of Alzheimer's disease (AD) patients. Neurology. 2005 64suppl 1. A144. [Google Scholar]
- Pollack SJ, Lewis H.. Secretase inhibitors for Alzheimer's disease: challenges of a promiscuous protease. Curr Opin Investig Drugs. 2005;6:35–47. [PubMed] [Google Scholar]
- Wong GT, Manfra D, and Poulet FM. et al. Chronic treatment with the γ-secretase inhibitor LY-411,575 inhibits β-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004 279:12876–12882. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan SL, and Miele L. et al. Involvement of Notch signaling in hippocampal synaptic plasticity. PNAS. 2004 101:9458–9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura CA, Choi S-Y, and Beglopoulos V. et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004 42:23–36. [DOI] [PubMed] [Google Scholar]
- Siemers E, Skinner M, and Dean RA. et al. Safety, tolerability, and changes in amyloid β concentrations after administration of a γ-secretase inhibitor in volunteers. Clin Neuropharmacol. 2005 28:126–132. [DOI] [PubMed] [Google Scholar]
- Siemers E, Quinn J, and Kaye J. et al. Effect of LY450139, a functional γ-secretase inhibitor, on plasma and cerebrospinal fluid concentrations A-β and cognitive functioning in patients with mild to moderate Alzheimer's disease. Neurology. 2004 62suppl 5. A174. [Google Scholar]
- Alzforum: Drugs in Clinical Trials. Beta- & Gamma-secretase inhibitors. 2005. Available at: http://www.alzforum.org/drg/drc/detail.asp?id=22. Accessed Jan 22, 2006. [Google Scholar]
- Serneels L, Dejaegere T, and Craessaerts K. et al. Differential contribution of the three Aph1 genes to gamma-secretase activity in vivo. Proc Natl Acad Sci U S A. 2005 102:1719–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barten DM, Guss VL, and Corsa JA. et al. Dynamics of β-amyloid reductions in brain, cerebrospinal fluid, and plasma of β-amyloid precursor protein transgenic mice treated with a γ-secretase inhibitor. J Pharmacol Exp Ther. 2005 312:635–643. [DOI] [PubMed] [Google Scholar]
- Rosenberg RN.. Explaining the cause of the amyloid burden in Alzheimer disease. Arch Neurol. 2002;59:1367–1368. doi: 10.1001/archneur.59.9.1367. [DOI] [PubMed] [Google Scholar]
- Luo Y, Bolon B, and Damore MA. et al. BACE1 (beta-secretase) knockout mice do not acquire compensatory gene expression changes or develop neural lesions over time. Neurobiol Dis. 2003 14:81–88. [DOI] [PubMed] [Google Scholar]
- Wong P. BACE. Alzheimer's & Dementia. 2005 1suppl 1. S3. [Google Scholar]
- Citron M.. β-Secretase inhibition for the treatment of Alzheimer's disease: promise and challenge. Trends Pharmacol Sci. 2004;25:92–97. doi: 10.1016/j.tips.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, and Sagi SA. et al. Aβ42-lowering nonsteroidal anti-inflammatory drugs preserve intramembrane cleavage of the amyloid precursor protein (APP) and ErbB-4 receptor and signaling through the APP intracellular domain. J Biol Chem. 2003 278:30748–30754. [DOI] [PubMed] [Google Scholar]
- Beher D, Clarke EE, and Wrigley JD. et al. Selected nonsteroidal anti-inflammatory drugs and their derivatives target γ-secretase at a novel site: evidence for an allosteric mechanism. J Biol Chem. 2004 279:43419–43426. [DOI] [PubMed] [Google Scholar]
- Lleo A, Berezovska O, and Herl L. et al. Nonsteroidal anti-inflammatory drugs lower Aβ42 and change presenilin 1 conformation. Nat Med. 2004 10:1065–1066. [DOI] [PubMed] [Google Scholar]
- Townsend KP, Praticò D.. Novel therapeutic opportunities for Alzheimer's disease: focus on nonsteroidal anti-inflammatory drugs. FASEB J. 2005;19:1592–1601. doi: 10.1096/fj.04-3620rev. [DOI] [PubMed] [Google Scholar]
- Golde TE, Eriksen J, and Nicolle M. et al. Selective Aβ42 modifying agents: effects on Aβ deposition and behavior in Tg2576 mice. Presented at the 9th International Conference on Alzheimer's Disease and Related Disorders; July 18, 2004; Philadelphia, Pa. [Google Scholar]
- Galasko D, Graff-Radford N, and Murphy MP. et al. Safety, tolerability, pharmacokinetics and Aβ levels following short-term administration of R-flurbiprofen in healthy elderly individuals: a phase 1 study. Presented at the 9th International Conference on Alzheimer's Disease and Related Disorders; July 18, 2004; Philadelphia, Pa. [Google Scholar]
- Press release. Myriad Genetics' follow-on study of Flurizan demonstrates cognitive improvement in Alzheimer's disease. Nov 15, 2005. Available at: http:/www.myriad.com/news/release/051115. Accessed Jan 22, 2006. [Google Scholar]
- Gervais F, Chalifour R, and Garceau D. et al. Glycosaminoglycan mimetics: a therapeutic approach to cerebral amyloid angiopathy. Amyloid. 2001 8suppl 1. 28–35. [PubMed] [Google Scholar]
- Garceau D, Gurbindo C, and Laurin J. Safety, tolerability and pharmacokinetic profile of Alzhemed, an anti-amyloid agent for Alzheimer's disease, in healthy subjects. Presented at the 7th International Geneva/Springfield Symposium on Advances in Alzheimer Therapy; April 3–6, 2002; Geneva, Switzerland. [Google Scholar]
- Geerts H.. NC-531 Neurochem. Curr Opin Investig Drugs. 2004;5:95–100. [PubMed] [Google Scholar]
- Aisen P, Mehran M, and Poole R. et al. Clinical data on Alzhemed after 12 months of treatment in patients with mild to moderate Alzheimer's disease. Presented at the 9th International Conference on Alzheimer's Disease and Related Disorders; July 18, 2004; Philadelphia, Pa. [Google Scholar]
- Gibson GL, Douraghi-Zadeh D, and Parsons RB. et al. Properties of ovine colostrinin (O-CLN) on the in vitro aggregation and toxicity of β -amyloid. Neurobiol Aging. 2004 25:592. [Google Scholar]
- Rattray M.. Technology evaluation: colostrinin, ReGen. Curr Opin Mol Ther. 2005;7:78–84. [PubMed] [Google Scholar]
- Bilikiewicz A, Gaus W.. Colostrinin (a naturally occurring, proline-rich, polypeptide mixture) in the treatment of Alzheimer's disease. J Alzheimer's Dis. 2004;6:17–26. doi: 10.3233/jad-2004-6103. [DOI] [PubMed] [Google Scholar]
- Leszek J, Inglot AD, and Janusz M. et al. Colostrinin proline-rich polypeptide complex from ovine colostrum—a long-term study of its efficacy in Alzheimer's disease. Med Sci Monit. 2002 8:PI93–PI96. [PubMed] [Google Scholar]
- Ritchie CW, Bush AI, and Mackinnon A. et al. Metal-protein attenuation with iodochlorhydroxyquin (Clioquinol) targeting Aβ amyloid deposition and toxicity in Alzheimer disease. Arch Neurol. 2003 60:1685–1691. [DOI] [PubMed] [Google Scholar]
- Shaw KTY, Utsuki T, and Rogers J. et al. Phenserine regulates translation of β-amyloid precursor protein mRNA by a putative interleukin-1 responsive element, a target for drug development. PNAS. 2001 98:7605–7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B. The efficacy of phenserine in the treatment of mild-to-moderate Alzheimer's disease. Presented at the 7th International Conference on Alzheimer's Disease and Parkinson's Disease; March 12, 2005; Sorrento, Italy. [Google Scholar]
- in't Veld BA, Ruitenberg A, and Hofman A. et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med. 2001 345:1515–1521. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Schulzer M, McGeer EG.. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- Szekely CA, Thorne JE, and Zandi PP. et al. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer's disease: a systematic review. Neuroepidemiology. 2004 23:159–169. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, and Grundman M. et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003 289:2819–2826. [DOI] [PubMed] [Google Scholar]
- Reines SA, Block GA, and Morris JC. et al. Rofecoxib: no effect on Alzheimer's disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004 62:66–71. [DOI] [PubMed] [Google Scholar]
- Scharf S, Mander A, and Ugoni A. et al. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer's disease. Neurology. 1999 53:197–201. [DOI] [PubMed] [Google Scholar]
- Rogers J, Kirby LC, and Hempelman SR. et al. Clinical trial of indo-methacin in Alzheimer's disease. Neurology. 1993 43:1609–1611. [DOI] [PubMed] [Google Scholar]
- Tabet N, Feldman H. Indomethacin for the treatment of Alzheimer's disease patients. Cochrane Database Syst Rev. 2002 CD003673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J, Mintzer J.. Alzheimer disease update: new targets, new options. Drug Benefit Trends. 2005;17:83–88. 91–95. [Google Scholar]
- Press release. Use of nonsteroidal anti-inflammatory drugs suspended in large Alzheimer's disease prevention trial. NIH News. Dec 20, 2004. Available at: http://www.nih.gov/news/pr/dec2004/od-20.htm. Accessed Jan 22, 2006. [Google Scholar]
- Hauss-Wegrzyniak B, Vraniak P, Wenk GL.. The effects of a novel NSAID on chronic neuroinflammation are age dependent. Neurobiol Aging. 1999;20:305–313. doi: 10.1016/s0197-4580(99)00028-7. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Kadish I.. Transgenic AD model mice, effects of potential anti-AD treatments on inflammation and pathology. Brain Res Brain Res Rev. 2005;48:370–378. doi: 10.1016/j.brainresrev.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Press release. NicOx announces successful phase I clinical results with HCT 1026 in development for Alzheimer's disease. May 13, 2003. Available at: http://www.nicox.com/upload/HCT%201026%20Final%20English.pdf. Accessed Jan 22, 2006. [Google Scholar]
- Fiorucci S, Santucci L, and Sardina M. et al. Effect of HCT1026, a nitric oxide (NO) releasing derivative of flurbiprofen, on gastrointestinal mucosa: a double blind placebo-controlled endoscopic study. Presented at Digestive Disease Week; May 17–22, 2003; Orlando, Fla. [Google Scholar]
- Derwent Information Ltd. Nitroflurbiprofen (oral), NicOx. April 26, 2004. Available at: http://www.bizcharts.com/pdfs/IDdbCox2Record.pdf. Accessed Dec 14, 2005. [Google Scholar]
- Wolozin B, Kellman W, and Ruosseau P. et al. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000 57:1439–1443. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Sabbagh MN, and Connor DJ. et al. Atorvastatin therapy lowers circulating cholesterol but not free radical activity in advance of identifiable clinical benefit in the treatment of mild-to-moderate AD. Curr Alzheimer Res. 2005 2:343–353. [DOI] [PubMed] [Google Scholar]
- Pedrini S, Carter TL, and Prendergast G. et al. Modulation of statin-activated shedding of Alzheimer APP ectodomain by ROCK. PLoS Med. 2005 2:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov. ALADDIN study, phase III: antigonadotropin-leuprolide in Alzheimer's disease drug investigation (VP-AD-301). Available at: http://www.clinicaltrials.gov/ct/show/NCT00231946?order=1. Accessed Jan 22, 2006. [Google Scholar]
- Bowen RL, Verdile G, and Liu T. et al. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-β precursor protein and amyloid-β deposition. J Biol Chem. 2004 279:20539–20545. [DOI] [PubMed] [Google Scholar]
- Golde TE.. Alzheimer disease therapy: can the amyloid cascade be halted? J Clin Invest. 2003;111:11–18. doi: 10.1172/JCI17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB. Risk factors for vascular dementia and Alzheimer disease. Stroke. 2004 35suppl 1. 2620–2622. [DOI] [PubMed] [Google Scholar]
- Jorm AF.. Cross-national comparisons of the occurrence of Alzheimer's and vascular dementias. Eur Arch Psychiatry Clin Neurosci. 1991;240:218–222. doi: 10.1007/BF02189530. [DOI] [PubMed] [Google Scholar]
- Poirier J, Davignon J, and Bouthillier D. et al. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993 342:697–699. [DOI] [PubMed] [Google Scholar]
- Lott IT, Head E.. Alzheimer disease and Down syndrome: factors in pathogenesis. Neurobiol Aging. 2005;26:383–389. doi: 10.1016/j.neurobiolaging.2004.08.005. [DOI] [PubMed] [Google Scholar]