Abstract
A number of observations and discoveries over the past 20 years support the concept of important physiological interactions between the endocrine and immune systems. The best known pathway for transmission of information from the immune system to the neuroendocrine system is humoral in the form of cytokines, although neural transmission via the afferent vagus is well documented also. In the other direction, efferent signals from the nervous system to the immune system are conveyed by both the neuroendocrine and autonomic nervous systems. Communication is possible because the nervous and immune systems share a common biochemical language involving shared ligands and receptors, including neurotransmitters, neuropeptides, growth factors, neuroendocrine hormones and cytokines. This means that the brain functions as an immune-regulating organ participating in immune responses. A great deal of evidence has accumulated and confirmed that hormones secreted by the neuroendocrine system play an important role in communication and regulation of the cells of the immune system. Among protein hormones, this has been most clearly documented for prolactin (PRL), growth hormone (GH), and insulin-like growth factor-1 (IGF-I), but significant influences on immunity by thyroid stimulating hormone (TSH) have also been demonstrated. Here we review evidence obtained during the past 20 years to clearly demonstrate that neuroendocrine protein hormones influence immunity and that immune processes affect the neuroendocrine system. New findings highlight a previously undiscovered route of communication between the immune and endocrine systems that is now known to occur at the cellular level. This communication system is activated when inflammatory processes induced by proinflammatory cytokines antagonize the function of a variety of hormones, which then causes endocrine resistance in both the periphery and brain. Homeostasis during inflammation is achieved by a balance between cytokines and endocrine hormones.
Introduction
The brain has the potential to orchestrate responses from leukocytes through the autonomic nervous system as well as through the endocrine system. Neuroendocrine interactions predominantly occur at the level of the hypothalamic-pituitary axis, so pituitary-derived hormones can clearly mediate effects of the central nervous system on immune responses. However, pituitary hormones may also affect the immune system independently of the the central nervous system. This occurs through autocrine or paracrine interactions within the immune system and as a result of regulation of pituitary hormones by cytokines that act directly at the pituitary level. A clear example is the abundant expression of IL-1 type I and II receptors specifically on GH-secreting cells in the murine anterior pituitary gland (French et al., 1996).
Prolactin (PRL) is a member of a class of related protein hormones that includes PRL and placental lactogens (PL). It is a pleiotropic hormone that is mainly produced in the pituitary. PRL secretion in the pituitary is under negative control by dopamine but can also be stimulated by thyrotropin releasing hormone. Although the main functions of PRL are mammary gland development and initiation and maintenance of lactation, PRL receptors (PRLR) are widely distributed throughout many different tissues. The fact that PRL subserves many different functions is in accordance with the expression of PRL in extra-pituitary tissues, the existence of several molecular variants of PRL with different activities and the tissue-specific regulation of PRL.
The PRLR belongs to a large, heterogeneous family of cytokine-hematopoietic receptors known as the class I cytokine receptor family. There is a strong homology between the growth hormone receptor (GHR) and the PRLR. The primary structural homology between the GHR or PRLR and other members of this family is restricted to two extracellular domains of 100 amino acids and intracellular motifs known as boxes. Rodents express three isoforms of the PRLR, whereas humans express four. One of these isoforms is unable to signal, suggesting that it might act as a decoy receptor. The PRLR is only known to bind PRL, placental lactogens and high concentrations of primate GH. Indeed, the priming actions of GH on human neutrophils are mediated not by the GHR but rather by the PRLR (Fu et al., 1992). PRLR homodimerization via two different sites on PRL leads to subsequent activation of associated kinases that phosphorylate downstream targets. Most signalling studies have focussed on the JAK-STAT signalling pathway, which is used by all hematopoietic cytokine receptors. Binding of PRL to its receptor predominantly evokes the activation of JAK-2 which leads to tyrosine phosphorylation of the PRLR, allowing recruitment of latent cytoplasmic transcription factors (STAT). JAK-2 activation by PRL mainly activates STAT-5 and to a lesser extent STAT-1 and -3. Common use of the JAK-STAT pathway by PRL, GH and other cytokines likely leads to redundancy in their actions.
GH is expressed primarily in the pituitary, but also by cells of the immune system, and is positively regulated by growth hormone releasing hormone (GHRH) and is negatively regulated by somatostatin in the pituitary. The main effect of GH is to promote postnatal longitudinal growth through interaction with the GHR, which is also a member of the class I cytokine receptor family. GH binding to the GHR causes receptor dimerization and activation of JAK2 and STAT proteins. GH is most well known for its regulation of carbohydrate, lipid, nitrogen and mineral metabolism. Many of the actions of GH are mediated by the induction of insulin-like growth factor-I (IGF-I) expression at local sites, but the primary source of circulating IGF-I is the liver. An evolving theme over the past 20 years is the idea that GH and IGF-I also play a role in the development, maintenance and function of the immune system. An overview of IGF-I receptor (IGF-1R) activation has recently appeared (McCusker et al., 2006).
Thyroid stimulating hormone (TSH) is a peptide hormone acting through a typical serpentine G-protein coupled receptor to stimulate the production of thyroxin (T4) by the thyroid gland. T4 is a prohormone to T3 that regulates oxygen consumption, lipid metabolism and carbohydrate metabolism, and is required for normal growth and maturation. T3 enters the cell and binds to a nuclear receptor which then acts as a transcription factor by binding to thyroxin response elements in the promoter region of target genes, including TSH and GH.
Prior to 1987
The first indication that pituitary hormones play a role in lymphopoiesis and effector functions of leukocytes came from experiments using pituitary-deficient rodents such as hypophysectomized rats and Snell-Bagg dwarf mice. Hypophysectomy leads to a combined deficiency in all pituitary hormones including the anterior pituitary hormones (TSH, GH, PRL, follicle-stimulating hormone, luteinizing hormone, and POMC-derived peptides such as adrenocorticopic hormone (ACTH) and α-melanocyte-stimulating hormone (α-MSH)) and hormones derived from the posterior lobe (oxytocin and vasopressin). In hypophysectomized rats, impaired development of different compartments of the immune system as well as cellular and humoral immune responses can be restored by administration of either PRL or GH (reviewed by Gala, 1991; Kelley, 1989; Kelley et al., 1992).
Snell-Bagg-dwarf mice are deficient in the anterior pituitary hormones PRL, GH and TSH due to a point mutation in the pituitary transcription factor Pit-1. Expression of these hormones, as well as the development of lactotropes, somatotropes and thyrotropes, depends on Pit-1. Therefore, immunological defects in these mice could be due to the deficiency of PRL, GH or TSH. However, an important advantage of this animal model over hypophysectomy is that the ACTH-adrenal axis, which is responsible for suppression of inflammation through the synthesis and release of endogenous adrenal glucocorticoids, remains intact. All three protein hormones have been shown to contribute, at least in part, to restoration of cellular depletion in different compartments of the immune system (Gala, 1991; Kelley et al., 1992; Kooijman et al., 1996). Although PRL, GH and T4 are all able to re-establish humoral and cellular immune responses in certain studies (Gala, 1991), others have found differential effects of PRL and GH. For example, treatment of Snell-Bagg dwarf mice with PRL enhances antigen-induced T cell proliferation which does not occur after GH treatment, whereas only GH is able to reverse the decrease in thymic cellularity (Murphy et al., 1993).
Since pituitary PRL production is under stringent inhibitive dopaminergic control, a specific reduction in serum PRL levels can be induced by the dopaminergic D2 agonist bromocryptine. In general, administration of bromocriptine is immunosuppressive. In mice, it reduces IFNγproduction and T cell-dependent killing of microorganisms by macrophages. Importantly, this effect is reversed by administration of PRL (Gala, 1991; Kelley et al., 1992). In rats, bromocryptine suppresses antibody production to sheep red blood cells. Remarkably, the negative effects of bromocryptine can be restored by either PRL or GH (Berczi, 1992), indicating that their roles in the immune system could be redundant.
The possibility of direct PRL effects on the immune system is indicated by the detection of high affinity PRLRs on human monocytes, T cells, B cells (Russell et al., 1985) and NK cells (Matera et al., 1988). Several in vitro effects of PRL on rodent or human leukocytes have been reported. For instance, PRL primes the oxidative burst in granulocytes and stimulates lymphocyte proliferation, NK cell activity and IL-2 receptor expression (Berczi, 1992; Kelley et al., 1992). However, results on primary cells are not always consistent. Sometimes high concentrations of PRL and certain in vitro effects of PRL are dependent on the hormonal status of the animal from which the cells are obtained (Berczi, 1992). These observations, in combination with the above-mentioned in vivo data, have led to the hypothesis that PRL plays a stimulatory role in the immune system.
The idea that GH also influences the immune system originated prior to 1987 from findings in hypophysectomized animals as well as in humans or animals with deficiencies in pituitary hormones (Gala, 1991). In general, defects in both humoral and cell-mediated immunity were observed in hypophysectomized rodents, including a reduction in the antibody response and an increase in graft survival. Similar findings were obtained in the Snell-Bagg and Ames dwarf mouse strains that lack both GH and PRL. GH hormone replacement studies evaluated the specificity of its effects on the immune response and showed that GH improves immunological deficiencies in hypophysectomized or dwarf animals. Thus, very early studies revealed that GH may be important in the regulation of the immune response. The specificity was confusing since in some cases PRL and GH appeared to be redundant in restoring immune deficiencies, while in other situations only one of the hormones was active. In an effort to sort out this confusion, more rigorous attempts were made after 1986 to identify specific receptors on cells of the immune system and to analyze their direct effects on cells of the immune system.
1987-1997
During this decade, further in vitro evidence for a role of PRL in the immune system was provided (Matera et al., 1992). Above all, it became clear that the immune system in rodents as well as in humans produces PRL, GH and IGF-I (Kooijman et al., 1996). Using antibodies against human and rodent PRL, it was shown that leukocyte-derived PRL is functional as these antibodies inhibit lymphocyte proliferation (Berczi, 1992). Although several immunoreactive PRL variants were detected in cells from the immune system, it was eventually established that leukocytes produce bona fide pituitary-like PRL with normal biological activity. The observation that in humans extrapituitary PRL expression, in the placenta as well as in the immune system, is differentially regulated at the molecular level through an upstream non-pituitary promoter (Berwaer et al., 1994;Gellersen et al., 1994) provided additional support for a specific role of PRL in the immune system.
It was shown that PRL gene expression in lymphoid cells is regulated independently of the pituitary transcription factor Pit-1. Due to the presence of a 5’-non-coding exon (exon 1a), the extrapituitary transcript of the PRL gene is 150 nucleotides longer than its pituitary counterpart. The PRL peptide produced from this mRNA is, however, indistinguishable from that of pituitary origin. As a result, PRL can no longer be considered as an immune stimulatory endocrine factor, but also as an autocrine or paracrine immune regulatory cytokine. Indeed, PRL in human immune cells can be regulated by cytokines and other immune-specific agonists.
Early work indicated a role for the pituitary-thyroid axis in the immune system by demonstrating that T4 treatment of Snell dwarf mice resulted in increased bone marrow cellularity (Pierpaoli et al., 1969). However, the exact effects of T4 on the immune system only became clear in the late nineties when more animal models and sophisticated techniques became available to detect different developmental stages of bone marrow cells. By flow cytometric analysis using lineage- and stage-specific antibodies it was shown that Snell-Bagg dwarf mice exhibit a deficiency in bone marrow CD45R+/surface IgM- pre-B cells (Murphy et al., 1992). More detailed analysis was done by exploring this phenomenon in mice with different hormone deficiencies, including Snell-Bagg dwarfs, IGF-I knock outs, lit/lit dwarf mice with a mutation in the GH-releasing hormone receptor (GHRHR) and hyperthyroid mice which poorly respond to TSH. It was shown that only Snell-Bagg dwarfs and hypothyroid mice showed the described pre-B cell depletion (Dorshkind & Horseman, 2000). Furthermore, the defects in Snell-Bagg dwarf mice could be restored by treatment with T4. These results implicated a role for the pituitary-thyroid axis in murine B cell development. However, the humoral immune response, as well as the cellular immune response, was not affected. Instead, the results suggested that innate immune response was impaired (Dorshkind and Horseman, 2000).
Although a report as early as 1973 indicated lymphocytes have high affinity receptors for GH, it took at least another 12 years for the presence of GHRs on cells of the immune system to become generally accepted. Both primary lymphocytes and lymphoid cell lines were studied and the results suggested 4,000-20,000 high affinity GH-binding sites per cell. GH bound to these sites could be partially displaced by high concentrations of PRL. The presence of specific GHRs was also indicated by in vitro biological studies (Kelley et al., 1992). Treatment of phagocytes with GH was shown to increase lysosomal enzyme content and superoxide anion production. It should be made clear, however, that the increase in superoxide production in GH-treated phagocytic cells could not be detected unless the cells were treated with a triggering stimulus, such as opsonized zymosan (Kelley, 1989). Therefore, phagocytic cells exposed to GH do not spontaneously release superoxide anions. It appeared that GH was able to influence many cell types of the immune system. Subsequently, it became clear that some of these effects of GH were indirectly caused by the actions of IGF-I. For example, culture of primary human bone marrow cells and also erythroleukemia cells with GH showed an increase in erythoid colony formation that could be blocked by antibodies to the IGF1R. It was also shown that GH could increase the proliferation of normal and leukemic human T cells as well as the generation of cytotoxic T cells.
After these initial findings, a substantial number of studies were conducted and further evidence was gathered supporting a role for GH in regulating activities of the immune system (Kooijman et al., 1996). In addition, the new knowledge gained during this decade about the crystal structure of GH, dimerization and intracellular signaling mechanisms, primarily from other cell systems, added greatly to the complexity of understanding GH and its role in immunoregulation. Discovery of plasma membrane bound GHRs, nuclear GHRs and Janus kinases suggested novel nuclear roles in addition to their cytoplasmic activities.
A significant body of work appeared on the GH binding protein (GHBP), which was first reported in 1964 and isolated from the serum of pregnant mice in 1977. Both alternative splicing of mRNA and limited proteolysis, depending upon the species, have been shown to generate the GHBP which corresponds to the extracellular domain of the GHR. IM-9 lymphocytes utilize proteolysis in the formation of the GHBP which appears to show antagonistic properties in vitro and agonistic effects in vivo. Major advances in the decade following 1986 appeared on the mechanism of GH at the cellular level. Thus, we learned that GH promotes the rapid association of the GHR with the tyrosine kinase JAK2. The phosphorylation and activation of JAK2/GHR complexes in turn binds and phosphorylates a number of proteins including signal transducers and activators of transcription (STATS), the insulin receptor substrates (IRS)-1 and -2 and Shc proteins that are upstream of RAS and the mitogen activated protein kinases (MAPK) (Argetsinger and Carter-Su, 1996).
In the early 1980s, it was shown that cells of the immune system produce the adrenocorticotropin, endorphins, thyroid stimulating hormone proteins as well as a prolactin/GH-related mRNA in mitogen-stimulated lymphocytes (Weigent and Blalock, 2007). Our own findings at this time on production of GH by cells of the immune system clearly showed that leukocytes contained the mRNA transcript and GH molecules identical to pituitary GH in terms of antigenicity and molecular weight (Weigent, 1996). This work was confirmed and reproduced in a number of lymphoid cell lines and primary lymphoid tissues from a handful of different species of animals. In our model system, the upregulation of IGF-I and the IGF-1R by endogenous GH was a critical component of the anti-apoptotic activity of GH. More recently, it has become clear that the effects of endogenous GH on cell growth are complex, as induction of the IGF-IIR and TGF-β1 are also observed in EL4 T cells, which could be viewed as limiting cellular proliferation (Farmer and Weigent, 2007). Thus, endogenous GH may influence survival of T cells during activation and/or maintenance of memory cells. All this news may not be good. These same properties acquired by certain T cells may contribute to autoimmunity while tumor cells may show enhanced survival. Subsequent studies established that both rat spleen cells and human lymphocytes synthesize and secrete growth hormone releasing hormone (GHRH) and IGF-I and express their respective receptors (reviewed by Farmer and Weigent, 2007). The predominant type of leukocyte that synthesizes and secretes IGF-I are macrophages, including microglia (Arkins et al, 1993). A number of reports by others on exogenous GH acting through the GHR and our own on endogenous GH from a cell line devoid of GHRs suggest an important relationship between GH and the regulation of cell death. During this decade, further studies showed that treatment of cells of the immune system with GH modulates humoral and cellular immune functions, including immunoglobulin secretion of B cells, thymulin secretion of thymic epithelial cells, NK cell activity, phagocytosis, oxidative burst and killing capacity of neutrophils and macrophages (Kooijman et al., 1996; Weigent, 1996). It is likely that at least upstream components of GH regulation in both T and B cells are similar in that JAK/STAT family members are involved, but much more work is needed to determine the specific details for every type of leukocyte and their functions. Despite this, however, no clinically significant immunodeficiency was found in GH-deficient patients, creating controversy regarding the physiological significance of GH effects in the immune system. Failure to find significant immunodeficiencies in GH-deficient patients could well be due to normal amounts of PRL in these subjects, particularly since human GH activates the PRLR on human neutrophils and PRL shares significant immunoregulatory properties with GH.
1997-2007
Prior to the turn of the century, it was considered that physiological concentrations of IGF-I did not have any major adverse consequences in the body. However, it has become increasingly clear that the concentration of circulating IGF-I is inversely related to life span and is positively correlated to progression of the development of several different types of tumors. We have shown that IGF-I acts in cycling cells via insulin receptor substrate-1, phosphatidylinositol 3′-kinase (PI3-K), AKT and cyclin-dependent kinase 2 (CDK2), and the former and latter proteins are inhibited by TNFα(Shen et al, 2004). It has recently been shown that CDK2 phosphorylates the Forkhead box O (FOXO) transcription factor, which activates a number of death genes (Huang et al, 2006). This phosphorylation of FOXO on serine causes it to translocate from the nucleus to the cytoplasm, where FOXO is inactive. For reasons as yet unknown, IGF-I inactivation of FOXO promotes cell survival, but at the same time seems to be associated with a reduction in life span (reviewed by Kenyon, 2005). Furthermore, the paradox that IGF-I, which is absolutely necessary for growth and development, is inversely correlated with lifespan of the whole organism and positively correlated with progression of many types of cancers is intriguing. It has been suggested that antagonistic pleiotrophy may explain these findings, whereby the cost of longer living is a reduction in both peripheral and central physiological functions (Sonntag et al, 2005). This hypothesis may explain why so many mechanisms have been identified that regulate IGF-I activity, including the IGF-1R, IGF-2R, insulin receptor, and the six high affinity IGF binding proteins. It is also important to note that nearly all mice with a null mutation in the IGF-1R die shortly after birth, and IGF-I deficient mutant mice die perinatally. Therefore, the long-term effects of IGF-I loss on immune events remains unknown. These collective considerations, along with the lack of serious immunological deficiencies in either GH- or PRL-deficient patients, limited the influx of new investigators and research support and led to slow progress during this decade.
A role for leukocyte-derived PRL as an autocrine or paracrine factor is supported by recent observations that prolactin regulates cytokine production (Dimitrov et al., 2004) and that PRL expression in T cells is regulated by IL-2, IL-4 and IL-1β(Gerlo et al., 2005). In the late nineties, experiments from two different groups using PRL (Horseman et al., 1997) and PRLR (Bouchard et al., 1999) knockout mice, respectively, revealed that neither PRL nor the PRLR are required for normal development or function of the immune system in mice. However, the in vitro immunomodulatory actions of PRL, both in rodents and humans, were established by many different groups. It is likely that redundancy in the effects of PRL, GH, IGF-I or specific cytokines is responsible for the absence of immune disorders in PRL or PRLR-deficient mice.
Comparing the role of PRL in the immune system in rodents with that in humans is difficult because an alternative extrapituitary promoter has not been detected in rodents. It cannot be excluded that development of an alternative molecular regulation system for PRL expression in the immune system in humans has led to the development of specific immunological functions for leukocyte-derived PRL that are absent in rodents and other non-primates which do not have the upstream promoter (Gerlo et al., 2006). For instance, the absence of immunological disorders in PRL-deficient women lacking pituitary PRL does not imply that PRL is not required for the function of the immune system. It is important to note that in leukocytes as well as in the placenta, the role of PRL expression under the control of the alternative promoter in humans needs further attention during the next decade (Gerlo et al., 2006).
The observation that hyperprolactinemia occurs in a fraction of patients with autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus (SLE), has drawn much attention to the putative role of PRL in autoimmunity. In rheumatoid arthritis, paracrine PRL produced by synovial T cells has been proposed to play a role in the pathophysiology of the disease (Nagafuchi et al., 1999). However, most studies on the role of PRL in autoimmunity has dealt with SLE. PRL exacerbates SLE in the New Zealand/Black mice strain, and it increases the survival of autoreactive B cells in susceptible strains (Peeva et al., 2004). Since hyperprolactenemia is observed in 20% of the SLE patients, it was proposed that a role for PRL in SLE in humans depends on the genetic background. Recent observations indicating that PRL influences the B cell repertoire of mice (Peeva et al., 2004) beckons for further research to elucidate the role PRL in SLE and other autoimmune disorders.
The last 10 years have provided additional cellular and molecular details of GH action as well as important developments in our understanding of GH and immunity through the use of transgenic and knockout animals (Savino et al., 2003). Thus, transgenic mice overexpressing GH or GHRH exhibit overgrowth of the thymus and spleen and display increases in mitogenic responses to concanavalin A. The enhancing effect of GH on thymocytes was initially reported from observations in old rats receiving syngeneic GH3 cell implants (Kelley et al, 1986). High thymulin serum levels were observed in GH-transgenic mice whereas low serum levels were seen in GHR knockout mice. The ability of GH to modulate thymocyte export suggests it may be of some utility as a therapeutic agent in T cell immunodeficiencies. Therapies for aging, AIDS, and transplant patients are suggested by the immune-enhancing effects of oral GH secretagogues. During the last decade, clinical trials involving GH and GH secretagogues, particularly in AIDS patients, imply they might be beneficial. Only more extensive clinical trials can validate the efficacy of using GH or GH secretagogues as immunotherapeutics. It should be noted, however, that in some cases the results with GH have not been impressive, particularly during inflammatory states. This is most likely the result of TNF-α-induced IGF-I resistance that occurs in AIDS patients (Kelley, 2004).
Important new technologies in genomic biology such as microarray analysis have just begun to be applied to various tissues in animals treated with GH and can be expected to be applied to cells of the immune system soon. In future experiments in animal models of aging, autoimmunity, and cancer, DNA and protein arrays will identify genes in terms of their regulation by GH. The GHBP is now known to be located intracellularly and can be translocated to the nucleus after ligand stimulation and function as a potent enhancer of STAT-5-mediated transcription. GH has been shown to promote tubulin polymerization, stabilizing the microtubule network that can protect cells against apoptosis. Indeed, our understanding of endogenous GH remains very limited. Lymphocyte-derived GH regulates lymphoid cell sensitivity and production of IGF-I and is an active participant in protecting cells from apoptosis. Lymphocyte GH stimulates interferon-γproduction and can be inhibited by cortisol and norepinephrine, whereas analysis of the GH promoter indicates that specificity protein 3 (SP3) acts as a negative transcription factor. It is anticipated that future experiments will identify the intracellular site of action of endogenous GH. This new knowledge will greatly aid in revealing the mechanism of action and provide further understanding of the control of the immune response by the local production of this hormone. The role of exogenous and/or endogenous GH in the development of neoplasia is not yet clear, but efforts are being made to identify the potential pathologies associated with GH production. There are recent studies to suggest that autocrine or endogenously produced GH, but not exogenous GH, may be an important promoter of oncogenic transformation (Zhu et al, 2005).
Variations in housing conditions, which could affect levels of both sanitation and stress, have also been proposed to account for the differences in immune status of pituitary dwarfs reported by various groups of investigators (for review see Dorshkind and Horseman, 2000). These findings reinforced the old and well-established concept that, under physiological conditions, PRL, GH and IGF-I normally function to counteract the effects of physiological or environmental stress (Kelley and Dantzer, 1991). This idea is attractive since GH and PRL have long been known to be stress-responsive hormones. For example, it is possible that both GH and PRL are responsible for a phenomenon discovered in the early 1980s that restraint, heat and cold stress increase contact sensitivity reactions to dinitofluorobenzene (Blecha et al, 1982 a,b). Most of the early data supporting the idea that GH and PRL counteract the suppressive effects of glucocorticoids were described in the early 90’s (Kelley and Dantzer, 1991). This concept is similar in principle to interferon-γreversing the suppressive effects of natural and synthetic glucocorticoids (Dunham et al, 1990). Indeed, GH improves several aspects of the immune response following in vivo treatment of rats with dexamethasone or after surgical stress (Hinton et al, 1995). It was later discovered that targeted disruption of the PRL gene impairs mitogen-induced proliferation of splenocytes in thermally-injured mice but not in normal controls (Dugan et al., 2002). Findings such as these led to the clinical use of GH to improve growth in children undergoing long-term-glucocorticoid treatment, which increases catabolism caused by its effects on tissue protein synthesis and degradation, following liver transplantation (Sarna et al, 1996) and to reduce muscle loss in wasting AIDS and cancer patients (reviewed by Kelley, 2004).
2007 and Beyond
The idea that GH, PRL and IGF-I interact with immunosuppressive hormones strongly suggests that there is a “two way street” in hormone-hormone interactions, as well as with hormone-immune system interactions. That is, not only should these protein hormones counteract the catabolic effects of glucocorticoids, but glucocorticoids are likely to impair the actions of GH, PRL and IGF-I. For example, IGF-I-induced activation of ERK 1,2 is significantly impaired by dexamethasone, which may be due to reduced expression of the critical IGF-1R intracellular messenger, insulin receptor substrate-1 (Hanssan, Hehenberger and Thoren, 1996). It is this homestatic balance that constitutes the yen and the yang of immunoregulation by hormones. That this balance plays a critical role in susceptibility to infectious disease was shown by increased survival of both pituitary-deficient and pituitary-intact rats with injections of GH into rats infected with Salmonella typhimurium (Edwards et al, 1991). By perturbing the hormonal balance in this way, GH increased the survival rate of infected rats to nearly the same extent as that achieved with interferon-γ(Edwards et al, 1992).
There is substantial new evidence that supports this concept of a “two way street” in not only hormone-hormone interactions but in hormone-immune system interactions as well. These new data point to the clinical importance of simultaneous costimulation of cells with hormones and cytokines, as normally occurs in animals and humans. This balance is perturbed during infectious and autoimmune diseases. A common clinical result of this change in the hormone-cytokine ratio is that the biological effect of the hormone is impaired by a proinflammatory cytokine, thereby leading to endocrine resistance. For example, dexamethasone inhibits proliferation of human T cells, and this effect is partially reversed by increasing the ratio of hormones by addition of exogenous GH and IGF-I (Dobashi et al, 2001). Unfortunately, we do not yet know the critical events that occur inside proliferating T cells when they are exposed to both IGF-I and dexamethasone.
Experiments conducted in other systems, such as cancer and muscle cells, are providing insights with other cells in other physiological systems. For example, IGF-I promotes the proliferation of breast cancer cells, and this effect is inhibited by picogram concentrations of both TNFα and IL-1 (Shen et al, 2004). Similarly, these low concentrations of both cytokines inhibit the ability of IGF-I to promote differentiation of muscle cell progenitors into more differentiated myotubes. This inhibitory property of proinflammatory cytokines has recently been shown to be due to activation of the intracellular mediator, JNK (Strle et al, 2006). Indeed, most cell culture studies are conducted in the presence of at least 5% fetal bovine serum. These sera contain significant quantities of IGF-I and IGF-II, as well as much smaller concentrations of GH and PRL. Therefore, many of the biological activities of proinflammatory cytokines that have been reported in vitro could actually occur because these proinflammatory cytokines antagonize the effects of hormones contained in the fetal bovine serum.
It is now widely recognized that the innate immune system in the brain is not only active and functional, but involved in all major disorders of the central nervous system, including Alzheimer’s disease, AIDS-associated dementia, multiple sclerosis and neuropathic pain. New evidence indicates that neuroinflammation is even involved in the pain-suppressing properties of morphine (Watkins et al, 2007). The role of IGF-I is intriguing, particularly since it is synthesized by mononuclear phagocytes, including microglia, in the brain (Arkins et al, 1993). The synthesis of IGF-I in the brain appears to be independent of GH regulation (Sun et al, 2005). Indeed, even though Ames dwarf mice have profound reductions in circulating GH and IGF-I, they have extended life spans and their cognitive functions are maintained until late in life. These longer-lived Ames mice do not display a reduction in brain IGF-I but instead express elevated IGF-I concentrations in the hippocampus compared to age-matched controls. This elevation in hippocampal IGF-I is associated with an increase in neurogenesis in the dentate gyrus of this dwarf mice (Sun et al, 2005). It could well be that the delay in age-related decline cognitive function in Ames mice is related to their youthful levels of brain IGF-I. A recent meta-analysis concluded that GH treatment also improves quality of life, including those psychological dimensions associated with clinical depression and anxiety, in adults with hypopituitarism (Deijen and Arwert, 2006).
In addition to improving cognition and memory and reducing symptoms of sickness behavior induced by proinflammatory cytokines, IGF-I is also being considered in the clinical setting for treatment of diseases of both the central (e.g., multiple sclerosis) and peripheral (e.g., diabetic neuropathy) nervous system (see McCusker et al., 2006). IGF-I has long been known to be an important neurotrophic factor that protects against excitotoxic/ischemic insults. Indeed, one component of healthy life styles is regular physical exercise. Exercise protects against a variety of brain insults in rodents, and this neuroprotection is mediated by IGF-I (Carro et al., 2001). It could well be that increasing the ratio of IGF-I to proinflammatory cyokines caused by exercise is an important underlying mechanism involved in exercise-induced neuroprotection and its antiinflammatory properties.
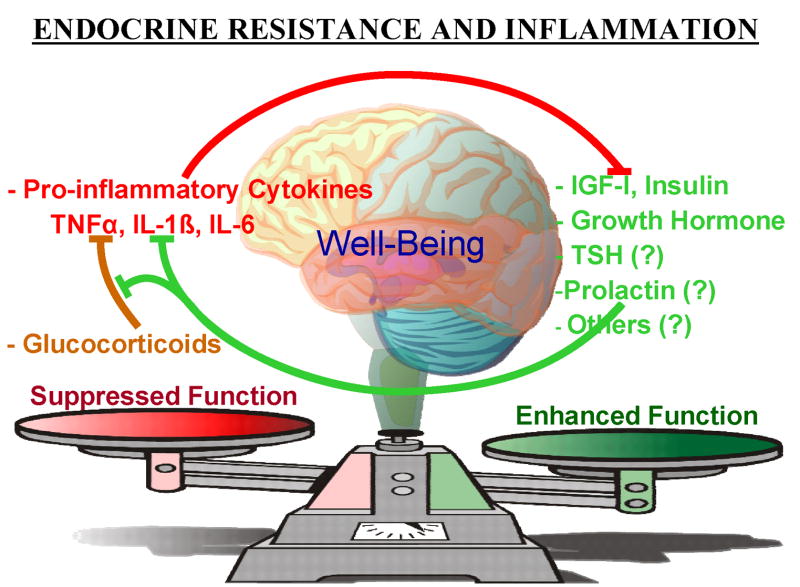
All these data point to the importance of a critical balance between protein hormones and cytokines from the immune system (Fig. 1). The role of proinflammatory cytokines in inducing resistance to IGF-I (Kelley, 2004), GH (Lang, Hong-Brown and Frost, 2005), glucocorticoids (Pace, Hu and Miller, 2007), G-protein coupled receptors such as catecholamines (Heijnen, 2007) and insulin (Hotamisligil, 2003) have been recently highlighted. Some of these hormone-cytokine receptor interactions are mediated by members of the family of suppressors of cytokine signaling (SOCS) proteins (reviewed by Auernhammer and Melmed, 2001). For example, lipopolysaccaride, IL-1β, TNFαand IL-6 all stimulate hepatic SOCS-3 expression and inhibit signaling via the GHR. These molecular events are likely to be some of the major causes leading to GH resistance and a reduction in growth of children and animals during inflammatory states. The emerging theme is that this crosstalk between receptors for protein hormones and proinflammatory cytokines can antagonize the function of hormones and lead to endocrine resistance. This crosstalk represents a previously undiscovered route of communication between the immune and endocrine systems. These new findings underscore the importance of understanding the molecular details of communication systems between the immune and endocrine systems. Closing this gap requires much more detailed knowledge of the intracellular crosstalk that occurs between heterogeneous receptors that are expressed in a single cell.
Figure 1.
A newly-discovered regulatory system between the endocrine and immune systems. Hormone and immune system crosstalk can antagonize the biological functions of hormones and cause endocrine resistance, which ultimately affects human health and well-being. During health, there is an absence of inflammation and an optimal balance of hormones and pro-inflammatory cytokines. The adrenal steroid glucocorticoid hormones act in many peripheral tissues to inhibit synthesis and action of proinflammatory cytokines. This balance is disrupted during inflammatory states in both the periphery and the brain. One mechanism by which this occurs is at the cellular level, whereby proinflammatory cytokines antagonize receptor-signaling pathways in muscle, brain and cancer cells. The body often responds by producing more hormones to overcome this cytokine-induced endocrine resistance. In this way, the endocrine-immune system communication pathway is reciprocal because the rise in concentration of hormones increases the hormone/cytokine ratio. This rise in the amount of hormone relative to cytokine partially overcomes the suppressive actions of both glucocorticoids and proinflammatory cytokines. This scenario even occurs in the brain, as demonstrated by abrogation of TNFα-induced sickness behavior following central administration of IGF-I.
Acknowledgments
We apologize to colleagues whose work, often primary relevant papers, could not be cited due to space limitations.
Footnotes
This research was supported by grants from the National Institutes of Health to K.W.K b(MH51569 and AI50442) and D.A.Wc (AI41651).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argetsinger LS, Carter-Su C. Mechanism of signaling by growth hormone receptor. Physiol Rev. 1996;76:1089–1107. doi: 10.1152/physrev.1996.76.4.1089. [DOI] [PubMed] [Google Scholar]
- Arkins S, Rebeiz N, Biragyn A, Reese DL, Kelley KW. Murine macrophages express abundant insulin-like growth factor-I class I Ea and Eb transcripts. Endocrinology. 1993;133:2334–2343. doi: 10.1210/endo.133.5.8404686. [DOI] [PubMed] [Google Scholar]
- Auernhammer CJ, Melmed S. The central role of SOCS-3 in integrating the neuro-immunoendocrine interface. J Clin Invest. 2001;108:1735–1740. doi: 10.1172/JCI14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berczi I. The immunology of prolactin. Seminars in Reproductive Endocrinology. 1992;10:196–219. [Google Scholar]
- Berwaer M, Martial JA, Davis JRE. Characterization of an up-stream promoter directing extrapituitary expression of the human prolactin gene. Mol Endocrinol. 1994;8:635–642. doi: 10.1210/mend.8.5.8058071. [DOI] [PubMed] [Google Scholar]
- Blecha F, Barry RA, Kelley KW. Stress-induced alterations in delayed-type hypersensitivity to SRBC and contact sensitivity to DNFB in mice. Proc Soc Exp Biol Med. 1982a;169:239–246. doi: 10.3181/00379727-169-41338. [DOI] [PubMed] [Google Scholar]
- Blecha F, Kelley KW, Satterlee DG. Adrenal involvement in the expression of delayed-type hypersensitivity to SRBC and contact sensitivity to DNFB in stressed mice. Proc Soc Exp Biol Med. 1982b;169:247–252. doi: 10.3181/00379727-169-41339. [DOI] [PubMed] [Google Scholar]
- Bouchard B, Ormandy CJ, Di Santo JP, Kelly PA. Immune system development and funciton in prolactin receptor-deficient mice. J Immunol. 1999;163:576–582. [PubMed] [Google Scholar]
- Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–6684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deijen JB, Arwert LI. Impaired quality of life in hypopituitary adults with growth hormone deficiency: can somatotropin replacement therapy help? Treat Endocrinol. 2006;5:243–250. doi: 10.2165/00024677-200605040-00005. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Fehm HL, Born J. A regulatory role of prolactin, growth hormone, and corticosteroids for human T-cell production of cytokines. Brain Behav Immun. 2004;18:368–374. doi: 10.1016/j.bbi.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Dobashi H, Sato M, Tanake T, Tokuda M, Ishida T. Growth hormone restores glucocorticoid-induced T cell suppression. FASEB J. 2001;15:1861–1863. doi: 10.1096/fj.00-0702fje. [DOI] [PubMed] [Google Scholar]
- Dorshkind K, Horseman ND. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocr Rev. 2000;21:292–312. doi: 10.1210/edrv.21.3.0397. [DOI] [PubMed] [Google Scholar]
- Dorshkind K, Horseman ND. Anterior pituitary hormones, stress, and immune system homeostasis. Bioessays. 2001;23:288–294. doi: 10.1002/1521-1878(200103)23:3<288::AID-BIES1039>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Dugan AL, Thellin O, Buckley DJ, Buckley AR, Ogle CK, Horseman ND. Effects of prolactin deficiency on myelopoiesis and splenic T lymphocyte proliferation in thermally injured mice. Endocrinology. 2002;143:4147–4151. doi: 10.1210/en.2002-220515. [DOI] [PubMed] [Google Scholar]
- Dunham DM, Arkins S, Edwards CK, III, Dantzer R, Kelley KW. Role of interferon-gamma in counteracting the suppressive effects of transforming growth factor-beta 2 and glucocorticoids on the production of tumor necrosis factoralpha. J Leukoc Biol. 1990;48:473–481. doi: 10.1002/jlb.48.6.473. [DOI] [PubMed] [Google Scholar]
- Edwards CK, III, Ghiasuddin SM, Yunger LM, Lorence RM, Arkins S, Dantzer R, Kelley KW. In vivo administration of recombinant growth hormone or interferon-γactivates macrophages: Enhanced resistance to experimental Salmonella typhimurium infection is correlated with the generation of reactive oxygen intermediates. Infection and Immunity. 1992;60:2514–2521. doi: 10.1128/iai.60.6.2514-2521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CK, III, Yunger LM, Lorence RM, Dantzer R, Kelley KW. The pituitary gland is required for protection against lethal effects of Salmonella typhimurium. Proc Natl Acad Sci USA. 1991;88:2274–2277. doi: 10.1073/pnas.88.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer JT, Weigent DA. Expression of insulin-like growth factor-2 receptors on EL4 cells overexpressing growth hormone. Brain Behav Immun. 2007;21 doi: 10.1016/j.bbi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- French RA, Zachary JF, Dantzer R, Frawley LS, Chizzonite R, Parnet P, Kelley KW. Dual expression of p80 type I and p68 type II interleukin-1 receptors on anterior pituitary cells synthesizing growth hormone. Endocrinology. 1996;137:4027–4036. doi: 10.1210/endo.137.9.8756580. [DOI] [PubMed] [Google Scholar]
- Fu YK, Arkins S, Fuh G, Cunningham BC, Wells JA, Fong S, Cronin MJ, Dantzer R, Kelley KW. Growth hormone augments superoxide anion secretion of human neutrophils by binding to the prolactin receptor. J Clin Inves. 1992;89:451–457. doi: 10.1172/JCI115605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gala RR. Prolactin and growth hormone in the regulation of the immune system. Proc Soc Exp Biol Med. 1991;198:513–527. doi: 10.3181/00379727-198-43286b. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Kempf R, Telgmann R, DiMattia GE. Nonpituitary human prolactin gene transcription is independent of Pit-1 and differentially controlled in lymphocytes and in endometrial stroma. Mol Endocrinol. 1994;8:356–373. doi: 10.1210/mend.8.3.8015553. [DOI] [PubMed] [Google Scholar]
- Gerlo S, Davis JRE, Mager D, Kooijman R. Prolactin in man: a tale of two promoters. Bioessays. 2006;28 doi: 10.1002/bies.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlo S, Verdood P, Hooghe-Peters EL, Kooijman R. Modulation of prolactin expression in human T lymphocytes by cytokines. J Neuroimmunol. 2005;162:190–193. doi: 10.1016/j.jneuroim.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Hansson A, Hehenberger K, Thoren M. Long-term treatment of Swiss 3T3 fibroblasts with dexamethasone attenuates MAP kinase activation induced by insulin-like growth factor-I (IGF-I) Cell Biochem Funct. 1996;14:121–129. doi: 10.1002/cbf.656. [DOI] [PubMed] [Google Scholar]
- Heijnen CJ. Receptor regulation in neuro-endocrine-immune communication: Current knowledge and Future perspectives . Brain, Behavior, and Immunity. 2007 doi: 10.1016/j.bbi.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Hinton PS, Peterson CA, Lo HC, Yang H, McCarthy D, Ney DM. Insulin-like growth factor-I enhances immune response in dexamethasone-treated or surgically stressed rats maintained with total parenteral nutrition. J Paranter Enteral Nutr. 1995;19:444–452. doi: 10.1177/0148607195019006444. [DOI] [PubMed] [Google Scholar]
- Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16:6926–6935. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):53–55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Brief S, Westly HJ, Novakofski J, Bechtel PJ, Simon J, Walker EB. GH3 pituitary adenoma implants can reverse thymic aging. Proc Natl Acad Sci USA. 1986;83:5663–5667. doi: 10.1073/pnas.83.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW. Growth hormone, lymphocytes and macrophages. Biochem Pharmacol. 1989;38:705–713. doi: 10.1016/0006-2952(89)90222-0. [DOI] [PubMed] [Google Scholar]
- Kelley KW. From hormones to immunity: the physiology of immunology. Brain Behav Immun. 2004;18:95–113. doi: 10.1016/j.bbi.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Arkins S, Li YM. Growth hormone, prolactin, and insulin-like growth factors: new jobs for old players. Brain Behav Immun. 1992;6:317–326. [PubMed] [Google Scholar]
- Kelley KW, Dantzer R. Growth hormone and prolactin as natural antagonists of glucocorticoids in immunoregulation. In: Plotnikoff N, Murgo A, Faith R, Wybran J, editors. Stress and Immunity. CRC Press; Boca Raton, Florida: 1991. pp. 433–452. [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kooijman R, Hooghe-Peters EL, Hooghe R. Prolactin, growth hormone and insulin-like growth factor-1 in the immune system. Adv Immunol. 1996;63:377–453. doi: 10.1016/s0065-2776(08)60860-3. [DOI] [PubMed] [Google Scholar]
- Lang CH, Hong-Brown L, Frost RA. Cytokine inhibition of JAK-STAT signaling: a new mechanism of growth hormone resistance. Pediatr Nephrol. 2005;20:306–312. doi: 10.1007/s00467-004-1607-9. [DOI] [PubMed] [Google Scholar]
- Matera L, Cesano A, Bellone G, Oberholtzer E. Modulatory effect of prolactin on the resting and mitogen- induced activity of T, B, and NK lymphocytes. Brain Behav Immun. 1992;6:409–417. doi: 10.1016/0889-1591(92)90039-q. [DOI] [PubMed] [Google Scholar]
- Matera L, Muccioli G, Cesano A, Bellussi G, Genazzani E. Prolactin receptors on large granular lymphocytes: dual regulation by cyclosporin A. Brain Behav Immun. 1988;2:1–10. doi: 10.1016/0889-1591(88)90001-3. [DOI] [PubMed] [Google Scholar]
- McCusker RH, Strle K, Broussard SR, Dantzer R, Bluthé RM, Kelley KW. Crosstalk between insulin-like growth gactors and proinflammatory cytokines In. In: Ader R, editor. Psychoneuroimmunology. Fourth Edition. Elsevier; Amsterdam, The Netherlands: 2006. pp. 171–192. [Google Scholar]
- Murphy WJ, Durum SK, Anver MR, Longo DL. Immunologic and hematologic effects of neuroendocrine hormones. Studies on DW/J dwarf mice. J Immunol. 1992;148:3799–3805. [PubMed] [Google Scholar]
- Murphy WJ, Durum SK, Longo DL. Differential effects of growth hormone and prolactin on murine T cell development and function. J Exp Med. 1993;178:231–236. doi: 10.1084/jem.178.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi H, Suzuki N, Kaneko A, Asai T, Sakane T. Prolactin locally produced by synovium infiltrating T lymphocytes induces excessive synovial cell functions in patients with rheumatoid arthritis. J Rheumatol. 1999;26:1890–1900. [PubMed] [Google Scholar]
- Pace T, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression . Brain, Behavior, and Immunity. 2007 doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeva E, Venkatesh J, Michael D, Diamond B. Prolactin as a modulator of B cell function: implications for SLE. Biomed Pharmacother. 2004;58:310–319. doi: 10.1016/j.biopha.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W, Baroni C, Fabris N, Sorkin E. Hormones and immunological capacity. II. Reconstitution of antibody production in hormonally deficient mice by somatotropic hormone, thyrotropic hormone and thyroxin. J Immunol. 1969;16:217–230. [PMC free article] [PubMed] [Google Scholar]
- Russell DH, Kibler R, Matrisian L, Larson DF, Poulos B, Magun BE. Prolactin receptors on human T and B lymphocytes: antagonism of prolactin binding by cyclosporine. J Immunol. 1985;134:3027–3031. [PubMed] [Google Scholar]
- Sarna S, Sipila I, Ronnholm K, Koistinen R, Holmberg C. Recombinant human growth hormone improves growth in children receiving glucocorticoid treatment after liver transplantation. J Clin Endocrinol Metab. 1996;81:1476–1482. doi: 10.1210/jcem.81.4.8636354. [DOI] [PubMed] [Google Scholar]
- Savino W, Smaniotto S, Binart N, Postel-Vinay MC, Dardenne M. In vivo effects of growth hormone on thymic cells. Ann NY Acad Sci. 2003;992:179–185. doi: 10.1111/j.1749-6632.2003.tb03148.x. [DOI] [PubMed] [Google Scholar]
- Shen WH, Yin Y, Broussard SR, McCusker RH, Freund GG, Dantzer R, Kelley KW. Tumor necrosis factor αinhibits cyclin A expression and retinoblastoma hyperphosphorylation triggered by insulin-like growth factor-I induction of new E2F-1 synthesis. J Biological Chemistry. 2004;279:7438–7446. doi: 10.1074/jbc.M310264200. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- Strle K, Broussard SR, McCusker RH, Shen WH, LeCleir JM, Johnson RW, Freund GG, Dantzer R, Kelley KW. C-jun N-terminal kinase mediates TNFαsuppression of differentiation in myoblasts. Endocrinology. 2006;147:4363–4373. doi: 10.1210/en.2005-1541. [DOI] [PubMed] [Google Scholar]
- Sun LY, Evans MS, Hsieh J, Panici J, Barke A. Increase neurogenesis in dentate gyrus of long-lived Ames dwarf mice. Endocrinology. 2005;146:1138–1144. doi: 10.1210/en.2004-1115. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Glia as the “bad guys”: Implications for improving clinical pain control and the clinical utility of opioids. Brain, Behavior, and Immunity . 2007 doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigent DA. Immunoregulatory properties of growth hormone and prolactin. J Pharmacol Therapeut. 1996;69:237–257. doi: 10.1016/0163-7258(96)00001-0. [DOI] [PubMed] [Google Scholar]
- Weigent DA, Blalock JE. Bidirectional communication between the brainand the immune system. In: Pandi-Perumal SR, Cardinali DP, Chrousos GP, editors. Neuroimmunology of Sleep. Springer; NY: 2007. In press. [Google Scholar]
- Zhu T, Starling-Emerald B, Zhang X, Lee K-O, Gluckman PD, Mertani HC, Lobie PD. Oncogenic transformation of human mammary epithelial cells by autocrine human growth hormone. Cancer Res. 2005;65:317–324. [PubMed] [Google Scholar]