Abstract
Objective
This pilot study investigated whether our previous findings of disrupted normal sexual brain dimorphisms in language-associated regions in schizophrenia were linked with our previously reported sex differences in language dysfunction in schizophrenia.
Method
Nineteen adults with schizophrenia and 15 normal comparisons were tested on phonology, semantics and grammar and underwent structural MRI.
Results
Among males, left hippocampal and left planum temporale (PT) abnormalities were associated with phonological, semantic and grammar deficits, accounting for 17-52% and 27-33%, respectively, of variance in diagnostic group differences. Anterior cingulate gyrus was significantly associated with semantics. Among females, right Heschl’s Gyrus (HG) and left PT were significantly associated with phonology, right HG with semantics and grammar and right hippocampus with semantics.
Conclusions
These preliminary findings suggest disrupted sexual brain dimorphisms in schizophrenia are associated with sex-specific language deficits, and left hippocampal abnormalities, in particular, contribute to language dysfunction among men. Abnormalities in right cortical temporal regions showed stronger associations with language dysfunction among females.
Keywords: language, schizophrenia, sex differences, MRI, morphometry, laterality
INTRODUCTION
Neuroimaging literature provides evidence of normal sex differences in region-specific structural brain volumes (Goldstein et al. 2001; Harasty et al. 1997; Schlaepfer et al. 1995) and function (Baxter et al. 2003; Goldstein et al. 2005; Kansaku et al. 2000; Pugh et al. 1997; Shaywitz et al. 1995) that implicates sex differences in the neuroanatomic organization of language. Specifically, superior temporal gyrus (STG) [including planum temporale (PT)] and inferior frontal gyrus [Broca’s area (BA)] tend to be larger in women than men relative to cerebrum size (Goldstein et al. 2001; Harasty et al. 1997). In some studies, activation is more strongly left lateralized in men particularly in posterior language areas, whereas women show greater right or bilateral representation (Baxter et al. 2003; Goldstein et al. 2005; Pugh et al. 1997). Conversely, a meta-analysis aimed at assessing sex differences in bilateral representation of language in the brain based on functional imaging studies of healthy individuals suggested absence of sexually differentiated language lateralization, in general, with differences possibly on particular tasks (Sommer et al. 2004). Further, during auditory verbal working memory, women showed greater signal intensity changes in bilateral prefrontal regions than men, in whom activations were more likely present in one hemisphere (Goldstein et al. 2005).
Neuroimaging studies examining sex differences in schizophrenia are inconsistent. Some structural findings suggest normal patterns of sexual differentiation may go awry in schizophrenia (Goldstein et al. 2002). Consistent with this, early regional cerebral blood flow functional imaging studies (Gur et al. 1983) evidenced lower language lateralization in female patients versus same-sex healthy comparisons, with no evidence of language lateralization in male patients or healthy comparisons. Other functional neuroimaging studies showed diminished lateralization in all patients versus same-sex healthy comparisons and no sex difference among patients (Sommer et al. 2003). The authors argued that though women overall demonstrated greater language lateralization than men, within-sex findings (patients vs. comparisons) were due to increased right hemisphere language activation whereas left hemisphere language activation was “normal”, accounting for the overall sex differences in lateralization (Sommer et al. 2003). They argued for an absence of gender specificity regarding observed decreases in language lateralization in schizophrenia (Sommer et al 2003) and that the absence of language lateralization among men in earlier studies (Gur et al 1983,1985) may have been due to relatively low signal to noise ratio (Sommer et al 2003). Thus, definitive conclusions in this realm are as yet premature warranting further examination.
We previously demonstrated disrupted normal volumetric sexual brain dimorphisms in language-associated regions in schizophrenia with greater Heschl’s gyrus (HG) abnormalities in men and PT abnormalities in women (Goldstein et al. 2002). Normal PT asymmetry (L>R among both sexes) was disrupted in male patients (smaller right PT, yielding exaggerated leftward asymmetry) and female patients (greater right PT, yielding greater symmetry) relative to healthy same-sex comparisons, with a significant sex by diagnostic group interaction effect (Goldstein et al. 2002). We also showed a more pronounced reduction in anterior (e.g., phonology) and posterior (e.g., semantics) language functions in male than female patients relative to same-sex matched comparisons, with relatively preserved function among female patients despite somewhat worse (albeit mildly so) phonology (Walder et al. 2006).
This pilot study was aimed at investigating whether sex differences in structural brain volume abnormalities were associated with sex differences in language dysfunction in phonology, semantics and grammar in schizophrenia. Hypothesized regions of interest (ROIs) were those that showed disrupted normal sexual dimorphisms in schizophrenia in our previous study (Goldstein et al. 2002) and were associated with language processing in previous research, including right HG, right PT, hippocampus (HIPP), right orbitofrontal cortex (OFC), and anterior cingulate gyrus (ACG). It was hypothesized that variability in diagnostic group differences within-sex across language domains would depend on variability in structural brain volume in language-associated regions, and that this pattern would be sexually differentiated.
MATERIALS AND METHODS
1.1 Subjects
Subjects included a sub-sample of the original cohort ascertained for a study of sex differences in neuropsychological deficits in schizophrenia (Goldstein et al. 1998). They were systematically ascertained and representative of an extensive outpatient treatment system in Boston, which ensured clinical stability upon participation. Of the 31 (17 male, 14 female) schizophrenia patients and 27 (13 male, 14 female) healthy comparisons included in (Goldstein et al. 1998) and (Walder et al. 2006), 34 underwent MRI and comprised the current sub-sample. Comparisons of sociodemographic and clinical characteristics of this sub-sample with the original sample revealed no statistically significant differences. Consensus diagnoses were based on DSM-III-R diagnostic criteria, made by experienced diagnosticians (JMG and LJS) who were unaware of neuropsychological data. Diagnosticians reviewed information obtained from structured research interviews (Schedule for Affective Disorders and Schizophrenia (Spitzer and Endicott 1978)) and systematic record reviews. Normal comparisons received a brief clinical interview and the MMPI-168 to screen for current psychopathology; they were included if T-score was less than or equal to 70 (Vincent et al. 1984).
Patients (SZ; n=19; 11 male/8 female) and normal comparisons (NC; n=15; 6 male/9 female) were proportionately comparable and not significantly different within-sex on age (NC male: 35.8 years ± 6.7, SZ male: 39.5 years ± 6.6; NC female: 42.5 years ± 9.5, SZ female: 41.2 years ± 5.5), handedness (right-handed: NC male: 67%; SZ male: 64%; NC female: 67%; SZ female: 63%), ethnicity (Caucasian: NC male: 90%; SZ male: 82%; NC female: 100%; SZ female: 63%), and parental education level (NC male: 14.8 years ± 3.0; SZ male: 13.1 years ± 1.7; NC female: 13.7 years ± 2.3; SZ female: 13.9 years ± 2.4). Estimated IQ for male patients (92.0 ± 10.3) was significantly lower than for female patients (104.9 ± 15.3; t(17)= -2.20, p=.04) and male comparisons (111.7 ± 5.2, p=.001; t(15)=4.34, p=.0006), which is consistent with our prior reports and which we argued reflected an illness effect (Goldstein et al. 1998). Estimated IQ for female patients (104.9 ± 15.3) was comparable to female comparisons (110.4 ± 12.1; t(15)=0.84, p=.42). There were no other significant sex differences on demographic characteristics. There were also no significant sex differences in medication level assessed as chlorpromazine equivalents (mean= 688; sd (±)= 509), duration of illness (mean= 16 ± 8 years), or number of hospitalizations (mean=6 ± 3). Patients were clinically stable (mild to moderate symptom ratings (.4-2.0) as measured by the SANS and SAPS (Andreasen 1983a; Andreasen 1983b) and by our ratings of cooperation and degree of overt psychotic agitation (Faraone et al. 1995). Subjects were also not hospitalized nor experiencing a current psychotic episode, also as reflected in mild to moderate symptomatology. Thus, sociodemographic and clinical characteristics of this subsample were similar to the original sample described in (Goldstein et al. 1998) and (Walder et al. 2006). Given that MRIs were conducted at a different time than neuropsychological assessments, brief symptom evaluations were conducted to confirm the absence of change in clinical status since the prior assessment. Written informed consent was obtained following a full explanation of study procedures. The study was approved by Human Studies Committees at Harvard Medical School and Massachusetts Department of Mental Health, Massachusetts Mental Health Center.
1.1.1 Procedures
An extensive language battery including measures of phonology (production and processing of individual speech sounds), semantics (meaning of words) and grammar (language structure) was administered in the context of a comprehensive neuropsychological battery (see (Goldstein et al. 1998; Walder et al. 2006) for measures comprising each language composite). The Phonology composite included: Roeltgen′s Nonwords Reading and Spelling (Roeltgen 1992), Auditory Blending, Rapid Automatized Naming (Katz et al. 1992), Wide Range Achievement Test-Revised (WRAT-R) single oral word Reading (Jastak and Wilkinson 1984), and Controlled Oral Word Association Test (Benton and Hamsher 1989). Semantics included Vocabulary and Similarities subtests of WAIS-R (Wechsler 1981), Boston Naming Test (Kaplan et al. 1983) and COWAT-Animals (Benton and Hamsher 1989). Grammar consisted of Syntactic Comprehension (Caplan and Hildebrant 1992), in which subjects identify the subject and object of actions based on varied placement of prepositional phrases. Internal consistency reliabilities were excellent (.72 to .81) (Walder et al. 2006).
MR images were acquired at the Athinoula Martinos Center for Biomedical Engineering at Massachusetts General Hospital (MGH), Boston, with a 1.5-Tesla scanner (Signa; General Electric Co, Milwaukee, WI). Contiguous 3.1-mm coronal spoiled gradient echo images of the entire brain were obtained (see (Goldstein et al. 2002) for parameters, segmentation and parcellation procedures). All images were processed and analyzed at the MGH Center for Morphometric Analysis as in our previous work with these subjects (Goldstein et al. 2001; Goldstein et al. 2002). These methods are based on a semi-automated system developed from an anatomically-based perspective (Caviness et al. 1995; Filipek et al. 1989; Rademacher et al. 1992). Very good interrater and intrarater reliabilities of cortical and subcortical regions were previously established (Caviness et al. 1996; Goldstein et al. 1999; Seidman et al. 1999). Concurrent, discriminant, and predictive validity of the volumetric measurements of our brain regions of interest in relation to illness factors and outcomes have been demonstrated in multiple previous studies (Caplan et al. 1995; Filipek et al. 1994; Goldstein et al. 1999; Rauch et al. 2000; Seidman et al. 1999; Vaina et al. 1998). Consistent with methods used in other imaging studies (Filipek et al. 1994; Goldstein et al. 2001; Goldstein et al. 2002), brain volumes were adjusted for cerebrum size given men tend to have larger cerebrums than women.
1.1.2 Data Analyses
Data analyses were aimed at assessing: 1) whether variability in diagnostic group differences across language domains among males (Walder et al. 2006) depended on structural brain volume abnormalities in language-associated regions; and 2) whether these same brain regions related to language function variability in females, even though females did not significantly differ from healthy counterparts across domains (Walder et al. 2006). General linear models (GLM) assessed which a priori ROIs (controlled for age) were associated with each language domain. Among males, stepwise regression analyses entered group effect first followed by ROIs, to test whether each ROI accounted for variability in the group effect that demonstrated poorer performance among male patients versus healthy males (Walder et al. 2006). That is, the regression coefficient (beta) for the group effect alone in males on the three language domains, controlled for age, was compared to the beta for the group effect in males on each language domain controlled for the targeted ROI and age. A reduction in beta for the group effect alone compared to beta controlled for the ROI would demonstrate an association between volumetric abnormalities and language dysfunction in men. The ROI could be interpreted as accounting for variance due to diagnostic group differences on that language domain. Given prior findings suggesting laterality effects in language-related brain regions, GLMs included left and right PT, HG, and HIPP. GLMs were repeated among females alone to examine which ROIs were associated with language performance. Given small sample size in this pilot study, interaction effects across sexes were not tested. Asymmetries were tested using a standard formula of [2(L-R) / L+R] (Geschwind and Galaburda 1985). Thus, a positive value represented greater left-sided volume; a negative value, greater right-sided volume; and around 0, symmetry.
RESULTS
An examination of the mean volumetric differences between patients and controls within gender resulted in similar directions of the effects reported in (Goldstein et al. 2002). As with the original sample (volume data that reflect stable estimates given the larger sample size), the subsample of male patients in this study had a smaller hippocampus, particularly on the left, Heschl’s gyrus, and PT and increased right orbitofrontal cortex compared with normal control men. Female patients in this study had smaller hippocampi, particularly on the left, cingulate gyrus, and larger PT volume. GLMs among males (patients versus normal comparisons) showed total HIPP was significantly associated with phonology (t=2.88, p=.01) and semantics (t=2.56, p=.02), particularly left HIPP [i.e., phonology (t=2.91, p=.01), semantics (t=2.59, p=.02) and grammar at (t=1.93, p<.08)]. Total ACG was significantly associated with semantics (t=2.17, p<.05) and at trend level with grammar (t=2.14, p<.06). Right OFC was not significantly associated with phonology (t = -.54, ns), semantics (t = -.82, ns), nor grammar (t = -1.14, ns).
Left (but not right) PT was significantly associated with phonology (t=2.44, p<.03), and at trend level with semantics (t=1.995, p<.07) and grammar (t=2.11, p<.06). There were no significant asymmetry differences between male patients (mean asymmetry = .23 ± .23) and male controls (.32 ± .25). All males showed larger left than right PT. PT asymmetry among male patients showed moderate correlations with semantics (Spearman correlation = .47) and grammar (r=.47) and low correlation with phonology (r=.15). Comparisons of these correlations between male patients and healthy males using Fisher’s z, showed a significant difference regarding semantics between healthy males (r = -.37) and male patients (r=.47); Fishers z = 1.97, p < .05.
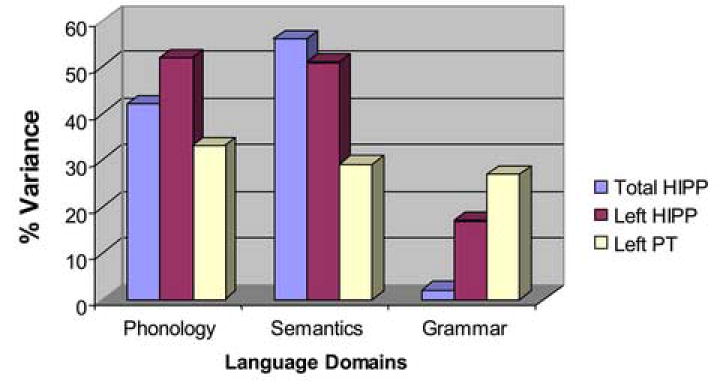
Among males alone, variability in hippocampal volume accounted for a substantial percent of variance (approximately 42% for total HIPP and 52% for left HIPP) in the group effect on phonology alone compared with the group effect controlled for HIPP, respectively (although not significantly different betas given the small sample size) [b=.76 (s.e.=.37) vs. b=.44 (s.e.=.36), 95% confidence interval (CI): (-0.1828, 0.8178)]; [for left HIPP: b=.36 (s.e.=.39), 95% CI: (-0.1752, 0.9727)]. Variability in hippocampal volume also accounted for a substantial percent of variance (approximately 56% for total HIPP; 51% for left HIPP) in the group effect on semantic processing comparing the group effect alone with group effect controlled for HIPP, respectively [b=.92 (s.e.=.49) vs. b=.55 (s.e.=.49), 95% CI: (-0.2511, 1.0066)]; [for left HIPP: b=.45 (s.e.=.53), 95% CI: (-0.2852, 1.2335)]. Left HIPP accounted for approximately 17% of variance in grammar comparing the group effect alone with group effect controlled for left HIPP [b=1.12 (s.e.=.54) vs. b=.93 (s.e.=.51), respectively, 95% CI: (-0.2549, 0.6342)]. Left PT accounted for approximately 33%, 29% and 27% of variance in phonology, semantics and grammar, respectively, comparing the group effect alone with group controlled for left PT [respectively, for phonology, group b=.76 (s.e.=.37) vs. b=.51 (s.e.=.37), 95% CI: (-0.1003, 0.6075); for semantics, group b=.92 (s.e.=.49) vs. b=.65 (s.e.=.51), 95% CI: (-0.2213, 0.7633); for grammar, group b=1.118 (s.e.=.54) vs. b=.82 (s.e.=.55), 95% CI: (-0.2238, 0.8299)].
GLMs among females (patients versus normal comparisons) showed that right HG (t=2.75, p<.02) and left PT (t=2.28, p<.04) were positively and significantly associated with phonology. Tests of asymmetries of HG and PT were not significant, although female patients (.02 ± .21) and healthy females (.16 ± 21) showed more symmetric PT volumes than the males patients and healthy males. Right HG was significantly associated with semantics (t=2.36, p=.03) and at trend level with grammar (t=1.98, t<.07). Larger right HIPP was significantly associated with poorer semantics among females (t=-2.41, p=.03). No ROIs were significantly associated with grammar.
DISCUSSION
Findings in this pilot study suggest disrupted normal patterns of sexual brain dimorphisms in schizophrenia were significantly associated with sexually divergent functional language deficits in schizophrenia. The relative salience of hippocampal (versus other a priori ROIs) contribution to diagnostic group differences across all language domains in males suggests a substantial role for hippocampal abnormalities in language dysfunction in males even for domains not necessarily highly associated with the hippocampus, such as phonology. In contrast, among females (patients versus normal comparisons), greater reliance on temporal cortical regions, particularly right HG and left PT, suggests relative prominence of cortical involvement in language among women with schizophrenia. This, together with a more prominent left (vs. right) hemisphere (i.e., PT, HG, HIPP) contribution across domains in males, is consistent with research implicating sexually differentiated neuroanatomic organization of language (Baxter et al. 2003; Goldstein et al. 2005; Goldstein et al. 2001; Harasty et al. 1997; Kansaku et al. 2000; Pugh et al. 1997; Schlaepfer et al. 1995; Shaywitz et al. 1995). In fact, the positive association of PT asymmetry with semantics and grammar among male patients versus an inverse association of PT asymmetry with semantics in male controls also suggests a possible laterality effect on language processing among males. Specifically, there may be a laterality effect whereby females may recruit more right hemisphere function relative to predominant reliance on left hemisphere for language processing among men. However, larger right hippocampal volume associated with poorer semantic processing among females suggests laterality abnormalities require future investigation. Findings point to the possibility of: 1) a differential reliance on cortical versus subcortical structures in aspects of language processing in male patients versus healthy comparisons; and 2) an important relationship between our previous findings in schizophrenia of disrupted normal sexual brain dimorphisms in language-related regions (Goldstein et al. 2002) and respective sex differences in language dysfunction (Walder et al. 2006).
The small sample size in this pilot study yielded limited statistical power preventing adjustment for multiple testing and direct assessment for interaction effects (sex by diagnostic group). As such, findings need to be interpreted with caution. Replication using larger samples is essential to better characterize these preliminary structure-function relationships, directly examine potential interaction and laterality effects, and validate these findings. Our findings extend previous work by suggesting a direct relationship between structural brain volume abnormalities and specific language dysfunctions, particularly left hemisphere among men with schizophrenia. This is consistent with research underscoring the role of left hippocampal abnormalities in schizophrenia among men (Shenton et al. 1992). Future studies may test for a more prominent role of left hippocampal abnormalities in language deficits in men with schizophrenia compared with women, whereas relative weaknesses in language processing among women may be more highly related to cortical temporal abnormalities in language-associated regions. An understanding of sex differences in structural brain laterality effects in schizophrenia may provide insights into the relative preservation of language functioning in women compared with men found in our (Walder et al. 2006) and others’ work. This may suggest etiological differences between the sexes with potential clinical implications, such as sex-specific rehabilitative strategies.
Figure 1. Percent Variance Accounted for by ROIs Between Male Patients and Same-Sex Comparisons on Language Functions.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health, SDA K21 MH00096 (JMG; ’92-94; for data collection) and RO1 MH56956 (JMG) which was, in part, supported by the N.I.H. Office of Research on Women’s Health. DJW’s time was supported by a National Institute of Mental Health postdoctoral fellowship in the Clinical Research Training Program in Biological Psychiatry, Department of Psychiatry, Harvard Medical School (T32 MH16259). We would like to thank Sara Cherkerzian, Sc.D. for help with some of the data analyses and Valerie Thompson for manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983a. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City : IA University of Iowa; 1983b. [Google Scholar]
- Baxter LC, Saykin AJ, Flashman LA, Johnson SC, Guerin SJ, Babcock DR, Wishart HA. Sex differences in semantic language processing: a functional MRI study. Brain and Language. 2003;84(2):264–72. doi: 10.1016/s0093-934x(02)00549-7. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City, Iowa: AJA Associates; 1989. [Google Scholar]
- Caplan D, Gow D, Makris N. Analysis of lesions by MRI in stroke patients with acoustic-phonetic processing deficits. Neurology. 1995;45:293–298. doi: 10.1212/wnl.45.2.293. [DOI] [PubMed] [Google Scholar]
- Caplan D, Hildebrant N. Modified Battery for the Assessment of Syntactic ComprehensionBoston. Massachusetts General Hospital, Department of Neurology, Neuropsychology Laborato: 1992. [Google Scholar]
- Caviness VS, Jr, Kennedy DN, Makris N, Bates J. Advanced application of magnetic resonance imaging in human brain science. Brain Dev. 1995;17(6):399–408. doi: 10.1016/0387-7604(95)00090-9. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Meyer J, Makris N, Kennedy DN. MRI-based topographic parcellation of human neocortex: an anatomically specified method with estimate of reliability. Journal of Cognitive Neuroscience. 1996;8:566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Lyons MJ, Tsuang MT. Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a diagnostic efficiency analysis. Journal of Abnormal Psychology. 1995;104:286–304. doi: 10.1037//0021-843x.104.2.286. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Kennedy DN, Caviness VSJ, Rossnick SL, Spraggins TA, Starewicz PM. Magnetic resonance imaging-based Brain morphometry: Development and application to normal subjects. Annals of Neurology. 1989;25(1):61–67. doi: 10.1002/ana.410250110. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric analysis. Cerebral Cortex. 1994;4(4):344–60. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda A. Cerebral lateralization biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Archives of Neurology. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Goodman JM, Seidman LJ, Kennedy D, Makris N, Lee H, Tourville J, Caviness VS, Faraone SV, Tsuang MT. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Archives of General Psychiatry. 1999;56(6):537–47. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack RA, Anagnoson R, Breiter HC, Makris N, Goodman JM, Tsuang MT, Seidman LJ. Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology. 2005;19(4):509–519. doi: 10.1037/0894-4105.19.4.509. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Goodman JM, Koren D, Lee H, Weintraub S, Tsuang MT. Are there sex differences in neuropsychological functions among patients with schizophrenia? American Journal of Psychiatry. 1998;155(10):1358–1364. doi: 10.1176/ajp.155.10.1358. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in-vivo magnetic resonance imaging. Cerebral Cortex. 2001;11(6):490–7. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O′Brien LM, Horton NJ, Kennedy DN, Makris N, Caviness VS, Jr, Faraone SV, Tsuang MT. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Archives of General Psychiatry. 2002;59(2):154–64. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Gur RE, Skolnick BE, Gur RC, al e. Brain function in schizophrenic disorders. I. Regional blood flow in medicated schizophrenics. Archives of General Psychiatry. 1983;40:1250–1254. doi: 10.1001/archpsyc.1983.01790100096013. [DOI] [PubMed] [Google Scholar]
- Harasty J, Double KL, Halliday GM, Kril JJ, McRitchie DA. Language-associated cortical regions are proportionally larger in the female brain. Archives of Neurology. 1997;54(2):171–6. doi: 10.1001/archneur.1997.00550140045011. [DOI] [PubMed] [Google Scholar]
- Jastak S, Wilkinson GS. Administration manual . Wilmington, DL: Jastak Associates; 1984. Wide Range Achievement Test - Revised. [Google Scholar]
- Kansaku K, Yamaura A, Kitazawa S. Sex differences in lateralization revealed in the posterior language areas. Cereb Cortex. 2000;10(9):866–72. doi: 10.1093/cercor/10.9.866. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weindtraub S. The Boston Naming Test. 1983 [Google Scholar]
- Katz WF, Curtiss S, Tallal P. Rapid automatized naming and gesture by normal and language-impaired children. Brain Lang. 1992;43(4):623–41. doi: 10.1016/0093-934x(92)90087-u. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Shankweiler DP, Katz L, Fletcher JM, Skudlarski P, Fulbright RK, Constable RT, Bronen RA, Lacadie C, Gore JC. Predicting reading performance from neuroimaging profiles: the cerebral basis of phonological effects in printed word identification. Journal of Experimental Psychology: Human Perception and Performance. 1997;23(2):299–318. doi: 10.1037//0096-1523.23.2.299. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Galaburda AM, Kennedy DN, Filipek PA, Caviness VS. Human cerebral cortex: localization, parcellation, and morphometry with magnetic resonance imaging. Journal of Cognitive Neuroscience. 1992;4:352–374. doi: 10.1162/jocn.1992.4.4.352. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Kim H, Makris N, Cosgrove GR, Cassem EH, Savage CR, Price BH, Nierenberg AA, Shera D, Baer L, Buchbinder B, Caviness VS, Jenike MA, Kennedy DN. Volume reduction in the caudate nucleus following stereotactic placement of lesions in the anterior cingulate cortex in humans: a morphometric magnetic resonance imaging study. J Neurosurg. 2000;93:1019–1025. doi: 10.3171/jns.2000.93.6.1019. [DOI] [PubMed] [Google Scholar]
- Roeltgen DP. Phonological error analysis, development and empirical evaluation. Brain Lang. 1992;43(2):190–229. doi: 10.1016/0093-934x(92)90128-2. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Harris GJ, Tien AY, Peng L, Lee S, Pearlson GD. Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance imaging study. Psychiatry Research: Neuroimaging. 1995;61(3):129–35. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Toomey R, Tourville J, Kennedy D, Makris N, Caviness VS, Tsuang MT. Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biological Psychiatry. 1999;46:941–954. doi: 10.1016/s0006-3223(99)00075-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz B, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. New England Journal of Medicine. 1992;327(8):604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Aleman A, Bouma A, Kahn RS. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127(Pt 8):1845–52. doi: 10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Mandl RC, Kahn RS. Language lateralization in female patients with schizophrenia: an fMRI study. Schizophr Res. 2003;60(23):183–90. doi: 10.1016/s0920-9964(02)00300-6. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Endicott J. Schedule for Affective Disorders and Schizophrenia (SADS) New York: Biometrics Research Dept., New York State Psychiatric Institute; 1978. [Google Scholar]
- Vaina L, Makris N, Kennedy D, Cowey A. The selective impairment of the perception of first-order motion by unilateral cortical brian damage. Visual Neuroscience. 1998;15:333–348. doi: 10.1017/s0952523898152082. [DOI] [PubMed] [Google Scholar]
- Vincent KR, Castillo IM, Hauser RI, Zapata JA, Stuart HJ, Cohn CK, O′Shanick GJ. MMPI - 168 Codebook . Norwood, NJ: Ablex Publishing Corporation; 1984. [Google Scholar]
- Walder D, Seidman LJ, Cullen N, Su J, Tsuang MT, Goldstein JM. Sex differences in language dysfunction in schizophrenia. Am J Psychiatry. 2006;163:470–477. doi: 10.1176/appi.ajp.163.3.470. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale . Revised San Antonio TX: Psychological Corporation; 1981. [Google Scholar]


