Abstract
Flight dispersal of Triatoma infestans Klug is probably the most important mechanism for house reinfestation at a village scale after residual spraying with insecticides. The aim of the current study was to estimate the flight initiation probability of field-collected T. infestans and to assess how this probability was affected by sex, adult age, partial bloodmeal, and the presence of a host inaccessible for feeding. Four experimental series, each consisting of three to six consecutive nights and repeated measurements of flight initiation on each individually marked bug, were carried out in experimental huts inside closed cages under natural climatic conditions. We demonstrate that flight initiation probability of T. infestans is much higher than previously reported, responds to temperature in a sigmoid manner, and is higher in females than males, and that the frequency distribution of the number of flights per individual is highly aggregated in female and male bugs. The age of adults had strong effects on flight initiation, whereas the presence of an inaccessible host and a partial bloodmeal exerted no significant effects in models controlling for the effects of bug weight-to-length ratio. The high flight potential found is consistent with the rapid changes in reinfestation patterns observed in the field. The present estimates of flight probabilities and the identification of factors modifying them provide essential knowledge for modeling reinfestation patterns and for improving control strategies of T. infestans.
Keywords: flight initiation, Triatominae, Chagas disease, reinfestation
THEORETICAL AND EMPIRICAL DATA suggest that flight dispersal by Triatoma infestans Klug, the main vector of Chagas disease in South America, is the most important mechanism for reinfestation of houses at a village scale after insecticide spraying (Schofield and Matthews 1985, Cecere et al. 2004, Vazquez-Prokopec et al. 2004, unpublished data; Ceballos et al. 2005). Yet a key reference source disregards flight dispersal in favor of passive transport by humans as a major driver of local reinfestation (WHO 2002). Such misconception probably arose from the typical nocturnal activity and sporadically observed flights of T. infestans. T. infestans may easily fly >550 m (Schofield et al. 1992) or reach 1,500 m (Schweigmann et al. 1988) in an open field, and it may sustain tethered flights for >20 min at speeds of 2 m/s (Ward and Baker 1982).
Flight initiation is associated with low nutritional status (estimated by weight-to-length ratio, W/L), high temperatures, and low wind speed (Lehane and Schofield 1982, Lehane et al. 1992, Vazquez-Prokopec et al. 2004, unpublished data). Flight initiation is positively associated with adult age for up to 40-60 d and then falls to lower constant levels (Lehane and Schofield 1982). In laboratory experiments, Williams and Schofield (1985) reported higher proportions of female T. infestans flying, whereas Lehane et al. (1992) found no significant differences between sexes. Although triatomine bugs are known to be attracted by host odors and radiant heat (Lazzari and Núñez 1989, Taneja and Guerin 1995), there are no published reports on the effects of these attractants or a live host on flight decision. Previous evidence suggests that sex would have negligible effects on flight initiation, whereas the presence of an inaccessible host and a partial bloodmeal would exert negative effects, and age would increase flight probability during the first 5 wk after adult emergence.
Decision-making processes, including those involving the triggering of complex motor displays, depend on the integration of information provided by different senses as well as the general motivational level of the animal (McFarland 1971). Addressing these behaviors requires analyzing responses under conditions that mimic natural situations as close as possible (Lorenz 1959). However, previous studies on T. infestans flight behavior were conducted under artificial test conditions and usually with triatomines reared in the laboratory for many generations (Lehane and Schofield 1981, 1982; Lehane et al. 1992; Schofield et al. 1992; McEwen et al. 1993). The behavior of laboratory bugs and natural populations may differ significantly, especially because size, weight, shape, and antennal sensilla have been shown to vary between them (Jaramillo et al. 2002, Catalá et al. 2004, Vazquez-Prokopec et al. 2004).
The aim of the current study was to assess experimentally the flight initiation probabilities of field-collected T. infestans under natural climatic conditions and how these probabilities were associated with sex, adult age, a recent partial bloodmeal, and the presence of a host inaccessible for feeding, while controlling for W/L ratio effects. Individual behavioral variability also was assessed by means of a repeated measures design.
Materials and Methods
Field Site
The experiments were carried out at the National Vector Control Coordination (NVCC) field station located in Santa María de Punilla (31° 14′ S, 64° 28′ W), Province of Córdoba, Argentina, which lies within the distribution range of T. infestans and has been used for investigations on bug population dynamics under natural climatic conditions (Cecere et al. 2003). The annual mean temperature is ≈15°C, with absolute maxima reaching 40°C in summer. Annual rainfall ranges between 500 and 600 mm from November to March.
Insects
For a pilot assay, adult T. infestans from a 2-yr-old colony kept at the insectary of the NVCC for three to four generations were used. This colony originated from wild-caught bugs from the Department of Cruz del Eje, Province of Córdoba, and was regularly fed on chickens. The adults used were 5-20 d old and were not fed after the final molt.
For the experiments, 360 adults and ≈500 fourth and fifth instars of T. infestans were collected in February 2004 from peridomestic sites in neighboring villages (Km 34, Km 40, Invernada Norte and La Loma) located in the Department of Figueroa (27° 23′ S, 63° 29′ W), Province of Santiago del Estero, Argentina. Bugs were collected by searches in chicken nests, kitchens, and storerooms and then transported to the field station in plastic jars with filter paper.
Experimental Design
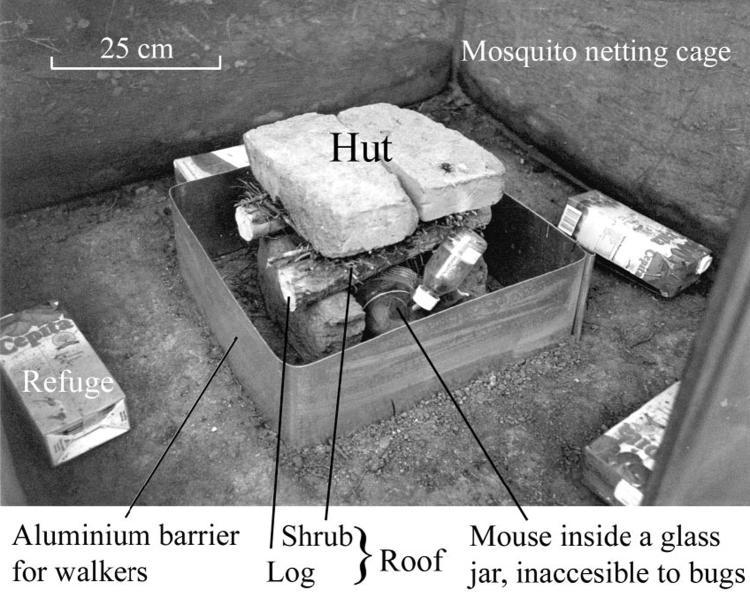
Each experimental device (Fig. 1) consisted of a small hut (30 cm in width, 25 cm in height) inside a cage made of plastic mosquito netting mounted on a metal frame, and covered with additional mosquito netting for further protection. Each hut had a 30-cm-wide, 5-cm-thick roof of shrubs (Larrea sp.) positioned on two logs of blackwood, Acacia melanoxylon R. Br. ex Ait. f., and tied with wire. The roof rested on two mud bricks (the hut walls) and was covered by a 25 by 25cm square piece of black nylon and two mud bricks. A surrounding barrier was 5 cm apart from the hut and consisted of a 15-cm-high aluminum plate that could not be climbed by the bugs. In the inner side of the cage roof and 15 cm away from the walls, another 7-cm-high aluminum plate prevented fliers from walking over the hut and falling back into it. Four pleated corrugated cardboard refuges (23 by 10 by 6 cm) on the floor close to the cage walls and another four refuges (40 by 5 by 5 cm) on the top corners provided shelter for fliers. A data logger (HOBO, Onset Co., Bourne, MA) placed inside each hut registered temperature (precision ± 0.7°C) and relative humidity (precision ± 5%) every 15 min. The cages were in a row, almost 3 m apart, and 10-20 m away from a chicken house and an inhabited house.
Fig.1.
Experimental device for registering flight initiation of T. infestans under natural climatic conditions.
The pilot assay was conducted to test whether the experimental device was effective for registering T. infestans flight initiation and to assess the effect of marking on flight initiation. About half of the bugs were marked on the pronotum with two dots of acrylic paint (Liquitex and Alba, Buenos Aires, Argentina), which exerted no adverse effects on the survival of T. infestans (Cecere et al. 2003). A group of T. infestans was released in each of two huts at 1300 hours and recovered the next morning at 1000 hours. Bugs recovered beyond the aluminum barrier were considered to have initiated flight. In total, 48 males and 42 females were released on 21 January and 70 males and 64 females on 22 January. An infrared videocamera (900 nm) placed in one of the top corners of a cage recorded bug activity in the exterior of the hut and within 20 cm around the aluminum barrier on the floor, from ≈2000 to 0400 hours. All the bugs that left the hut were observed to do so flying and none of them returned, thus demonstrating the effectiveness of the experimental hut design for present purposes. No significant effects on flight initiation of the acrylic paint mark were found (data not shown).
Four experimental series (A-D) were conducted (Table 1). Each series consisted of four groups of 31-41 adult T. infestans with a sex ratio of two males per female, as in the original field collection. Bugs were individually identified with two dots of acrylic paint (six colors) in two of five different positions on the pronotum. Two of the four huts harbored a male mouse kept in a glass jar closed with metal mosquito netting to prevent the bugs from reaching the host. A group of bugs was released in each of the huts at ≈2000hours. For the next two nights, a mosquito net held to the aluminum barrier prevented bugs from leaving the hut either by walking or flying, allowing the bugs to adapt to the new environment. After the two confinement nights, the mosquito net was removed and during the next three to six measurement nights (depending on the series; Table 1), bugs were allowed to fly out of the hut. After each measurement night, the color mark of the bugs found beyond the aluminum fence was registered early in the morning. The bugs were immediately returned to the hut, except after the last night of each series when all bugs were recovered. In series D, most parts of the huts were affected by a heavy rain on the second confinement night; some of the wet mud bricks were replaced by dry bricks after the second night of measurement.
Table 1.
Details of the four experimental series carried out for registering flight initiation of field-collected T. infestans
| Series | No. of bugs | Release-recovery date | Nights of measurement | Maximum daily temp | Daily temp at sunset | Bug characteristics |
|---|---|---|---|---|---|---|
| A | 156 | 15-22 Feb. | 5 | 16-25 | 19-23 | Adults used 5 d after field collection and kept unfed since then |
| B | 142 | 22-27 Feb. | 3 | 28-34 | 26-30 | As in series A, used 10 d after collection |
| C | 158 | 24-29 Mar. | 3 | 30-31 | 26-30 | Recruited from field-collected fifth instars; used 1 mo after being fed to repletion on chickens; adult age of each bug known with a 1-wk precision |
| D | 131 | 29 Mar.-6 April | 6 | 21-22 (first and second night) | 19-20 (first and second night) | Previously used in series A; fully engorged 15 d after collection; after 1.5 mo of starvation, onethird of females and males were again allowed to feed partially on chickens for ≈5 min |
| 25-28 (third-sixth night) | 23-25 (third -sixth night) |
Temperatures express the range observed for the measurement nights in each series.
All recovered bugs from series B-D were individually weighed on an electronic balance (Shimadzu Libror, AEG-220, Duisburg, Germany) (precision ± 0.1 mg) and measured from clypeus to abdominal tip with a hand-held vernier caliper (precision ± 0.05 mm). The W/L ratio was assumed approximately constant during the measurement nights because several days had passed since the last bloodmeal (Lehane and Schofield 1982). Mortality was 1.4% (2/144) in series B, 0% in series C, and 1.5% (2/133) in series D. In series A, bug nutritional status was only estimated qualitatively (Montenegro 1983, Ceballos et al. 2005), and mortality was not recorded.
Data Analysis
Repeated measures multiple logistic regression was used within each series to assess the effects on the binary response variable (bug flying on a given night) of sex, W/L ratio (continuous variable), a partial bloodmeal (categorical variable, series D), adult age (categorical variable with four levels, series C), and the presence of a mouse not accessible for feeding (categorical variable, series B-D). All replicates within a series were pooled for analysis because no significant effect of each particular setup was found. Unless stated otherwise and because of the low temperatures registered in series A and during the first two nights of series D (Table 1), averages and regressions were calculated only for all three measurement nights of series B and C and the last four measurement nights of series D. Generalized estimating equations method (GEE) was used to estimate and control for the correlation between repeated measures (Liang and Zeger 1986). The group variable was the individual bug and the time variable was the measurement night. An unstructured correlation matrix and the robust variance estimator were used. Differences in the proportions of flight initiation between the pilot assay and series B-D were tested for using repeated measures multiple logistic regression as stated above; the independent variables were sex and the specific series (the pilot assay being the reference category). To test whether flight initiation probabilities differed among individual bugs of the same sex, the observed frequency distribution of the number of nights that each bug flew was compared with the expected distribution under the null hypothesis that the probability was the same for all nights and bugs. The difference in the flight proportions between nights for each series and sex was evaluated using a chi-square test. All analyses were run on Stata 7.0 (StataCorp 1999).
Results
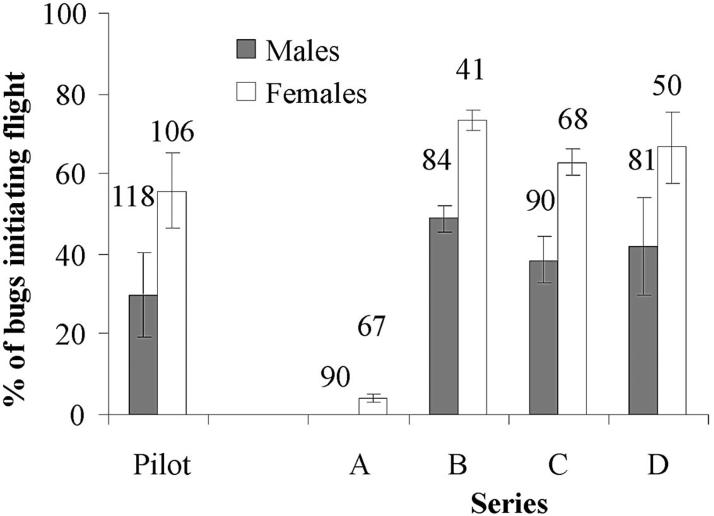
In the pilot assay with insectary bugs, an average of 30% of male and 56% of female T. infestans initiated flight each night (Fig. 2). For series B-D, an average of 43% (range 25-52%) of 255 male and 67% (range 54-76%) of 159 female bugs collected in the field initiated flight each night. Female bugs were consistently more likely to initiate flight than males in all nights and series. Logistic regression analysis showed that the bugs from the pilot assay flew significantly less frequently than the bugs from series B (P < 0.001) and D(P = 0.01) and marginally less frequently than bugs from series C (P = 0.07).
Fig.2.
Sex-specific average percentage of T. infestans that initiated flight in the pilot assay and the four experimental series. The numbers above the bars indicate the total number of bugs tested in each series. Lines indicate ± 1 SD.
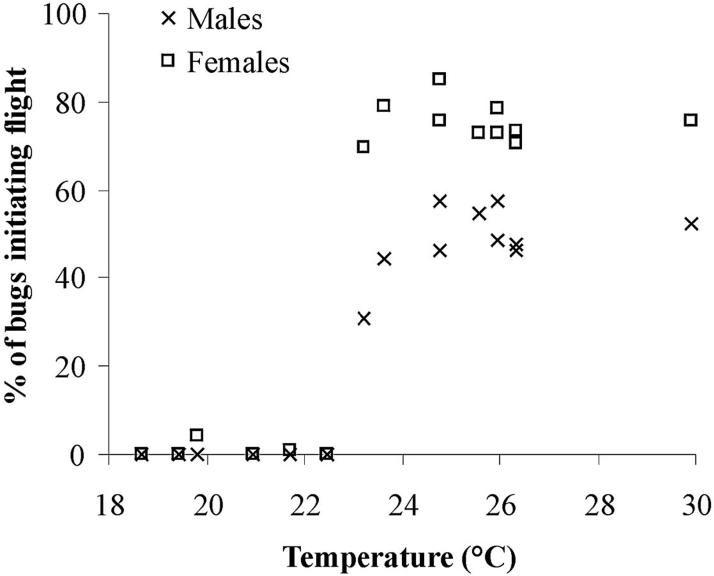
Flight initiation was practically absent up to 23° C at sunset (2000 hours) (series A and the first two nights of measurement of series D; Table 1), increased sharply above 23°C and remained fairly constant with increasing temperatures above 23°C (Fig. 3). This suggests an upper limit or asymptote of ≈50% for males and 75% for females. In the pilot assay, the temperature at sunset was 24.5°C on both nights. Wind speed was always negligible.
Fig.3.
Flight initiation of field-collected T. infestans according to the temperature at sunset for every measurement night of all experimental series (A-D). The values shown correspond to relatively comparable bugs: all bugs from series A and B, bugs ≥4 wk old from series C, and unfed bugs from series D.
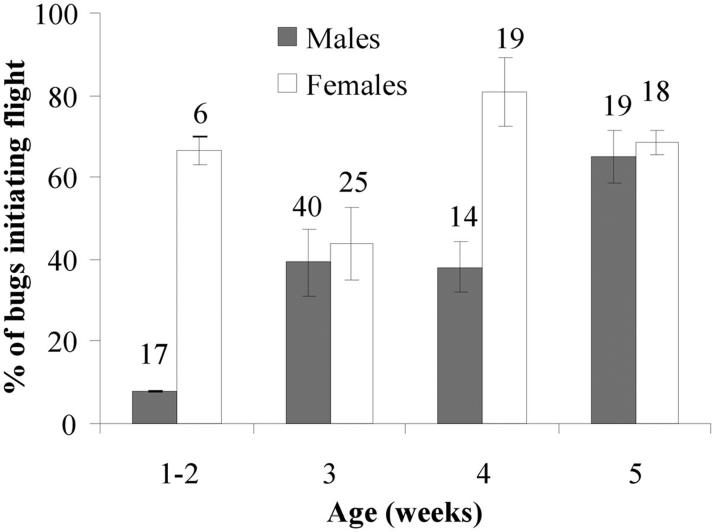
Table 2 presents the multiple regression analysis assessing the effects of different factors on flight initiation. Female bugs were consistently more likely to initiate flight than males in series B-D (Table 2) and the pilot assay (P < 0.01). In series C, the significant interaction between sex and age was consistent with the main effects observed in series B and D, with females showing a higher flight initiation probability than males (Fig. 4). Therefore, age was analyzed for each sex separately while controlling for W/L effects. Taking the most abundant age group (i.e., 3-wk-old bugs) as the reference class, 1- and 2-wk-old males initiated flight significantly less frequently than 3-wk-old males (OR, 0.16; 95% CI, 0.03-0.78), and these did not initiate flight significantly more often than 4-(OR, 1.15; 95% CI, 0.38-3.41) and 5-wk-old males (OR, 1.95; 95% CI, 0.73-8.26). Only 4-wk-old females had a flight initiation proportion significantly different from 3-wk-old females (OR, 6.89; 95% CI, 2.27-20.92). The presence of an inaccessible host exerted no significant effects on flight initiation in any series (Table 2).
Table 2.
Repeated measures logistic regression analyses of factors associated with flight initiation of field-collected T. infestans under
| Series | Independent variable | Odds ratio ± SE | P (z) | 95% CI | P (Wald) |
|---|---|---|---|---|---|
| B | Sex | 0.33 ± 0.11 | 0.001 | 0.17-0.64 | |
| W/L | 0.91 ± 0.11 | 0.47 | 0.72-1.17 | ||
| Host | 1.41 ± 0.44 | 0.27 | 0.77-2.58 | 0.007 | |
| C | Sex | 0.85 ± 0.37 | 0.72 | 0.36-2.01 | |
| W/L | 0.48 ± 0.08 | <0.001 | 0.35-0.65 | ||
| Age 1-2 wk | 0.16 ± 0.14 | 0.32 | 0.03-0.86 | ||
| Age 4 wk | 1.16 ± 0.64 | 0.79 | 0.39-3.45 | ||
| Age 5 wk | 1.98 ± 1.04 | 0.20 | 0.70-5.55 | ||
| Sex × age 1-2 wk | 13.2 ± 14.6 | 0.02 | 1.46-116 | ||
| Sex × age 4 wk | 5.96 ± 4.40 | 0.02 | 1.40-25.4 | ||
| Sex × age 5 wk | 0.99 ± 0.78 | 0.99 | 0.21-1.64 | ||
| Host | 0.98 ± 0.18 | 0.93 | 0.69-1.41 | <0.001 | |
| D (all bugs) | Sex | 0.38 ± 0.11 | 0.001 | 0.22-0.67 | |
| W/L | 0.82 ± 0.12 | 0.17 | 0.62-1.09 | ||
| Partial meal | 0.62 ± 0.23 | 0.20 | 0.30-1.28 | ||
| Host | 1.29 ± 0.35 | 0.35 | 0.76-2.19 | <0.001 | |
| D (W/L 6-9) | Sex | 0.34 ± 0.11 | 0.001 | 0.18-0.55 | |
| W/L | 1.10 ± 0.26 | 0.69 | 0.59-1.75 | ||
| Partial meal | 0.60 ± 0.24 | 0.19 | 0.28-1.29 | 0.012 |
P(z), significance of odds ratio; P(Wald), model significance; and W/L, weight-to-length ratio.
Fig.4.
Average flight initiation of field-collected T. infestans according to sex and age of adults in series C. The numbers above the bars indicate the total number of bugs tested in each class. Lines indicate ± 1 SD.
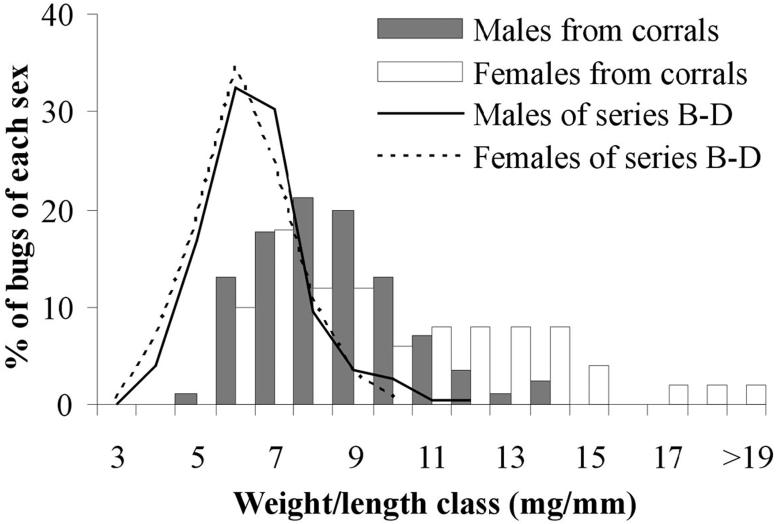
The effects of the W/L ratio were only significant in series C (Table 2). In series D, W/L effects were significant only when the effects of a partial bloodmeal were removed from the regression model (data not shown). The absence of a clear negative effect of the W/L ratio on flight initiation is probably because of the narrow, low range of W/L values in the study bugs. Comparing the current W/L distribution with that of a natural population of T. infestans with a general poor nutritional status evidences how starved all the bugs used were (Fig. 5).
Fig.5.
Sex-specific W/L distribution of the T. infestans bugs used in series B-D (lines) compared with the distribution corresponding to a bug population from goat and pig corrals (bars), typically with a poor nutritional status at the end of summer in northern Argentina (Ceballos et al. 2005).
The recent partial bloodmeal increased moderately the bug W/L ratio (mean ± SD for fed bugs, 8.6 ± 1.3 mg/mm; unfed bugs, 6.9 ± 0.9 mg/mm) (analysis of variance [ANOVA] of recent bloodmeal nested in sex, F = 43.4; df = 1, 127; P < 0.001) and decreased flight initiation chances only when the effects of the W/L ratio were removed from the regression model (data not shown). To avoid the unbalanced numbers of bugs between the extreme W/L classes of recently fed and unfed bugs, only bugs with W/L ratios between 6 and 9 mg/mm were included in another regression analysis (Table 2). Neither W/L ratios nor a recent partial bloodmeal had significant effects on flight initiation in this subset of bugs.
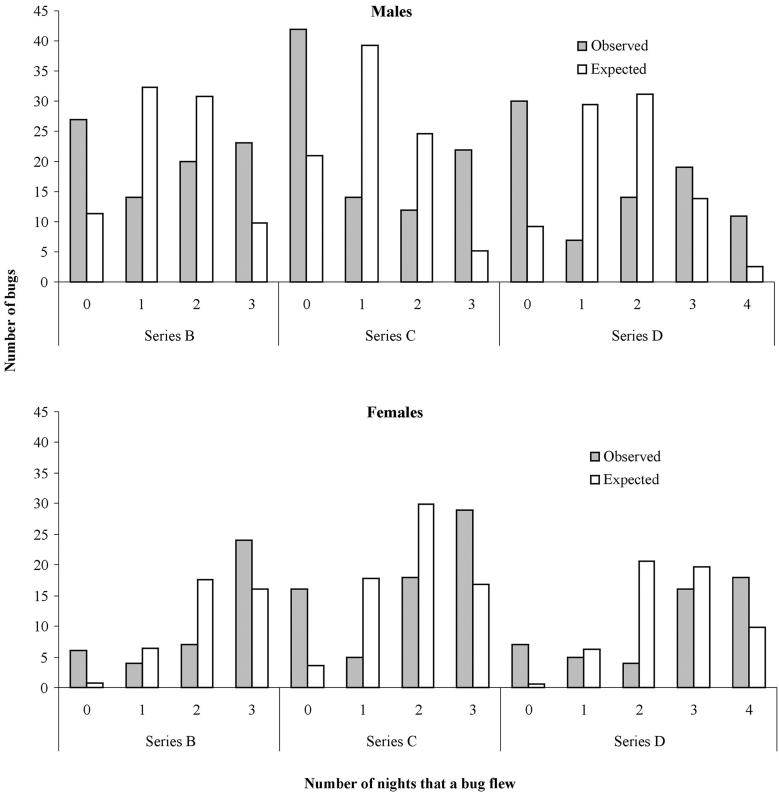
The observed frequency distributions of the number of nights that a bug flew differed significantly from the expected values (assuming the same flight probability for all nights and bugs within a series) for both sexes in all series (in all cases, χ2 > 45, df = 3, P < 0.001) (Fig. 6). No significant differences were found in flight initiation between nights within the same series (P > 0.15; Fisher’s test), except for males in series D (P = 0.002), probably because of the lower temperature during the third night of measurement. Therefore, the flight probability differed significantly among individual bugs of the same sex and series, providing evidence for the existence of bugs that were clearly fliers or nonfliers. Female bugs presented always higher proportions of always-fliers and lower proportions of never-fliers than males.
Fig.6.
Sex-specific observed and expected frequency distribution of the number of nights that each individual T. infestans bug flew. Expected values were calculated under the hypothesis that flight probability was the same for all bugs of the same sex over all nights within a series.
Discussion
Our study demonstrates that flight initiation probability of T. infestans is much higher than previously reported, responds to temperature in a sigmoid way, is higher in females than males and that the frequency distribution of the number flights per individual is highly aggregated in females and males. The probabilities of flight initiation here recorded for field-collected T. infestans exceed all previous reports, even though most of these earlier studies were conducted under higher temperatures (Table 3). Differences in size, shape, weight, and antennal sensilla between field and insectary-reared triatomines (Jaramillo et al. 2002, Catalá et al. 2004, Vazquez-Prokopec et al. 2004) suggest that these bugs also may present behavioral differences. In fact, the insectary bugs used in the pilot assay flew significantly less frequently than the field-collected bugs, despite having similar qualitative nutritional status and environmental conditions. The observed constancy of flight initiation proportions within a given temperature range suggests that differences in protocols and experimental conditions (e.g., uncontrolled variation in device materials, nearby lights and hosts) across series were of negligible importance. Thus, field T. infestans seem more prone to fly than bugs reared for successive generations in glass jars and subject to inbreeding effects. Other factors (availability of space and food, feeding frequency, and constancy of environmental conditions) also may be contributing to the observed differences.
Table 3.
Comparison of the highest probability values for flight initiation of T. infestans from all published studies
| % flight initiation |
|||||||
|---|---|---|---|---|---|---|---|
| Study | Experimental setting | Origin of bugs | Males | Females | W/L | Temp(°C) | Differences between sexes |
| Lehane and Schofield (1981) | Salt flat (Có rdoba) | 1-yr-old colony | 19 | ND | 23.5 | ND | |
| 10-yr-old colony | 5 | ND | 19 | ND | |||
| Lehane and Schofield (1982) | Laboratory | 10- and 45-yr-old colonies | 22 ± 10a | 30 ± 15a | 5/6b | 24-31 | NT |
| Lehane et al. (1992) | Laboratory | ≈10-yr-old colony | 5 | 5 | 25 | No | |
| 70 | 5 | 33 | No | ||||
| Schofield et al. (1992) | Salt flat (Có rdoba) | 2-yr-old colony and experimental hut bugs | 45 | 65 | 5.5/4.5b | 29 | NT |
| McEwen et al. (1993) | Laboratory | ≈-yr-old colony | 20-60 | 6.5 | 24-28 | NT | |
| This study | Punilla Valley (Córdoba) | 2-yr-old colony | 35 | 55 | <10c | 25 | Yes |
| Field collection | 57d | 76d | 5/5 | 25.7d | Yes | ||
ND, no data; NT, not tested.
Mean ± 1 SD (%).
W/L value for males/value for females.
Course estimate based on observation of midgut content and shape.
Average value among series B-D.
The apparent temperature threshold for flight initiation of ≈23°C is consistent with light trap collections of T. infestans (Vazquez-Prokopec et al., unpublished data) and contrasts with the reported linear relationship between flight and temperature (Williams and Schofield 1985), although reanalysis of the data showed it to be also consistent with a sigmoid relationship (C. J. Schofield, personal communication). Similar findings in other triatomines (Ekkens 1981) and various insects lend more support to a threshold response than to a linear relationship between temperature and flight initiation. Further work is needed to establish more precisely the existence of a temperature threshold and the factors affecting it.
The consistently higher flight initiation probabilities for female bugs agree with all trends reported in previous studies that discriminated between T. infestans sexes, except for one study (Lehane et al. 1992), although these were not tested for statistical significance (Table 3). Moreover, a replicated field experiment found that 82% of female T. infestans and 29% of males flied away from open, inhabited chicken coops after 2 mo (Canale and Carcavallo 1988). These findings contrast with the male-biased light trap collections of T. infestans (Vazquez-Prokopec et al. 2004, unpublished data), but this apparent contradiction disappears when the sex ratio of the putative bug sources is considered.
The peak flight initiation in females aged 4 wk and males older than 3 wk is notably consistent with most previous results, despite differences in the insects’ origin and experimental setup (Lehane and Schofield 1982, Ward and Baker 1982). The only exception (Lehane et al. 1992) may be explained by the old bug colony used. In addition, the present results suggest an interaction effect between age and sex. The peak flight probability around three weeks of age as adults in T. infestans coincides with a two-fold increase in mass of flight muscles and a five-fold increase in their lipid content (Ward et al. 1982). In some heteropterans, the histolysis of flight muscles occurs only in females, increases with age, is usually stimulated by feeding and copulation, and is coupled with oogenesis (Nair and Prabhu 1985). These physiological changes may be the mechanisms underlying the observed interaction between age and sex on flight initiation.
Blood ingestion on its own did not modify flight initiation probabilities under our experimental conditions. A recent partial bloodmeal exerted no effects on flight initiation 5-10 d after feeding, whereas fully engorged T. infestans did not initiate flight until 10-15 d after feeding, when body weight had declined sufficiently (Lehane and Schofield 1982). These results jointly suggest that the size of the partial bloodmeal was not sufficient for producing a significant increase in W/L above 10 to 11 mg/mm, which would deter bugs from flying (Lehane et al. 1992), because weight consistently limits take-off in a wide spectrum of species (Marden 1987). Regarding physiological effects of blood ingestion, the time elapsed since partial feeding may not have been sufficient for a change in metabolism to occur, as suggested by results on fat body mass and lipid content in Panstrongylus megistus (Burmeister) (Canavoso and Rubiolo 1998, Canavoso et al. 1998). The relative role of mechanical (e.g., weight increase, crop or body wall distension) and physiological factors on flight initiation requires further research.
Although mice attract bugs under laboratory (Taneja and Guerin 1995) and field conditions (Noireau et al. 1998), the presence of an inaccessible mouse had no effects on flight initiation in the current study. The most conservative explanation might be that stronger stimuli with positive effects, such as the unavoidable manipulation that bugs experienced since field collection, the novel environment, surrounding sensorial cues, and bug density, masked the effects of host presence. Alternatively, if none of these or other factors were involved, the results would suggest that the sensorial cues associated with an inaccessible host have no effects on bug flight decision.
The distribution of individual flight initiation was highly aggregated, with most of the bug population divided into fliers (bugs with a very high probability of initiating flight) and nonfliers (those with a very low or null probability of flying) across all experimental series. This also explains the upper limit reached by flight initiation proportions with increasing temperature (Fig. 4). Sex, age, or W/L variations could not discriminate between fliers and nonfliers. Previous studies that reported on flier and nonflier bugs only assessed for differences in W/L of T. infestans (Lehane and Schofield 1982) or glycerol-3-phosphate dehydrogenase activity (an essential enzyme for obtaining energy) in P. megistus and Triatoma sordida (Stål) (Soares and Santoro 2000). Other factors also may influence flight initiation in T. infestans.
The high probabilities of flight initiation found, especially in females, demonstrate that T. infestans has a higher flight potential than previously recognized. This supports the importance of flight dispersal as a major driver of the reinfestation process after insecticide spraying at a village-wide scale. Moreover, the observed effects of temperature and age may assist in identifying an appropriate time window for flight dispersal and founding of new bug colonies. Temperatures >23°C and wind speed <5 km/h are necessary for flights to occur, but bugs also need to be light enough, either because of starvation or lack of a full bloodmeal. When these conditions combine together with maximum abundance of adult bugs aged 3-5 wk old, peak flight probabilities should be expected. Determining this time window could assist in the optimal timing of bug control operations.
Acknowledgments
We thank Raúl Stariolo and Isaac Ochoa for assistance in fieldwork. We also thank Carla Cecere, Gonzalo Vázquez-Prokopec, Rodrigo De Marco, Gideon Wasserberg, Gabriel Manrique, Daniel Salomón, Nicolás Schweigmann, and the ECLAT network for helpful discussions. This project was supported by National Institutes of Health Research Grant R01 TW05836, funded by the Fogarty International Center and the National Institute of Environmental Health Sciences to U.K. and R.E.G and by grants from the University of Buenos Aires and Agencia Nacional de Promoción Científica y Técnica (Argentina) to R.E.G. R.E.G. is member of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina Researcher’s Career.
References Cited
- Canale DM, Carcavallo RU. Triatoma infestans (Klug). Factores biológicos y ecológicos en la Enfermedad de Chagas. In: Carcavallo RU, Rabinovich JE, Tonn RJ, editors. Ministerio de Salud y Acción Social de Argentina. Buenos Aires; Argentina: 1988. [Google Scholar]
- Canavoso LE, Rubiolo ER. Metabolic postfeeding changes in fat body and hemolymph of Dipetalogaster maximus (Hemiptera: Reduviidae) Mem. Inst. Oswaldo Cruz. 1998;93:225–230. doi: 10.1590/s0074-02761998000200018. [DOI] [PubMed] [Google Scholar]
- Canavoso LE, Bertello LE, Lederkremer RM, Rubiolo ER. Effect of fasting on the composition of the fat body lipid of Dipetalogaster maximus, Triatoma infestans and Panstrongylus megistus (Hemiptera: Reduviidae) J. Comp. Physiol. B. 1998;168:549–554. doi: 10.1007/s003600050176. [DOI] [PubMed] [Google Scholar]
- Catalá SS, Maida DM, Caro-Riano H, Jaramillo N, Moreno J. Changes associated with laboratory rearing in antennal sensilla patterns of Triatoma infestans, Rhodnius prolixus, and Rhodnius pallescens (Hemiptera, Reduviidae, Triatominae) Mem. Inst. Oswaldo Cruz. 2004;99:25–30. doi: 10.1590/s0074-02762004000100005. [DOI] [PubMed] [Google Scholar]
- Ceballos LA, Vazquez-Prokopec GM, Cecere MC, Gürtler RE. Seasonal variations and density-dependence of nutritional state and feeding rate of Triatoma infestans (Heteroptera; Reduviidae) in peridomestic ecotopes from northwestern Argentina. Acta Trop. 2005;95:149–159. [Google Scholar]
- Cecere MC, Canale DM, Gürtler RE. Effects of refuge availability on the population dynamics of Triatoma infestans in central Argentina. J. Appl. Ecol. 2003;40:742–756. [Google Scholar]
- Cecere MC, Vazquez-Prokopec GM, Gürlter RE, Kitron U. Spatio-temporal analysis of reinfestation by Triatoma infestans (Hemiptera: Reduviidae) following insecticide spraying in a rural community in northwestern Argentina. Am. J. Trop. Med. Hyg. 2004;71:803–810. [PMC free article] [PubMed] [Google Scholar]
- Ekkens D. Nocturnal flights of Triatoma (Hemiptera: Reduviidae) in Sabino Canyon, Arizona: I. Light collections. J. Med. Entomol. 1981;18:211–227. [Google Scholar]
- Jaramillo N, Castillo D, Wolff M. Geometric differences between Panstrongylus geniculatus from field and laboratory. Mem. Inst. Oswaldo Cruz. 2002;97:667–673. doi: 10.1590/s0074-02762002000500015. [DOI] [PubMed] [Google Scholar]
- Lazzari CR, Núñez JA. The response to radiant heat and the estimation of the temperature of distant sources in Triatoma infestans. J. Insect Physiol. 1989;35:525–529. [Google Scholar]
- Lehane MJ, McEwen PK, Whitaker CJ, Schofield CJ. The role of temperature and nutritional status in flight initiation by Triatoma infestans. Acta Trop. 1992;52:27–38. doi: 10.1016/0001-706x(92)90004-h. [DOI] [PubMed] [Google Scholar]
- Lehane MJ, Schofield CJ. Field experiments of dispersive flight by Triatoma infestans. Trans. R. Soc. Trop. Med. Hyg. 1981;75:399–400. doi: 10.1016/0035-9203(81)90103-6. [DOI] [PubMed] [Google Scholar]
- Lehane MJ, Schofield CJ. Flight initiation in Triatoma infestans (Klug) (Hemiptera: Reduviidae) Bull. Entomol. Res. 1982;72:497–510. [Google Scholar]
- Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lorenz K. Methods of approach to the problem of behaviour. Academic; New York: 1959. (The Harvey Lectures, Ser. 54.). [Google Scholar]
- Marden JH. Maximum lift production during takeoff in flying animals. J. Exp. Biol. 1987;130:235–258. [Google Scholar]
- McEwen PK, Lehane MJ, Whitaker CJ. The effect of adult population density on flight imitation in Triatoma infestans (Klug) (Hem., Reduviidae) J. Appl. Entomol. 1993;116:321–325. [Google Scholar]
- McFarland DJ. Feedback mechanisms in animal behaviour. Academic; London, United Kingdom: 1971. [Google Scholar]
- Montenegro S. Determinación de las reservas alimenticias en Triatoma infestans Klug, 1834 (Hemiptera, Reduviidae) en base a caracteres externos. I. Adultos Physis. 1983;41:159–167. [Google Scholar]
- Nair CRM, Prabhu VKK. The role of feeding, mating and ovariectomy on degeneration of indirect flight muscles of Dysdercus cingulatus (Heteroptera: Pyrrhocoridae) J. Insect Physiol. 1985;31:35–39. [Google Scholar]
- Noireau F, Flores R, Vargas F. Trapping of silvatic Triatominae (Reduviidae) in hollow trees. Trans. R. Soc. Trop. Med. Hyg. 1998;93:13–14. doi: 10.1016/s0035-9203(99)90161-x. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Matthews JNS. Theoretical approach to active dispersal and colonization of houses by Triatoma infestans. J. Trop. Med. Hyg. 1985;88:211–222. [PubMed] [Google Scholar]
- Schofield CJ, Lehane MJ, McEwen P, Catala SS, Gorla DE. Dispersive flight by Triatoma infestans under natural climatic conditions in Argentina. Med. Vet. Entomol. 1992;6:51–56. doi: 10.1111/j.1365-2915.1992.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Schweigmann N, Vallvé S, Muscio O, Guillini M, Alberti A, Wisnivesky-Colli C. Dispersal flight by Triatoma infestans in an arid area of Argentina. Med. Vet. Entomol. 1988;2:401–404. doi: 10.1111/j.1365-2915.1988.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Soares RPP, Santoro MM. α-Glycerophosphate dehydrogenase activity in flight muscles of triatomine bugs Panstrongylus megistus and Triatoma sordida. Mem. Inst. Oswaldo Cruz. 2000;95:707–709. doi: 10.1590/s0074-02762000000500016. [DOI] [PubMed] [Google Scholar]
- StataCorp . Stata statistical software: release 7.0. Stata Corporation, College Station; TX: 1999. [Google Scholar]
- Taneja J, Guerin PM. Oriented response of the triatomine bugs Rhodnius prolixus and Triatoma infestans to vertebrate odours on a servosphere. J. Comp. Physiol. A. 1995;176:455–464. [Google Scholar]
- Vazquez-Prokopec GM, Ceballos LA, Kitron U, Gürtler GE. Active dispersal of natural populations of Triatoma infestans (Hemiptera: Reduviidae) in rural northwestern Argentina. J. Med. Entomol. 2004;41:614–621. doi: 10.1603/0022-2585-41.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JP, Baker PS. The tethered flight performance of a laboratory population of Triatoma infestans Klug (Hemiptera: Reduviidae) Bull. Entomol. Res. 1982;72:17–28. [Google Scholar]
- Ward JP, Candy DJ, Smith SN. Lipid storage and changes during flight by triatomine bugs (Rhodnius prolixus and Triatoma infestans) J. Insect Physiol. 1982;28:527–534. [Google Scholar]
- Williams NG, Schofield CJ. The role of temperature in flight initiation of triatomine bugs. Trans. R. Soc. Trop. Med. Hyg. 1985;79:282. [Google Scholar]
- [WHO] World Health Organization . Control de la enfermedad de Chagas. Serie de Informes Técnicos 905. Comité de Expertos de la OMS en Control de la Enfermedad de Chagas. Ginebra, WHO; Geneva, Switzerland: 2002. [Google Scholar]