Abstract
Integrin signaling modulates trophoblast adhesion to extracellular matrices during blastocyst implantation. Fibronectin (FN)-binding activity on the apical surface of trophoblast cells is strengthened after elevation of intracellular Ca2+ downstream of integrin ligation by FN. We report here that phosphoinositide-specific phospholipase C (PLC) mediates Ca2+ signaling in response to FN. Pharmacological agents used to antagonize PLC (U73122) or the inositol phosphate receptor (Xestospongin C) inhibited FN-induced elevation of intracellular Ca2+ and prevented the upregulation of FN-binding activity. In contrast, inhibitors of Ca2+ influx through either voltage-gated or non-voltage-gated Ca2+ channels were without effect. Inhibition of protein tyrosine kinase activity by genistein, but not G-protein inhibition by suramin, blocked FN-induced intracellular Ca2+ signaling and upregulation of adhesion, consistent with involvement of PLC-γ. Confocal immunofluorescence imaging of peri-implantation blastocysts demonstrated that PLC-γ2, but not PLC-γ1 nor PLC-β1, accumulated near the outer surface of the embryo. Phosphotyrosine site-directed antibodies revealed phosphorylation of PLC-γ2, but not PLC-γ1, upon integrin ligation by FN. These data suggest that integrin-mediated activation of PLC-γ to initiate phosphoinositide signaling and intracellular Ca2+ mobilization is required for blastocyst adhesion to FN. Signaling cascades regulating PLC-γ could, therefore, control a critical feature of trophoblast differentiation during peri-implantation development.
Keywords: Blastocyst implantation, trophoblast, phospholipase C, Ca2+ signaling, signal transduction, integrins, fibronectin, extracellular matrix, cell adhesion, tyrosine phosphorylation
INTRODUCTION
Blastocyst implantation in mice and humans depends on the interaction of differentiated, adhesion-competent trophoblast cells with extracellular matrix (ECM) components of the receptive uterus (Carson et al., 2000; Wang and Armant, 2002; Armant, 2005). Achievement of the adhesion-competent stage by mouse blastocysts correlates with trafficking of α5β1 integrins and the concomitant acquisition of fibronectin (FN)-binding activity on the apical surface of trophoblast cells (Schultz and Armant, 1995; Schultz et al., 1997). Blastocysts developing to the adhesion-competent stage exhibit strong FN-binding activity only after exposure to FN or an active fragment, FN-120, through a process dependent on energy, intracellular trafficking and actin microfilament integrity, but requiring no new protein synthesis (Schultz and Armant, 1995). The strengthening of trophoblast adhesion to FN is mediated by ligation of integrins that contain the α5, αv, β1 and β3 subunits and is strongly correlated with subsequent trafficking of the αIIb integrin subunit to the apical plasma membrane (Wang et al., 2002; Rout et al., 2004). Therefore, both “outside-in” and “inside-out” integrin signaling (Burridge and Chrzanowska-Wodnicka, 1996; Aplin et al., 1998; Giancotti and Ruoslahti, 1999) appear to be required by trophoblast cells in order to achieve strong adhesion to FN and progress through the periimplantation developmental program (Armant, 2005). Evidence suggests that α5β1 is primarily responsible for outside-in signaling, while inside-out signaling positions αIIbβ3 on the surface of the blastocyst to strengthen adhesion (Rout et al., 2004).
Integrin ligation by FN transiently elevates intracellular Ca2+ levels in mouse blastocysts and interference with this Ca2+ signaling blocks the upregulation of FN-binding activity (Wang et al., 2002). Conversely, direct elevation of intracellular Ca2+ with ionomycin strengthens trophoblast adhesion to FN to the same degree as integrin ligation. The role of intracellular Ca2+ in integrin-mediated signal transduction has been described in several cell types. Mobilization of intracellular Ca2+ followed by protein-tyrosine phosphorylation occurs when cells expressing αIIbβ3 integrin adhere to FN or fibrinogen (Pelletier et al., 1992). Signal transduction stimulated in endothelial cells also involves an interaction between integrins present in focal adhesion complexes and the Ca2+-dependent kinases, protein kinase C (PKC) and Ca2+-calmodulin-dependent protein kinase (Berk et al., 1995). Ca2+-related transducers of intracellular signaling, including inositol 1,4,5 trisphosphate (Ins(1,4,5)P3)-specific phospholipase C (PLC) and PKC, are recruited to the plasma membrane by α5β1 integrins when ligated by either FN or specific antibodies (Miyamoto et al., 1995). In adhesion-competent trophoblast cells, elevation of intracellular Ca2+ to strengthen trophoblast adhesion to FN appears to require the downstream factors, PKC and calmodulin, (Wang et al., 2002). How intracellular Ca2+ signaling is initiated by integrin-ligation in mouse blastocysts has not been delineated.
Mechanisms responsible for elevation of intracellular Ca2+ include 1) release from intracellular stores through either the Ins(1,4,5)P3 or ryanodine receptors, 2) influx through voltage-gated Ca2+ channels, and 3) capacitative Ca2+ entry, in which Ca2+ influx occurs subsequent to the release of intracellular Ca2+ stores (store-operated Ca2+ entry) (Putney et al., 2001). Integrin ligation can elevate intracellular Ca2+ levels through Ca2+ influx (Leavesley et al., 1993; Schwartz, 1993; Schwartz, 1993), activation of PLC to generate Ins(1,4,5)P3 and mobilize intracellular Ca2+ stores (Somogyi et al., 1994; Chan et al., 2001), or initiation of capacitative Ca2+ influx (Schottelndreier et al., 2001). The capacity for both Ca2+ influx and mobilization of intracellular Ca2+ stores exist in mouse preimplantation embryos. During early preimplantation development, the activities of PLC and the Ins(1,4,5)P3 receptor are operative during capacitative Ca2+ entry in response to platelet activation factor (Emerson et al., 2000). Evidence also exists for the presence of Ins(1,4,5)P3-sensitive intracellular Ca2+ stores at the 8-cell and blastocyst stages (Stachecki and Armant, 1996; Liu and Armant, 2004), and Ca2+ influx through L- and N-type voltage-gated calcium channels after stimulation by cannabinoids or heparin-binding EGF-like growth factor (HBEGF), respectively, at the blastocyst stage (Wang et al., 2003; Wang et al., 2000b). In the present study, we have examined the mechanism underlying integrin-mediated Ca2+ mobilization in adhesion-competent trophoblast cells during a period when these cells differentiate from an epithelial phenotype into motile, invasive cells.
MATERIALS AND METHODS
Production and Culture of Mouse Embryos
Female NSA or MF1 mice (Harlan Sprague Daly, Indianapolis, IN) 5-8 weeks old were superovulated, as previously described (Armant, 2006). Eggs were recovered by flushing oviducts with M2 medium (Sigma Chemical Company, St Louis, MO) the morning after injection of human chorionic gonadotropin. For generation of embryos, female mice were mated with B6SJLF1/J males (Jackson Laboratory, Bar Harbor, ME) and embryos were collected by flushing the oviducts with M2 on gestation day (GD) 1-3 or the uterus on GD 4 (where GD1 is the day of the vaginal plug), as previously described (Armant, 2006). All experiments using adhesion-competent blastocysts were conducted by first culturing blastocysts from GD 4 to GD 7 in Ham's F10 medium containing 4 mg/ml BSA, 100 U/ml penicillin and 0.1 mg/ml streptomycin (all from Sigma) at 37°C in a 5% CO2/air incubator.
Preparation of Cell Extracts
Monolayers of mouse B16-F10 melanoma cells (American Type Culture Collection, Rockville, MD), mouse trophoblast stem (TS) cells and human HTR-8/Svneo cytotrophoblast cells were grown as previously described (Yelian et al., 1995; Kilburn et al., 2000; Tanaka et al., 1998) and harvested in SDS sample buffer containing 1 mM sodium orthovanadate, and protease inhibitors (1 mM PMSF, 25 KIU/ml aprotinin, 2 μM leupeptin, 2 μM antipain, 10 μM benzamidine, 1 μM pepstatin, and 1 μM chymostatin; all from Sigma). Mouse spleen was cut into small pieces, rinsed in ice-cold PBS three times and dissociated to prepare a single cell suspension using a Polytron (Omni International, Inc. Waterbury, CT) at 7500 rpm. Spleen cells were collected by centrifugation at 1000 × g and rinsed three times using cold PBS. Cells were lysed using cold NP-40 lysis buffer (137 mM NaCl, 20 mM Tris, 1% NP-40, 10 % glycerol, pH 8.0) containing orthovanadate and protease inhibitors described above. After centrifuging at 13,000 × g for 3 min, the supernatant was recovered and the protein concentration was determined using a Bio-Rad (Rockville Center, NY) DC protein assay kit.
FN-Binding Activity
FN-binding activity was upregulated by exposing blastocysts to 50 μg/ml FN-120 for 1 h, as previously described (Wang et al., 2000b; Armant, 2006). In some experiments, the blastocysts were treated at 37°C for 1 h before and during exposure to FN-120 by addition of the following inhibitors to the culture medium: 10 μM U73122, 10 μM U73343, 1 μM Xestospongin C, 10 μM flunarizine, 10 μM bepridil, 10 μM genistein, 10 μM daidzein or 10 μM suramin, (all from EMD Biosciences, Inc., San Diego, CA). Similar treatments were also carried out using 2 μM FTX-3.3, 3 μM ω-conotoxin GVIA, 1 μM ω-conotoxin MVIIC or 1 μM calciseptine (all from Alomone Labs, Jerusalem, Israel).
FN-binding activity was assayed, as previously described (Schultz and Armant, 1995; Armant, 2006), using 1.0 μm fluorescent-green polystyrene microspheres (Polyscience) coated with FN-120. The fluorescence intensity of the bound microspheres was quantified over the abembryonic pole of each blastocyst using computer-based image analysis, as described by Schultz and Armant (1995). Basal FN-binding activity, determined by exposing embryos to BSA in place of FN-120, was subtracted from all values obtained with embryos exposed for 1 h to 50 μg/ml FN-120. The derived change (Δ) in FN-binding activity values were normalized to the mean control values obtained in each experiment and reported as mean ± SEM.
Intracellular Ca2+ Mearurements
For estimation of the intracellular Ca2+ concentration, blastocyst cells loaded for 1 h with 5 μM fluo-3 acetoxymethyl ester (fluo-3-AM) or fluo-4-AM (Molecular Probes, Inc., Eugene, OR) were subjected to epifluorescence microscopy and image analysis, as previously described (Wang et al., 1998). After dye loading, some embryos were treated for 15 min with 2 μM FTX-3.3, 3 μM ω-conotoxin GVIA, 1 μM ω-conotoxin MVIIC, 1 μM calciseptine, 10 μM flunarizine, 10 μM bepridil, 10 mM NiCl2, 10 μM caffeine (Sigma), 10 μM thapsigargin (EMD Biosciences), 10 μM U73122, 10 μM U73343, 1 μM Xestospongin C, 10 μM genistein or 10 μM daidzein. Embryos were imaged individually at 37°C in 5 μl drops of culture medium with or without 50 μg/ml FN-120 on Petri-dishes flooded with mineral oil. To monitor Ca2+ mobilization, embryos were briefly illuminated every 0.25 min for fluorescence imaging. All data presented depict single embryos that are representative of a minimum of 10 embryos for each treatment.
Immunological Identification of PLC Isoforms
Immunological procedures were conducted using polyclonal antibodies that recognize specific PLC isoforms. Rabbit IgGs recognizing PLC-β1 (G-12), PLC-γ1 (530) or PLC-γ2 (Q-20) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Tyrosine phosphorylation of the two PLC-γ isoforms was detected using antibodies that recognize specific tyrosine-phosphorylated epitopes. PLC-γ1 phosphorylated at tyrosine 783 was labeled using either a goat antibody (Tyr 783) from Santa Cruz or a rabbit antibody (Tyr783) from Cell Signaling Technologies, Inc. (Danvers, MA). PLC-γ2 phosphorylated at tyrosine 1217 was labeled with a rabbit antibody (Tyr1217) from Cell Signaling.
For immunofluorescence microscopy, blastocysts were fixed at room temperature for 30 min in 3% paraformaldehyde, washed through 2 drops of 150 mM glycine, pH 7.2, and permeabilized by treatment with 0.1% Triton X100 (Sigma) for 15 min at room temperature. Five drops of PBS containing 10 mg/ml BSA (PBS/BSA) were used to rinse embryos after all incubations throughout the procedure. Blastocysts were incubated overnight at 4°C with primary antibodies prepared at 10 μg/ml in PBS/BSA. As primary antibody controls, some embryos were incubated with 10 μg/ml non-immune rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Primary antibodies were detected using 10 μg/ml Texas-Red-conjugated goat anti-rabbit IgG (Jackson Immunoresearch) incubated with embryos at 37°C for 1 h.
For confocal imaging, Texas-Red-labeled blastocysts were mounted on slides with permanent mounting medium (Chemicon, Temecula, CA) and stored at 4°C. Embryos were viewed on a Zeiss (Thornwood, NY) 310 confocal scanning laser microscope, using identical contrast and gain settings to generate all images. Blastocysts labeled with anti-phospho-PLC antibodies were viewed using epifluorescence microscopy (Leica DM IRB; Wetzlar, Germany). Images captured with an Orca digital camera (Hamamatsu City, Japan) were deconvolved from 1 μm serial optical sections using SimplePCI (C-Imaging system, Cranberry Township, PA) imaging software and a “nearest neighbor” algorithm. The fluorescence intensities of labeled blastocysts were quantified from these images using SimplePCI software, which measured the total fluorescence (grey level) of an area at the outer edge of each embryo delineated within a digital image. The mean intensity of control embryos exposed to FN-120 for 30 min and labeled with non-immune IgG was subtracted from the intensity for each embryo to derive the specific phospho-PLC staining intensity.
Western blotting was used to validate all primary antibodies. Proteins extracted from B16, TS, HTR-8/Svneo and mouse spleen cells were diluted in SDS sample buffer (125 mM Tris pH 6.8, 4% SDS, 10% glycerol, 0.006% bromophenol blue, 2% β-mercaptoethanol) and electrophoresed (30 μg/lane) on a 7.5% SDS polyacrylamide gel. Separated proteins were then transferred electrophoretically from the gel to polyvinylidene difluoride or nitrocellulose membranes (Millipore, Burlington, MA), and blocked overnight at room temperature with 5% (w/v) nonfat dry milk prepared in PBS. Membranes were incubated overnight at 4°C with 1 μg/ml primary antibody diluted in TTBS (20 mM Tris-HCl, pH 7.6, 145 mM NaCl, and 0.1% Tween-20) containing 1% nonfat dry milk. After rinsing three times with TTBS, the membranes were incubated for 1 h at room temperature with 1:3000 horseradish peroxidase-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch) in TTBS/1% nonfat dry milk. After three rinses in TTBS, the probe was visualized using enhanced chemiluminensence, as previously described (Kilburn et al., 2000).
Statistical Analysis
All experiments were repeated at least three times. Values reported for FN-binding activity were obtained using at least 15 blastocysts per treatment group. Differences between treatment groups in the FN-binding activity and PLC staining intensity were tested for significance using a one-way ANOVA and the Bonferroni/Dunn post-hoc test.
RESULTS
Upregulation of FN-Binding Activity Does Not Depend on Ca2+ Influx
To investigate whether FN-120 induces an intracellular Ca2+ transient in blastocysts by initiating Ca2+ influx through voltage-gated Ca2+ channels, we have examined the effects of Ca2+ channel (L-, N-, P-, Q- or T- type) blockers on FN-120-mediated Ca2+ signaling and upregulation of FN-binding activity. A similar approach was used previously for blastocysts treated with HBEGF to demonstrate that inhibitors of N-type Ca2+ channels significantly attenuates the acceleration of development by 50-75% (Wang et al., 2000b). None of the blockers tested significantly inhibited the ability of FN-120 to induce a Ca2+ transient or upregulate FN-binding activity (Table 1), suggesting that voltage-gated Ca2+ channels are not required for integrin signaling in trophoblast cells.
Table 1.
Influence of voltage-gated Ca2+ channel blockers on upregulation of FN-binding activity and increased cytoplasmic Ca2+ in blastocysts treated with FN-120
| Ca2+ Channel Blockers | Channel Type | ΔFN-binding activitya (% of control) |
Ca2+ elevatedb |
|---|---|---|---|
| Control | 99.9 ± 15 | Yes | |
| 2 μM FTX-3.3 | P-/Q- | 90.0 ± 15 | Yes |
| 1 μM ω-conotoxin MVIIC | Q- | 102 ± 18 | Yes |
| 3 μM ω-conotoxin GVIA | N- | 110 ± 15 | Yes |
| 1 μM Calciseptiine | L- | 71.3 ± 16 | Yes |
| 10 μM Flunarizine | T- | 73.4 ± 10 | Yes |
| 10 μM Bepridil | T- | 73.1 ± 20 | Yes |
Blastocysts were treated with 50 μg/ml FN-120 in the absence (Control) or presence of the indicated inhibitors and assayed for ΔFN-binding activity and Ca2+ signaling, as detailed in the Materials and Methods section.
Values (mean ± SEM) were not significantly different according to ANOVA.
Intracellular Ca2+ increased from about 200 nM to at least 365 nM after adding FN-120 where scored as elevated. At least 10 embryos all tested positively for each treatment.
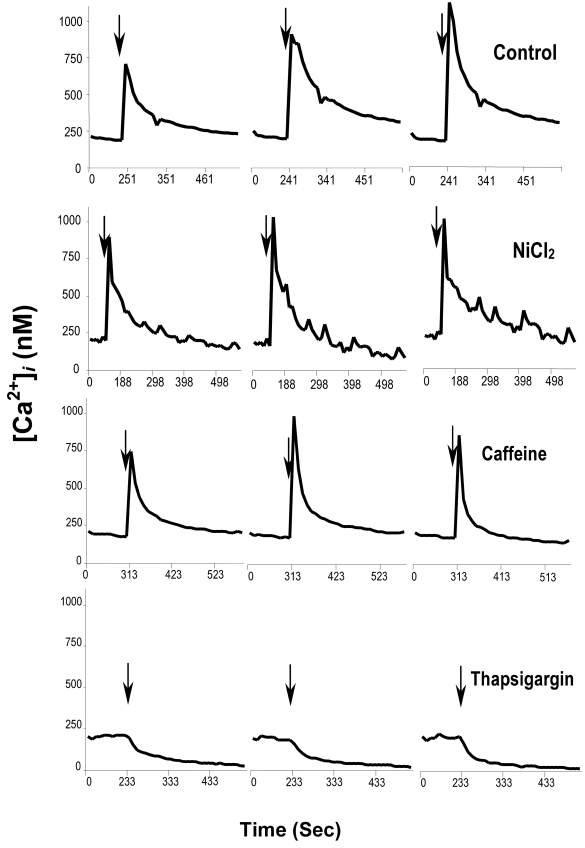
Integrins can induce Ca2+ influx through non-voltage gated Ca2+ channels (Schottelndreier et al., 2001). It was considered inadvisable to chelate extracellular Ca2+ because of possible interference with integrin binding to FN-120, as found by Schultz and Armant (1995) using EDTA. As an alternate approach, Ca2+ influx was inhibited by NiCl2. Blastocysts were first treated with 10 mM NiCl2 and then exposed to FN-120 in the continued presence of inhibitor prior to assessing intracellular Ca2+ or FN-binding activity. NiCl2 did not reduce FN-induced elevation of intracellular Ca2+ (Fig. 1) or upregulation of FN-binding activity (not shown), suggesting that influx of extracellular Ca2+ is not the primary source for elevation of intracellular Ca2+ levels during this process.
Figure 1.
Regulation of intracellular Ca2+ downstream of integrin ligation. Blastocysts were treated with vehicle (Control), 10 mM NiCl2, 10 μM thapsigargin or 10 μM caffeine and then exposed to 50 μg/ml FN-120 in the continued presence of inhibitor. Intracellular Ca2+ concentration was monitored by immunofluorescence microscopy, as detailed in the Materials and Methods. Each graph depicts the average concentration integrated over an entire representative embryo.
FN Elevates Cytoplasmic Free Ca2+ through Release from Ins(1,4,5)P3-Sensitive Ca2+ Stores
To examine whether FN-120 elevates intracellular Ca2+ through release from intracellular Ca2+ stores, FN-induced Ca2+ signaling was examined in blastocysts treated with either caffeine or thapsigargin to deplete ryanodine- or Ins(1,4,5)P3-sensitive intracellular Ca2+ stores, respectively. FN-120 induced an intracellular Ca2+ transient in caffeine-treated blastocysts, but not in thapsigargin-treated embryos (Fig. 1), suggesting that integrin signaling triggered by FN-120 primarily targets Ins(1,4,5)P3-sensitive intracellular Ca2+ stores.
Integrin Ligation Mobilizes Intracellular Ca2+ Stores through Activation of PLC
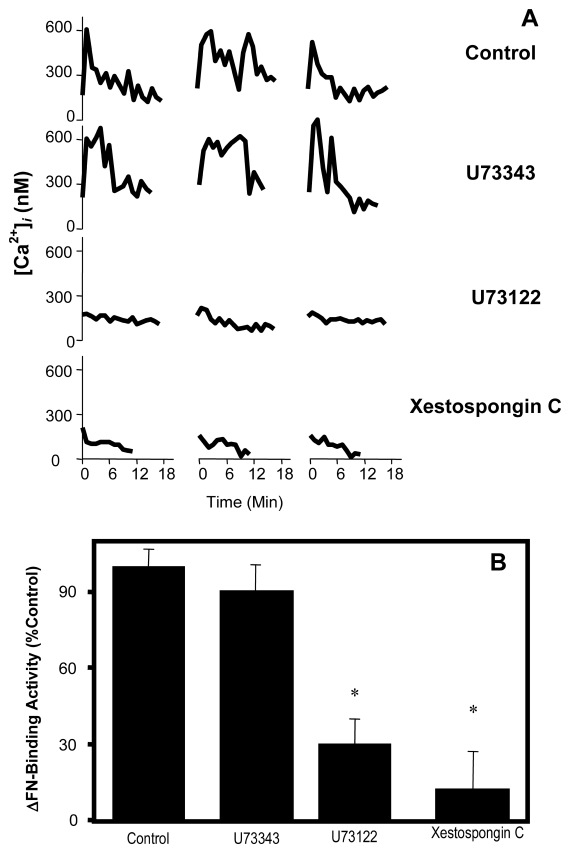
Ins(1,4,5)P3 is produced by activation of phosphoinositide-specific PLC and the subsequent hydrolysis of phosphatidylinositol 4,5-bisphosphate (Rhee and Bae, 1997). To examine the role of PLC activity and Ins(1,4,5)P3 signaling after ligation of integrins by FN, blastocysts were treated before exposure to FN-120 with 10 μM U73122, a potent inhibitor of phosphoinositide-specific PLC, or 1 μM Xestospongin C, an Ins(1,4,5)P3 receptor antagonist (Gafni et al., 1997). Both inhibitors prevented the appearance of a Ca2+ transient (Fig. 2A) and blocked the upregulation of FN-binding activity (Fig. 2B), suggesting that activation of the PLC/Ins(1,4,5)P3 signaling pathway is required for both events. U73343, a less active structural analog of U73122, inhibited neither the upregulation of FN-binding activity nor induction of intracellular Ca2+ transients (Fig. 2A,B). It can be concluded that PLC operates upstream to Ca2+ mobilization in the integrin signaling pathway initiated by FN-120.
Figure 2.
Dependence of FN-induced intracellular Ca2+ signaling on the PLC-Ins(1,4,5)P3 pathway. Blastocysts were treated with vehicle (Control), 10 μM U73343, 10 μM U73122 or 1 μM Xestospongin C and then exposed to FN-120. Intracellular Ca2+ levels (A) were monitored and reported as in Fig. 1 and ΔFN-binding activities (B) were normalized to the Control group and reported as mean ± SEM. *, P < 0.05, compared to Control.
PLC-γ Mediates Outside-In Signaling during Upregulation of FN-Binding Activity
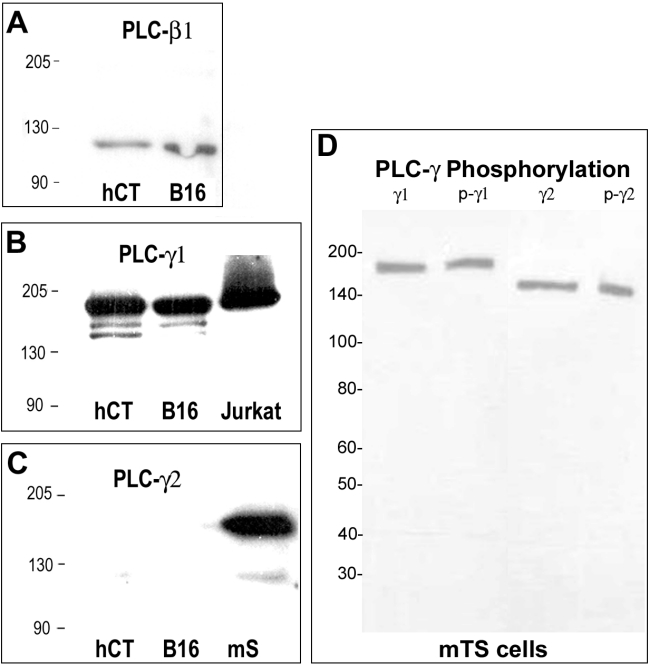
Six major families of PLC isoforms (PLC-β, -γ, -δ, -ε, -ζ , and -η) have been identified in mammalian tissues that include at least 13 different isozymes (Rebecchi and Pentyala, 2000; Harden and Sondek, 2006). PLC-γ isoforms are activated by protein tyrosine kinases (PTK), while PLC-β, PLC-δ and the other isoforms are activated through pathways dependent on heterotrimeric G proteins or small GTPases of the Ras framily (Rebecchi and Pentyala, 2000; Harden and Sondek, 2006). We have examined the expression of three PLC isozymes in mouse embryos using antibodies that recognize proteins of the expected molecular weights and tissue distribution. As expected, PLC-β1 and PLC-γ1 were detected by western blot in melanoma and trophoblast cells, while PLC-γ2 was only detected in spleen (Fig. 3A-C). Trophoblast cells in adhesion-competent mouse blastocysts expressed both PLC-β and PLC-γ isoforms (Fig. 4), indicating that either a tyrosine kinase or G protein-based PLC-activation pathway could link integrin ligation to Ca2+ mobilization.
Figure 3.
Specificity of antibodies against PLC isoforms. Western blotting was conducted, as described in the Materials & Methods, using antibodies against PLC-β1 (panel A), PLC-γ1 (panel B) or PLC-γ2 (panel C). The lanes contained proteins extracted from human HTR-8/SVneo cytotrophoblast cells (hCT), mouse B16 melanoma cells (B16), mouse spleen (mS; positive control for PLC-γ2) and a commercial Jurkat cell lysate preparation (Jurkat; BD Biosciences, San Jose, CA; positive control for PLC-γ1). In panel D, antibodies against PLCγ1 (γ1) and PLCγ2 (γ2) were compared to antibodies recognizing the tyrosine phosphorylated forms of each enzyme (p-γ1, p-γ2) using a lysate prepared from mouse TS cells. Molecular weights (kDa) are indicated to the left in each panel.
Figure 4.
Expression of PLC-β1 and PLC-γ1 in adhesion-competent trophoblast cells. Blastocysts cultured to GD 7 were fixed, permeabilized, and labeled by immunofluorescence using non-immune IgG (IgG) or antibodies against PLC-γ1 or PLC-β1, as detailed in the Materials & Methods. Representative 1 μm optical sections are shown for antigen visualized using Texas-Red-conjugated secondary antibody and scanning laser confocal microscopy.
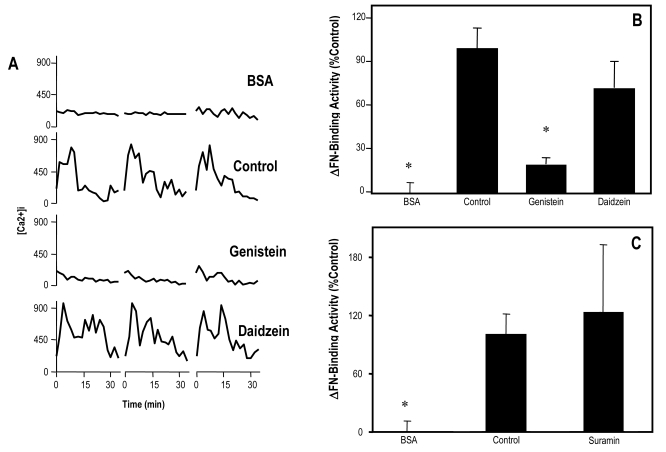
In an effort to resolve the principal signaling pathway upstream to PLC, blastocysts were treated with suramin to inhibit G-protein activity, as well as genistein to block activation of PTK. Genistein effectively blocked the elevation of intracellular Ca2+ induced by FN-120 (Fig. 5A) and the upregulation of FN-binding activity (Fig. 5B), while the G-protein inhibitor had no effect on the ability of FN-120 to upregulate FN-binding activity (Fig. 5C). As a positive control, suramin blocked the upregulation of FN-binding activity by lysophosphatidic acid (data not shown), an inducer of Ca2+ mobilization in blastocysts (Liu and Armant, 2004) that binds to a G-protein-coupled receptor (van Corven et al., 1989). The less active structural analogue of genistein, daidzein, did not inhibit the effects of FN-120 treatment (Fig.5A and B). These findings suggest that the PTK-dependent PLC-γ isoforms, but not PLC-β1 or other G protein-dependent isozymes, are responsible for Ca2+ signaling during trophoblast interaction with FN.
Figure 5.
Dependence of of FN-induced intracellular Ca2+ signaling on PTK and G-proteins. Blastocysts were treated with 10 μM genistein, daidzein or suramin and then exposed to FN-120 in the continued presence of the same inhibitor. Embryos treated with vehicle served as controls. Some embryos were not exposed to FN-120 or inhibitor (BSA). Intracellular Ca2+ levels (A) and ΔFN-binding activities (B, C) were monitored and reported as in Fig. 2. *, P < 0.05, compared to Control.
Expression of PLC-γ during Preimplantation Development
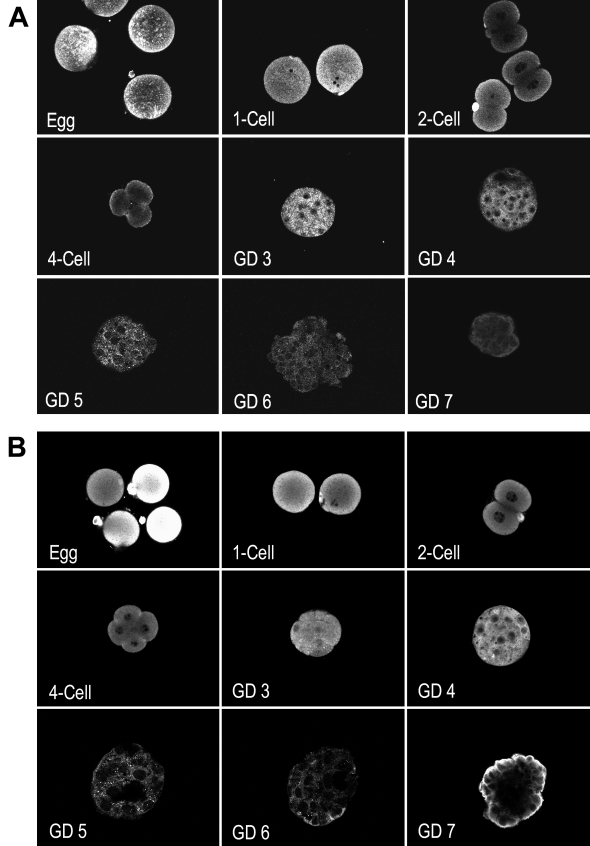
To delineate the expression of the two PLCγ isoforms during mouse preimplantation development, unfertilized eggs and embryos at the 1-cell stage to the adhesive blastocyst stage (cultured to GD 7) were assessed by immunofluorescence labeling and scanning laser confocal microscopy. PLC-γ1, a ubiquitously expressed isoform (Rebecchi and Pentyala, 2000), was detected at a relatively high level in unfertilized eggs and zygotes (Fig. 6A). PLC-γ1 declined during preimplantation development, with only trace levels observed in adhesion-competent blastocysts. In contrast, PLC-γ2, which is primarily expressed in the lymphatic system (Rebecchi and Pentyala, 2000), was highly expressed in preimplantation mouse embryos (Fig. 6B). Similar to PLC-γ1, high levels of PLC-γ2 were found in unfertilized eggs and zygotes with decreasing expression after blastocyst formation. However, a relatively high level of PLC-γ2 appeared in adhesive blastocysts on GD7 localized primarily at the apical surface of trophoblast cells, making it accessible to integrin signaling complexes.
Figure 6.
Expression of PLC-γ1 and PLC-γ2 during preimplantation development. Eggs and embryos at the indicated stages were fixed, permeablized, and labeled by immunofluorescence using antibody against PLC-γ1 (A) or PLC-γ2 (B). Antigen was visualized and imaged as in Fig. 4, which shows a control stained with non-immune rabbit IgG in place of primary antibody.
PLC-γ Phosphorylation during Integrin Signaling
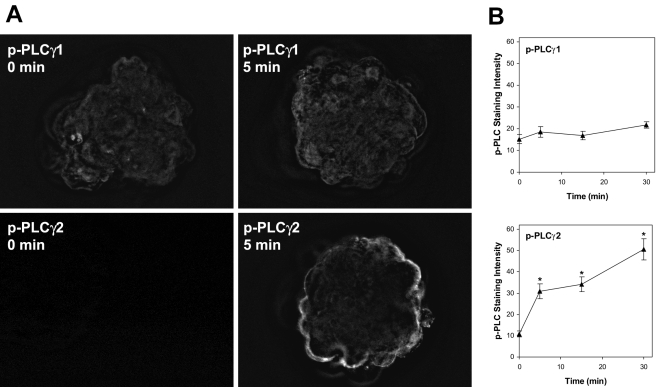
Activation of PLC-γ is accomplished through tyrosine phosphorylation (Harden and Sondek, 2006) and can be monitored using phosphorylation-specific antibodies. We examined PLC-γ1 and PLC-γ2 phosphorylation in adhesion-competent blastocysts during the period immediately following exposure to FN-120 using antibodies directed against phosphorylated tyrosine residues at positions 783 and 1217 in the respective proteins. Western blotting of mouse TS cell extracts demonstrated that antibodies against both phosphorylated proteins specifically detected bands at the expected molecular sizes, matching antibodies against PLC-γ1 and PLC-γ2 (Fig. 3D). The phosphorylation-specific antibodies were then used to detect PLC activation in blastocysts by immunofluorescence (Fig. 7A). Five min after exposure to FN-120, phospho-PLC-γ1 staining remained at or near background levels, while phospho-PLC-γ2 labeling became intense near the apical surface of the trophoblast. Quantification of the fluorescence intensity indicated no change in PLC-γ1 phosphorylation over a 30-min period; however, phospho-PLCγ2 labeling increased significantly within 5 min of FN-120 treatment and continued to rise over the next 25 min (Fig. 7B). Specific phospho-PLC staining shown in Fig. 7B was calculated by subtracting the background staining of non-immune IgG, which was low (6.0 ± 0.8). While not ruling out a role for PLC-γ1 or other PLC isozymes, these findings provide strong evidence that PLC-γ2 has a major role in transducing integrin-initiated signals in mouse trophoblast cells.
Figure 7.
Phosphorylation of PLC-γ1 and PLC-γ2 during exposure to FN-120. Adhesion-competent blastocysts cultured to GD7 were exposed to FN-120 for 0, 5, 15 or 30 min and then fixed, permeablized, and labeled by immunofluorescence using an antibody against either PLC-γ1 phosphorylated at tyrosine 783 or PLC-γ2 phosphorylated at tyrosine 1217. Panel A shows deconvolved images of embryos exposed to FN-120 for 0 or 5 min. Panel B shows the specific phospho-PLC staining quantified as described in the Materials and Methods section for each antibody over a 30 min period of exposure to FN-120. The mean ± SEM is shown at each time for 10-12 embryos. *, P < 0.05, compared to Control.
DISCUSSION
We previously reported that ligation of integrins on the apical surface of adhesion competent trophoblast cells by FN initiates intracellular Ca2+ signaling and upregulates FN-binding activity (Wang et al., 2002). These biochemical events could be critical for blastocyst adhesion to endometrial ECM during implantation and may modulate adhesive interactions with ECM components as trophoblast cells pass through the basal lamina, invade the decidua and remodel the uterine vascular system. We now present evidence demonstrating that PLC transduces outside-in signaling by integrins upstream to Ca2+ mobilization, and that the responsible isozyme could be PLC-γ2.
Activation of integrins by ECM components frequently initiates intracellular Ca2+ signaling (Ng-Sikorski et al., 1991; Pardi et al., 1989; Smith et al., 1991; Pelletier et al., 1992; Schwartz, 1993; Berk et al., 1995; Sjaastad et al., 1996; Ng-Sikorski et al., 1991; Smith et al., 1991; Pelletier et al., 1992; Schwartz, 1993; Berk et al., 1995; Sjaastad et al., 1996). We have previously reported that RGD-containing peptides and ECM components, as well as activating antibodies against αv, α5, β1 and β3 integrins upregulate trophoblast adhesion to FN (Wang et al., 2002). Since intracellular Ca2+ signaling is required for this process, FN-120 could, in binding to these integrins, initiate Ca2+ influx through specific Ca2+ channels located on the apical surface of adhesive trophoblast cells. Ligation of αvβ3 and α5β1 integrins by RGD-containing ECM components produces Ca2+ influx through L-type Ca2+ channels (Wu et al., 1998). However, antagonists of most known voltage-dependent Ca2+ channels were unable to abolish the ability of FN-120 to elevate either intracellular Ca2+ or FN-binding activity of peri-implantation mouse blastocysts. Treatment with all channel blockers simultaneously was also without inhibitory effect (data not shown), and general inhibition of Ca2+ influx by NiCl2 was ineffective, arguing against the involvement of multiple channels that compensate for one another. Therefore, Ca2+ influx does not appear to participate in integrin-mediated Ca2+ signaling that leads to strong adhesion of mouse blastocysts to FN.
Thapsigargin, which depletes the Ins(1,4,5)P3-sensitive intracellular Ca2+ stores (Lewis and Cahalan, 1995), abolished the ability of FN-120 to elevate intracellular Ca2+, while depletion of stores regulated by the ryanodine receptor with caffeine did not interfere. These findings indicate that trophoblast adhesion to FN induces Ca2+ release from Ins(1,4,5)P3 receptor-operated Ca2+ stores, with or without subsequent capacitative Ca2+ influx. The lack of inhibition by NiCl2 suggests that capacitative Ca2+ influx is not a requirement for integrin signaling in this case, although it could facilitate subsequent trophoblast development. A role for the Ins(1,4,5)P3 receptor in mobilizing intracellular Ca2+ was supported by the observed inhibition by Xestospongin C. Production of Ins(1,4,5)P3 depends on hydrolysis of phosphatidylinositol 4,5-bisphosphate by PLC (Rhee and Bae, 1997; Rebecchi and Pentyala, 2000; Rebecchi and Pentyala, 2000), which is activated by integrins in several cell types (Kanner et al., 1993; Cybulsky et al., 1993; Cybulsky et al., 1993). PLC activity was required for Ca2+ signaling and the upregulation of FN-binding activity in mouse trophoblast cells, as demonstrated by specific inhibition with U73122.
Adhesion of fibroblasts to FN or treatment with monoclonal antibodies against FN-binding integrins recruits PLC-γ into a membrane-associated complex in a PTK-dependent manner (Miyamoto et al., 1995). Activation of PLC-γ through tyrosine phosphorylation occurs when platelets bind to collagen (Blake et al., 1994; Ichinohe et al., 1995) and tyrosine phosphorylation of PLC-γ is widely reported during integrin-mediated outside-in signaling (Kanner et al., 1993; Blake et al., 1994; Ichinohe et al., 1995; Blake et al., 1994; Ichinohe et al., 1995). When activation of PLC is blocked using PTK inhibitors, both intracellular Ca2+ signaling and strengthening of cell adhesion are prevented (Ichinohe et al., 1995; Melford et al., 1997; Melford et al., 1997). In peri-implantation blastocysts, inhibition of PTK activity blocked the ability of FN-120 to elevate intracellular Ca2+ and upregulate FN-binding activity, which strongly favors a role for PLC-γ in mediating integrin signaling by trophoblast cells adhering to FN. Indeed, recent investigations have established a central role for PLC-γ in the tyrosine kinase-based signaling cascades that regulate cell adhesion via integrins (Jones et al., 2005; Tvorogov et al., 2005; Watson et al., 2005). Depending on cell type and the initiating signaling source, various PTKs were found to participate in PLC-γ phosphorylation, as well as upstream and downstream transduction of signaling. The cytoplasmic PTK, Src, is required for activation of PLC-γ1 in fibroblasts (Tvorogov et al., 2005) and a variety of other cell types (Jones et al., 2005) cultured on fibronectin, laminin, collagen or complex basement membrane, as well as in platelets adhering to collagen or fibrinogen (Watson et al., 2005). Cell and platelet spreading in response to integrin engagement, which involves formation of lamellipodia, fails in the absence of PLC-γ activation (Jones et al., 2005; Tvorogov et al., 2005; McCarty et al., 2004; Wonerow et al., 2003). Strong adhesion to FN at the apical surface of trophoblast cells appears to require both PTK and PLC activities, and subsequently leads to cell spreading and migration on the ECM. Our findings suggest that invasive trophoblast differentiation requires the acquisition of integrin signaling pathways that share many elements with other cell types, particularly PLC-γ activation.
In adhesion-competent trophoblast cells, both PLC-β and PLC-γ isoforms were expressed. The finding that suramin did not interfere with upregulation of FN-binding activity diminishes the likelihood of a role in this pathway for PLC-β or other PLC isoforms activated by heterotrimeric G proteins. Suramin inhibits GDP-GTP exchange, the rate limiting step in the activation of Gα-subunits, making it a fairly broad inhibitor of heterotrimeric G proteins (Freissmuth et al., 1996). Suramin has poor specificity in that it can inhibit other signaling molecules, but its lack of effect on FN-binding activity supports the interpretation that Gα proteins are not involved. However, we cannot exclude the possible involvement of suramin-resistant PLC isozymes that are activated by small GTPases. Due to complex crosstalk, it is conceivable, for example, that PLC-ε could be activated by Ras family GTPases downstream of PTK (Harden and Sondek, 2006). Rho expression and function has been reported as early as the 8-cell stage during mouse development (Clayton et al., 1999). The small GTPases Rho, Rac and Cdc42 are all highly expressed by rat Rcho-1 choriocarcinoma cells and mouse secondary trophoblast giant cells, and appear to be developmentally regulated during trophoblast differentiation (Parast et al., 2001; El Hashash and Kimber, 2006). At this time, the expression of additional PLC isoforms by mouse blastocysts and the role of small GTPases in the upregulation of FN-binding activity are unknown.
The pattern of the expression of both PLC-γ1 and PLC-γ2 during preimplantation development has not previously been investigated, although it is known that both isozymes are expressed in mouse eggs (Mehlmann et al., 1998). We report that the relative levels of both isozymes decrease at the blastocyst stage, a pattern suggesting that both are initially maternal gene products. In adhesion-competent trophoblast cells of late stage blastocysts, levels of PLC-γ2 specifically increased, although both isozymes were detectable. Unlike PLC-γ1, which was distributed primarily within the cytoplasm, and PLC-β1, which localized near cell-cell junctions, PLC-γ2 was located near the apical surface of trophoblast cells. Therefore, PLC-γ2 was strongly expressed in the vicinity of integrins that mediate blastocyst interaction with maternal ECM. Activation of PLC-γ2 immediately after exposure to FN-120 was established by monitoring its phosphorylation at tyrosine 1217. No evidence of PLC-γ1 phosphorylation was found using antibodies from two commercial sources that recognize an epitope containing phosphorylated tyrosine at position 783. We conclude that PLC-γ2 is a prime candidate to mediate FN-induced Ca2+ signaling required for strong adhesion to FN during blastocyst implantation.
Knockout of the PLC-γ1 gene in mice causes significant embryonic growth retardation, although no specific systemic defects were noted (Ji et al., 1997). Interestingly, developmental defects, including embryonic death, occur only after GD 8.5, and no placental defects were described. PLC-γ2 deficient mice are viable and apparently have a normal implantation phenotype (Wang et al., 2000a). The possibility that the two PLC-γ isozymes are able to compensate for one another during implantation has not been investigated and no information is yet available regarding a double knockout. Signaling molecules that regulate integrin-mediated adhesion are numerous and frequently have overlapping functions, reducing the likelihood that deletion of a single component will generate a phenotype (Watson et al., 2005). Within the enedometrium, the blastocyst encounters a complex matrix that contains not just FN, but numerous components. We have examined FN as an archetypical ECM component, demonstrating its ability to precipitate intracellular signaling that directs invasive trophoblast differentiation. It remains to be determined whether this response is typical of interaction with other ECM components or if there is diversity among the effects of each element to fine tune trophoblast phenotype.
Our study strongly suggests a critical role for PLC-γ in FN-mediated intracellular Ca2+ signaling that leads to strengthening of trophoblast adhesion to FN. A deeper understanding of the developmental mechanism that controls blastocyst implantation awaits elaboration of the molecular cascade that regulates PLC-γ activity in trophoblast cells. The biochemical data presented here clearly indicate that PLC-γ activity plays a major role in early trophoblast differentiation and its conversion to an invasive phenotype as directed through interactions with endometrial ECM components.
ACKNOWLEDGEMENTS
This research was supported by National Institutes of Health (NIH) grants HD 36764 and AA12057 to D.R.A., a grant from the Department of Obstetrics and Gynecology at Wayne State University and, in part, by NIH center grants P30ES06639 and P30CA22453. Mouse trophoblast stem cells were generously provided by Dr. Janet Rossant, University of Toronto and the HTR-8/Svneo cells were a gift from Dr. Charles Graham, Queen's University. We thank Brian Kilburn for technical assistance and Dr. Daniel Rappolee for help with trophoblast stem cell culture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulincell adhesion molecules, and selectins. Pharm.Rev. 1998;50:197–263. [PubMed] [Google Scholar]
- Armant DR. Blastocysts don't go it alone. Extrinsic signals fine-tune the intrinsic developmental program of trophoblast cells. Dev.Biol. 2005;280:260–280. doi: 10.1016/j.ydbio.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armant DR. Blastocyst culture. In: Soares MJ, Hunt JS, editors. Placenta and trophoblast methods and protocols, volume I. Humana Press; Totowa, NJ: 2006. pp. 35–56. [Google Scholar]
- Berk BC, Corson MA, Peterson TE, Tseng H. Protein kinases as mediators of fluid shear stress stimulated signal transduction in endothelial cells: a hypothesis for calcium-dependent and calcium-independent events activated by flow. Journal of Biomechanics. 1995;28:1439–1450. doi: 10.1016/0021-9290(95)00092-5. [DOI] [PubMed] [Google Scholar]
- Blake RA, Schieven GL, Watson SP. Collagen stimulates tyrosine phosphorylation of phospholipase C-gamma 2 but not phospholipase C-gamma 1 in human platelets. FEBS Lett. 1994;353:212–216. doi: 10.1016/0014-5793(94)01037-4. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu.Rev.Cell Dev.Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo Implantation. Dev.Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- Chan WL, Holstein-Rathlou NH, Yip KP. Integrin mobilizes intracellular Ca(2+) in renal vascular smooth muscle cells. Am.J.Physiol.Cell Physiol. 2001;280:C593–C603. doi: 10.1152/ajpcell.2001.280.3.C593. [DOI] [PubMed] [Google Scholar]
- Clayton L, Hall A, Johnson MH. A role for Rho-like GTPases in the polarisation of mouse eight-cell blastomeres. Dev.Biol. 1999;205:322–331. doi: 10.1006/dbio.1998.9117. [DOI] [PubMed] [Google Scholar]
- Cybulsky AV, Carbonetto S, Cyr MD, McTavish AJ, Huang Q. Extracellular matrix-stimulated phospholipase activation is mediated by beta 1-integrin. Am.J.Physiol. 1993;264:C323–C332. doi: 10.1152/ajpcell.1993.264.2.C323. [DOI] [PubMed] [Google Scholar]
- El Hashash AH, Kimber SJ. PTHrP induces changes in cell cytoskeleton and Ecadherin and regulates Eph/Ephrin kinases and RhoGTPases in murine secondary trophoblast cells. Dev.Biol. 2006;290:13–31. doi: 10.1016/j.ydbio.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Emerson M, Travis AR, Bathgate R, Stojanov T, Cook DI, Harding E, Lu DP, O'Neill C. Characterization and functional significance of calcium transients in the 2-cell mouse embryo induced by an autocrine growth factor. J.Biol.Chem. 2000;275:21905–21913. doi: 10.1074/jbc.M001719200. [DOI] [PubMed] [Google Scholar]
- Freissmuth M, Boehm S, Beindl W, Nickel P, Ijzerman AP, Hohenegger M, Nanoff C. Suramin analogues as subtype-selective G protein inhibitors. Mol.Pharmacol. 1996;49:602–611. [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Harden TK, Sondek J. Regulation of phospholipase C isozymes by ras superfamily GTPases. Annu.Rev.Pharmacol.Toxicol. 2006;46:355–379. doi: 10.1146/annurev.pharmtox.46.120604.141223. [DOI] [PubMed] [Google Scholar]
- Ichinohe T, Takayama H, Ezumi Y, Yanagi S, Yamamura H, Okuma M. Cyclic AMP-insensitive activation of c-Src and Syk protein-tyrosine kinases through platelet membrane glycoprotein VI. J.Biol.Chem. 1995;270:28029–28036. doi: 10.1074/jbc.270.47.28029. [DOI] [PubMed] [Google Scholar]
- Ji QS, Winnier GE, Niswender KD, Horstman D, Wisdom R, Magnuson MA, Carpenter G. Essential role of the tyrosine kinase substrate phospholipase C-gamma1 in mammalian growth and development. Proc.Natl.Acad.Sci.USA. 1997;94:2999–3003. doi: 10.1073/pnas.94.7.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NP, Peak J, Brader S, Eccles SA, Katan M. PLCgamma1 is essential for early events in integrin signalling required for cell motility. J.Cell Sci. 2005;118:2695–2706. doi: 10.1242/jcs.02374. [DOI] [PubMed] [Google Scholar]
- Kanner SB, Grosmaire LS, Ledbetter JA, Damle NK. Beta 2-integrin LFA-1 signaling through phospholipase C-gamma 1 activation. Proc.Natl.Acad.Sci.USA. 1993;90:7099–7103. doi: 10.1073/pnas.90.15.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLAG and integrins in a human trophoblast cell line. Biol.Reprod. 2000;62:739–747. doi: 10.1095/biolreprod62.3.739. [DOI] [PubMed] [Google Scholar]
- Leavesley DI, Schwartz MA, Rosenfeld M, Cheresh DA. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J.Cell Biol. 1993;121:163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS, Cahalan MD. Potassium and calcium channels in lymphocytes. Annu.Rev.Immunol. 1995;13:623–653. doi: 10.1146/annurev.iy.13.040195.003203. [DOI] [PubMed] [Google Scholar]
- Liu Z, Armant DR. Lysophosphatidic acid regulates murine blastocyst development by transactivation of receptors for heparin-binding EGF-like growth factor. Exp. Cell Res. 2004;296:317–326. doi: 10.1016/j.yexcr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- McCarty OJ, Zhao Y, Andrew N, Machesky LM, Staunton D, Frampton J, Watson SP. Evaluation of the role of platelet integrins in fibronectin-dependent spreading and adhesion. J.Thromb.Haemost. 2004;2:1823–1833. doi: 10.1111/j.1538-7836.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA. SH2 domain-mediated activation of phospholipase Cgamma is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev.Biol. 1998;203:221–232. doi: 10.1006/dbio.1998.9051. [DOI] [PubMed] [Google Scholar]
- Melford SK, Turner M, Briddon SJ, Tybulewicz VL, Watson SP. Syk and Fyn are required by mouse megakaryocytes for the rise in intracellular calcium induced by a collagen-related peptide. J.Biol.Chem. 1997;272:27539–27542. doi: 10.1074/jbc.272.44.27539. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J.Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng-Sikorski J, Andersson R, Patarroyo M, Andersson T. Calcium signaling capacity of the CD11b/CD18 integrin on human neutrophils. Exp.Cell Res. 1991;195:504–508. doi: 10.1016/0014-4827(91)90402-g. [DOI] [PubMed] [Google Scholar]
- Parast MM, Aeder S, Sutherland AE. Trophoblast giant-cell differentiation involves changes in cytoskeleton and cell motility. Dev.Biol. 2001;230:43–60. doi: 10.1006/dbio.2000.0102. [DOI] [PubMed] [Google Scholar]
- Pardi R, Bender JR, Dettori C, Giannazza E, Engleman EG. Heterogeneous distribution and transmembrane signaling properties of lymphocyte function-associated antigen (LFA-1) in human lymphocyte subsets. J.Immunol. 1989;143:3157–3166. [PubMed] [Google Scholar]
- Pelletier AJ, Bodary SC, Levinson AD. Signal transduction by the platelet integrin alpha IIb beta 3: induction of calcium oscillations required for protein-tyrosine phosphorylation and ligand-induced spreading of stably transfected cells. Mol.Biol.Cell. 1992;3:989–998. doi: 10.1091/mbc.3.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JWJ, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J.Cell Sci. 2001;114:2223–2229. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol.Rev. 2000;80:1291–1335. [Google Scholar]
- Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J.Biol.Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- Rout UK, Wang J, Paria BC, Armant DR. alpha5beta1, alphaVbeta3 and the platelet-associated integrin alphaIIbbeta3 coordinately regulate adhesion and migration of differentiating mouse trophoblast cells. Dev.Biol. 2004;268:135–151. doi: 10.1016/j.ydbio.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Schottelndreier H, Potter BV, Mayr GW, Guse AH. Mechanisms involved in alpha6beta1-integrin-mediated Ca(2+) signalling. Cell.Signal. 2001;13:895–899. doi: 10.1016/s0898-6568(01)00225-x. [DOI] [PubMed] [Google Scholar]
- Schultz JF, Armant DR. Beta1- and beta3-class integrins mediate fibronectin binding activity at the surface of developing mouse peri-implantation blastocysts. Regulation by ligand-induced mobilization of stored receptor. J.Biol.Chem. 1995;270:11522–11531. doi: 10.1074/jbc.270.19.11522. [DOI] [PubMed] [Google Scholar]
- Schultz JF, Mayernik L, Rout UK, Armant DR. Integrin trafficking regulates adhesion to fibronectin during differentiation of mouse peri-implantation blastocysts. Dev.Genet. 1997;21:31–43. doi: 10.1002/(SICI)1520-6408(1997)21:1<31::AID-DVG4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Schwartz MA. Spreading of human endothelial cells on fibronectin or vitronectin triggers elevation of intracellular free calcium. J.Cell Biol. 1993;120:1003–1010. doi: 10.1083/jcb.120.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjaastad MD, Lewis RS, Nelson WJ. Mechanisms of integrin-mediated calcium signaling in MDCK cells: regulation of adhesion by IP3- and store-independent calcium influx. Mol.Biol.Cell. 1996;7:1025–1041. doi: 10.1091/mbc.7.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JB, Dangelmaier C, Selak MA, Daniel JL. Facile platelet adhesion to collagen requires metabolic energy and actin polymerization and evokes intracellular free calcium mobilization. J.Cell.Biochem. 1991;47:54–61. doi: 10.1002/jcb.240470108. [DOI] [PubMed] [Google Scholar]
- Somogyi L, Lasic Z, Vukicevic S, Banfic H. Collagen type IV stimulates an increase in intracellular Ca2+ in pancreatic acinar cells via activation of phospholipase C. Biochem.J. 1994;299:603–611. doi: 10.1042/bj2990603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachecki JJ, Armant DR. Transient release of calcium from inositol 1,4,5-trisphosphate- specific stores regulates mouse preimplantation development. Development. 1996;122:2485–2496. doi: 10.1242/dev.122.8.2485. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- Tvorogov D, Wang XJ, Zent R, Carpenter G. Integrin-dependent PLC-gamma1 phosphorylation mediates fibronectin-dependent adhesion. J.Cell Sci. 2005;118:601–610. doi: 10.1242/jcs.01643. [DOI] [PubMed] [Google Scholar]
- van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- Wang D, Feng J, Wen R, Marine JC, Sangster MY, Parganas E, Hoffmeyer A, Jackson CW, Cleveland JL, Murray PJ, Ihle JN. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity. 2000a;13:25–35. doi: 10.1016/s1074-7613(00)00005-4. [DOI] [PubMed] [Google Scholar]
- Wang H, Matsumoto H, Guo Y, Paria BC, Roberts RL, Dey SK. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc.Natl.Acad.Sci.U.S.A. 2003;100:14914–14919. doi: 10.1073/pnas.2436379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Armant DR. Integrin-mediated adhesion and signaling during blastocyst implantation. Cells Tissues Organs. 2002;172:190–201. doi: 10.1159/000066970. [DOI] [PubMed] [Google Scholar]
- Wang J, Mayernik L, Armant DR. Integrin signaling regulates blastocyst adhesion to fibronectin at implantation: intracellular calcium transients and vesicle trafficking in primary trophoblast cells. Dev.Biol. 2002;245:270–279. doi: 10.1006/dbio.2002.0644. [DOI] [PubMed] [Google Scholar]
- Wang J, Mayernik L, Schultz JF, Armant DR. Acceleration of trophoblast differentiation by heparin-binding EGF-like growth factor is dependent on the stage-specific activation of calcium influx by ErbB receptors in developing mouse blastocysts. Development. 2000b;127:33–44. doi: 10.1242/dev.127.1.33. [DOI] [PubMed] [Google Scholar]
- Wang J, Rout UK, Bagchi IC, Armant DR. Expression of calcitonin receptors in mouse preimplantation embryos and their function in the regulation of blastocyst differentiation by calcitonin. Development. 1998;125:4293–4302. doi: 10.1242/dev.125.21.4293. [DOI] [PubMed] [Google Scholar]
- Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J.Thromb.Haemost. 2005;3:1752–1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- Wonerow P, Pearce AC, Vaux DJ, Watson SP. A critical role for phospholipase Cgamma2 in alphaIIbbeta3-mediated platelet spreading. J.Biol.Chem. 2003;278:37520–37529. doi: 10.1074/jbc.M305077200. [DOI] [PubMed] [Google Scholar]
- Wu X, Mogford JE, Platts SH, Davis GE, Meininger GA, Davis MJ. Modulation of calcium current in arteriolar smooth muscle by alphav beta3 and alpha5 beta1 integrin ligands. J.Cell Biol. 1998;143:241–252. doi: 10.1083/jcb.143.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelian FD, Yang Y, Hirata JD, Schultz JF, Armant DR. Molecular interactions between fibronectin and integrins during mouse blastocyst outgrowth. Mol.Reprod.Dev. 1995;41:435–448. doi: 10.1002/mrd.1080410406. [DOI] [PubMed] [Google Scholar]