Abstract
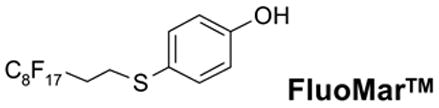
The fluorous counterpart of the Marshall resin, 4-(1H,1H,2H,2H-perfluorodecylsulfanyl)phenol (FluoMar™) is prepared by S-alkylation of 4-mercaptophenol with C8F17CH2CH2I and employed in the synthesis of amide and diamide analogs. The final products are purified by solid-phase extraction (SPE) over FluoroFlash™ silica cartridges.
Resin-based solid-phase organic synthesis (SPOS) is popular in drug discovery.1 Its advantage of easy separation, however, is usually counterbalanced by the time-consuming method development, the limitation of reaction scope, and the difficulty of analysis and purification of attached intermediates. Recently Curran and coworkers developed a fluorous tag strategy to overcome some disadvantages associated with the SPOS.2 Functionalized perfluoroalkyl groups instead of polymer supports are employed as the “phase tags”.2b,3 The separation of fluorous-tagged molecules is carried out over fluorous silica gel based on the strong and selective fluorine-fluorine interaction.4
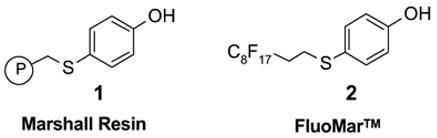
The Marshall resin 1 has been widely used as a carbonate and carbamate linker in solid-phase syntheses.5 The linker can be cleaved with primary and secondary amines to afford the corresponding amides either directly or after oxidation of the sulfide to the sulfone.6 Described in this paper is the synthesis of fluorous version of Marshall resin, perfluoroalkylsulfanylphenol 2 (FluoMar™). The utility of this compound is illustrated by the solution-phase synthesis of amides and diamides.
The FluoMar™ 2 was readily prepared by S-alkylation of 4-mercaptophenol with C8F17CH2CH2I and purified by flash column chromatography on normal silica gel (Scheme 1).7,8 The ethylene spacer between the C8F17 tag and the sulfur is expected to minimize the strong electron-withdrawing effect from the perfluoroalkyl group and maintain the nucleophilicity of the hydroxy group. This compound has the general features of organic molecules; it dissolves well in common solvents such as CH2Cl2, THF, and AcOEt, and can be analyzed by traditional chromatographic and spectroscopic methods.
Scheme 1.
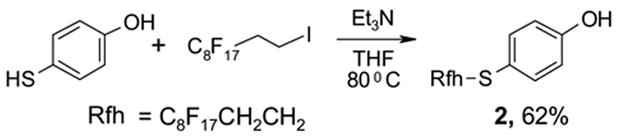
Preparation of FluoMar™
With compound 2 in hand, we first validated the attachment to carboxylic acids 3 and the tag cleavage by the amine displacement (Scheme 2). The coupling of 2 with indole-5-carboxylic acid 3{1} (2.0 equiv) or 7-methoxy-2-benzofurancarboxylic acid 3{2} was carried out under a standard solution-phase conditions with 2.0 equiv of diisopropylcarbodiimide (DIC) and 1.0 equiv of dimethylaminopyridine (DMAP) in DMF. These intermediates were purified by regular flash column chromatography. Compounds 4{1} and 4{2} were each split to three portions and directly displaced with three primary amines 5{1-3} without oxidation of the sulfur to give the corresponding amides 6{1-2,1-3}. After a quick acidic workup with 1.0 N HCl to remove the unreacted amine,9 the crude product was loaded onto a FluoroFlash™ cartridge and the MeOH/H2O fraction was collected to give analytically pure product. The FluoMar™ tag 2 was recovered in the MeOH fraction in 65–70% yield.
Scheme 2.
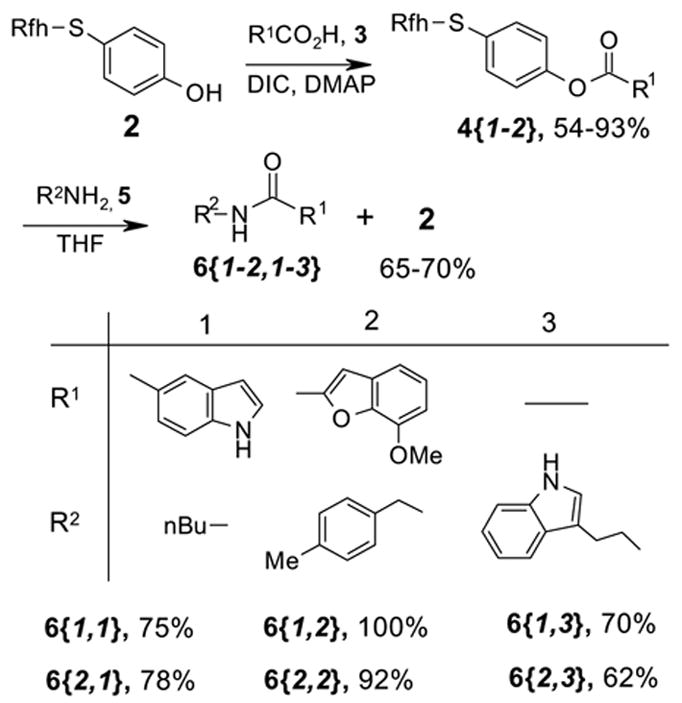
Synthesis of 2x3 array of amides 6{R1,R2}
Encouraged by the preliminary results, we next explored the use of FluoMar™ 2 in a multi-step parallel synthesis of diamides (Scheme 3). The N-Boc isonipecotic acid 7 was coupled with 2 followed by deprotection with TFA and N-acylation with three different acid halides. The resulting compounds 9{1-3} were each split into three portions and displaced by three amines resulting in a demonstration library of diamides 10{1-3,1-3}.11 The final products were purified by SPE and cleaved FluoMar™ 2 was recovered in an average yield of 65%. Figure 1 shows a typical 1H NMR trace of the products prior and after SPE; the crude mixture containing 10{1,2} and cleaved tag 2 (top trace of Figure 1), the MeOH/H2O fraction containing 10{1,2} (middle trace of Figure 1), and the MeOH fraction containing recovered FluoMar 2 (bottom trace of Figure 1).
Scheme 3.
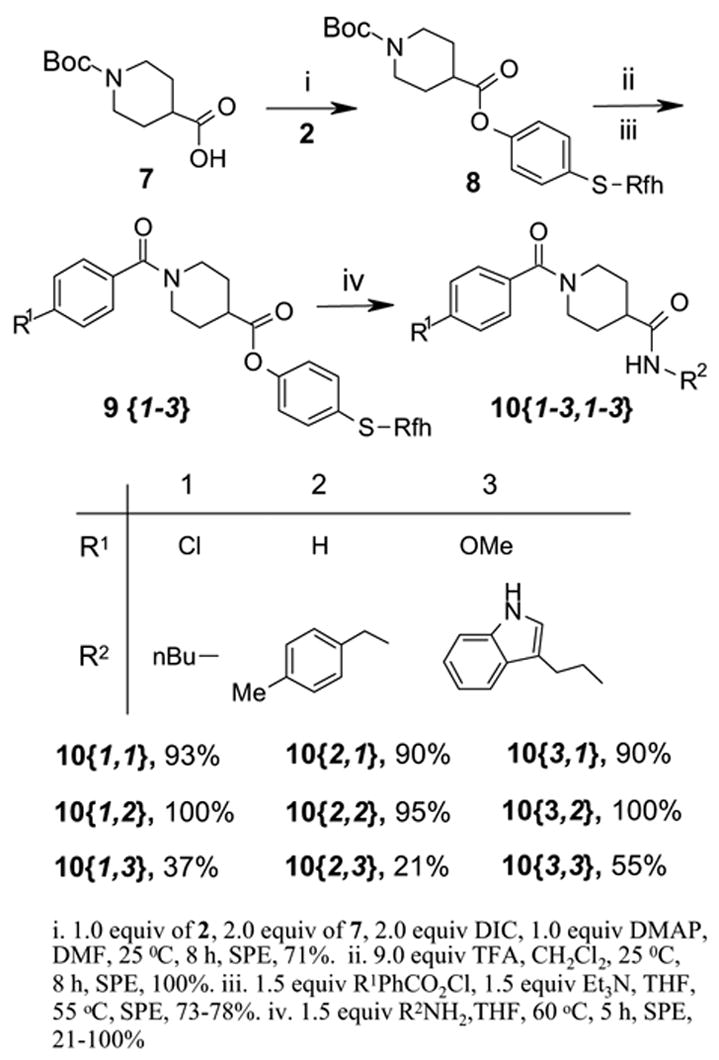
Synthesis of 3x3 array of diamides 10{R1,R2}
Figure 1.
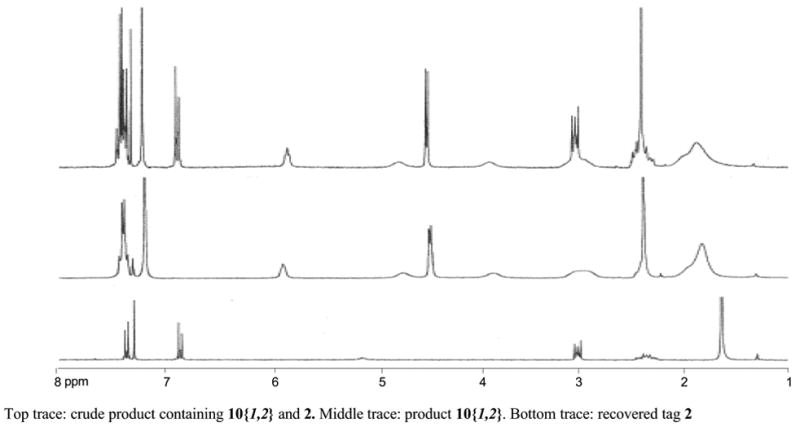
1H NMR spectra of product 10{1,2} and recovered 2
We also carried out a simple experiment to estimate the reactivity difference between the Marshall resin 1 and FluoMar™ 2 towards a typical carboxylic acid. Equimolar amounts of 112 and 2 (1.0 equiv each) were mixed with 1.0 equiv of benzofuran carboxylic acid under a standard coupling condition used in the synthesis of amide 4 and diamides 8 (Scheme 4). The reaction was stopped after 8 h when all the acid was consumed as indicated by TLC analysis. The resin was filtered off from the reaction mixture and washed with DMF and CH2Cl2. The filtrate was analyzed by the HPLC and the ratio of product 11 to the unreacted 2 was 73:27 with the assumption that the 27% unreacted FluoMar™ 2 was due to a competitive reaction of the acid 3 with the Marshall resin. This result suggested that despite the electron withdrawing effect of the fluorous chain, fluorous tag still reacted as least 2.7 times faster than the resin.
Scheme 4.
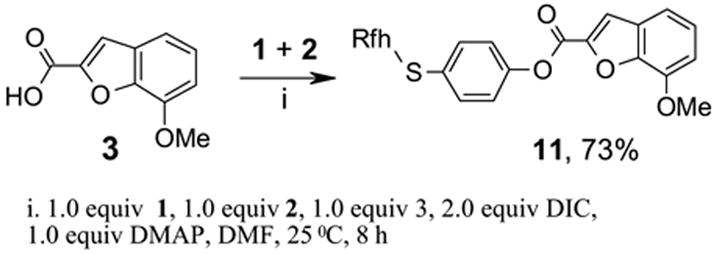
Competitive tagging of 3 with Marshall resin 1 and FluoMar™ 2
recyclable phase tag in the solution-phase synthesis of amides and diamides. This reagent can be used as an alternative to the Marshall resin in combinatorial and parallel synthesis.
Acknowledgments
We thank Dr. John Hodges and Professor Dennis Curran for important suggestions and helpful discussions.
References
- 1.Reviews: ; (a) Guillier F, Orain D, Bradley M.Chem Rev> 20001002091. [DOI] [PubMed] [Google Scholar]; (a) Dorwald FZ. Organic Synthesis on Solid Phase. Wiley-VCH; Weinheim: 2000. [Google Scholar]; (b) Burgess K. Solid Phase Organic Synthesis. John Wiley & Sons; New York: 2000. [Google Scholar]; (c) Nicolaou KC, Hanko R, Hartwig W, editors. Handbook of Combinatorial Chemistry. 1–2 Wiley-VCH; Weinheim: 2002. [Google Scholar]
- 2.Curran DP. Chemtracts-Org Chem. 1996;9:75. [Google Scholar]; (b) Curran DP. Angew Chem Int Ed Eng. 1998;37:1175. [Google Scholar]; (c) Curran DP. In: In Stimulating Concepts in Chemistry. Stoddard F, Reinhoudt D, Shibasaki M, editors. Wiley-VCH; New York: 2000. p. p25. [Google Scholar]; (d) Curran DP, Hadida S, Studer A, He M, Kim S-Y, Luo Z, Larhed M, Hallberg M, Linclau B. In: In Combinatorial Chemistry: A Practical Approach. Fenniri H, editor. Vol. 2. Oxford University Press; Oxford: 2001. p. 327. [Google Scholar]
- 3.(a) Yoshida J-I, Itami K. Chem Rev. 2002;102:3693. doi: 10.1021/cr0103524. [DOI] [PubMed] [Google Scholar]; (b) Tzschucke CC, Markert C, Bannwarth W, Roller S, Hebel A, Haag R. Angew Chem Int Ed. 2002;41:3964. doi: 10.1002/1521-3773(20021104)41:21<3964::AID-ANIE3964>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Curran DP. Synlett. 2001:1488. [Google Scholar]
- 5.Marshall DL, Liener IE. J Org Chem. 1970;35:867. [Google Scholar]
- 6.(a) Johnson CR, Zhang B, Fantauzzi P, Hocker M, Yager KM. Tetrahedron. 1998;54:4097. [Google Scholar]; (b) Breitenbucher JG, Johnson CR, Haight M, Phelan JC. Tetrahedron Lett. 1998;39:1295. [Google Scholar]; (c) Fantauzzi PP, Yager KM. Tetrahedron. 1998;39:1291. [Google Scholar]; (d) Dressman B, Singh U, Kaldor SW. Tetrahedron Lett. 1998;39:3631. [Google Scholar]; (a) Breitenbucher JG, Hui HC. Tetrahedron Lett. 1998;39:8207. [Google Scholar]; (b) Yan B, Nguyen N, Liu L, Holland G, Raju B. J Com Chem. 2000;2:66. doi: 10.1021/cc990051w. [DOI] [PubMed] [Google Scholar]; (c) Beech C, Coope J, Fairley G, Gilgert P, Main B, Ple K. J Org Chem. 2001;66:2240. doi: 10.1021/jo0012086. [DOI] [PubMed] [Google Scholar]; (d) Fang L, Demee M, Sierra T, Kshirsagar T, Celebi AA, Yan B. J Comb Chem. 2002;4:362. doi: 10.1021/cc020010r. [DOI] [PubMed] [Google Scholar]
- 7.Selective S-alkylation of 4-mercaptophenol see: ; Breitenbucher JG, Johnson CR, Haight M, Phelan JC. Tetrahedron Lett. 1998;39:1295. [Google Scholar]
- 8. Perfluoroalkylsulfanylphenol 2 has been used as a photoimaging compound see: Wakamatsu, K.; Wakata, Y.; Satomura, M.; Namiki, T. European Patent 468531 A1, 1992.
- 9. Our recent study and an independent work from Lindsley (ref 10) demonstrated that amines could be retained on the SPE cartridge by adding acidic ion exchange resin on top of the fluorous silica. No more acidic workup was needed prior to the SPE.
- 10.Lindsley CW, Zhao Z, Leister WH, Strauss KA. Tetrahedron Lett. 2002;43:6319. [Google Scholar]
- 11. A general protocol for the synthesis of diamide 10 from 9. FluoMar™ bound intermediate 9 (0.127 mmol) was dissolved in THF (1.0 mL) in a capped vial. The amine (1.2 equiv) was added and the reaction mixture was stirred at 60 °C for 5 h. An aqueous solution of 1.0 N HCl (1.0 ml) was added and the vial was shaken with 0.5 ml of EtOAc. The EtOAc layer was loaded onto a 2 g FluoroFlash™ cartridge and eluted with MeOH/H2O (80/20). The first 8 mL fraction contained the desired product and the subsequent fraction eluted with MeOH contained the displaced fluorous tag.
- 12. Purchased from Aldrich, loading 1.0–1.5 mmol/g, polystyrene with 1% cross linking with DVB. An average loading of 1.25 mmol/g was used for the calculation.


