Abstract
Coupling of microwave reactions with fluorous separations can dramatically increase the efficiency of high-speed synthesis. Described in this paper is a fluorous synthesis of aryl sulfides by palladium-catalyzed cross-coupling of aryl perfluoroalkylsulfonates (C8F17O2SOAr) with thiols (RSH) under microwave irradiation. Fluorous solid-phase extractions (F-SPE) are employed for the purification of reaction mixtures. No fluorous solvents are involved in reaction and separation processes. The fluorous synthesis is further extended to the multi-step synthesis of substituted hydantoin and amide scaffolds.
Keywords: microwave reaction, fluorous synthesis, solid phase extraction, cross coupling, perfluoroalkylsulfonates, palladium catalysts, aryl sulfides
Microwave irradiation has been recognized as an alternative to conventional heating in organic synthesis.1 With the enhanced efficiency, microwave reactions can finish in minutes and frequently with improvements in reaction selectivity and yield. Combination of microwave technology with tag-based separations2 such as solid-phase3 and fluorous synthesis provides a good opportunity to further improve the productivity in high-speed synthesis.
Fluorous synthesis is a complementary type of liquid-phase synthesis that employs perfluoroalkyl groups as a “phase tag” to facilitate the separation.4 Fluorous tags have good thermostability and solution-phase reactivity, which are free from some constraints of polymer supports under microwave irradiation. Larhed, Hallberg and Curran first introduced fluorous strategy into microwave synthesis.5 In their work of the Stille-coupling of organohalides with PhSn(CH2CH2C10F21)3, a “heavy fluorous” tag containing three C10F21 groups was employed to ensure the partition of the fluorous species during the fluorous liquid-liquid extraction. We recently explored the Suzuki coupling of fluorous aryl perfluorosulfonates with organoboronic acids6 and found that perfluoroalkylsulfonates (C8F17O2SOAr) had similar reactivity as the aryl triflates. The C8F17 chain can be utilized as a fluorous tag for the F-SPE separation.7
Aryl triflates, just like halides, are important species for both solution-phase and solid-phase palladium-catalyzed reactions.1c,8 The aryl triflates can easily be prepared from a large selection of commercially available phenols. Using aryl triflates as starting materials, Zheng and coworkers reported the synthesis of aryl sulfides under thermal conditions.9 The reaction conditions were mild (Pd(dba)3, Tol-BINAP, NaOt-Bu or NaHMDS, toluene, 100 °C), but reactions were relatively slow (12–24 h). Herein we report two improvements to this reaction: 1) use of microwave to speed up the reaction; and 2) use of fluorous tag to simplify the separation.
Two commercially available phenols were converted to the sulfonates 1 by reacting with C8F17SO2F under a general condition (K2CO3, DMF, 70 °C, 8h) (Scheme 1). The resulting F-sulfonates 1a and 1b were each reacted with four different thiols including aryl, benzyl, hexyl, and cyclohexyl thiols under microwave irradiation. We selected a Suzuki-type coupling condition according to a literature procedure10 using Pd(dppf)Cl2 (0.1 equiv) as a catalyst, K2CO3 (2.0 equiv) as a base, and acetone/toluene/H2O (4:4:1) as a co-solvent. The reaction temperature was in the range of 100 to 150 °C and the reaction time was between 5–10 min. To prevent the unreacted thiol from contaminating the product in MeOH/H2O fraction, a slight excess of F-sulfonate 1 was used in the coupling reaction. After an aqueous workup, the reaction mixture was loaded on the FluoroFlash™ cartridge and purified by SPE. The product was collected at the 80:20 MeOH-H2O elution, while unreacted F-sulfonate and the cleaved tag were retained on the cartridge. The fluorous species were washed out from the cartridge with a more fluorophilic solvent such as MeOH or acetone. The F-SPE cartridge can be conditioned and reused. Scheme 2 shows the structures and yields of aryl sulfides. The purities of the product after the F-SPE were greater than 90% by 1H NMR analysis. Figure 1 shows a typical 1H NMR spectrum of the product after the F-SPE separation.11
Scheme 1.
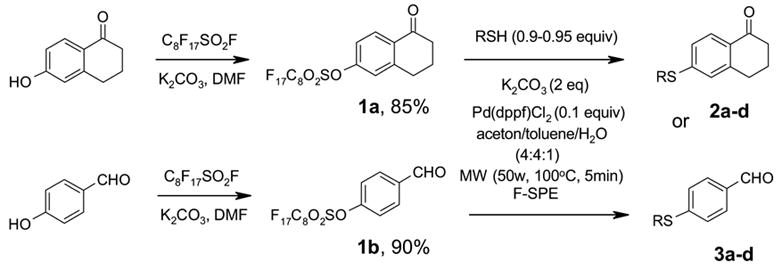
Microwave –assisted fluorous synthesis of aryl sulfides 2 and 3.
Scheme 2.
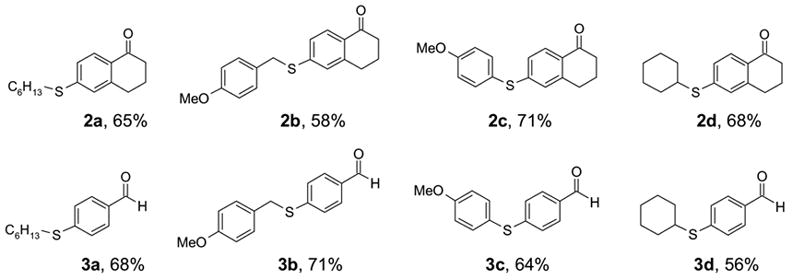
Structures and yields of aryl sulfides
Figure 1.
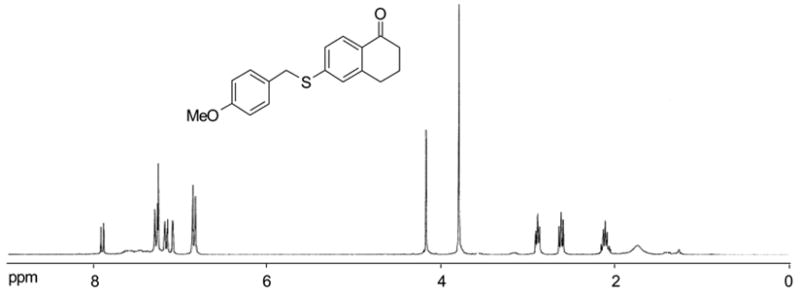
1H NMR (CDCl3) spectrum of 2b after F-SPE
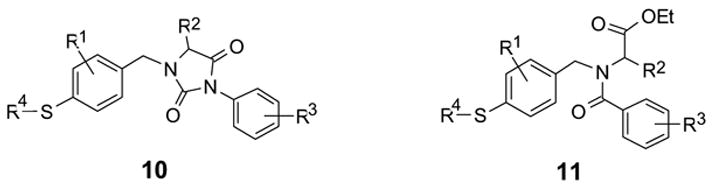
The fluorous tag strategy can be better utilized in multi-step synthesis since all the intermediates bearing the fluorous tags can be purified by F-SPE.14 The synthesis of substituted hydantoin 8 and amide 9 are two examples outlined in Scheme 3. Intermediate 5 was prepared by reductive amination of 1b.15 This compound was then reacted with an isocyanate to form substituted hydantoin 6 or with a benzoyl chloride to form amide 7. Both 6 and 7 were purified by F-SPE. The non-fluorous compounds were collected in the 80:20 MeOH-H2O fraction, while fluorous compounds were collected in the MeOH fraction. Similar fluorous palladium-catalyzed conditions described above were employed to convert F-sulfonates 6 and 7 to the corresponding sulfides 8 and 9, respectively. The substituted hydantoin 10 and amide 11 possesses four points of diversity which are useful scaffolds for parallel and combinatorial synthesis (Scheme 4).
Scheme 3.
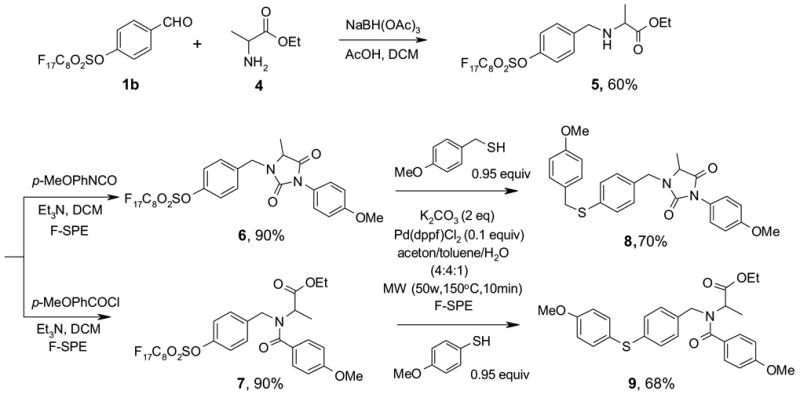
F-Sulfonate-tagged multi-step synthesis of substituted hydantoin 8 and amide 9
Scheme 4.
In summary, we have developed a new path to aryl sulfides by palladium-catalyzed cross coupling of fluorous sulfonates with thiols under microwave irradiation. The combination of microwave and fluorous technologies speeds up both the reaction and the purification processes.
Acknowledgments
We thank the National Institutes of General Medical Sciences for the SBIR funding (1R43GM066415-01).
Notes and references
- 1.Recent reviews on microwave synthesis Loupy A, editor. Microwaves in Organic Synthesis. Wiley-VCH; Weinheim: 2002. Hayes BL. Microwave Synthesis: Chemistry at the Speed of Light. CEM Publishing; Matthews, NC: 2002. Larhed M, Moberg C, Hallberg A. Microwave-Accelerated Homogeneous Catalysis in Organic Chemistry. Acc Chem Res. 2002;35:717–727. doi: 10.1021/ar010074v.Wathey B, Tierney J, Lidström P, Westman J. The Impact of Microwave-Assisted Organic Chemistry on Drug Discovery. Drug Discovery Today. 2002;7:373–380. doi: 10.1016/s1359-6446(02)02178-5.Kappe CO. High-Speed Combinatorial Synthesis Utilizing Microwave Irradiation. Curr Opin Chem Biol. 2002;6:314–320. doi: 10.1016/s1367-5931(02)00306-x.Larhed M, Hallberg A. Microwave-Assisted High-Speed Chemistry: A New Technique in Drug Discovery. Drug Discovery Today. 2001;6:406–416. doi: 10.1016/s1359-6446(01)01735-4.Lidstrom P, Tierney J, Wathey B, Westman J. Microwave Assisted Organic Synthesis - A Review. Tetrahedron. 2001;57:9225–9283.Varma RS. Solvent-Free Accelerated Organic Synthesis Using Microwaves. Pure Appl Chem. 2001;73:193–198.
- 2.Curran DP. Strategy-Level Separations in Organic Synthesis: From Planning to Practice. Angew Chem, Int Ed Eng. 1998;37:1175–1196. doi: 10.1002/(SICI)1521-3773(19980518)37:9<1174::AID-ANIE1174>3.0.CO;2-P. See also Yoshida J, Itami K. Tag Strategy for Separation and Recovery. Chem Rev. 2002;102:3693–3716. doi: 10.1021/cr0103524.Tzschucke CC, Markert C, Bannwarth W, Roller S, Hebel A. Modern Separation Techniques for the Efficient Workup in Organic Synthesis. Angew Chem Int Ed. 2002;41:3964–4000. doi: 10.1002/1521-3773(20021104)41:21<3964::AID-ANIE3964>3.0.CO;2-3.Flynn D. Phase-Trafficking Reagents and Phase-Switching Strategies for Parallel Synthesis. Med Res Rev. 1999;19:408–432. doi: 10.1002/(sici)1098-1128(199909)19:5<408::aid-med7>3.0.co;2-j.
- 3.a) Lidström P, Westman J, Lewis A. Enhancement of Combinatorial Chemistry by Microwave-Assisted Organic Synthesis. Comb Chem High Throughput Screening. 2002;5:441–448. doi: 10.2174/1386207023330147. [DOI] [PubMed] [Google Scholar]; b) Kappe CO. Speeding up Solid-Phase Chemistry by Microwave Irradiation. A Tool for High-Throughput Synthesis. Am Lab. 2001;33:13–19. [Google Scholar]; c) Larhed M, Lindeberg G, Hallberg A. Rapid Microwave-Assisted Suzuki Coupling on Solid-Phase. Tetrahedron Lett. 1996;37:8219–8222. [Google Scholar]
- 4.a) Zhang W. Fluorous Technologies for Solution-Phase High-Throughput Organic Synthesis. Tetrahedron. 2003;59:4475–4489. [Google Scholar]; b) Gladysz JA, Curran DP. Fluorous Chemistry: From Biphasic Catalysis to a Parallel Chemical Universe and Beyond. Tetrahedron. 2002;58:3823–3825. [Google Scholar]; c) Dobbs AP, Kimberley MR. Fluorous Phase Chemistry: A New Industrial Technology. J Fluorine Chem. 2002;118:3–17. [Google Scholar]; d) Curran DP. In: In Stimulating Concepts in Chemistry. Stoddard F, Reinhoudt D, Shibasaki M, editors. Wiley-VCH; New York: 2000. pp. 25–37. [Google Scholar]; e) Curran DP, Hadida S, Studer A, He M, Kim S-Y, Luo Z, Larhed M, Hallberg A, Linclau B. Experimental Techniques in Fluorous Synthesis: A User's Guide. In: Fenniri H, editor. Combinatorial Chemistry: A Practical Approach. Vol. 2. Oxford Univ. Press; Oxford: 2000. pp. 327–352. [Google Scholar]
- 5.a) Larhed M, Hoshino M, Hadida S, Curran DP, Hallberg A. Rapid Fluorous Stille Coupling Reactions Conducted Under Microwave Irradiation. J Org Chem. 1997;62:5583–5587. [Google Scholar]; b) Olofsson K, Kim SY, Larhed M, Curran DP, Hallberg A. High-Speed, Highly Fluorous Organic Reactions. J Org Chem. 1999;64:4539–4541. [Google Scholar]
- 6.Zhang W, Chen CH-T, Lu Y. The palladium-catalyzed microwave reactions were conducted under a license form Personal Chemistry. unpublished results. [Google Scholar]
- 7.Curran DP. Fluorous Reverse Phase Silica Gel. A New Tool for Preparative Separations in Synthetic Organic and Organofluorine Chemistry. Synlett. 2001:1488–1496. [Google Scholar]
- 8.a) Bräse S, Kirchhoff JH, Kobberling J. Palladium-Catalyzed Reaction in Solid Phase Organic Synthesis. Tetrahedron. 2003;59:885–939. [Google Scholar]; b) Negishi E, editor. Hankbook of Palladium Chemistry. Wiley; New York: 2002. [Google Scholar]; c) Beletskaya IP, Cheprakov AV. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem Rev. 2000;100:3009–3066. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]; d) Lorsbury BA, Kurt MJ. Carbon-Carbon Bond Forming Solid-Phase Reactions. Chem Rev. 1999;99:1549–1581. doi: 10.1021/cr970109y. [DOI] [PubMed] [Google Scholar]; e) Bräse S, de Meijere A. In: In Metal-Catalyzed Cross-Coupling Reactions. Diederich F, Stang PJ, editors. Wiley-VCH; Weinheim: 1997. [Google Scholar]
- 9.a) Zheng N, McWilliams JC, Fleitz FJ, Armstrong JD, III, Volante RP. Palladium-Catalyzed Synthesis of Aryl Sulfides from Aryl Triflates. J Org Chem. 1998;63:9606. [Google Scholar]; b) Denmark SE, Sweis RF. Preparation of n-Butyl 4-Chlorophenyl Sulfide. Org Synth. 2002;79:43–51. [Google Scholar]
- 10.Pridgen LN, Huang GK. An Optimized Palladium Catalyzed Cross-Coupling of Nonracemic Trifluoromethylsulfonyl and Fluorosulfonyl Enol Ethers to Arylboronic Acids. Tetrahedron Lett. 1998;39:8421–8424. [Google Scholar]
- 11.General procedures for fluorous palladium-catalyzed cross-coupling reactions under microwave irradiation. A septum-sealed microwave tube charged with F-sulfonate 1 (1.0 mmol), a thiol (0.95 mmol), Pd(pddf)Cl2 (0.1 mmol), K2CO3 (2.0 mmol) in 0.5 mL of a co-solvent acetone-toluene-H2O (4:4:1) was irradiated in a monomode microwave cavity (50w, 100–150 °C, 5–10 min). The reaction mixture was washed with 1 mL of H2O. The organic layer was loaded onto a 5 g FluoroFlash™ cartridge,12 eluted with 10 mL of 80:20 MeOH-H2O.13 The collected fraction was concentrated to give an aryl sulfide. The fluorous species were washed out from the cartridge with 15–20 mL of MeOH. The cartridge can be conditioned for reuse.
- 12.FluoroFlash™ SPE cartridges are available from FTI: www.fluorous.com
- 13.For more information on F-SPE, please log on to: http://www.fluorous.com/download/fspe.pdf
- 14.For fluorous tags in multi-step synthesis, see Zhang W, Lu Y. Fluorous Synthesis of Hydantoins and Thiohydantoins. Org Lett. 2003;5:2555–2558. doi: 10.1021/ol034854a.Zhang W. Fluorous Synthesis of Disubstituted Pyrimidines. Org Lett. 2003;5:1011–1014. doi: 10.1021/ol027469e.
- 15.Because the reaction mixture was relatively simple, intermediate 5 was purified by normal phase SPE instead of F-SPE.


