Abstract
The effect of spinal cord injury (SCI) on the expression levels and distribution of water channel aquaporin 4 (AQP4) has not been studied. We have found AQP4 in gray and white matter astrocytes in both uninjured and injured rat spinal cords. AQP4 was detected in astrocytic processes that were tightly surrounding neurons and blood vessels, but more robustly in glia limitans externa and interna, which were forming an interface between spinal cord parenchyma and cerebrospinal fluid (CSF). Such spatial distribution of AQP4 suggests a critical role that astrocytes expressing AQP4 play in the transport of water from blood/CSF to spinal cord parenchyma and vice versa. SCI induced biphasic changes in astrocytic AQP4 levels, including its early down-regulation and subsequent persistent up-regulation. However, changes in AQP4 expression did not correlate well with the onset and magnitude of astrocytic activation, when measured as changes in GFAP expression levels. It appears that reactive astrocytes began expressing increased levels of AQP4 after migrating to the wound area (thoracic region) two weeks after SCI, and AQP4 remained significantly elevated for months after SCI. We also showed that increased levels of AQP4 spread away from the lesion site to cervical and lumbar segments, but only in chronically injured spinal cords. Although overall AQP4 expression levels increased in chronically-injured spinal cords, AQP4 immunolabeling in astrocytic processes forming glia limitans externa was decreased, which may indicate impaired water transport through glia limitans externa. Finally, we also showed that SCI-induced changes in AQP4 protein levels correlate, both temporally and spatially, with persistent increases in water content in acutely and chronically injured spinal cords. Although correlative, this finding suggests a possible link between AQP4 and impaired water transport/edema/syringomyelia in contused spinal cords.
Keywords: spinal cord injury, astrocytes, Aquaporin 4, water content, glia limitans
INTRODUCTION
The aquaporins (AQPs) are a family of at least 11 homologous water channel proteins that provide the major route for water movement in all tissues, including the nervous system (Manley et al., 2000; Agre et al., 2002; Badaut et al., 2002; Amiry-Moghaddam and Ottersen, 2003; Manley 2004). AQPs are small hydrophobic membrane proteins (28 kDa monomers; Borgnia et al., 1999) that assemble in homotetramers and facilitate bi-directional water transport across membranes in response to osmotic gradients. AQP4 is the most abundant AQP in the CNS (Manley et al., 2004).
Role of AQP4 in the CNS
Analysis of AQP4-null mice has shown that AQP4 provides a principal molecular pathway for water permeability in the brain (Ma et al., 1997; Verkman et al., 2006). Phenotype analysis of transgenic mice lacking AQP4 also shows involvement of AQP4 in the migration of astrocytes (Saadoun et al. 2005; Verkman et al., 2006). AQP4 deficiency slows astrocyte migration in response to a chemotactic stimulus in vitro, and AQP4 deletion impairs glial scar progression following injury in vivo (Saadoun et al. 2005). However, the best understood and most often studied is the connection between AQP4 expression changes and accumulation of water within the brain, i.e., edema ( for a review see Unterberg et al., 2004). Cerebral edema is a key contributor to brain injury-associated morbidity and mortality (Marmarou, 1994; Graham et al., 1995; Papadopoulos et al., 2002). There is no efficient treatment for brain/spinal cord edema due, in part, to a lack of understanding of the mechanisms of water accumulation and clearance. AQP4 has different roles in vasogenic versus cytogenic edema in the central nervous system (CNS). Vasogenic edema primarily results from the disruption of the blood-brain-barrier (BBB) and subsequent accumulation of water in the extracellular spaces. In experimental models of vasogenic edema (in brain freeze-injury or in brain tumors), a lack of AQP4 in AQP4-null mice worsens outcome (Papadopoulos et al., 2004). This suggests that removal of excess water from the extracellular compartments requires the presence of AQP4. It appears that water enters the brain parenchyma independently of AQP4, but exits the brain through AQP4.
There are three main barriers across which edema fluid can be eliminated from the brain: ependyma, glia limitans and BBB. All three barriers express AQP4 protein, although the individual contributions to the clearance of vasogenic brain edema fluid are not clear. On the contrary, the deletion of AQP4 in AQP4-null mice reduces brain water content and significantly improves the survival rate of injured mice after the induction of cytogenic edema by water intoxication (Manley et al., 2004). Cytogenic edema occurs when water accumulates in intracellular brain compartments, while the BBB remains intact. Neurons are outnumbered by astrocytes (which can swell to five times their normal size), so it is obvious that glial swelling is the main mediator of brain edema (Kimelberg, 1995). It also appears that the absence of AQP4 in AQP-null mice worsens vasogenic, but improves cytogenic edema. Therefore, the predominantly astrocytic localization of AQP4 can have dual consequences - it facilitates water removal in vasogenic edema, and it may contribute to astrocytic swelling in cytogenic edema. The mechanisms underlying those contrasting functions of AQP4 channels remain to be characterized.
Water accumulation has been documented in the acute phase after contusion spinal cord injury (SCI; Li and Tator, 1999; Sharma et al., 2005) and has been attributed to the formation of vasogenic edema. Wagner and Stewart (1981) report that edema is directly related to the amount of initial trauma (Wagner and Stewart, 1981), while Sharma et al. (2005) find that the extent of edema is closely associated with the amount of SCI-induced motor dysfunction. This is not surprising, since CNS edema results in the compression of adjacent tissues and ischemic cell death, both significant contributors to secondary tissue damage. However, the effect of SCI on AQP4 expression and function, and the possible role of AQP4 in the formation of SCI-induced edema have not been studied.
Impaired function of AQP4 and the resulting disturbance in water transport is not only directly damaging to the surrounding tissue in the injured CNS, but also can affect neuronal excitability. Astrocytic regulation of water transport is tightly linked to the maintenance of ion homeostasis and neurotransmitter release and uptake (Simard and Nedergaard, 2004).The subcellular co-localization of AQP4 with the inwardly rectifying potassium channel Kir4.1 (Connors et al., 2004; Nagelhus et al., 2004) suggests that AQP4 may participate in the coupled influx of water and K+ into astrocytes that occurs after neural activity (Manley et al., 2004). Binder et al., (2006) report increased seizure duration and slowed potassium kinetics in mice lacking AQP4 channels, while increases in AQP4 are associated with hyperexcitability in epileptic human hippocampi (Lee et al, 2004), thus implicating AQP4 in direct modulation of neuronal excitability,.
Here we report acute and chronic changes in AQP4 expression in injured spinal cords, which might affect edema formation, consequent tissue damage, glial migration and neuronal excitability- all processes critically involved in defining final functional deficits after SCI.
METHODS
Rat Model of Spinal Cord Injury
Male Sprague-Dawley rats weighing 225-250g were anesthetized by i.p. injection of pentobarbital (50 mg/kg). Anesthesia was considered complete when animals did not display hindlimb withdrawal in response to a noxious foot pinch. A partial laminectomy of the 13th thoracic vertebrae was also conducted before the animals were contused with the Infinite Horizons (IH) Impactor using a force of 150 kdyn with 1 second dwell time. The surgical site was closed by suturing the muscle and fascia and stapling the skin; which was followed by the superficial application of 0.3mL of 1% lidocaine. Animals were then injected subcutaneously with 2mL of 0.9% sterile saline and placed on a heating pad to maintain body temperature until they revived. SCI rats received the analgesic buprenorphin (0.1mg/kg) s.c. twice a day for 3d. Sham and SCI rats received the baytril (2.7mg/kg) s.c. twice a day until bladder function returned, which typically occurred two weeks after SCI. Bladders of injured animals were manually emptied twice daily until normal function returned. All procedures complied with the recommendations in the NIH Guide for the Care and Use of Laboratory Animals and are approved by the UTMB Animal Care and Use Committee. Detailed description of the SCI model was given in Nesic-Taylor et al., 2005.
Protein Extraction
200μl of whole cell extraction buffer (10mM HEPES, 10mM KCl, 0.1mM EDTA, 0.1mM EGTA, 1mM DTT, 0.5mM PMSF, 2ug/mL antipain, 2ug/mL chymostatin, 2ug/mL pepstatin, 2ug/mL leupeptin) is added to each spinal cord segment for homogenization on ice with a clear grinder that tightly fits inside the 1.5ml tubes. After homogenization, samples are vortexed for 5 seconds and then centrifuged at 8,000rpm for 8 minutes at 4°C. The resulting supernatant is the whole cell protein extract. Protein concentrations are determined using the BioRad Protein Assay, according to the manufacture's instructions (500-0006; BioRad).
Electrophoresis and Western Blotting
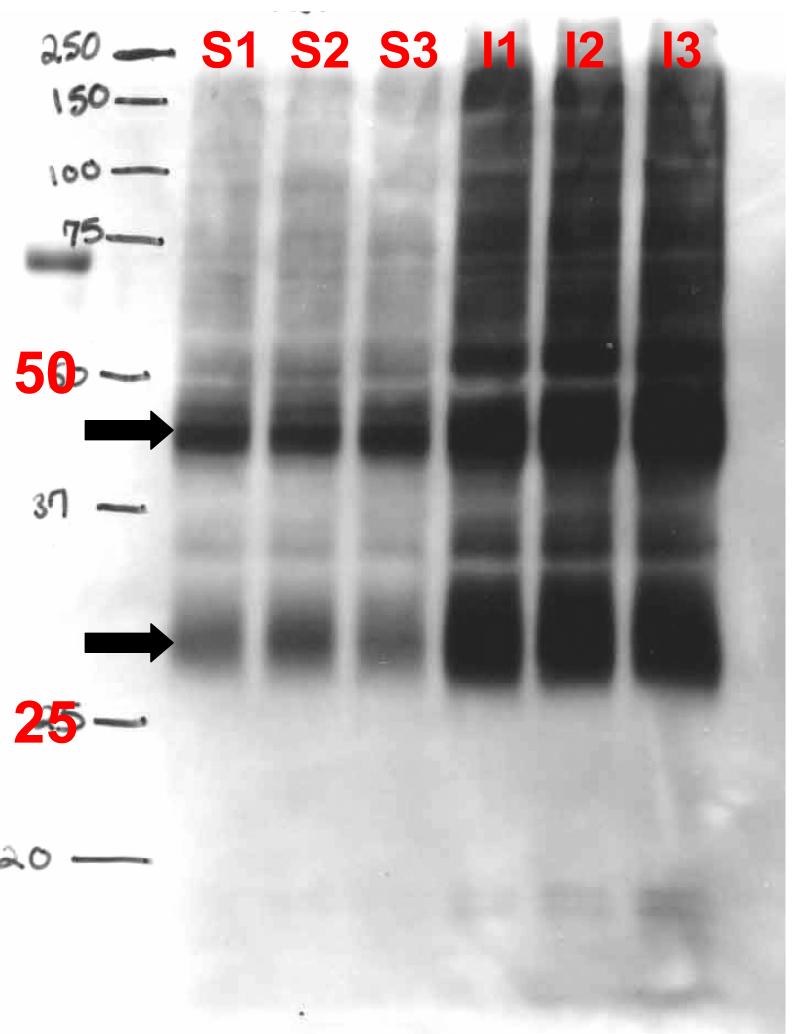
Samples containing 40 μg of protein were boiled for 10 minutes at 100°C with an appropriate volume of 6 X sample buffer ( 350 mM Tris HCl , pH 6.8, 1M Urea, 1 % 2-mercaptoethanol, 9.3% DTT, 13% SDS, 0.0 6 % bromophenol blue, 30% glycerol). Samples were then placed on ice to cool, and subsequently centrifuged at 313g for 30 seconds before being loaded onto a sodium dodecyl sulfate-polyacrylamide gel. Detailed procedure is described in Nesic et al., 2005. Membranes were reversibly stained with Ponceau S to confirm the transfer of proteins, and destained in water. Peroxidase activity was detected using the Amersham enhanced chemiluminescence lighting system (ECL) (RPN2209; Amersham Biosciences). Antibodies used: Aquaporin 4 (Chemicon AB3594, 1: 3 000); GFAP (Chemicon MAB360 , 1:60.000 and Dako Cytomation # Z0334 1:1000; both antibodies gave identical results). As described in Nesic et al (2005), both GFAP antibodies gave several GFAP bands in Western blots. We quantified 50kD band, which is the molecular weight of intact GFAP. The AQP4 gene encodes proteins of ∼31 and 34 kDa with distinct N-terminal sequences (Umenishi and Verkman (1998). Both isoforms are expressed in brain, but mainly the smaller isoform is found in other tissues. An AQP4 antibody (Chemicon) that recognizes the C-terminus of AQP4 produced several bands in AQP4 Western blots, with ∼30 and ∼45 kD being the most robust (see Fig. M1). Several groups of investigators using Abs specific for the C-terminus of AQP4 have shown the presence of a major band at ∼30 kDa on immunoblots from all tissues where AQP4 is expressed (Frigeri et al., 1995; Neely et al., 1999). Many other bands ranging from 25 to >60 kDa were observed but were poorly resolved (Lu et al., 1996). Different AQP4 isoforms may have different functions or regulatory mechanisms determining water permeability, as reported by Neeely et al. (1999).Thus, in the rest of our preliminary data we quantified only the ∼30kD band. Thus, in this report we quantified only the ∼30kD band. β-Actin is used as a control for loading (anti-β- actin, Sigma; 1:10,000) in all Western blots, in addition to checking Ponceau staining.
Fig. M1.
Representative AQP4 Western blot showing several bands, the most intense at ∼30kD and ∼45 kD (arrow), in three sham samples (S1-S3) and three injured samples (I1-I3) 46d after SCI (T10).
To establish spatial distribution of both, AQP4 expression and water content changes in injured spinal cords, we isolated three spinal cord regions: (a) thoracic, that consisted of the site of injury (T10), and adjacent segments T9 and T11; (b) cervical, consisting of pooled C7 and C8 segments relevant for the function of fore-limbs, that is preserved after T10 contusion, and (c) lumbar, consisting of pooled L4 and L5 segments relevant to the control of hindlims, paralyzed after T10 contusion.
To establish the time course of SCI-induced AQP4 expression changes we measured AQP4 levels at multiple time points after SCI: 12h, 24h, 3d, 7d. 35d, 40d, 56d, 3.5m, 5m and 9m. We relied on the quantitative measurements using Western blot in all time points, except 5m, when semi-quantitative analysis of immunolabeled AQP4 was employed. To correlate water content changes after SCI, we measured water content at 3d, 60d and 5m.
Double immunofluorescence staining
Animals were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer via the aorta. The thoracic spinal cord segments were removed, postfixed for 2-4 hours at 4°C, cryoprotected in 30% sucrose in phosphate buffer overnight, and embedded in OCT compound. Tissue sections were cut transversely at 30 μm on a sliding microtome. Immunofluorescence staining proceeded according to the proceduresdescribed in Nesic et al., 2005. . Omission of the primary antibodies or use of non-specific secondary IgG's in the immunostaining process resulted in negative staining of the tissue. Antibodies used: GFAP (mouse monoclonal, Chemicon;1:500), AQP4 (rabbit polyclonal Chemicon; 1:1000) and OX-42 (Serotec, Raleigh, NC, USA; 1:200); TUJ1: mouse monoclonal at 1:3,000 Covance (Richmond, CA) , NeuN : mouse monoclonal at 1:5,000 Chemicon International (Temecula, CA) , RECA-1: mouse monoclonal at 1:200 Serotoec (Oxford, UK). Confocal laser scanning microscopy. Stained sections were scanned with a confocal laser scanning system (BIO-RAD Radiance 2100, K-2 system). For double immunofluorescent staining, data from two channels were collected by sequential scans to avoid bleeding through between two channels. Images were collected with Krypton lasers of 488 nm excitation and 568 nm excitation. The overlay of the two channels showing the co-localization of the two antigens was indicated in yellow. Digital images were saved and processed with Adobe Photoshop for final editing. For quantitative analysis of intensity of immunolabeling, all images were taken or captured on a Nikon E1000 microscope at the same exposure time and the average density was measured with MetaMorph software. Five randomly selected sections from the spinal cord segment in each animal were analyzed and the relative optical densities were averaged to provide a mean number for each rat.
Water content
Water content was determined according to the protocol described in Li and Tator (1999) Immediately after sacrificing rats, three regions of spinal cords were removed: cervical, thoracic and lumbar. Those spinal cord segments were weighed on aluminum foil, dried at 105°C for 24 h, and reweighed. The percent water content was calculated as follows: water content (%) = [(wet weight-dry weight)/ wet weight] ×100.
Statistical Analysis
All statistical tests were evaluated at the alpha level of significance of 0.05, two-tailed. All of the experiments have similar structures in that the effects of a manipulation on the level of the factors were measured. We used parametric methods (t-test) for our analyses. However, if the assumptions for these tests were not met, we proceeded with non-parametric analyses (Mann-Whitney). Likewise, we used non-parametric methods to check all parametric results as a safeguard of assumptions. If the results were not consistent, we reported the results from non-parametric tests. For multiple-group comparisons, data were analyzed using one way analysis of variance (one way ANOVA). The LSD multiple comparisons post-hoc test was used to determine p values. p values less than 0.05 were considered significant.
RESULTS
1. Astrocytic localization of AQP4 in uninjured and injured spinal cords
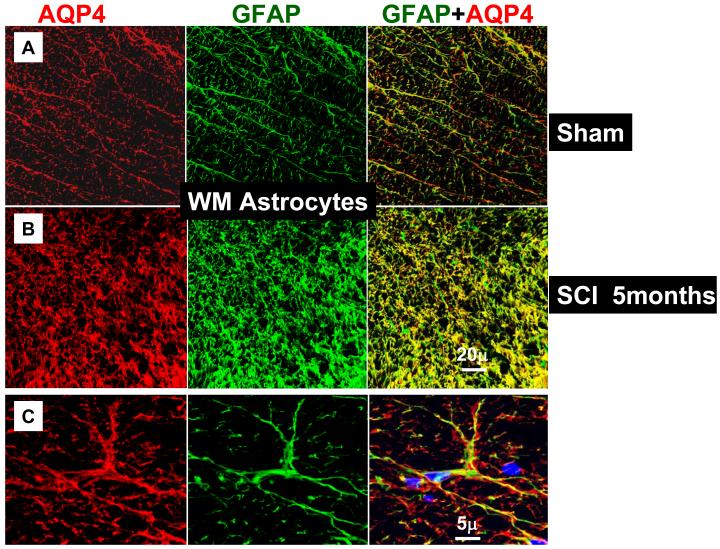
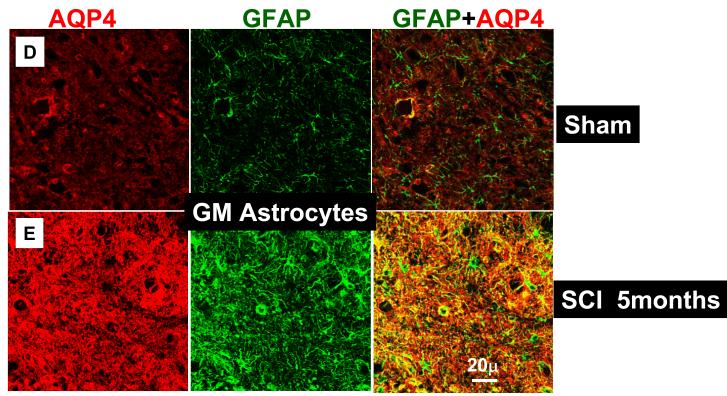
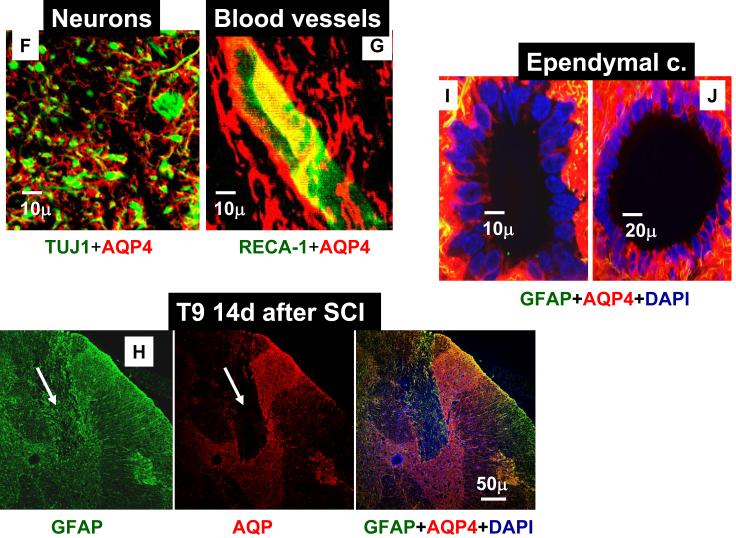
AQP4 was primarily expressed in gray and white matter astrocytes (immunolabeled with GFAP), in both uninjured (n=16) and injured spinal cords (n=17). The same result was obtained irrespective of the region of spinal cord examined (thoracic, lumbar or cervical) or the time after SCI (from 24h to 5 months after SCI). An image presented in Fig. 1A illustrates representative co-localization of GFAP-labeled astrocytes (green) with AQP4 labeling (red) in the white matter of the spinal cord segment T9, 5 months after sham treatments, while Fig. 1B represents co-localization of GFAP and AQP4 in the white mater of injured T9 segment, 5 months after SCI in the (SCI injury was at T10). The co-localization of GFAP and AQP4 was also evident in the high magnification image (Fig. 1C) that showed GFAP-positive cells in a sham-treated spinal cord (the same one presented in 1A) that expressed AQP4. The most intense AQP4 immunolabeling was detected in the gray matter astrocytes in T9 of sham–treated (Fig. 1D) or injured spinal cords (Fig. 1E). Since AQP4 immunolabeling was dense and did not completely co-localize with GFAP labeling (see Fig. 1C: red AQP4 staining that was not co-labeled with green GFAP staining), we tested the possibility that neurons also express AQP4. However, both NeuN labeling (staining neuronal nuclei/somata; data not shown; n=3) and TUJ1 (labeling neurites; Fig. 1F; n=3 for sham and n=3 for injured spinal cords) did not indicate any neuronal expression of AQP4. Double labeling with CC1 for oligodendrocytes (n=3 for sham and n=3 for injured spinal cords; data not shown) and OX-42 for microglia (n=3 for sham and n=3 for injured spinal cords; data not shown), also showed a lack of AQP4 expression in those non-astrocytic glial cells.
Fig. 1.
Astrocytic localization of AQP4- Co-localization of AQP4 and GFAP. A) Sham-treated spinal cords. AQP4 (red) and GFAP (green) immunolabeling in the white matter of the sham-treated spinal cords (T9; 5 months after sham treatment; n=3). Yellow color in the merged image represents co-localization of AQP4 and GFAP. B) Injured spinal cords. AQP4 and GFAP immunolabeling in white matter of the injured spinal cord (T9; isolated 5 months after SCI; n=3) also showed complete co-localization. C) High magnification image of a GFAP-labeled cell that expresses AQP4 in sham-treated spinal cords, 5m after sham treatment in T9 shows AQP4 that overlaps with GFAP immunolabeling, but also AQP4 labeling that likely shows astrocytic processes not stained with GFAP. D), E) AQP4 and GFAP immunolabeling in the gray mater of sham (C; n=3) and injured spinal cords (D; n=3) in T9 section, 5 months after SCI. F) TUJ1 immunolabeling against beta-tubulin (green) illustrates how tightly astrocytic processes expressing AQP4 (red) surrounded neurites in uninjured spinal cords (T9; n=3). G) RECA-1 immunolabeling (endothelial cell marker, green) showed a close proximity of astrocytic processes expressing AQP4 and blood vessels in uninjured spinal cords (T9; n=3), similarly to injured spinal cords (not shown; n=6). H) Astrocytes not expressing AQP4. GFAP-positive cells that are present in the damaged area of spinal cords, 14 days after SCI (in T9 segment) do not express AQP4 (marked with white arrow). In sham samples at different time points after sham treatment we have not found GFAP-positive cells that did not express AQP4. I, J) AQP4 in ependymal cells. AQP4 was also expressed in ependymal cells surrounding the central canal in sham (H), or injured (I) spinal cords in T9, 5 months after SCI (n=3), but with lower intensity than in astrocytes. AQP4 was visible in the apical part of cells, facing the central canal.
The dense immunolableng of AQP4 in the gray matter can be explained by assuming that astrocytic processes expressing AQP4 are tightly surrounding neuronal cell bodies/neurites, as shown in Fig. 1F, and blood vessels (Fig. 1G), but the same processes were not labeled with GFAP (Fig. 1C) This is consistent with the report of Simard and Nedergaard (2004) – who demonstrate that many fine leaflet astrocytic processes are not labeled with GFAP antibodies.
In contrast, early after SCI, there were some GFAP positive cells in the injured regions of the spinal cord that did not express AQP4, as shown in Fig. 1H (marked with arrows). This figure shows injured T9 segment 14 days after SCI. It might be that these GFAP-positive cells, which did not express AQP4 14 days after SCI; are astrocytes that migrated into the lesion site. The result presented in Fig. 1H was consistent with the Western blot results presented in Fig. 3B: the expression changes in AQP4 up to 7d after SCI did not correspond to the changes in GFAP. However, reactive astrocytes within the lesion site (see arrow in Fig. 2) started displaying robust expression of AQP4 at later times after SCI. These later robust increases in AQP4 levels paralleled those in GFAP levels (Fig. 3D).
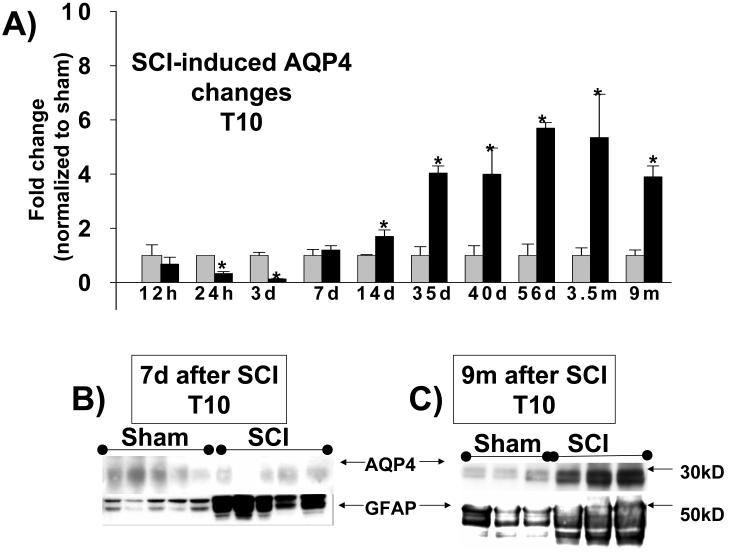
Fig. 3.
Time course of AQP4 expression changes after SCI. A) Quantitative analyses of immunoblotted AQP4 expression (Western blots) in uninjured and injured spinal cords (at the site of injury:T10) at different time points after SCI: 12h (n=5 for both sham and SCI), 24h (n=6 for both sham and SCI), 72h (n=5 for both sham and SCI), 7 d (n=5 for both sham and SCI); 14d (n=3 for both sham and SCI); 35 d (n= 12 for both sham and SCI); 40d (n=3 for sham and n=4 for SCI); 56d (n=4 for sham and n=5 for SCI) and 3.5 mo ( n=3 for sham and n=5 for SCI) and 9 mon (9m; n=6 for sham n=6 for SCI), shown as equidistant points on the X-axis. The Y-axis represents the relative intensity of the 30kD AQP4 band normalized to β-actin, and than to sham values (set to =1). AQP4 protein levels showed significant down-regulation in the first week after SCI, but robust and chronic up-regulation in the months after SCI (Bonferroni multiple comparisons tests; *=p<0.05). Representative AQP4 Western blots were shown above bar graphs for two sham samples (S1, S2) and two injured samples (I1, I2) for chronically injured spinal cords. B) Representative examples of AQP4 and GFAP Western blots 12h after SCI showed a significant increase in GFAP expression (∼50 kD), and no change for AQP4 expression in five injured samples (SCI) in T10. C) A representative example of AQP4 and GFAP Western blots 9 months after SCI show significant 4 fold increases in AQP4 expression (see Fig. 3A), and 1.4 fold increases in GFAP in injured T10. p<0.05. (Mean +/− SD).
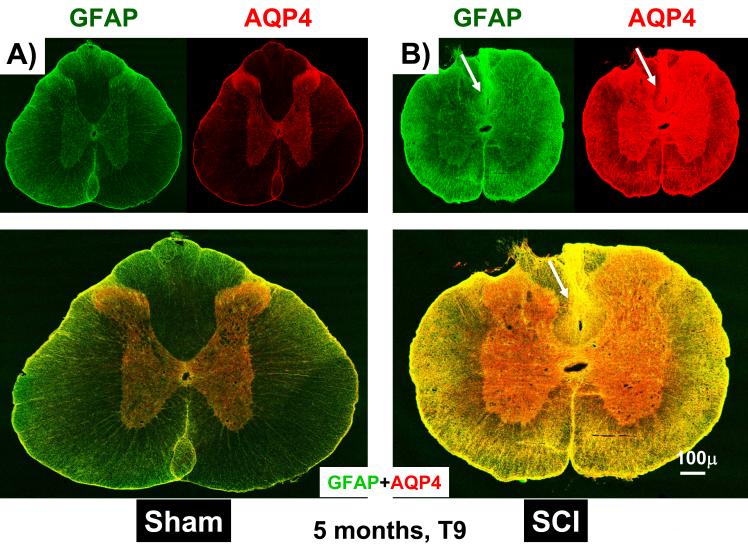
Fig. 2.
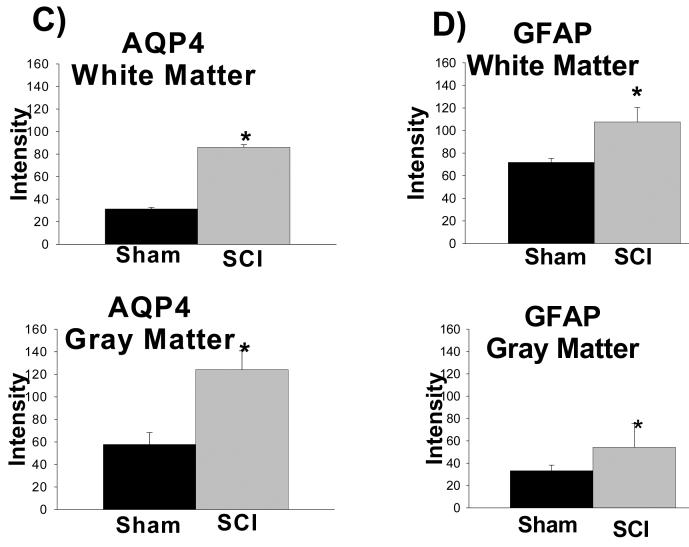
AQP4 immunolabeling increases in chronically injured spinal cords. A) A representative example of GFAP (green) and AQP4 (red) immunolabeling in sham-treated spinal cords (T9), extracted 5 months after sham treatments. B) A representative example of GFAP (green) and AQP4 (red) immunolabeling in injured spinal cords (T9), 5 months after SCI. C) Semi-quantitative analysis of the intensity of AQP4 immunolabeling, in gray and white matter, before and after SCI (n=3 per group). D) Semi-quantitative analysis of the intensity of GFAP immunolabeling, in gray and white matter, before and after SCI (n=3 per group). (Mean +/− SD; p<0.05.).
Our results showed that AQP4 was abundantly expressed around blood vessels (Fig. 1G), in the glia limitans externa facing CSF in subdural spaces (Fig. 4) and in ependymal cells surrounding central canal filled with cerebrospinal fluid (CSF) (Fig. 1I, J), thus supporting the hypothesis that AQP4 mediates water transport between the spinal cord parenchyma and blood or CSF.
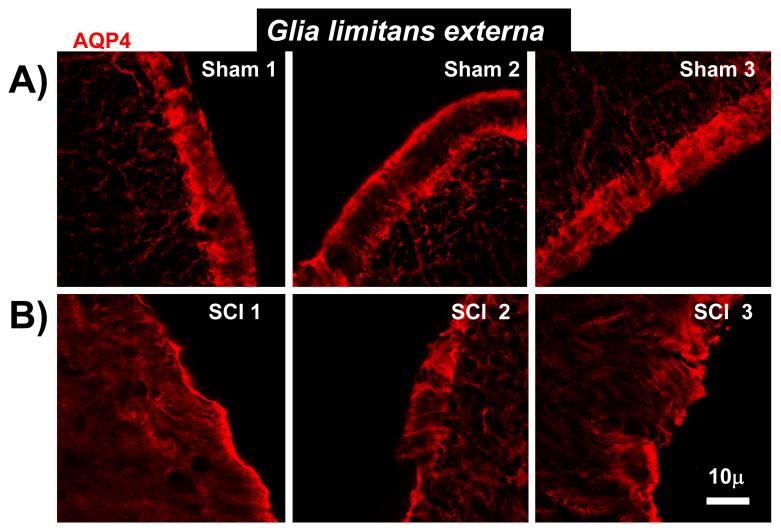
Fig. 4.
Decreased AQP4 in Glia limitans externa. A) Representative examples of AQP4 immunolabeling in glia limitans externa in three sham-treated spinal cords, 5 months after the sham treatment (T9 segments). The images were taken in three different regions of uninjured spinal sections (dorsal and ventral horns). B) Representative examples of AQP4 immunolabeling in glia limitans in three contused spinal cords, 5 months after SCI. (in corresponding regions to T9 spinal sections presented in A; n=3).
Given that AQP4 immunolabeling visibly increased after SCI (Figs. 1B and D), we performed a semi-quantitative analysis, shown in Fig. 2. Using 5 randomly chosen sections from the spinal segment T9 (3 rats per group), we found statistically significant increases in AQP4 labeling in white matter (2.1 fold; Fig. 2C, upper graph) and in gray matter (2.7 fold; Fig. 2C, bottom graph), 5 months after SCI, in agreement with our previous report (Nesic et al., 2005). Similar results were obtained after analyses of other spinal cord regions (cervical, C7-C8 and lumbar, L4-L5) 5 months after SCI (not shown). Interestingly, increases in GFAP immunolabeling 5 months after SCI were smaller: 1.6 fold in gray and 1.7 fold in white matter (Fig. 2D) in T9, and also in lumbar and cervical regions (not shown).
2. Time course of SCI-induced AQP4 expression changes in different regions of injured spinal cords
To quantitatively analyze SCI-induced changes in AQP4 and GFAP over time, we used Western blot analyses. We measured AQP4 expression levels at 12h, 24h, 3d, 7d, 35d, 40d, 56d, 3.5m, 5m and 9m after SCI. As shown in Fig. 3, AQP4 expression at the site of injury (T10) was down-regulated during the first week after SCI, started increasing at 2 weeks after SCI and then was persistently increased during the chronic phase of post-injury SCI.
Interestingly, the changes in AQP4 expression during the first 7 days after SCI did not correlate with the changes in GFAP expression, which significantly increased 3.9 fold by 12 h (Fig. 3B) and 2.4 fold after 7 days post-injury (data not shown), while AQP4 decreased at 12h (not significantly) or remained unchanged at 7 days. However, increases in AQP4 levels beyond one month after SCI, paralleled increases in GFAP levels, but were more robust than SCI-induced GFAP expression changes. For example, AQP4 protein levels increased 4 fold at 9 months after SCI versus 1.4 fold increases of GFAP at the same time (Fig. 3C).
AQP4 expression levels were consistently increased in chronically injured spinal cords, in both gray and white matter, except for the glia limitans externa region. As shown in Fig. 4A, AQP4 immunostaining was robust and well defined in glia limitans externa regions facing the cerebrospinal fluid (CSF) in sham-treated spinal cords. However, in chronically injured spinal cords (Fig. 4B), the orderly organization of astrocytic AQP4 in glia limitans externa disappeared and AQP4 expression decreased. The same result was obtained in injured spinal cords 2 months after SCI (n=3; not shown).
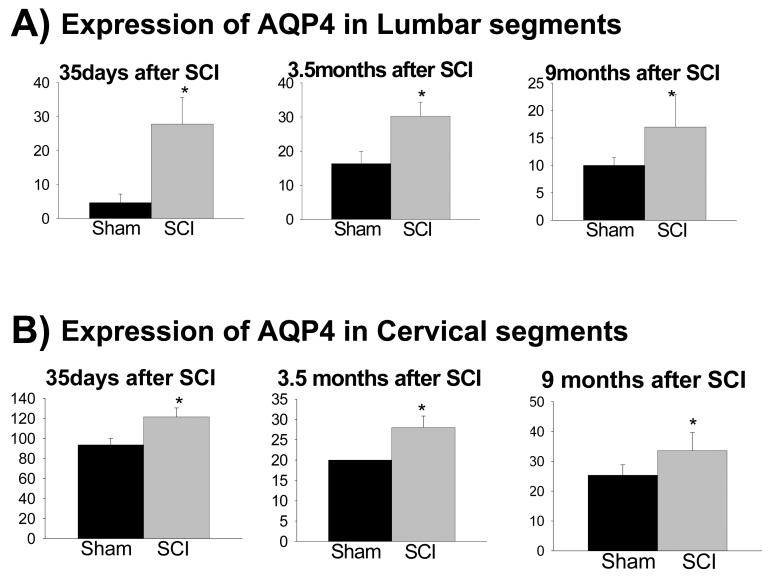
SCI-induced AQP4 expression changes were not found only at the site of injury (T10), but also away from the injury site, in lumbar (pooled L4+L5; Fig. 5A) and in cervical regions (pooled C7+C8; Fig. 5B), but only in chronically injured spinal cords (1month and later). However, early after SCI, AQP4 expression changes were localized only to the site of injury. While AQP4 levels decreased at the site of injury 3d after SCI (Fig. 3), they were unchanged in lumbar (Fig. 6) or cervical regions at that time (not shown).
Fig. 5.
AQP4 expression changes spread to cervical and lumbar segments. Quantitative Western blot analysis showed significantly higher expression levels of AQP4 in: A) lumbar (consisting of pooled L4 and L5 segments), and B) cervical regions (consisting of pooled C7 and C8 segments), at 35d (n=3 for sham and n=5 for SCI), 3.5 months (n=3 for sham and n=5 for SCI) and 9 months after SCI. (n=3 for sham and n=5 for SCI; p<0.05. (Mean +/− SD).
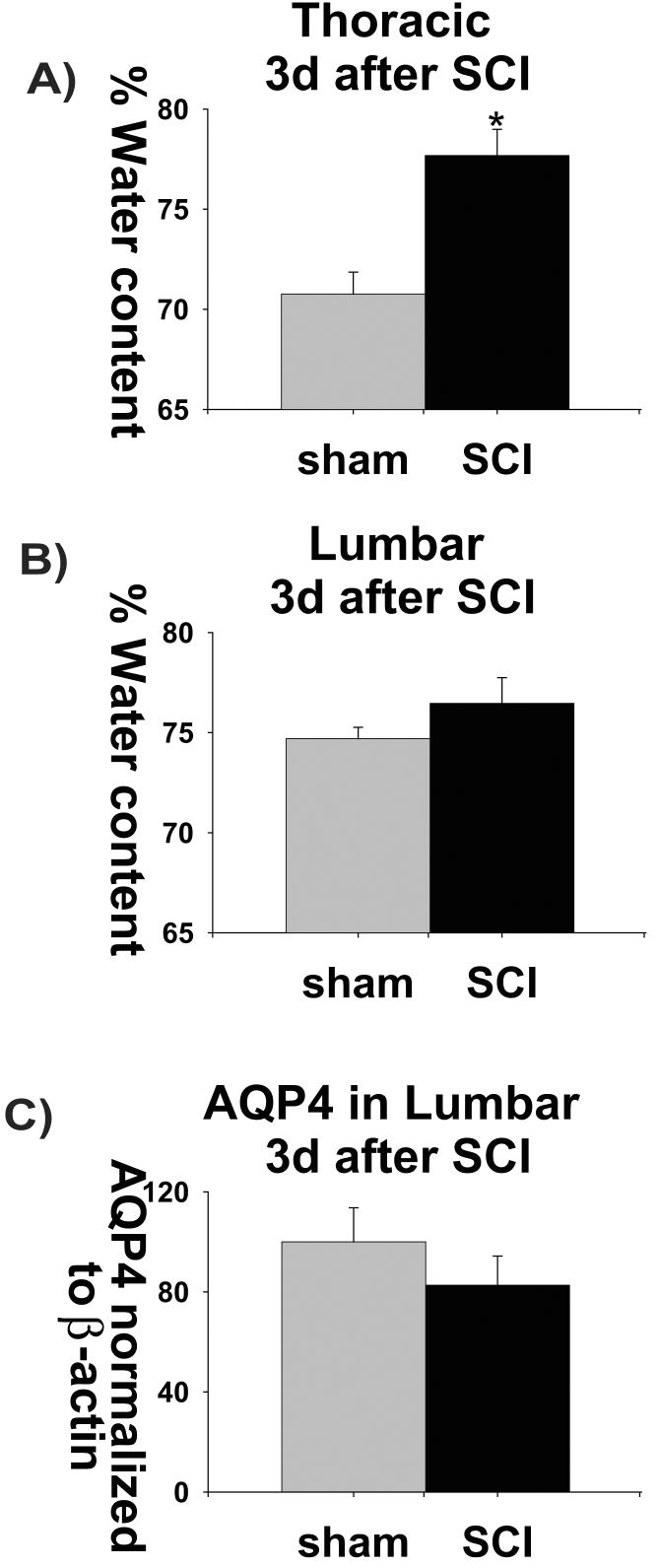
Fig. 6.
Increased water content in acutely injured spinal cords spatially and temporally correlate with AQP4 expression changes. A) The water content was significantly increased (6%) in the thoracic spinal region 3 days after SCI. Three thoracic segments (T9+T10+T11) from sham-treated (n=4) and injured spinal cords (n=5) were pooled and water content measured using dry weight method. B) Water content was also measured in lumbar (L4+L5 pooled) of sham-treated (n= 4) and injured spinal cords (n=5). There was no change in the water content 3d after SCI in the lumbar or cervical regions (C7+C8 pooled; not shown n=the same as in lumbar). C) AQP4 expression levels were measured in L4+L5, also at 3d after SCI, using Western blot analysis (n=5 for both sham and SCI).
3. SCI-induced changes in water content correlate spatially and temporally with SCI-induced changes in AQP4 expression
As discussed in the Introduction, the changes in AQP4 expression suggest the possibility that the water content of injured spinal cords also changes. Therefore, we measured spinal cord water content at 3d, 60d and 5months after SCI (Figs. 6 and 8) in spinal cord regions that displayed AQP4 changes at those corresponding time points (Figs. 3 and 5).
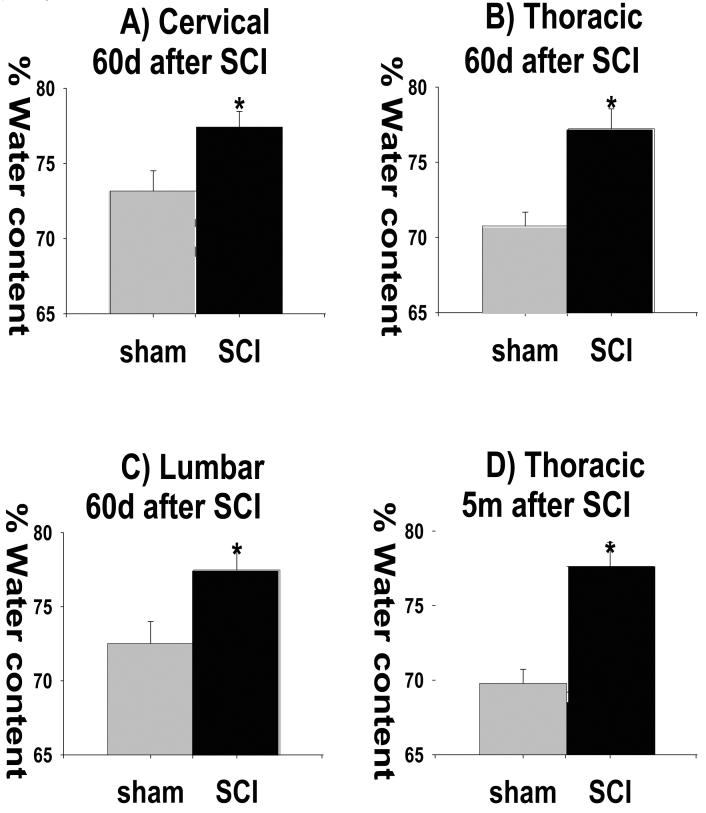
Fig. 8).
Increased water content in chronically injured spinal cords. A-C) Water content was also increased 2 months after SCI in all three spinal regions, cervical (C7+C8), thoracic (T9+T10+T11) and lumbar (L4+L5; n=7 for sham and n=11 for SCI; Mean +/− SD; p<0.05). D) Water content increases in injured spinal cords 5 months after SCI (n=7 for sham and n=11 for SCI; Mean +/− SD; p<0.05).
We found significantly increased water content (5.99% increase) in thoracic regions 3d after SCI (Fig. 6A), which corresponded to the well-established early formation of edema at the site of injury (Li and Tator, 1999; Sharma et al., 2005). This early edema did not spread to lumbar regions (Fig. 6B), which paralleled the lack of AQP4 expression changes in the lumbar regions (Fig. 6C). Also, there were no changes either in the percentage of water content, or in AQP4 protein levels, in cervical segments 3d post-contusion (data not shown).
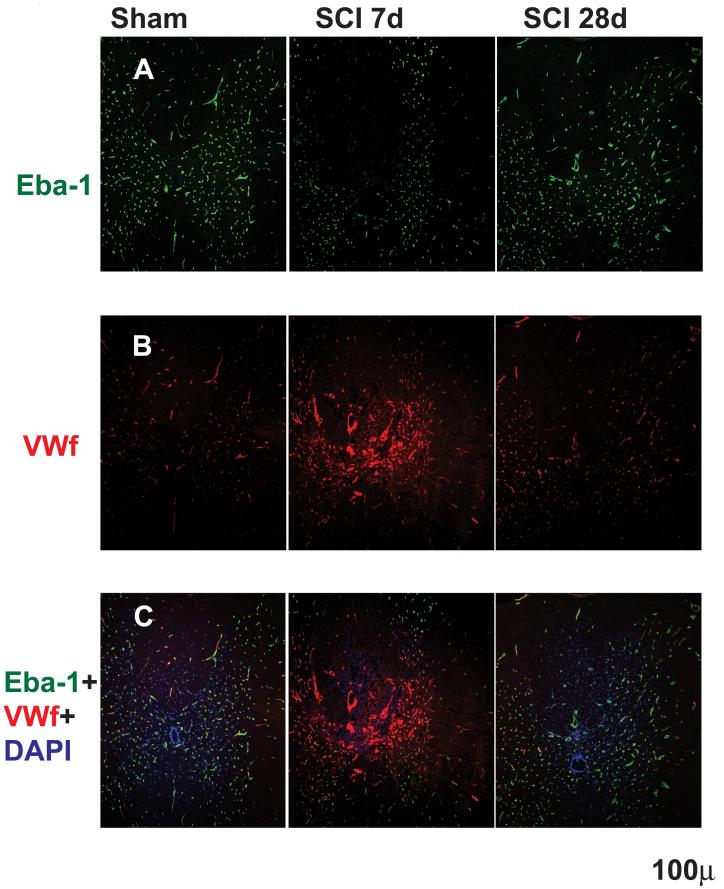
Early edema after SCI is attributed to the well characterized disruption of the blood spinal cord barrier (BSCB; Noble and Wrathall 1989; Popovich et al., 1996; Mautes et al. 2000; Whetstone et al., 2003). Our results (Fig. 7) illustrated an early breakdown of BSCB (7d) and its recovery by day 28, and are in agreement with earlier reports (Noble and Wrathall 1989; Popovich et al., 1996; Mautes et al. 2000; Whetstone et al., 2003). We showed significantly decreased expression of Endothelial barrier antigen (Eba-1) 7d after SCI, (Fig. 7A), in agreement with others (Perdiki et al., 1998; Sharma et al., 2005). However, Eba-1 levels returned to control levels by 28d after SCI (Fig. 7A), suggesting that there was recovery of BSCB properties in the chronic phase of SCI, also consistent with other reports (Noble and Wrathall 1989; Popovich et al., 1996; Mautes et al. 2000; Whetstone et al., 2003). The decrease in Eba-1 immunolabeling 7d after SCI did not result from the absence/destruction of vasculature (Fig. 7B). The same sections stained for Eba-1 were immunolabeled with von Willebrand factor (vWf) known to recognize endothelial cells (Ulger et al., 2002). As shown in Fig. 7B, vWf intensity even increased 7d after SCI, but returned to control (sham) levels 28d after SCI. Vasculature that was intensely labeled with vWf at 7d after SCI was not co-labeled with Eba-1, suggesting that vWf stained newly formed blood vessels after SCI that lacked BSCB properties, in agreement with Loy et al (2003). As expected, Eba-1 and vWf labeling in sham - treated spinal cords (Fig. 7C) and in injured spinal cords 28 days after SCI were co-localized almost 100%. This result suggests that maturation of the new vasculature and recovery of BSCB properties were completed by 4 weeks after SCI.
Fig. 7.
Blood spinal cord barrier (BSCB) breakdown and recovery after SCI. A) Top panel: Eba-1 (Endothelial barrier antigen) immunolabeling in sham spinal cord segment T9 (left column; n=3) and in injured T9 segment, 7 (middle column; n=3) and 28d after SCI (right column; n=3). There were visible decreases in Eba-1 staining in injured spinal cords 7d after SCI, but not at 28d after SCI, suggesting disruption of BSCB properties at 7d and recovery at 28d after SCI. B) Middle panel: Von Willebrandt factor labeling of endothelial cells, in sham spinal cord segment T9 and in injured T9 7d after SCI and 28d after SCI (the same sections used in A). Von Willebrand factor labeling increases at 7d after SC indicated angiogenesis and formation of newly formed blood vessels, in agreement with other reports. C) Bottom panel: Images show dual labeling of Eba-1 and vWf in the same sections as presented in above pictures. The absence of co-localization of the vWf and Eba-1 labeling in the putative new blood vessels (intense red vWf staining around the site of injury in the dorsal horns) suggests that newly formed blood vessels after SCI do not form BSCB at 7d, but, they mature by day 28 post-injury, and Eba-1 expression appears and co-localizes with vWf labeling.
We also found that initial water content increases of 5.99% at 3 d after SCI persisted in chronically injured spinal cords; at 2 months after SCI water content in the thoracic regions was increased by 5.02% (Fig. 8B), and by 5.76% at 5 months after SCI (Fig. 8D), a novel finding. In these chronically injured spinal cords, increased water content was detected throughout the spinal cords, including cervical and lumbar segments (Fig. 8A and C), corresponding to an increased expression of AQP4 in the same segments (Fig. 5). Therefore, it appears that early down-regulation of AQP4 corresponded to the formation of vasogenic edema; while late up-regulation of AQP4 also correlated with the accumulation of water in the contused spinal cords, which however cannot be attributed to the vasogenic edema.
DISCUSSION
Astrocytic localization of AQP4 in the uninjured and injured spinal cords
All reports have shown that AQP4 water channels are primarily expressed in spinal cord astrocytes, surrounding blood vessels and neurons (Vitellaro-Zuccarello et al., 2005; Rash et al., 1998, 1999; Oshio et al., 2004). Consistent with those reports, we found robust expression of AQP4 in astrocytes forming barriers between the spinal cord parenchyma and blood or cerebrospinal fluid: in astrocytic processes surrounding blood vessels or in glia limitans externa and interna. Such cellular localization clearly suggests a role for AQP4 in the regulation of water transport among the different spinal cord compartments - with astrocytes having a central role in mediating those transport processes in both uninjured and injured spinal cords.
SCI-induced changes in AQP4 expression
Several groups have reported changes in AQP4 expression after brain injury, or in brain tumors (Vajda et al., 2000; Lo et al., 2005; Zhao et al., 2005; Saadoun et al., 2002). Brain injury, depending on the type, can induce up- or down-regulation of AQP4. Early after traumatic brain trauma, there is decreased AQP4 levels (Zhao et al, 2005), while focal cerebral ischemia increases AQP4 levels (Taniguchi et al., 2000). This discrepancy is attributed to the opposing roles of AQP4 in the formation of vasogenic vs. cytotoxic edema in different types of brain injuries. We found that SCI induces both early down-regulation and late up-regulation of AQP4. Similar observations were reported in brain ischemic models; AQP4 was reduced within the first 48 h after onset (Sato et al., 2000; Yamamoto et al., 2001) while marked upregulation occurs at later time points (Taniguchi et al., 2000).
Since astrocytic population after SCI undergoes depletion early after SCI (Zai et al., 2005), followed by their proliferation and activation (Zai et al., 2005; Popovich et al., 1997; Nesic et al., 2005), it is possible that the changes in AQP4 expression passively reflect SCI-induced changes in the population of astrocytes. However, we found that SCI-induced changes in AQP4 protein levels did not reflect SCI-induced changes in GFAP protein levels. Early after SCI, GFAP was up-regulated, while AQP4 was down-regulated. This is consistent with Zhao et al. (2005), who found that GFAP levels did not change after traumatic brain injury, while AQP4 levels decreased for several days after trauma. Furthermore, we showed that GFAP-positive cells that migrated into the damaged regions of spinal cords one week after SCI did not express AQP4, in contrast to the finding of Sadaaoun et al, (2005), that AQP4 participates in the migration of astrocytes to the wound. Our results indicated that increased AQP4 expression had started 2 weeks after SCI, after the migration of GFAP-positive cells to the site of injury had begun. It is possible, however, that reactive GFAP-positive cells in injured spinal cords change their phenotype over time, and that those transformations involve transcriptional regulation of AQP4, consistent with findings of Nomura et al (2006), who showed that GFAP-expressing cells are cells with heterogeneous phenotypes. However, it is unknown what upstream signaling may lead to AQP4 expression changes in astrocytes after CNS injury. It has been reported that exposing the brain to hypo-osmotic shock increases AQP4 protein levels (Vajda et al., 2000), and that endothelin-1, which increases after stroke, induces AQP4 expression and edema (Lo et al., 2005). Zhao et al (2005) shows that sulforaphane, an anti-oxidant gene activator, can also stimulate AQP4 induction, which suggests that AQP4 expression may react to oxidative stress conditions in injured CNS. The signals that trigger AQP4 expression changes after SCI remain to be identified.
Possible connection between AQP4 expression changes and water accumulation in injured spinal cords
Spinal cord edema has been well documented in the acute phase (hours/days) after SCI (Li and Tator, 1999; Sharma et al., 2005). Our results showing significant 6% increases in the water content at the site of injury corresponded well to the percentage of water content increases (edema) recorded in brain injury or SCI (Zhao et al., 2005; Li and Tator, 1999; Sharma et al., 2005). Early edema after SCI has been attributed to the well-characterized disruption of the blood spinal cord barrier (BSCB), which is known to recover by 4 weeks after SCI (Noble and Wrathall 1989; Popovich et al., 1996; Mautes et al. 2000; Whetstone et al., 2003). This is consistent with our findings that showed a disappearance of barrier properties at 7 d after SCI in spinal cord vasculature, as well as re-expression of endothelial barrier antigen in spinal cord vasculature at 4 weeks after SCI. Given that the lack of AQP4 worsens vasogenic edema (Papadopoulos et al, 2004; Bloch et al., 2005), we can speculate that decreased AQP4 expression within the first 7 days after SCI contributes to the formation of vasogenic edema after SCI: water enters spinal cord tissue through dysfunctional BSCB and independently of AQP4. However, due to reduced APQ4 expression early after SCI, water removal from the spinal cord parenchyma into the blood/CSF is impaired, resulting in edema after SCI.
Since BSCB properties seem to return to normal by 1 month after SCI, late water accumulation in chronically injured spinal cords may be attributed to cytotoxic edema and accumulation of water within astrocytes. It has been shown that AQP4-expressing astrocytes had a three-fold higher water permeability than astrocytes not expressing AQP4 (Gunnarson et al., 2005), and that a lack of AQP4 improves cytotoxic edema (Manley et al., 2004). These results suggest that the robustly increased expression of AQP4 in chronically injured spinal cords may be a sign of water accumulation in astrocytes. Given that the up-stream pathways leading to changes in AQP4 expression in contused spinal cords are unknown, we could not directly test a possible causal relationship between AQP4 and edema after SCI. An alternative explanation for increased water content in chronically injured spinal cords may be the formation of cavities of variable forms and dimensions filled with fluid (syringomyelia). Syringomyelia in SCI patients is a serious condition associated with different pathological outcomes (Carroll and Brackenridge, 2005) for which we still do not know the mechanism. It may be that SCI-induced changes in AQP4 levels and/or distribution contribute to the formation of fluid filled cavities.
Regardless of the mechanisms underlying water accumulation in injured spinal cords, the question remains as to why increased expression of AQP4 in chronically injured spinal cords does not facilitate water extrusion into blood and/or CSF. Elimination of excess water requires passage across three AQP4-rich barriers: a) the glia limitans externa, b) the glia limitans interna/ependyma, and c) the BSCB (Kimelberg, 2004). Given that AQP4 expression decreases in glia limitans externa, we hypothesize that mislocalization and damaged anchoring of AQP4 proteins in glia limitans impairs excess water clearance in chronically injured spinal cords. It has been shown that a shift in the localization of AQP4 significantly impairs its water channel function. For example, cytoskeletal protein dystrophin is involved in the maintenance of the polarized expression of AQP4. Analyses of dystrophin-null brains revealed a dramatic reduction of AQP4 in the astrocytes that surround capillaries and at the glia limitans, although total AQP4 expression was unchanged (Vajda et al., 2004). The aberrant localization of AQP4 protein in dystrophin-null brains affects the formation of edema, as well as mouse survival time. Similar studies using mice deficient in α-syntrophin show a lack of perivascular AQP4, which resulted in edema formation (Amiry-Moghaddam et al., 2004). The same authors have shown that the astrocyte membrane domain contacting the pial basal lamina differs from the perivascular membrane domain, pointing to the existence of different anchoring proteins/ mechanisms for the different domains. Possible changes in perivascular localization of AQP4, dystrophyn or alpha-syntrophin expression/distribution after SCI have not been studied.
Although descriptive and correlative, our data strongly suggest that the impaired regulation of astrocytic AQP4 expression may contribute to the water accumulation in acutely and chronically contused spinal cords, which may result in a plethora of pathophysiological changes after SCI. We believe that identifying interventions that could manipulate expression levels of AQP4 would be doubly useful. Such interventions would allow for the testing of causality between AQP4 and water accumulation/edema/ syringomyelia, and would also offer therapeutic approaches to counter the development of edema or syringomyelia, for which we still lack effective treatment.
Acknowledgement
This work was supported by grants by Mission Connect of TIRR-Houston, and NIH grant NS39161.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agre L.S. King, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. Aquaporin water channels–from atomic structure to clinical medicine. J Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Xue R, Haug FM, Neely JD, Bhardwaj A, Agre P, Adams ME, Froehner SC, Mori S, Ottersen OP. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004 Mar;18(3):542–4. doi: 10.1096/fj.03-0869fje. [DOI] [PubMed] [Google Scholar]
- Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002 Apr;22(4):367–78. doi: 10.1097/00004647-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using NYU weight drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006 Apr 15;53(6):631–6. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- Bloch O, Papadopoulos MC, Manley GT, Verkman AS. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J Neurochem. 2005 Oct;95(1):254–62. doi: 10.1111/j.1471-4159.2005.03362.x. [DOI] [PubMed] [Google Scholar]
- Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- Carroll AM, Brackenridge P. Post-traumatic syringomyelia: a review of the cases presenting in a regional spinal injuries unit in the north east of England over a 5-year period. Spine. 2005 May 15;30(10):1206–10. doi: 10.1097/01.brs.0000162277.76012.0b. [DOI] [PubMed] [Google Scholar]
- Chebabo SR, Hester MA, Aitken PG, Somjen GG. Hypotonic exposure enhances synaptic transmission and triggers spreading depression in rat hippocampal tissue slices. Brain Res. 1995;695:203–216. doi: 10.1016/0006-8993(95)00778-o. [DOI] [PubMed] [Google Scholar]
- Christensen, Hulsebosch CE. Chronic central pain after spinal cord injury. J. Neurotrauma. 1997;14:517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Obenhaus A, Tasker JG. Osmolality-induced changes in extracellular volume alter epileptiform bursts independent of chemical synapses in the rat: importance of non-synaptic mechanisms in hippocampal epileptogenesis. Neurosci Lett. 1990;120:267–270. doi: 10.1016/0304-3940(90)90056-f. [DOI] [PubMed] [Google Scholar]
- Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci. 1995;108(Pt 9):2993–3002. doi: 10.1242/jcs.108.9.2993. [DOI] [PubMed] [Google Scholar]
- Graham DI, Adams JH, Nicoll JA, Maxwell WL, Gennarelli TA. The nature, distribution and causes of traumatic brain injury. Brain Pathol. 1995;5:397–406. doi: 10.1111/j.1750-3639.1995.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Gunnarson E, Axehult G, Baturina G, Zelenin S, Zelenina M, Aperia A. Lead induces increased water permeability in astrocytes expressing aquaporin 4. Neuroscience. 2005;136(1):105–114. doi: 10.1016/j.neuroscience.2005.07.027. Epub 2005 Oct 3. [DOI] [PubMed] [Google Scholar]
- Zhang Haijun, Xie Wenrui, Xie Yikuan. Spinal cord injury triggers sensitization of wide dynamic range dorsal horn neurons in segments rostral to the injury. Brain Research. 2005 September 7;1055(12):103–110. doi: 10.1016/j.brainres.2005.06.072. [DOI] [PubMed] [Google Scholar]
- Hains W.D. Willis, Hulsebosch CE. Temporal plasticity of dorsal horn somatosensory neurons after acute and chronic spinal cord hemisection in rat. Brain Res. 2003;970:238–241. doi: 10.1016/s0006-8993(03)02347-3. [DOI] [PubMed] [Google Scholar]
- Han Z, Wax MB, Patil RV. Regulation of aquaporin-4 water channels by phorbol ester-dependent protein phosphorylation. J Biol Chem. 1998;273:6001–6004. doi: 10.1074/jbc.273.11.6001. [DOI] [PubMed] [Google Scholar]
- Hebel R, Stromberg M. Anatomy of the Laboratory Rat. Williams & Wilkins Company; Baltimore: 1976. p. 120. [Google Scholar]
- Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004 Dec;27(12):735–43. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Imura T, Nakano I, Kornblum HI, Sofroniew MV. Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia. 2006 Feb;53(3):277–93. doi: 10.1002/glia.20281. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Current concepts of brain edema: review of laboratory investigations. J Neurosurg. 1995;83:1051–1059. doi: 10.3171/jns.1995.83.6.1051. [DOI] [PubMed] [Google Scholar]
- Li S, Tator CH. Effects of MK801 on evoked potentials, spinal cord blood flow and cord edema in acute spinal cord injury in rats. Spinal Cord. 1999 Dec;37(12):820–32. doi: 10.1038/sj.sc.3100941. [DOI] [PubMed] [Google Scholar]
- Lien YH, Shapiro JI, Chan L. Study of brain electrolytes and organic osmolytes during correction of chronic hyponatremia. Implications for the pathogenesis of central pontine myelinolysis. J Clin Invest. 1991 Jul;88(1):303–9. doi: 10.1172/JCI115292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AC, Chen AY, Hung VK, Yaw LP, Fung MK, Ho MC, Tsang MC, Chung SS, Chung SK. Endothelin-1 overexpression leads to further water accumulation and brain edema after middle cerebral artery occlusion via aquaporin 4 expression in astrocytic end-feet. J Cereb Blood Flow Metab. 2005 Aug;25(8):998–1011. doi: 10.1038/sj.jcbfm.9600108. [DOI] [PubMed] [Google Scholar]
- Loy DN, Crawford CH, Darnall JB, Burke DA, Onifer SM, Whittemore SR. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 2002 Apr 15;445(4):308–24. doi: 10.1002/cne.10168. [DOI] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000 Feb;6(2):159–63. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Manley GT, Binder DK, Papadopoulos MC, Verkman AS. New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience. 2004;129(4):983–91. doi: 10.1016/j.neuroscience.2004.06.088. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Hochwald G, Nakamura T, Tanaka K, Weaver J, Dunbar J. Brain edema resolution by CSF pathways and brain vasculature in cats. Am J Physiol. 1994;267(H):514–520. doi: 10.1152/ajpheart.1994.267.2.H514. [DOI] [PubMed] [Google Scholar]
- Massieu L, Montiel T, Robles G, Quesada O. Brain amino acids during hyponatremia in vivo: clinical observations and experimental studies. Neurochem Res. 2004 Jan;29(1):73–81. doi: 10.1023/b:nere.0000010435.06586.e2. [DOI] [PubMed] [Google Scholar]
- Mautes AE, Weinzierl MR, Donovan F, Noble LJ. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther. 2000 Jul;80(7):673–87. [PubMed] [Google Scholar]
- McAdoo DJ, Xu G-Y, Robak G, Hughes MG. Changes in amino acid concentrations over time and space around an impact injury and their diffusion through the rat spinal cord. Exp. Neurol. 1999;159:538–544. doi: 10.1006/exnr.1999.7166. [DOI] [PubMed] [Google Scholar]
- de Montigny P, Stobaugh F, Givens RS, Carlson RG, Srinivasachar K, Sternson LA, Higuchi T. Naphthalene-2,3-dicarboxaldehyde/cyanide ion: A rationally designed fluorogenic reagent for primary amines. Anal. Chem. 1987;59:1098–1101. [Google Scholar]
- Nesic-Taylor O, Cittelly D, Ye Z, Xu GY, Unabia G, Lee JC, Svrakic NM, Liu XH, Youle RJ, Wood TG, McAdoo D, Westlund KN, Hulsebosch CE, Perez-Polo JR. Exogenous Bcl-x(l) fusion protein spares neurons after spinal cord injury. J Neurosci Res. 2005;79(5):628–37. doi: 10.1002/jnr.20400. [DOI] [PubMed] [Google Scholar]
- Nesic O, Lee J, Johnson KM, Ye Z, Xu GY, Unabia GC, Wood TG, McAdoo DJ, Westlund KN, Hulsebosch CE, Regino Perez-Polo J. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J Neurochem. 2005 Oct 10; doi: 10.1111/j.1471-4159.2005.03462.x. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res. 1989 Mar 13;482(1):57–66. doi: 10.1016/0006-8993(89)90542-8. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979 Feb 2;161(2):303–10. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Oshio K, Binder DK, Yang B, Schecter S, Verkman AS, Manley GT. Expression of aquaporin water channels in mouse spinal cord. Neuroscience. 2004;127:685–693. doi: 10.1016/j.neuroscience.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Pan E, Stringer JL. Influence of osmolality on seizure amplitude and propagation in the rat dentate gyrus. Neurosci Lett. 1996;207:9–12. doi: 10.1016/0304-3940(96)12477-0. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Krishna S, Verkman AS. Aquaporin water channels and brain edema. Mt Sinai J Med. 2002;69:242–248. [PubMed] [Google Scholar]
- Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin 4 facilitates the reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H. Volume regulation in brain cells: cellular and molecular mechanisms. Metab Brain Dis. 1996;11:187–204. doi: 10.1007/BF02237957. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Cardin V, Tuz K. Signaling events during swelling and regulatory volume decrease. Neurochemistry. 2000;25:1301–1314. doi: 10.1023/a:1007652330703. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Franco R, Ochoa L, Ordaz B. Osmosensitive release of neurotransmitter amino acids: revelance and mechanisms. Neurochem Res. 2002;27:59–65. doi: 10.1023/a:1014850505400. [DOI] [PubMed] [Google Scholar]
- Perdiki M, Farooque M, Holtz A, Li GL, Olsson Y. Expression of endothelial barrier antigen immunoreactivity in blood vessels following compression trauma to rat spinal cord. Temporal evolution and relation to the degree of the impact. Acta Neuropathol (Berl) 1998 Jul;96(1):8–12. doi: 10.1007/s004010050854. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Horner PJ, Mullin BB, Stokes BT. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp Neurol. 1996 Dec;142(2):258–75. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997 Jan 20;377(3):443–64. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T. Direct immunogold labeling of connexins and aquaporin-4 in freeze-fracture replicas of liver, brain, and spinal cord: factors limiting quantitative analysis. Cell Tissue Res. 1999;296:307–321. doi: 10.1007/s004410051291. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of asytrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci USA. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SN, Obenhaus A, Dudek FE. Osmolality and nonsynaptic epileptiform bursts in rat CA1 and dentate gyrus. Ann Neurol. 1992;31:81–85. doi: 10.1002/ana.410310115. [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Zai LJ, Wrathall JR. Chronic alterations in the cellular composition of spinal cord white matter following contusion injury. Glia. 2005 Jan 1;49(1):107–20. doi: 10.1002/glia.20096. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;72(2):262–5. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005 Dec 15;118(Pt 24):5691–8. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- Sato, et al. Sato S, Umenishi F, Inamasu G, Sato M, Ishikawa M, Nishizawa M, Oizumi T. Expression of water channel mRNA following cerebral ischemia. Acta Neurochir Suppl. 2000;76:239–241. doi: 10.1007/978-3-7091-6346-7_48. 2000. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Badgaiyan RD, Alm P, Mohanty S, Wiklund L. Neuroprotective effects of nitric oxide synthase inhibitors in spinal cord injury-induced pathophysiology and motor functions: an experimental study in the rat. Ann N Y Acad Sci. 2005 Aug;1053:422–34. doi: 10.1111/j.1749-6632.2005.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129(4):877–96. doi: 10.1016/j.neuroscience.2004.09.053. Review. [DOI] [PubMed] [Google Scholar]
- Sterns RH, Baer J, Ebersol S, Thomas D, Lohr JW, Kamm DE. Organic osmolytes in acute hyponatremia. Am J Physiol. 1993 May;264(5 Pt 2):F833–6. doi: 10.1152/ajprenal.1993.264.5.F833. [DOI] [PubMed] [Google Scholar]
- Sykova E. Glial diffusion barriers during aging and pathological states. Prog Brain Res. 2001;132:339–63. doi: 10.1016/S0079-6123(01)32087-3. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Yamashita T, Kumura E, Tamatani M, Kobayashi A, Yokawa T, Maruno M, Kato A, Ohnishi T, Kohmura E, Tohyama M, Yoshimine T. Induction of aquaporin-4 water channel mRNA after focal cerebral ischemia in rat. Brain Res Mol Brain Res. 2000;78:131–137. doi: 10.1016/s0169-328x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Ulger H, Karabulut AK, Pratten MK. Labelling of rat endothelial cells with antibodies to vWF, RECA-1, PECAM-1, ICAM-1, OX-43 and ZO-1. Anat Histol Embryol. 2002 Feb;31(1):31–5. doi: 10.1046/j.1439-0264.2002.00357.x. [DOI] [PubMed] [Google Scholar]
- Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129(4):1019–1027. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Vajda Z, Promeneur D, Doczi T, Sulyok E, Frokiaer J, Ottersen OP, Nielsen S. Increased aquaporin-4 immunoreactivity in rat brain in response to systemic hyponatremia. Biochem Biophys Res Commun. 2000 Apr 13;270(2):495–503. doi: 10.1006/bbrc.2000.2472. [DOI] [PubMed] [Google Scholar]
- Vajda Z, Pedersen M, Fuchtbauer EM, Wertz K, Stodkilde-Jorgensen H, Sulyok E, Doczi T, Neely JD, Agre P, Frokiaer J, Nielsen S. Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):13131–6. doi: 10.1073/pnas.192457099. Epub 2002 Sep 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta. 2006 Mar 10; doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Vitellaro-Zuccarello L, Mazzetti S, Bosisio P, Monti C, De Biasi S. Distribution of Aquaporin 4 in rodent spinal cord: relationship with astrocyte markers and chondroitin sulfate proteoglycans. Glia. 2005 Aug 1;51(2):148–59. doi: 10.1002/glia.20196. [DOI] [PubMed] [Google Scholar]
- Wagner FC, Jr, Stewart WB. Effect of trauma dose on spinal cord edema. J Neurosurg. 1981 Jun;54(6):802–6. doi: 10.3171/jns.1981.54.6.0802. [DOI] [PubMed] [Google Scholar]
- Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003 Oct 15;74(2):227–39. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai LJ, Yoo S, Wrathall JR. Increased growth factor expression and cell proliferation after contusive spinal cord injury. Brain Res. 2005 Aug 9;1052(2):147–55. doi: 10.1016/j.brainres.2005.05.071. [DOI] [PubMed] [Google Scholar]
- Zelenina M, Zelenin S, Bondar AA, Brismar H, Aperia A. Water permeability of aquaporin-4 is decreased by protein kinase C and dopamine. Am J Physiol Renal Physiol. 2002 Aug;283(2):F309–18. doi: 10.1152/ajprenal.00260.2001. [DOI] [PubMed] [Google Scholar]
- Zhao J, Moore AN, Clifton GL. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res. 2005 Oct 6;82(4):499–506. doi: 10.1002/jnr.20649. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xie W, Xie Y. Spinal cord injury triggers sensitization of wide dynamic range dorsal horn neurons in segments rostral to the injury. Brain Res. 2005 Sep 7;1055(12):103–10. doi: 10.1016/j.brainres.2005.06.072. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Yoneda K, Asai K, Sobue T, Tada Y, Fujita H, Katsuya M, Fujita N, Aihara M, Mase K, Yamada Y. Miura, Kato T. Alterations in the expression of the AQP family in cultured rat astrocytes during hypoxia and reoxygenation. Brain Res Mol Brain Res. 2001;90:26–38. doi: 10.1016/s0169-328x(01)00064-x. [DOI] [PubMed] [Google Scholar]