Abstract
Verbal fluency is a widely used neuropsychological paradigm. In fMRI implementations, conventional unpaced (self-paced) versions are suboptimal due to uncontrolled timing of responses, and overt responses carry the risk of motion artifact. We investigated the behavioral and neurofunctional effects of response pacing and overt speech in semantic category-driven word generation. Twelve right-handed adults (8 female) ages 21–37 were scanned in four conditions each: Paced-Overt, Paced-Covert, Unpaced-Overt, and Unpaced-Covert. There was no significant difference in the number of exemplars generated between overt versions of the paced and unpaced conditions. Imaging results for category-driven word generation overall showed left-hemispheric activation in inferior frontal cortex, premotor cortex, cingulate gyrus, thalamus, and basal ganglia. Direct comparison of generation modes revealed significantly greater activation for the paced compared to unpaced conditions in right superior temporal, bilateral middle frontal, and bilateral anterior cingulate cortex, including regions associated with sustained attention, motor planning, and response inhibition. Covert (compared to overt) conditions showed significantly greater effects in right parietal and anterior cingulate, as well as left middle temporal and superior frontal regions. We conclude that paced overt paradigms are useful adaptations of conventional semantic fluency in fMRI, given their superiority with regard to control over and monitoring of behavioral responses. However, response pacing is associated with additional non-linguistic effects related to response inhibition, motor preparation, and sustained attention.
Keywords: Language, Lexical, Brain imaging
1. Introduction
Verbal fluency paradigms have traditionally been applied as a factor in the assessment of verbal intelligence (Thurstone, 1938) and as an index of frontal lobe functioning (Milner, 1964). More recently however, verbal fluency paradigms have been used in functional magnetic resonance imaging (fMRI) studies to identify dominance for language in clinical populations (e.g., Hertz-Pannier et al., 1997; Lehericy et al., 2000; Yetkin et al., 1998), to help define the roles of frontal areas involved in normal language processing (Cuenod et al., 1995; Pujol et al., 1999), and to give insight into the functional maturation of language systems in children (Gaillard et al., 2000; Gaillard et al., 2003).
The most common measures of verbal fluency are phonemic (letter-based) fluency and semantic (category-based) fluency. In the conventional version of a verbal fluency paradigm, production of exemplars is unpaced (self-paced). Subjects are given a period of time (typically 1 or 2 minutes) in which to generate freely as many exemplars as possible from a given category (e.g., ‘words that begin with s’ for phonemic fluency, ‘animals’ for semantic fluency).
Functional neuroimaging studies of unpaced verbal fluency paradigms in healthy adults have shown involvement of a variety of regions, including premotor cortex, superior and middle temporal gyri, and anterior cingulate gyrus (e.g., Parks et al., 1988; Rueckert et al., 1994). The most consistently reported regions are in left frontal cortex, and more specifically in the left inferior frontal gyrus, (e.g., Gaillard et al., 2000; Gaillard et al., 2003; Hinke et al., 1993; Schlosser et al., 1998). This finding is not surprising in view of the decades of neuropsychological studies that have associated performance on verbal fluency tasks with frontal lobe functioning (cf. Stuss & Benson, 1986). Involvement of left frontal regions in verbal fluency is also consistent with a large body of data from lesion-behavior studies (e.g., Luria, 1966/1980; Milner, 1982; Stuss & Benson, 1984), event-related potential (ERP) studies (e.g., Markela-Lerenc et al., 2004; Posner & Rothbart, 1994; Swainson et al., 2003), and functional neuroimaging studies (e.g., Corbetta et al., 1991; Raichle et al., 1994) that have demonstrated the importance of the frontal lobes, especially dorsolateral prefrontal regions, in organizing and carrying out controlled processing, as required during verbal fluency performance (cf., (Duncan & Owen, 2000; Miller & Cohen, 2001).
1.1 Effects of response pacing
While exemplar production has typically been unpaced in behavioral verbal fluency paradigms, the lack of control over the timing of the responses can be problematic for the detection of task-related BOLD responses with fMRI. From this perspective, paced versions of verbal fluency may be better suited for study with fMRI. To make this determination, it is critical to consider the potential behavioral and functional neuroanatomical consequences of pacing responses in this task.
To date, there has not been an explicit comparison of BOLD responses during paced versus unpaced verbal fluency tasks. However, data from two fMRI studies on the effects of pacing visuospatial paradigms suggested that applying a fixed pace in a blocked fMRI design does indeed result in a greater detected BOLD signal (D’Esposito et al., 1997; Seurinck et al., 2005). Tieleman et al. (2005) observed greater medial temporal lobe activity in unpaced versions of semantic and perceptual categorization tasks than in paced versions, but noted that this finding could be a consequence of the greater number of items completed by subjects in the unpaced condition. Two event-related fMRI studies that used unpaced responses – one in a verbal recognition paradigm (Daselaar et al., 2001) and one in same/different judgments on rotated figures (Maccotta et al., 2001) – showed patterns of activation similar to those reported for paced versions of similar tasks. These earlier results suggest that systematic investigation of the effects of pacing on BOLD activity is warranted, as these effects may differ based on the type of task and fMRI design.
In addition to methodological considerations related to optimized detection of BOLD activity, it is important to investigate differences in task demands and the associated functional neuroanatomy that might result from paced (as compared to unpaced) production on verbal fluency tasks. For example, paced production of exemplars is likely to require greater inhibitory control and place greater demands on working memory than unpaced production. As such, brain regions such as the anterior cingulate cortex that have been implicated in cognitive control processes (cf. (Kerns et al., 2004) may show greater involvement in a paced version of the task than in an unpaced version. Paced production may also affect behavioral measures, specifically the number of exemplars generated for a given category. Investigation and consideration of each of these issues will be critical for determining whether paced verbal fluency should be adopted for use during fMRI scanning.
1.2 Effects of response mode
In most fMRI studies of language, overt verbal responses have been avoided due to the risk of motion artifacts that can both mask and mimic the BOLD signal of interest (Birn et al., 1999). Such artifacts can arise either from changes in head position during speech, or from speech-related changes in the airways and vocal apparatus that introduce variance in magnetic susceptibility (Barch et al., 1999; Gracco et al., 2005; Heim et al., 2006; Kemeny et al., 2005; Mehta et al., 2006). Indeed, even in the absence of significant head movement during speech, artifacts and distortions related to changes in magnetic susceptibility can be detected in BOLD images (Kemeny et al., 2005).
Without spoken responses, however, the scope of language tasks that can be implemented in fMRI is reduced, and the investigators’ ability to assess subject compliance and to obtain direct measures of behavior is often limited. Previous fMRI studies of verbal fluency and word generation have mostly relied upon covert responses (e.g., Friedman et al., 1998; Gaillard et al., 2003; Hugdahl et al., 1999), assessing compliance from post-scan subject report, if at all. This method can be unreliable even in the subject groups most likely to be compliant, and may be of little value in children, patients, and other groups for which compliance is often a concern. Further, without speech there is no direct way to determine the number or accuracy of exemplars generated during the scans.
Overt verbal responses during fMRI scans are therefore desirable, and some progress has been made in designing studies that minimize the detrimental effects of speech on fMRI data. Barch and colleagues (1999) found that interpretable data could be obtained from scans that used overt responding as long as the primary comparison was between two conditions that both used overt verbal responses, and the analyses collected were on pooled group data as opposed to individual data. Some research groups have successfully used compressed or clustered acquisition designs (e.g., Abrahams et al., 2003; Edmister et al., 1999; Fu et al., 2002) or interleaved gradient techniques (e.g., Eden et al., 1999) in which the timing of the spoken responses is offset relative to data acquisition so as to reduce the overlap between them. Such approaches, however, require longer scanning time, which may be problematic in clinical or pediatric studies. Other studies have demonstrated that the impact of speech-related motion can be minimized by using fMRI paradigms with certain characteristics (Birn et al., 2004; Palmer et al., 2001). For example, Birn et al. (2004) demonstrated that an event-related design with varying intervals between stimulus onsets optimizes signal detection while keeping data free from significant motion artifact. Such “jittered” trial designs are, however, less than ideally compatible with some types of task. For example, insertion of substantially varying inter-trial intervals in semantic fluency or paced word generation would result in a highly artificial word production paradigm, presumably associated with robust executive components that would confound the study of lexical semantics. In spite of such methodological concerns, the need for tightly monitored behavior during verbal fluency tasks calls for an evaluation of the effects of overt verbal responses on BOLD data in this paradigm, through a direct comparison of overt and covert versions of the task.
1.3 Present Study
The goals of the present study were to examine the behavioral and neurofunctional effects of generation mode in a 2×2 design, with factors of response pacing and response mode, resulting in overall four conditions of word generation: Paced-Overt; Paced-Covert; Unpaced-Overt; and Unpaced-Covert. Based on the limited prior work and the theoretical considerations reviewed above, we anticipated that effects of category-driven word generation would be overall more robust for paced than for unpaced conditions, and for overt than for covert conditions. In addition, we expected that the paced conditions of the category-driven word generation task would be associated with additional prefrontal brain activations beyond left inferior frontal gyrus, due to the increased executive demands of the paced relative to the unpaced conditions.
2. Methods
A group of 12 right-handed adults (4 males, 8 females) ages 21–37 years (mean = 25.6) participated in the fMRI experiment. Participants were students from San Diego State University who had acquired English as their first language (with no second language exposure before age 5 years), were right-handed, and had no history of head injury or psychiatric conditions. The study was approved by the Internal Review Boards of San Diego State University and the University of California, San Diego, and all subjects provided informed consent.
2.1 fMRI Experiment
Images were acquired on a 3 Tesla Varian MRI scanner at the Center for Functional MRI of the University of California, San Diego. In each functional run, four experimental blocks (42 seconds each) alternated with four control blocks (28 seconds each) for a total duration of 4 minutes and 48 seconds per run (four initial volumes were discarded to allow magnetization to reach equilibrium). Four functional runs (144 time points each, TR = 2000 ms) were acquired, one for each of the four conditions of the category-driven word generation paradigm: Paced-Overt; Paced-Covert; Unpaced-Overt; and Unpaced-Covert. Order of the functional runs was counterbalanced across subjects. For the control blocks subjects generated the word “nothing” according to the experimental condition (Paced-Overt, Paced-Covert, Unpaced-Overt, Unpaced-Covert).
At the beginning of each run, subjects were instructed as to whether to generate items silently or aloud for the upcoming run. At the beginning of each task block within the run, a pre-recorded auditory prompt (e.g., “Tell me body parts”) was played, indicating the category for which exemplars were to be generated during that block. The use of Presentation software (Neurobehavioral Systems, Inc., 2003) ensured exact timing of prompts. For each subject, a total of 16 semantic categories was presented across the four different conditions, four categories per condition: animals, academic subjects, body parts, car parts, colors, drinks, food, furniture, hobbies, musical instruments, occupations, shapes, sports, tools/appliances, things you wear, and transportation. The assignment of categories to different conditions was randomized. Other categories were used for practice with each of the four experimental conditions before the scanning session.
Overt responses were obtained via a Commander XG MRI-compatible patient response and sound system (Resonance Technology, Inc., Northridge, CA), which includes a microphone attached to headphones worn by the subject during the MR scans. Responses were recorded on a laptop computer using SoundEdit 16 software (Macromedia, Inc., 1995) at a sampling rate of 44.1 kHz. Recordings were subsequently played back for transcription using Audacity open source sound recording and editing software (http://audacity.sourceforge.net/).
Recordings of overt responses were obtained from ten of the twelve subjects (technical difficulties did not allow recording of the other two subjects). Because the recordings contained high-volume scanner noise in addition to spoken responses, attempts were made to filter the recordings to remove scanner noise and improve intelligibility of the vocal responses. Two methods developed specifically for fMRI were employed, one using adaptive spectral subtraction (ASSERT; Nelles et al., 2003), and one using a cancellation procedure based on an estimate of scanner noise generated from a sample TR (Cusack et al., 2005). While both of these methods were effective in reducing scanner noise, they also removed frequencies that were part of the speech responses, rendering them unintelligible. This was not surprising for the ASSERT method, which is very effective for measuring vocal response latencies but is not necessarily designed to improve intelligibility of responses (Nelles et al., 2003). For the cancellation method, improved intelligibility was reported by Cusack et al. (2005), but it is likely that the success of this method depends upon the particular frequencies of the gradient coils and pulse sequences being used, and the degree to which they overlap with the human voice frequency spectrum.
The most effective approach for our recordings turned out to be using an equalizer function in the Audacity program to increase frequencies less than 300 Hz and to decrease frequencies greater than 400 Hz. This decreased the volume of the scanner noise considerably, which contributed to increased audibility of the responses. Still, in two of the ten subjects for whom recordings were available, the responses remained unintelligible even after equalization, perhaps due to placement of the microphone. Thus, behavioral results are reported from eight of the twelve subjects below.
2.2 Paced versions
In the paced conditions (Paced-Overt; Paced-Covert) subjects were presented with an exclamation mark (!) on the screen to prompt them to produce one item every 3 seconds, aloud in the overt condition and silently in the covert condition. At the start of a category a fixation crosshair appeared on the screen for 4 seconds during which a prompt played for 2 seconds (e.g., “Tell me body parts”) followed by a 2-second pause. The crosshair was then replaced by an exclamation mark (!) for 1.5 seconds to signal the subjects to produce one exemplar. This was followed by the crosshair for 1.5 seconds before the next exclamation mark appeared. Twelve exclamation marks were presented for each category, thus subjects could produce up to 12 items. Subjects were instructed to say the word “nothing” if they were unable to generate an exemplar when they saw an exclamation mark. Asking subjects to produce the word “nothing” (rather than simply remain silent) served to better match the articulatory components of the task and control conditions.
2.3 Unpaced versions
In the unpaced conditions (Unpaced-Overt; Unpaced-Covert), the exclamation point – rather than appearing on the screen every 3 seconds – stayed continuously on the screen for 36 seconds. Subjects were instructed to name (aloud or silently, depending on the condition) as many exemplars as possible at their own pace for as long as the exclamation point was on the screen (i.e., 36 seconds). Similar to the paced versions, subjects were instructed to say the word “nothing” when they could not produce exemplars, but at their own pace.
2.4 Analyses
Data for each of the twelve subjects were preprocessed using the FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl/). Functional runs underwent brain extraction (BET), then image time series were motion corrected (FEAT), registered to the high-resolution structural volume of the individual subject, normalized to standard space (FLIRT), and concatenated for statistical analysis. Using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996), the concatenated data were spatially smoothed (6 mm Gaussian kernel) and hemodynamic changes for alternating task and control blocks were fitted with hemodynamic models for conditions of interest. Multiple regression and general linear tests were performed in each subject for fluency overall (collapsed across all four conditions), for each factor (i.e., mode of generation: paced, unpaced; covert, overt), and for direct-factor comparisons (paced vs. unpaced, overt vs. covert). Time series of detected motion for each axis and rotation were entered as orthogonal regressors. For groupwise analyses, one-sample t-tests were carried out entering fit coefficients from intraindividual analyses. To correct for multiple comparisons, cluster significance was determined by Monte Carlo-type alpha simulations (Forman et al., 1995) for a corrected significance threshold of p<.05.
3. Results
3.1 Behavioral Data
Response pacing may conceivably result in decreased numbers of exemplars generated during a fixed block duration, either due to greater task difficulty or ceiling effects for the limited number of prompts per block. Any significant difference in the number of exemplars produced in the two conditions could complicate the interpretation of imaging data.
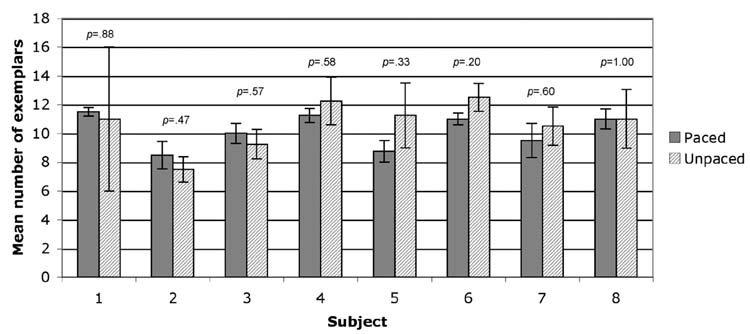
Intelligible recordings of overt responses were obtained from eight of the subjects (see Methods for a complete description). Responses from the overt conditions were transcribed and scored to exclude any exemplars that were repeated within a category. For each of the eight subjects, the mean number of exemplars was computed for the overt paced condition (4 categories) and for the overt unpaced condition (4 categories). These means and their associated standard errors are shown in Figure 1. A one-tailed paired t-test revealed that the mean number of exemplars did not differ significantly between the paced (mean across subjects = 10.19; SD = 1.17) and unpaced (mean = 10.66; SD = 1.62) conditions, t (7) = −1.08, p=.16. No significant performance differences in any of the participants were found either when numbers of exemplars per block were compared intraindividually (see Figure 1).
Fig. 1.
Mean number of exemplars generated by each of subjects in the paced overt (solid) and unpaced overt (hatched) conditions. Error bars are standard error of the mean. The inserted p-values result from 2-tailed t-tests performed for each subject comparing exemplars generated in four paced versus four unpaced blocks.
3.2 Imaging Data
Head motion
Rigid body registration carried out during preprocessing of the imaging data provided estimates of translation and rotation of the head in the x, y, and z planes at each time point in a run, relative to the position of the head on the 70th time point of that run. To assess differences in head motion among the 4 experimental conditions, the standard deviation of each of the 6 resulting measures (translation and rotation in the x, y, and z planes) across all time points in a run was computed for each of the 4 runs, for each of the 12 subjects. The mean deviation in head position across the 6 directions was then computed for each subject, for each of the 4 experimental conditions. The resulting mean values were subjected to a 2-factor ANOVA with Pacing (paced or unpaced) and Response Mode (overt or covert) as within-subjects factors.
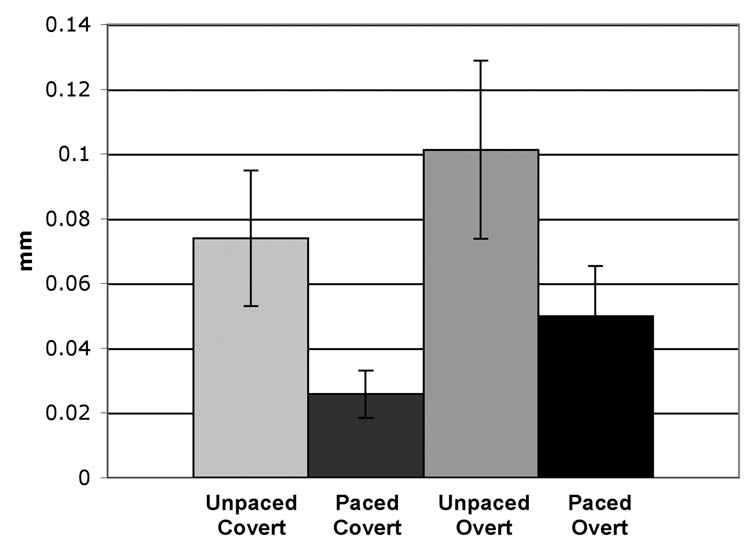
Across subjects, the mean deviations in head position for the Unpaced-Covert, Paced-Covert, Unpaced-Overt, and Paced-Overt conditions were 0.074 mm, 0.026 mm, 0.101 mm, and 0.050 mm, respectively (Fig. 2). The main effect of Response Mode was significant (p = .024), with greater motion in the overt than covert conditions. With regard to the main effect of Pacing, there was a trend of greater head motion during unpaced conditions, which approached significance (p = .054). There was no significant interaction between Response Mode and Pacing (p = .70).
Fig. 2.
Mean deviation in head position for each of the four generation modes, collapsed across twelve subjects and six movement directions (translation and rotation in the x, y, and z planes). Error bars are standard error of the mean.
Effects for word generation overall
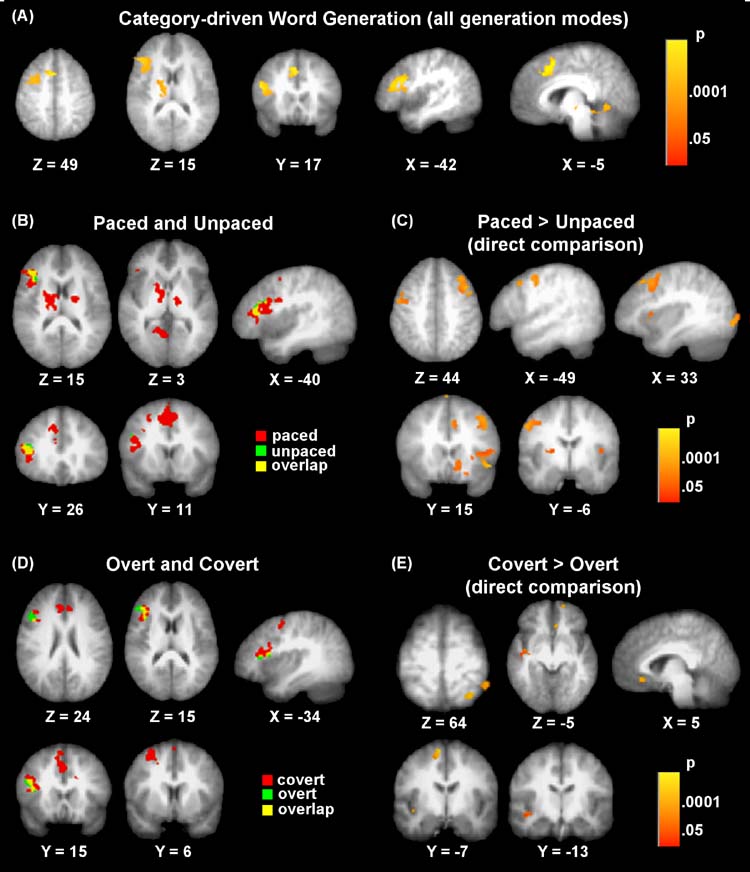
As anticipated, category-driven word generation overall was associated with activation in left inferior frontal gyrus (approximate Brodmann areas 44, 45), in addition to left cingulate gyrus (area 32), left middle frontal gyrus (area 6), left lingual gyrus (area 19), and left thalamus (see Table 1 and Fig. 3A).
Table 1.
Overall Effects for Category-driven word generation (across all four conditions)
| Talairach Coordinates
|
|||||
|---|---|---|---|---|---|
| Location | |||||
| Cluster Size (ml) | x | y | z | Peak t value | (approximate Brodmann Area) |
| 4568 | −13 | −17 | 15 | 8.9 | Left thalamus |
| −16 | −6 | 13 | 8.0 | Left lentiform nucleus | |
| 4336 | −34 | 17 | 17 | 12.5 | Left inferior frontal (44/45) |
| 2608 | −4 | 17 | 39 | 9.3 | Left cingulate (32) |
| 2336 | −24 | 2 | 52 | 9.5 | Left middle frontal (6) |
| 1752 | −9 | −58 | −2 | 7.7 | Left lingual (19) |
Note. All clusters p < .05 corrected.
Fig. 3.
T-maps showing clusters of significant activation for (A) word generation overall, collapsed across generation modes. Effects associated with Response Pacing are shown in (B) for Paced and Unpaced conditions and for the direct comparison between Paced and Unpaced conditions (C), which yielded greater activity in Paced conditions (but no inverse effects). Effects associated with Response Mode are seen in (D) for the Overt and Covert conditions and the direct comparison between the two modes (E), which resulted exclusively in greater effects for the Covert conditions.
Effects for generation modes (factors)
For paced versions, significant activations were identified in left inferior frontal (area 45), left middle frontal (area 6), left superior medial frontal/anterior cingulate (area 32), and left posterior cingulate/lingual gyri (areas 30, 18), as well as left thalamus/lentiform nucleus and midbrain (see Table 2 and Fig. 3B). The unpaced version showed less robust brain activation patterns with only one significant cluster in left inferior frontal gyrus (areas 44, 45). Despite the added risk of motion confounds, overt speech was associated with activation in left inferior frontal gyrus (areas 44, 45, 46). However, more extensive brain activation was seen for covert responses with activations in right cingulate (area 32), left inferior frontal (areas 45, 46), and left middle frontal gyri (area 6; see Table 2 and Fig. 3D).
Table 2.
Factor-Specific Effects for Paced, Unpaced, and Covert
| Talairach Coordinates
|
|||||
|---|---|---|---|---|---|
| Peak | Location | ||||
| Cluster Size (μl) | x | y | z | t value | (Brodmann Area) |
| Paced | |||||
| 3120 | −14 | −9 | 17 | 11.3 | Left thalamus/lentiform nucleus |
| 2760 | −5 | 15 | 37 | 10.3 | Left superior medial frontal (32)/anterior cingulate |
| 2664 | 2 | −30 | −8 | 10.4 | Midbrain |
| 2360 | −9 | −54 | 7 | 10.6 | Left posterior cingulate (30)/lingual (18) |
| 2320 | −38 | 28 | 15 | 9.3 | Left inferior frontal (45) |
| −40 | 13 | 15 | 8.6 | Left inferior frontal (44) | |
| 1424 | −24 | 6 | 49 | 12.9 | Left middle frontal (6) |
| Unpaced | |||||
| 1968 | −34 | −17 | 17 | 9.5 | Left inferior frontal (44/45) |
| Overt | |||||
| 2464 | −43 | 30 | 15 | 8.8 | Left inferior frontal (44/45) |
| −42 | 17 | 24 | 7.8 | Left inferior frontal (44/46) | |
| Covert | |||||
| 4176 | 5 | 26 | 25 | 11.8 | Right cingulate (32) |
| 2768 | −38 | 24 | 22 | 9.7 | Left inferior frontal (45/46) |
| 1624 | −24 | 4 | 52 | 8.8 | Left middle frontal (6) |
Note. All effects p < .05 corrected.
Direct factor (mode) comparisons
As expected, the paced conditions were associated with additional prefrontal activation relative to the unpaced conditions, with significant activation clusters seen in right middle frontal gyrus (area 6), left middle frontal gyrus (areas 9, 6), left superior frontal gyrus (area 6), and right anterior cingulate gyri (area 32). In addition to these prefrontal regions, greater activation for the paced conditions was seen in right superior temporal gyrus (areas 22, 42), right caudate nucleus, right inferior occipital gyrus (area 18), left cingulate (areas 23, 24) gyrus, left putamen, and cerebellar vermis. These regions are presented in Table 3 and Fig. 3C. No significant inverse effects (unpaced > paced) were found.
Table 3.
Direct Factor Comparisons of Paced Versus Unpaced and Covert Versus Overt
| Talairach Coordinates
|
|||||
|---|---|---|---|---|---|
| Peak | Location | ||||
| Cluster Size (μl) | x | y | z | t value | (approximate Brodmann Area) |
| Paced > Unpaced | |||||
| 6832 | 56 | −34 | 17 | 8.6 | Right superior temporal (22) |
| 3952 | 7 | 15 | − 5 | 7.6 | Right caudate nucleus |
| 3720 | 33 | 4 | 50 | 7.3 | Right middle frontal (6) |
| 3192 | 29 | −90 | 0 | 8.0 | Right inferior occipital (18) |
| 1968 | −47 | 9 | 39 | 7.4 | Left middle frontal (9/6) |
| 952 | −27 | 0 | 10 | 6.2 | Left putamen |
| 880 | 51 | −15 | 10 | 7.5 | Right superior temporal (42) |
| 848 | − 7 | 6 | 66 | 7.1 | Left superior frontal (6) |
| 840 | − 2 | −64 | −17 | 5.9 | Cerebellar vermis |
| 624 | − 9 | −11 | 30 | 6.8 | Left cingulate (23/24) |
| 528 | 7 | 15 | 39 | 9.4 | Right anterior cingulate (32) |
| Covert > Overt | |||||
| 448 | 22 | −52 | 66 | −6.5 | Right superior parietal lobule (7) |
| 376 | −43 | −21 | − 3 | −7.3 | Left middle temporal (21) |
| 344 | −14 | − 7 | 54 | −5.4 | Left superior frontal (6) |
| 320 | 5 | 24 | −10 | −6.3 | Right cingulate (32) |
| 256 | 14 | 54 | −12 | −6.8 | Right superior frontal (11) |
| 224 | 40 | −58 | 47 | −5.5 | Right inferior parietal lobe (40) |
| 224 | 33 | −45 | 64 | −6.2 | Right superior parietal lobe (7) |
| 200 | −27 | −19 | −18 | −6.1 | Left parahippocampal (35/36) |
Note. All clusters p < .05 corrected. No significant effects unpaced > paced or overt > covert were found.
For the comparison of overt versus covert conditions, our findings were contrary to our predictions. Significantly greater activation (p < .05) was seen for covert conditions relative to overt conditions in left middle temporal gyrus (area 21), left superior frontal gyrus (area 6), right cingulate gyrus (area 32), right superior frontal gyrus (area 11), right inferior and superior parietal lobes (areas 40, 7), as well as left parahippocampal gyrus (areas 35, 36) (see Table 3 and Fig. 3E). No significant inverse effects (Overt > Covert) were found.
4. Discussion
Our imaging results corroborate those of previous functional imaging studies of verbal fluency (e.g., Abrahams et al., 2003; Gaillard et al., 2003; Phelps et al., 1997), with fluency overall (regardless of pacing or mode of response) activating large portions of the left inferior frontal gyrus (areas 44 and 45). These regions are believed to contribute to word retrieval and working memory in verbal fluency tasks. Additional cortical activation clusters were seen in left anterior cingulate gyrus (area 32), which most likely reflects the attentional demands of the task (cf. (Abrahams et al., 2003; Phelps et al., 1997). Subcortical activation occurred in the thalamus and basal ganglia, consistent with previous imaging studies documenting participation of these structures in lexical generation (e.g., Crosson, 1999; Crosson et al., 2003).
When examining effects of the four different generation modes (paced, unpaced; overt, covert), the benefits of pacing were evidenced by robust brain activation patterns associated with this factor (Table 2; Fig. 3). Activation clusters were seen in left inferior frontal (areas 45, 44), left middle frontal (area 6), and left superior medial frontal/anterior cingulate gyri (area 32). These brain areas are consistent with the reported brain activation patterns found in previous conventional semantic fluency imaging studies (Gaillard et al., 2003; Hugdahl et al., 1999; Paulesu et al., 1997). Our results support the hypothesis that experimental control over response timing yields a maximally sustained hemodynamic response and robust brain activation patterns (D’Esposito et al., 1997). Moreover, the cluster activations associated with the overall fluency analysis were very similar to the robust activations found in the paced versions indicating that effects for fluency overall were mostly driven by the paced versions. As for unpaced versions, activation was overall less robust. Thus, our results strongly indicate that the fMRI adaptation of a paced version of the category-driven word generation task is preferable.
A direct comparison of generation mode factors revealed greater activity in a number of prefrontal regions for the paced relative to the unpaced conditions (Table 3; Fig. 3C). In support of our hypothesis, these regions were observed outside left inferior frontal gyrus, i.e., in right middle frontal gyrus (area 6), left middle and superior frontal gyri (areas 9, 6), and right anterior cingulate gyrus (area 32). It is not surprising that these regions showed greater involvement in the paced conditions, since they are known to be involved sustained attention (Lawrence et al., 2003), working memory (Belger et al., 1998), motor planning (Murphy et al., 1997), and response inhibition (de Zubicaray et al., 2000). The direct factor comparison did not reveal any regions in which activity was greater during the unpaced conditions relative to the paced conditions.
The regions that differed between the paced and unpaced conditions differed from those that were observed for fluency overall, suggesting that paced and unpaced fluency share a core set of regions, with additional regions recruited for the paced conditions. As an obvious possibility, activational differences between paced and unpaced conditions might be due to greater numbers of exemplars participants may generate when not required to pace their responses. However, our behavioral results indicate only very small performance differences between the two versions with regard to the number of exemplars produced. These differences were not significant in a groupwise analysis, nor were they significant in any single subject when performance for the four paced and four unpaced blocks was compared intraindividually. Since there were no robust or consistent performance differences between the two conditions, it is extremely unlikely that performance variability might have resulted in any significant activational differences detected in our analyses.
The rate of speech production has been shown in previous parametric studies to be associated with increased activity in motor regions, such as cerebellum, primary motor cortex, supplementary motor area, and thalamus (Riecker et al., 2005; Wildgruber, Ackermann & Grodd, 2001), i.e., in regions that mostly differed from those showing effects for paced (compared to unpaced) conditions in the current study. Possible exceptions are the cerebellum and medial superior frontal area 6. Note, however, that rate-related effects in these previous studies (Riecker et al., 2005; Wildgruber et al., 2001) occurred in the cerebellar hemispheres, whereas paced conditions in our study activated the vermis. Three further clusters observed in our study could be speech-rate related: Right superior temporal activity could be associated with monitoring of one’s own speech (Price et al., 1996); and activity in the right caudate nucleus and left putamen occurred in regions known to participate in speech motor functions (Price et al., 1996; Riecker et al., 2005). However, since word production rate was overall minimally lower for paced than for unpaced conditions, it remains unclear how detected effects could be motor-related. It is possible that regular timing of articulatory processes in paced conditions increased the overall detectability of speech motor effects. Although paced experimental conditions were matched with analogously paced control conditions (saying “nothing” repeatedly), the observed effects could be related to greater articulatory effort during novel word generation.
Response pacing was also not associated with increased head motion; on the contrary, we found a non-significant trend for reduced head motion in the paced compared to the unpaced conditions, which may have contributed to the overall more robust activation effects for the paced conditions. The comparatively robust activation effects for the paced conditions are thus partly explained by methodological advantages (more optimal distribution of exemplar production throughout task blocks, possibly reduced head movement), but also by additional cognitive demands (such as working memory and response inhibition), compared to unpaced conditions.
The differences between overt and covert conditions were not consistent with our expectations. Direct factor comparisons revealed a number of regions with greater activity for the covert compared to the overt conditions (Table 3 and Fig. 3E), whereas no significant inverse effects were found. The regions showing greater activity during covert conditions included the right superior and inferior parietal cortex, the left anterior cingulate gyrus, the superior frontal gyrus (area 6 on the left and area 11 on the right), and the left middle temporal gyrus (area 21). The finding of greater activity during covert than overt conditions differs from some previous results. For example, Barch and colleagues (Barch et al., 1999) found that in a Stroop paradigm, expected effects were seen only for overt – but not covert – responses. Shuster and Lemieux (Shuster & Lemieux, 2005) directly compared overt and covert word repetition and found numerous sites in precentral, insular, lateral temporal, and occipital regions showing greater activation for their overt condition.
Increased activity in covert compared to overt word generation could reflect greater overall signal variance in overt conditions due to greater head motion. Although detected head motion was generally low in all four conditions of our study, it was as expected significantly greater for overt than for covert conditions. Orthogonal regressors based on detected motion were used for protection against type-I error resulting from changes in head position. It should also be noted that in our study, absence or presence of overt speech was matched across task and control conditions, i.e., effects for overt generation were detected in comparison to an overt speech baseline whereas the baseline condition for covert generation was also covert. This stands in contrast to some previous studies that directly compared overt and covert conditions and reported stronger activity mostly in motor-related regions for overt conditions (Palmer et al., 2001; Shuster & Lemieux, 2005). Such effects were not to be expected for our paradigm and did indeed not occur. In view of previous findings (Barch et al., 1999), activation images based on comparisons of two conditions with similar speech production is expected to limit artifacts induced by magnetic susceptibility changes.
Further, within the overt conditions of our paradigm, articulation was well matched between task and control blocks. While this is to be expected for the paced responses given that subjects say a word at each prompt, it would not necessarily be the case for unpaced responses in which subjects establish their own pace of responding during the task and control periods. To determine how well matched the task and control blocks in the unpaced conditions were, the rates of responding in the task and control periods were compared. There was no significant difference between the mean response rates during the task and control periods.
If increased head motion had been a primary factor explaining our findings, it would have resulted in stronger detectable activation effects for covert conditions in regions known to be involved in lexical retrieval, such as inferior frontal cortex. While increased head motion during overt speech cannot be ruled out as a factor accounting for effects in left middle temporal gyrus (but see below) and parahippocampal regions, this explanation would not account for the majority of clusters showing such effects. Greater effects for covert conditions were seen in several right hemisphere regions, such as the anterior cingulate gyrus and the superior parietal lobe. Even a left hemispheric effect in superior frontal area 6 is unlikely to reflect activity associated with lexical retrieval, but rather explained by sustained and divided attention and movement-related conflict (Diedrichsen et al., 2006), given that our task required task performance (word generation) in the context of a conflicting instruction (not to articulate). Response conflict (Fan et al., 2003) and inhibition (Booth et al., 2003; de Zubicaray et al., 2000; Mostofsky et al., 2003; Rubia et al., 2000) have also been found to be associated with activity in inferior and superior parietal, superior frontal, anterior cingulate, and middle temporal regions in the vicinity of effects for covert versus overt production identified in the present study. It is therefore likely that these effects were predominantly related to the functional components mentioned above, which are orthogonal to lexical retrieval and word generation. Nonetheless, it cannot be ruled out that greater susceptibility artifact (related to movement of the oral cavity) in the overt conditions may have contributed to greater effects for covert conditions on direct comparison.
Our finding of relatively strong effects for covert compared to overt conditions is consistent with previous studies showing that covert word generation cannot be simply equated to overt responses minus articulatory motor execution, but instead contains additional extralinguistic functional components (Barch et al., 1999; Huang et al., 2002). It is also consistent with a recent review by Indefrey and Levelt (2004) showing that covert word generation is reliably associated with activity in various brain regions that activate less consistently in overt speech paradigms (even those with silent control conditions). Among these regions are the left middle temporal gyrus and the medial portion of the left superior frontal cortex, which were also found to show greater effects for covert than overt conditions in the present study.
As a general conclusion, our results suggest that specific task conditions chosen for eliciting a given linguistic process (category-driven word generation) have substantial impact on observed brain activation patterns. More specifically, our findings corroborate previous studies suggesting that covert versions of word generation paradigms do not only suffer from limitations in response monitoring, but also include extralinguistic functional components (related to divided attention, response conflict, and inhibition), which may confound the linguistic interpretation of regional activations. Our results suggest that extralinguistic components are also at work in paced generation paradigms, which put higher demands on working memory and transient response inhibition compared to unpaced generation. These components necessitate careful interpretation of regional hemodynamic effects. However, despite such disadvantages paced paradigms also have important strengths, such as tighter control over and reduced individual variability of task performance as well as potentially reduced head motion, resulting in an overall more robust pattern of language-related hemodynamic responses. Paced overt paradigms may be combined with sparse acquisition techniques to minimize motion and susceptibility artifact (e.g., Schwarzbauer et al., 2006). However, it is beyond the scope of the present study to examine the relative trade-offs related to increased session length and interrupted task performance in such techniques.
Acknowledgments
This study was supported by the National Institutes of Health, grant R01-NS43999 (S.B., E.D.P., M.A.R., B.W., R.-A.M). Thanks to Sarah N. Mattson for insightful comments, and to Giedrius Buracas and Akiko Mizuno for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams S, Goldstein LH, Simmons A, Brammer MJ, Williams SC, Giampietro VP, et al. Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Hum Brain Mapp. 2003;20(1):29–40. doi: 10.1002/hbm.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DC, Cohen JD. Overt verbal responding during fMRI scanning: Empirical investigations of problems and potential solutions. NeuroImage. 1999;10:642–657. doi: 10.1006/nimg.1999.0500. [DOI] [PubMed] [Google Scholar]
- Belger A, Puce A, Krystal JH, Gore JC, Goldman-Rakic P, McCarthy G. Dissociation of mnemonic and perceptual processes during spatial and nonspatial working memory using fMRI. Hum Brain Mapp. 1998;6(1):14–32. doi: 10.1002/(SICI)1097-0193(1998)6:1<14::AID-HBM2>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Human Brain Mapping. 1999;7(2):106–114. doi: 10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Experimental designs and processing strategies for fMRI studies involving overt verbal responses. Neuroimage. 2004;23(3):1046–1058. doi: 10.1016/j.neuroimage.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, et al. Neural development of selective attention and response inhibition. Neuroimage. 2003;20(2):737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: Functional anatomy by positron emission tomography. Journal of Neuroscience. 1991;11(8):2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crosson B. Subcortical mechanisms in language: lexical-semantic mechanisms and the thalamus. Brain Cogn. 1999;40(2):414–438. doi: 10.1006/brcg.1999.1088. [DOI] [PubMed] [Google Scholar]
- Crosson B, Benefield H, Cato MA, Sadek JR, Moore AB, Wierenga CE, et al. Left and right basal ganglia and frontal activity during language generation: contributions to lexical, semantic, and phonological processes. J Int Neuropsychol Soc. 2003;9(7):1061–1077. doi: 10.1017/S135561770397010X. [DOI] [PubMed] [Google Scholar]
- Cuenod CA, Bookheimer SY, Hertz-Pannier L, Zeffiro TA, Theodore WH, Le Bihan D. Functional MRI during word generation, using conventional equipment: a potential tool for language localization in the clinical environment. Neurology. 1995;45(10):1821–1827. doi: 10.1212/wnl.45.10.1821. [DOI] [PubMed] [Google Scholar]
- Cusack R, Cumming N, Bor D, Norris D, Lyzenga J. Automated post-hoc noise cancellation tool for audio recordings acquired in an MRI scanner. Hum Brain Mapp. 2005;24(4):299–304. doi: 10.1002/hbm.20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Zarahn E, Aguirre GK, Shin RK, Auerbach P, Detre JA. The effect of pacing of experimental stimuli on observed functional MRI activity. Neuroimage. 1997;6(2):113–121. doi: 10.1006/nimg.1997.0281. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SA, Veltman DJ, Raaijmakers JG, Lazeron RH, Jonker C. Parahippocampal activation during successful recognition of words: a self-paced event-related fMRI study. Neuroimage. 2001;13(6 Pt 1):1113–1120. doi: 10.1006/nimg.2001.0758. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, Zelaya FO, Andrew C, Williams SC, Bullmore ET. Cerebral regions associated with verbal response initiation, suppression and strategy use. Neuropsychologia. 2000;38(9):1292–1304. doi: 10.1016/s0028-3932(00)00026-9. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Grafton S, Albert N, Hazeltine E, Ivry RB. Goal-Selection and Movement-Related Conflict during Bimanual Reaching Movements. Cereb Cortex. 2006 doi: 10.1093/cercor/bhj108. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eden GF, Joseph JE, Brown HE, Brown CP, Zeffiro TA. Utilizing hemodynamic delay and dispersion to detect fMRI signal change without auditory interference: The behavior interleaved gradients technique. Magnetic Resonance Medicine. 1999;41(1):13–20. doi: 10.1002/(sici)1522-2594(199901)41:1<13::aid-mrm4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Edmister WB, Talavage TM, Ledden PJ, Weisskoff RM. Improved auditory cortex imaging using clustered volume acquisitions. Hum Brain Mapp. 1999;7(2):89–97. doi: 10.1002/(SICI)1097-0193(1999)7:2<89::AID-HBM2>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18(1):42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friedman L, Kenny JT, Wise AL, Wu D, Stuve TA, Miller DA, et al. Brain activation during silent word generation evaluated with functional MRI. Brain Lang. 1998;64(2):231–256. doi: 10.1006/brln.1998.1953. [DOI] [PubMed] [Google Scholar]
- Fu CH, Morgan K, Suckling J, Williams SC, Andrew C, Vythelingum GN, et al. A functional magnetic resonance imaging study of overt letter verbal fluency using a clustered acquisition sequence: greater anterior cingulate activation with increased task demand. Neuroimage. 2002;17(2):871–879. [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003;18(3):176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracco VL, Tremblay P, Pike B. Imaging speech production using fMRI. Neuroimage. 2005;26(1):294–301. doi: 10.1016/j.neuroimage.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Heim S, Amunts K, Mohlberg H, Wilms M, Friederici AD. Head motion during overt language production in functional magnetic resonance imaging. Neuroreport. 2006;17(6):579–582. doi: 10.1097/00001756-200604240-00005. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Gaillard W, Mott SH, Cuenod DA, Bookheimer Sy, Weinstein D, et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology. 1997;48:1003–1012. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- Hinke RM, Hu X, Stillman AE, Kim SG, Merkle H, Salmi R, et al. Functional magnetic resonance imaging of Broca’s area during internal speech. Neuroreport. 1993;4(6):675–678. doi: 10.1097/00001756-199306000-00018. [DOI] [PubMed] [Google Scholar]
- Huang J, Carr TH, Cao Y. Comparing cortical activations for silent and overt speech using event-related fMRI. Hum Brain Mapp. 2002;15(1):39–53. doi: 10.1002/hbm.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K, Lundervold A, Ersland L, Smievoll AI, Sundberg H, Barndon R, et al. Left frontal activation during a semantic categorization task: an fMRI-study. Int J Neurosci. 1999;99(1–4):49–58. doi: 10.3109/00207459908994312. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1–2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Kemeny S, Ye FQ, Birn R, Braun AR. Comparison of continuous overt speech fMRI using BOLD and arterial spin labeling. Hum Brain Mapp. 2005;24(3):173–183. doi: 10.1002/hbm.20078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15(7):1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Cohen L, Bazin B, Samson S, Giacomini E, Rougetet R, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54(8):1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- Luria AR. In: Higher Cortical Functions in Man. 2. Haigh B, translator. New York: Basic Books, Inc; 1966. 1980. [Google Scholar]
- Maccotta L, Zacks JM, Buckner RL. Rapid self-paced event-related functional MRI: feasibility and implications of stimulus- versus response-locked timing. Neuroimage. 2001;14:1105–1121. doi: 10.1006/nimg.2001.0912. [DOI] [PubMed] [Google Scholar]
- Markela-Lerenc J, Ille N, Kaiser S, Fiedler P, Mundt C, Weisbrod M. Prefrontal-cingulate activation during executive control: which comes first? Brain Res Cogn Brain Res. 2004;18(3):278–287. doi: 10.1016/j.cogbrainres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Mehta S, Grabowski TJ, Razavi M, Eaton B, Bolinger L. Analysis of speech-related variance in rapid event-related fMRI using a time-aware acquisition system. Neuroimage. 2006;29(4):1278–1293. doi: 10.1016/j.neuroimage.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milner B. Some Effects of Frontal Lobectomy in Man. In: Warren JM, Akert K, editors. The Frontal Granular Cortex and Behavior. New York: McGraw-Hill; 1964. [Google Scholar]
- Milner B. Some cognitive effects of frontal lobe lesions in man. Philosphical Transactions of the Royal Society of London. Series B: Biological sciences. 1982;298:211–226. doi: 10.1098/rstb.1982.0083. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Res Cogn Brain Res. 2003;17(2):419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Murphy K, Corfield DR, Guz A, Fink GR, Wise RJ, Harrison J, et al. Cerebral areas associated with motor control of speech in humans. J Appl Physiol. 1997;83(5):1438–1447. doi: 10.1152/jappl.1997.83.5.1438. [DOI] [PubMed] [Google Scholar]
- Nelles JL, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. An automated method for extracting response latencies of subject vocalizations in event-related fMRI experiments. NeuroImage. 2003;20(3):1865–1871. doi: 10.1016/j.neuroimage.2003.07.020. [DOI] [PubMed] [Google Scholar]
- Palmer ED, Rosen HJ, Ojemann JG, Buckner RL, Kelley WM, Petersen SE. An event-related fMRI study of overt and covert word stem completion. Neuroimage. 2001;14(1 Pt 1):182–193. doi: 10.1006/nimg.2001.0779. [DOI] [PubMed] [Google Scholar]
- Parks RW, Loewenstein DA, Dodrill KL, Barker WW, Yoshii F, Chang JY, et al. Cerebral metabolic effects of a verbal fluency test: a PET scan study. J Clin Exp Neuropsychol. 1988;10(5):565–575. doi: 10.1080/01688638808402795. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Goldacre B, Scifo P, Cappa SF, Gilardi MC, Castiglioni I, et al. Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport. 1997;8:2011–2016. doi: 10.1097/00001756-199705260-00042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Hyder F, Blamire AM, Shulman RG. FMRI of the prefrontal cortex during overt verbal fluency. NeuroReport. 1997;8:561–565. doi: 10.1097/00001756-199701200-00036. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Constructing neuronal theories of mind. In: Koch C, Davis JL, editors. Large-scale neuronal theories of the brain. Cambridge, MA, US: The MIT Press; 1994. [Google Scholar]
- Price CJ, Wise RJS, Warburton E, Moore CJ, Howard D, Patterson K, Frackowiak RSJ, Friston KJ. Hearing and saying: The functional neuroanatomy of auditory word processing. Brain. 1996;119:919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional fMRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AK, Pardo JV, Fox PT, et al. Practice-related changes in human brain functional anatomy during nonmotor learning. Cerebral Cortex. 1994 Jan/Feb;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64:700–6. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24(1):13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rueckert L, Appollonio I, Grafman J, Jezzard P, Johnson RJ, Le BD, et al. Magnetic resonance imaging functional activation of left frontal cortex during covert word production. J Neuroimaging. 1994;4(2):67–70. doi: 10.1111/jon19944267. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, et al. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry. 1998;64(4):492–498. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer C, Davis MH, Rodd JM, Johnsrude I. Interleaved silent steady state (ISSS) imaging: a new sparse imaging method applied to auditory fMRI. Neuroimage. 2006;29(3):774–782. doi: 10.1016/j.neuroimage.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Seurinck R, Vingerhoets G, Vandemaele P, Deblaere K, Achten E. Trial pacing in mental rotation tasks. Neuroimage. 2005;25(4):1187–1196. doi: 10.1016/j.neuroimage.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Shuster LI, Lemieux SK. An fMRI investigation of covertly and overtly produced mono- and multisyllabic words. Brain Lang. 2005;93(1):20–31. doi: 10.1016/j.bandl.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychological Bulletin. 1984;95(1):3–28. [PubMed] [Google Scholar]
- Stuss DT, Benson DF. The Frontal Lobes. New York: Raven Press; 1986. [Google Scholar]
- Swainson R, Cunnington R, Jackson GM, Rorden C, Peters AM, Morris PG, et al. Cognitive control mechanisms revealed by ERP and fMRI: evidence from repeated task-switching. J Cogn Neurosci. 2003;15(6):785–799. doi: 10.1162/089892903322370717. [DOI] [PubMed] [Google Scholar]
- Thurstone LL. Primary Mental Abilities. Chicago: Chicago University Press; 1938. [Google Scholar]
- Tieleman A, Seurinck R, Deblaere K, Vandemaele P, Vingerhoets G, Achten E. Stimulus pacing affects the activation of the medial temporal lobe during a semantic classification task: an fMRI study. Neuroimage. 2005;26(2):565–572. doi: 10.1016/j.neuroimage.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Ackermann H, Grodd W. Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: effects of syllable repetition rate evaluated by fMRI. Neuroimage. 2001;13:101–9. doi: 10.1006/nimg.2000.0672. [DOI] [PubMed] [Google Scholar]
- Yetkin FZ, Swanson S, Fischer M, Akansel G, Morris G, Mueller W, et al. Functional MR of frontal lobe activation: comparison with Wada language results. AJNR Am J Neuroradiol. 1998;19(6):1095–1098. [PMC free article] [PubMed] [Google Scholar]