Abstract
Objective
To determine whether African-American adolescents have endothelial dysfunction compared to Caucasians and whether differences are due to differences in insulin sensitivity (SI) or secretion.
Study design
Thirty-three Caucasian (age, 13.6±2.6 yr; BMI 21.6±4.4 kg/m2 mean ±SD) and 25 African-American (13.3±2.9 yr; 24.0±4.4 kg/m2) adolescents were studied. Forearm blood flow (FBF, plethysmography) was measured before and after 5 min of arterial occlusion. SI and acute insulin response to glucose (AIRG) were measured using intravenous glucose tolerance tests and minimal modeling.
Results
Baseline FBF did not differ between races. Post-occlusion FBF was lower in African-Americans (17.2±1.2 versus 22.6±1.2 ml/dl/min, p=0.006). AIRG was higher in African-Americans (6050±940 versus 2410±30 μU min/ml, p=0.001). Pubertal stage had no effect. SI did not differ by race or pubertal stage. In African-Americans, percent fall in forearm vascular resistance following arterial occlusion correlated (r=0.67 p=0.001) with logAIRG. No relationships were found between percent fall in FVR and SI in either race.
Conclusion
African-American adolescents have decreased endothelial function. This may be due to increased insulin secretion. Endothelial dysfunction in African-American adolescents may predispose to cardiovascular and type 2 diabetes.
Keywords: insulin sensitivity, insulin secretion, puberty
African-American adults have increased morbidity and mortality from most of the diseases associated with the metabolic syndrome, including myocardial infarction at younger ages (1–4), stroke (5,6), hypertension (3), and type 2 diabetes in both adults and adolescents (3,7,8). Multiple differences have been demonstrated between African Americans and Caucasians in the underlying pathophysiologic features of the metabolic syndrome. African-American adults have impaired endothelial function compared to Caucasian adults, specifically, endothelin 1, a potent vasoconstrictor, is increased (9) and flow mediated brachial artery vasodilation (10) is diminished. Hinderliter et al (11,12) found higher minimum FVR following vascular occlusion in African American, young adults compared to similar aged. Caucasian subjects. Endothelial dysfunction is an early predictor of each of both cardiovascular disease and type 2 diabetes (13). African-American adults have increased carotid artery intima-medial thickness (14). Adolescent African Americans with a family history of essential hypertension have increased basal and stress-stimulated endothelin 1 levels compared to similarly-selected, Caucasian adolescents (15).
Mechanistically, there is a close link between endothelial function and insulin sensitivity in both normotensive and hypertensive subjects (16,17). This is important because African American adolescents are more frequently insulin resistant compared to Caucasians (18,19). We therefore hypothesized that: 1) Since insulin sensitivity is decreased in African-American adolescents endothelial function would also be lower in African-American adolescents; 2) Since insulin sensitivity is lower in pubertal than in prepubertal and postpubertal subjects (20), endothelial function would also be lower in pubertal subjects than in prepubertal or postpubertal subjects, and 3) Endothelial function would be positively associated with muscle insulin sensitivity.
Methods
Subjects
Healthy, adolescent volunteers between 8 and 18 years of age were recruited for participation. They were taking no medications and were free from chronic and acute disease at the time of study. Pregnancy tests were done on all females and all were negative. Subjects were recruited into one of the following groups, prepubertal (Tanner stage 1 breast or genital development depending on sex, AA n=3, C, n=11), pubertal (Tanner stage 2,3, or 4 breast or genital development, AA, n=11, C, n=11) or postpubertal (Tanner stage 5 breast or genital development, AA, n=11, C=11). Tanner staging was done by the principal investigator (RPH), an experienced pediatric endocrinologist. The study was approved by the Ohio State University Office of Responsible Research. Informed consent was obtained from a parent or legal guardian and assent from each subject.
Protocol
Subjects were admitted to the Clinical Research Center of the Ohio State University at 8AM. They were instructed not to eat or drink anything except water after 10PM the evening before. Height and weight were measured and endothelial function was tested, as described below. Endothelial function of the resistance vessels was assessed by measuring forearm blood flow (FBF) before and after upper arm arterial occlusion (21–24). The stable isotope labeled frequently-sampled intravenous glucose tolerance test and minimal modeling were used to assess insulin secretion and secretion (25).
Endothelial Function
FBF was measured in the dominant arm using venous-occlusion plethysmography with indium-in-silastic strain gauge and a Hokanson EC6 plethysmograph (DE Hokanson Inc, Bellevue WA). Sphygomanometric cuffs were placed on the arm at the wrist and on the upper arm. During measurement the wrist cuff was inflated to 200 mmHg to occlude flow to the hand which is primarily skin blood flow and the upper arm cuff was inflated to 40 mmHG for 10 out of every 15 second to occlude venous return. Data were recorded using PowerLab and Chart 4.0 (AD Instruments, Grand Junction, CO) on a Power Mac G4 (Apple, Cupertino, CA). FBF is expressed as ml blood per dl arm tissue per min. Arterial blood pressure was continuously monitored by arterial tonometry in the nondominant arm. (Model 7000, Colin Medical Instruments, San Antonio TX). Forearm vascular resistance (FVR) was calculated by dividing mean arterial pressure by FBF. For each subject two minutes of FBF were recorded and then the upper arm cuff was inflated to 200 mmHg pressure for five minutes to occlude flow to the arm. It was then released and FBF was again measured over the next minute. All studies were scored twice by a single observer (RPH) on separate occasions and the mean value was used for analysis. Mean intra-observer coefficient of variation for FBF before upper arm occlusion was 5.1% and was 7.4% after upper arm occlusion. To confirm differences found between races in FBF post vascular occlusion, we also assessed the percent change in forearm vascular resistance following upper occlusion
Intravenous glucose tolerance test
After completion of the test of endothelial function, intravenous catheters were placed in each arm for the frequently- sampled intravenous glucose tolerance test and to obtain the fasting blood sample for measurement of the fasting lipid profile. A bolus of 25% dextrose in water with approximately 13% [6–6]D2 glucose was given. The total glucose dose was 250 mg/kg. Three milliliter blood samples were taken at -10, 0, 2, 4, 6, 8, 12, 14, 16, 19, 22, 27, 32, 42, 52, 62, 72, 82, 92, 102, 122, 142, 162, and 182 minutes relative to a glucose bolus for measurement of plasma glucose, insulin, and [6,6]D2 glucose concentrations.
Total body (SI) and peripheral insulin sensitivity (SI*) were calculated using the one compartment minimal model for total and labeled glucose concentrations, respectively, using the program Minmod (Minmod, Inc, Los Angeles, CA) (25,26). The program was also used to calculate the acute insulin response to glucose (AIRG) over the first 19 minutes of the test. Hepatic glucose production was calculated through the use of Steele’s equations (27) over the last hour of the study when the system had returned to a quasi steady state. To adjust for differing insulin levels between subjects during the time period when hepatic glucose production was determined, hepatic insulin resistance (HIR) was calculated by multiplying hepatic glucose production by the mean insulin level over the same hour, since higher insulin levels should suppress hepatic glucose production (25,26).
Assays
Plasma glucose and insulin concentrations were measured in the CORE lab of the Clinical Research Center of the Ohio State University and mole percent excess of [6,6] D2 glucose was measured by Metabolic Solutions (Cambridge MA). Plasma lipids were measured in the Clinical Laboratory of the Ohio State University Hospital.
Statistical Analysis
Analysis of variance and analysis of variance of repeated measures were used to determine differences in FBF and FVR responses to across-race and pubertal status. Two-tailed, planned contrasts were used for individual group comparisons. Pearson correlation coefficients and multiple linear regression were used to assess the relationships between reactive hyperemia and insulin sensitivity, secretion, BMI and lipids. Systat 10 (Systat Software Inc, Point Richmond, CA) was be used to perform all statistical analysis. Data are expressed as mean±SE. Differences were considered significant at p<0.05 and tendencies are mentioned as p<0.1.
Results
Subject characteristics
BMI significantly differed by pubertal group (Table I, p<0.001) but did not differ between the races. Systolic blood pressure tended to be higher in African-American than Caucasian subjects (p=0.056) although no differences were seen for diastolic pressure. Neither systolic nor diastolic pressure varied by pubertal group. For plasma lipid levels no differences were seen between races or pubertal groups except for a trend toward higher LDL levels in African Americans (p=0.071). The race by group interaction approached significance (p=0.064) indicating the effect of pubertal stage on LDL cholesterol may vary between the races. Specifically, LDL cholesterol was increased in prepubertal African American subjects. Fasting glucose and insulin levels did not differ between races or pubertal stages.
Endothelial function and insulin sensitivity differences
Baseline FBF was not different between the races. However, post-occlusion FBF was significantly lower in African-Americans (Figure 1, p=0.006). The FBF increase following upper arm occlusion in Caucasian adolescents was greater than in African-American adolescents (time x race interaction, p=0.004). The time by pubertal stage effect (p=0.10) and time by pubertal stage by race effects (p=0.28) were not significant indicating that pubertal stage did not alter post-occlusive FBF or the effect of race.
Figure 1.
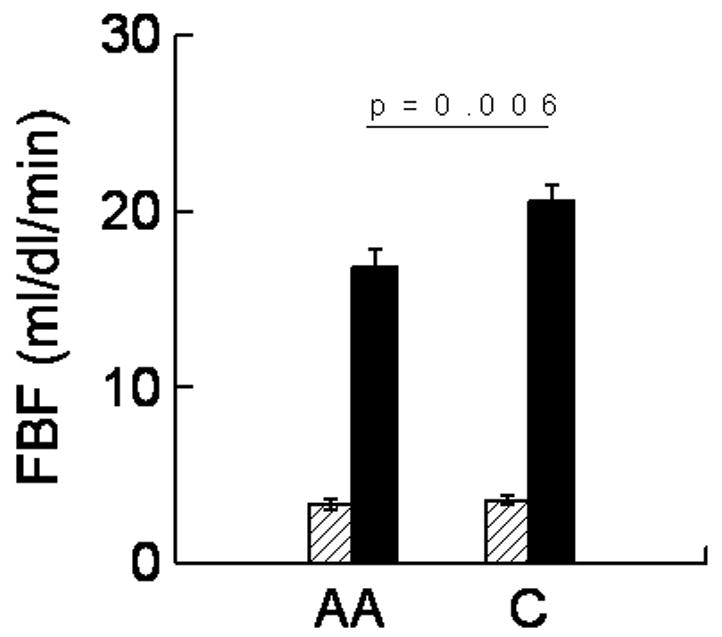
Pre (hatched bars) and post upper arm occlusion FBF (solid bars) in African-American (AA) and Caucasian (C) adolescents. FBF increased significantly more in Caucasians (time by race interaction p=0.004). Error bars represent SE.
The greater post-occlusion vasodilation in Caucasian adolescents was confirmed by the significant differences in percent change in FVR between the races (Table II, p=0.018). The pubertal stage effect and race by pubertal stage interaction were not significant. When the subjects were analyzed by pubertal stage, there was a trend toward significant racial difference in the postpubertal subjects (p=0.057).
AIRG was significantly greater (p=0.001) and SI* was significantly lower (p=0.023) in African-Americans than in Caucasians. There were no differences in SI or HIR between the races. When the subjects were compared within each pubertal stage group, the difference in AIRG was significant only in the postpubertal subjects (p=0.001), although the differences in the prepubertal (p=0.064) and pubertal (p=0.051) groups approached significance. For SI*, the difference between African-Americans and Caucasians was significant only in the prepubertal group (p=0.004). No pubertal stage effects were seen.
Because of the small number of African-American prepubertal subjects and because these subjects had an increased BMI, separate analysis was done on just the pubertal and postpubertal subjects. Baseline FBF did not differ by race pubertal stage. Post-occlusion FBF was lower in African-Americans (p=0.033) and the time by race interaction for FBF was again significant (p=0.014) indicating the increase in FBF was lower in African-Americans. This was confirmed by assessing the post-occlusion percent fall in FVR (p=0.041). Interestingly, with prepubertal subjects excluded, the post-occlusion FBF was significantly lower in postpubertal than pubertal subjects (p=0.009) and the time by puberty interaction was also significant (p=0.023). The pubertal differences in percent fall FVR did not quite reach statistical significance (p=0.084). Results from the intravenous glucose tolerance tests again revealed only a significant racial difference in AIRG.
Relationship of endothelial function to insulin secretion, sensitivity, and lipids
In the group as a whole no correlations were present between percent change in FVR and any of the plasma lipid measurement, SI, SI*, or HIR. There was a correlation between percent change in FVR and log AIRG (r=0.41, p=0.004). The percent change in FVR also correlated with BMI (r=0.31, p=0.017). Only the effect of logAIRG was significant (p=0.042) when all three were included in the regression equation.
Inclusion of log AIRG in the equation with race eliminated the racial effects and the effect of log AIRG tended to remain significant (Table III). The racial effect was nearly significant when BMI was included in the equation and remained significant when log triglycerides were included.
When the races were analyzed individually, significant relationships were found in African-Americans only. Specifically, percent change in FVR (Figure 2) correlated with BMI (r=0.52, p=0.008), log plasma triglyceride levels (r=0.49, p=0.016), and log AIRG (r=0.67, p=0.001). No relationships were found between RH and fasting glucose, fasting insulin, SI, SI*, or HIR in either race.
Figure 2.
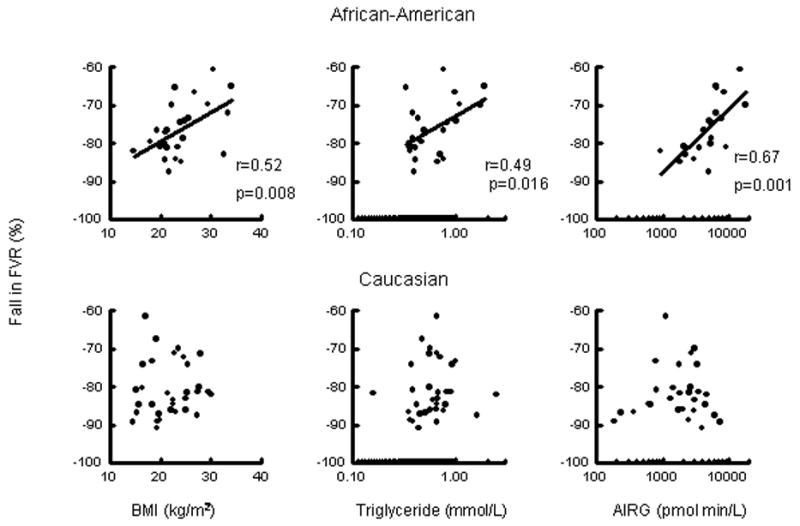
Relationship of percent fall in forearm vascular resistance following upper arm occlusion to body mass index (BMI), triglycerides, and acute insulin response to glucose in African-American (AA) and Caucasian (C) adolescents. For African-Americans: BMI r=0.52, p=0.008; log plasma triglyceride levels r=0.49, p=0.016; log AIRG r=0.67, p=0.001.
Multiple linear regression with percent change in FVR as the dependent variable and BMI, log plasma triglycerides and log AIRG as independent variables was also performed in each race separately. In African-Americans the relationships of percent change in FVR to BMI and triglycerides were no longer significant. The relationship to AIRG remained significant (p=0.012). This relationship retained significance if BMI or triglycerides were included, independently. No relationships were found in Caucasians.
Discussion
These results demonstrate that impaired endothelial function is present in African-American adolescents. Specifically, we found diminished maximal post-occlusion FBF compared to pubertal stage-matched Caucasian subjects which was confirmed by the greater fall in FVR in Caucasians. The percent change in FVR in the Caucasian adolescents in our study was nearly identical to that in non-diabetic adolescents found by Newkumett et al (28). We believe that this impairment in endothelial function may predispose African-American adolescents to future cardiovascular complications of the metabolic syndrome as adults.
In adults, there is an interrelationship between endothelial function and insulin sensitivity (16,17). Insulin-induced vasodilation (29–31) is mediated by endothelial nitric oxide release (32). Diminished endothelial function, thus, decreases insulin-induced vasodilation and glucose delivery to and usage by muscle tissue. We, however, did not find the hypothesized relationship between percent change in FVR and insulin sensitivity. These results confirm the findings of Singhal et al (23) using more rigorous measures of insulin sensitivity than that derived from fasting glucose and insulin levels using the homeostatic method in their study.
We did find a significant relationship between percent change in FVR and AIRG in the all subjects and in the African-Americans alone. The positive correlations indicate increased insulin secretion is associated with decreased endothelial function since percent change in FVR is negative. In the group as whole the racial difference in percent change in FVR was eliminated by controlling for increased insulin secretion. Interestingly, however this effect was primarily present only in the African-Americans indicating hyperinsulinism may have differing effects in the two races or there may be a threshold effect with manifestation in the African-American adolescents alone due to their higher insulin secretion. Since insulin, itself, causes endothelial-induced vasodilation (32), persistent exposure to higher insulin levels may exhaust nitric oxide availability. On the other hand, it is also possible that the reverse is true and that endothelial dysfunction is the cause of the increased insulin secretion in African-American adolescents. Diminished insulin-induced endothelial vasodilation could lead to decreased glucose delivery and insulin resistance with compensatory increases in insulin secretion, as described above. This is less likely since insulin sensitivity did not differ between the races in this study.
We, also, found positive relationships between percent change in FVR and BMI and triglyceride levels in African-American, but not Caucasian, adolescents. Multiple linear regression revealed that only the relationship to AIRG remained relevant. Thus, the relationship between percent change in FVR and AIRG does not appear to be due to increased body size or triglyceride levels
Evidence of insulin resistance in African-American adolescents includes higher insulin to glucose ratios following oral glucose in (33) compared to Caucasians in all Tanner stages and in both sexes have increased and higher glucose requirementr during insulin clamp in African-American pubertal (11) and prepubertal children (12). The differences are not explained by differences in body composition, although obesity in African-American children worsens insulin resistance more than in Caucasians (34). African-American children compensate for the decreased insulin sensitivity by both increasing insulin secretion (35) and decreasing insulin clearance (35,36).
In contrast to these studies which suggest that the increased insulin secretion in African-American adolescents is due to insulin resistance, Preeyasombat et al (37) suggested that increased insulin secretion may be the primary defect and insulin resistance secondary, at least in obese, African-American adolescents compared to obese, Caucasian adolescents. This was based on findings from oral glucose tolerance tests which demonstrated that insulin sensitivity was not different between the two groups when corrected for insulin secretion, but the latter was increased in African-Americans. Our findings support this hypothesis. We found markedly increased AIRG in all pubertal stages but a decrease in SI* only in prepubertal African American adolescents.
The study has two significant limitations. The first is the inclusion of subjects of all weights. Because of this we cannot categorically say that our results would be present in just lean adolescents. However, when BMI was included as a covariate, the race and pubertal stage effects remained significant. Second, we did not study subjects by sex. Differences in endothelial function and insulin sensitivity have been reported between males and females (26,38,39). Each of our groups contained nearly equal numbers of boys and girls. Including sex in the analysis of variance however did not eliminate the racial difference (data not shown).
Lastly, it would be useful to confirm our findings with other methods of measuring endothelial function, specifically, flow-mediated vasodilation of the brachial artery as measured by ultrasound. The two techniques measure endothelial function in different types of blood vessels. The technique we used function examines resistance vessels (23), as opposed to a conduit vessel. We believed this to be more clinically significant.
In conclusion, African-American adolescents have impaired endothelial function of the resistance vessels. Additional study will be needed to determine whether this dysfunction predisposes to future cardiovascular disease and type 2 diabetes in African-American adults. The impairment may be due to increased insulin secretion.
Table 1.
Baseline data in African American and Caucasian Adolescents
| ANOVA (p-value) | |||||||
|---|---|---|---|---|---|---|---|
| Prepubertal | Pubertal | Postpubertal | Race Effect | Puberty Effect | Interaction Effect | ||
| BMI (kg/m2) | African American | 21.5±1.3 (n=3) | 22.6±1.5 (n=11) | 26.2±1.4 (n=11) | 0.14 | <0.001 | 0.17 |
| Caucasian | 17.3±0.7 (n=11) | 23.5±0.9 (n=11) | 24.7±1.1 (n=11) | ||||
| Systolic Pressure (mmHg) | African American | 106±9 | 118±4 | 111±3 | 0.056 | 0.12 | 0.38 |
| Caucasian | 93±8 | 105±3 | 110±3 | ||||
| Diastolic Pressure (mmHg) | African American | 55±6 | 58±3 | 56±3 | 0.25 | 0.75 | 0.52 |
| Caucasian | 52±2 | 52±2 | 56±2 | ||||
| Cholesterol (mmol/L) | African American | 4.7±0.3 | 4.0±0.3 | 4.1±0.2 | 0.23 | 0.40 | 0.33 |
| Caucasian | 4.0±0.1 | 4.0±0.2 | 160±0.3 | ||||
| HDL (mmol/L) | African American | 1.3±0.2 | 1.2±0.1 | 1.2±0.1 | 1 | 0.19 | 0.76 |
| Caucasian | 1.5±0.1 | 1.1±0.1 | 1.2±0.1 | ||||
| LDL (mmol/L) | African American | 3.2±0.2* | 2.5±0.2 | 2.6±0.2 | 0.078 | 0.46 | 0.71 |
| Caucasian | 2.2±0.1 | 2.5±0.1 | 2.7±0.2 | ||||
| Triglycerides (mmol/L) | African American | 0.5±0.1 | 0.5±0.1 | 0.8±0.2 | 0.71 | 0.15 | 0.90 |
| Caucasian | 0.5±0.1 | 0.6±0.1 | 0.8±0.2 | ||||
| Fasting glucose (mmol/L) | African American | 4.8±0.3 | 4.6±0.2 | 4.6±0.1 | 0.55 | 0.11 | 0.61 |
| Caucasian | 4.6±0.1 | 4.6±0.2 | 4.3±0.1 | ||||
| Fasting insulin (pmol/L) | African American | 76±22 | 49±9 | 73±14 | 0.80 | 0.95 | 0.14 |
| Caucasian | 83±50 | 135±66 | 58±18 | ||||
Mean ±SE
p<0.05 versus Caucasians for same pubertal stage.
Table 2.
Endothelial function and stable labeled frequently sampled intravenous glucose tolerance test results in African-American and Caucasian adolescents.
| ANOVA (p-values) | |||||||
|---|---|---|---|---|---|---|---|
| Prepubertal | Pubertal | Postpubertal | Race effect | Puberty Effect | Interaction Effect | ||
| Change in FVR (%) | African American | −74.1±3.1 (n=3) | −79.5±1.9 (n=11) | −74.2±2.3 (n=11) | 0.018 | 0.17 | 0.76 |
| Caucasian | −80.2±2.7 (n=11) | −82.4±2.2 (n=11) | −80.0±1.6 (n=11) | ||||
| AIRG pmol min/L) | African American | 5880±610 | 4240±770 | 8350±1900* | 0.001 | 0.36 | 0.60 |
| Caucasian | 2080±770 | 2472±550 | 2380±430 | ||||
| SI (l/pmol min) | African American | 0.608±0.213 | 1.49±0.85 | 0.902±0.258 | 0.32 | 0.60 | 0.28 |
| Caucasian | 3.60±1.00 | 1.91±0.98 | 1.22±0.38 | ||||
| SI* (l/mU min) | African American | 0.330±0.37* | 1.06±0.54 | 0847±0.192 | 0.023 | 0.19 | 0.027 |
| Caucasian | 1.57±0.60 | 1.01±0.24 | 0.903±0.25 | ||||
| HIR (mmol mol/kg min L) | African American | 0.637±0.19 | 0.282±0.043 | 0.266±0.80 | 0.58 | 0.47 | 0.081 |
| Caucasian | 0.244±0124 | 0263±0.047 | 0.583±280 | ||||
Mean±SE
p<0.05 vs Caucasians in same pubertal group.
Table 3.
Effect of AIRG, BMI, triglycerides on racial difference in endothelial function as assessed by multiple linear regression analysis.
| Secondary effect | ||||||
|---|---|---|---|---|---|---|
| Dependent Variable | Constant | Racial effect AA=1 C=2 | Variable | Coefficient | R2 | P |
| Fall in FVR(%) | −87.8
p<0.001 |
−3.14
p=0.161 |
Log(AIRG) | 2.26
p=0.068 |
0.20 | 0.006 |
| −82.5
p<0.001 |
−3.67
p<0.059 |
BMI | 0.41
p=0.041 |
0.155 | 0.010 | |
| −86.8
p<0.001 |
−4.67
p=0.019 |
Log(Triglycerides) | 3.831
p=0.054 |
0.154 | 0.012 | |
Acknowledgments
The authors would like to thank the nurses of the Clinical Research Center for their help with the glucose tolerance tests.
Abbreviations
- AIRG
acute insulin response to glucose
- FBF
forearm Blood flow
- FVR
forearm vascular resistance
- HIR
hepatic insulin resistance
- SI
insulin sensitivity calculated from total glucose
- SI*
insulin sensitivity calculated from labeled glucose
- SG
glucose effectiveness
This research was funded by a grant from the Ohio Valley Affiliate of the American Heart Association and the National Institute of Health (M01-RR00034) and is the work of the investigators only.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alderman MH, Cohen HW, Madhavan S. Myocardial infarction in treated hypertensive patients: the paradox of lower incidence but higher mortality in young blacks compared with whites. Circulation. 2000;101:1109–14. doi: 10.1161/01.cir.101.10.1109. [DOI] [PubMed] [Google Scholar]
- 2.Manhapra A, Canto JG, Vaccarino V, Parsons L, Kiefe CI, Barron HV, et al. Relation of age and race with hospital death after acute myocardial infarction. Am Heart J. 2004;148:92–8. doi: 10.1016/j.ahj.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Ferdinand KC, Clark LT. The epidemic of diabetes mellitus and the metabolic syndrome in African Americans. Rev Cardiovasc Med. 2004;5(Suppl 3):S28–33. [PubMed] [Google Scholar]
- 4.Williams JE, Massing M, Rosamond WD, Sorlie PD, Tyroler HA. Racial disparities in CHD mortality from 1968–1992 in the state economic areas surrounding the ARIC study communities. Atherosclerosis Risk in Communities. Ann Epidemiol. 1999;9:472–80. doi: 10.1016/s1047-2797(99)00029-0. [DOI] [PubMed] [Google Scholar]
- 5.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–31. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 6.Yang D, Howard G, Coffey CS, Roseman J. The confounding of race and geography: how much of the excess stroke mortality among African Americans is explained by geography? Neuroepidemiology. 2004;23:118–22. doi: 10.1159/000075954. [DOI] [PubMed] [Google Scholar]
- 7.Macaluso CJ, Bauer UE, Deeb LC, Malone JI, Chaudhari M, Silverstein J, et al. Type 2 diabetes mellitus among Florida children and adolescents, 1994 through 1998. Pub Health Rep. 2002;117:373–9. doi: 10.1093/phr/117.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBean AM, Li S, Gilbertson DT, Collins AJ. Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: whites, blacks, Hispanics, and Asians. Diabetes Care. 2004;27:2317–24. doi: 10.2337/diacare.27.10.2317. [DOI] [PubMed] [Google Scholar]
- 9.Evans RR, Phillips BG, Singh G, Bauman JL, Gulati A. racial and gender differences in endothelin-1. Am J Cardiol. 1996;78:486–8. doi: 10.1016/s0002-9149(96)00344-x. [DOI] [PubMed] [Google Scholar]
- 10.Perregaux D, Chauduri A, Rao S, Airen A, Wilson M, Sung BH, Dandona D. Brachial vascular reactivity in blacks. Hypertens. 2000;36:866–71. doi: 10.1161/01.hyp.36.5.866. [DOI] [PubMed] [Google Scholar]
- 11.Hinderliter AL, Sager AR, Sherwood A, Light KC, Gridler SS, Willis PW. Ethnic differences in forearm vasodilator capacity. Am J Cardiolol. 1996;78:208–11. doi: 10.1016/s0002-9149(96)90397-5. [DOI] [PubMed] [Google Scholar]
- 12.Hinderliter AL, Blumenthal JA, Waugh R, Chlukuri M, Sherwood A. Ethnic differences in left ventricular structure: relations to hemodynamics and diurnal blood pressure variation. Am J Hypertens. 2004;17:43–9. doi: 10.1016/j.amjhyper.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Vita JA, Keany JF., Jr Hormone replacement therapy and endothelial function: the exception that proves the rule? Arterioscler Thromb Vasc Biol. 2004;21:1867–69. [PubMed] [Google Scholar]
- 14.Lange LA, Bowden DW, Langefeld CD, Wagenknecht LE, Carr JJ, Rich SS, et al. Heritability of carotid artery intima-medial thickness in type 2 diabetes. Stroke. 2002;33:1876–81. doi: 10.1161/01.str.0000019909.71547.aa. [DOI] [PubMed] [Google Scholar]
- 15.Serne EH, Gans ROB, ter Maaten JC, ter Wee PM, Donker AJM, Stehouwer CDA. Capillary recruitment is impaired in essential hypertension and relates to insulin’s metabolic and vascular actions. Cardiovascular Res. 2001;49:161–168. doi: 10.1016/s0008-6363(00)00198-x. [DOI] [PubMed] [Google Scholar]
- 16.Serne EH, Stehouwer CDA, ter Maaten JC, ter Wee PM, et al. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation. 1999;99:896–902. doi: 10.1161/01.cir.99.7.896. [DOI] [PubMed] [Google Scholar]
- 17.Petrie JR, Ueda S, Webb DJ, Elliott HL, Connell JM. Endothelial nitric oxide production and insulin sensitivity. A physiologic link with implications for pathogeneisis of cardiovascular disease. Circulation. 1996;33:1331–1333. doi: 10.1161/01.cir.93.7.1331. [DOI] [PubMed] [Google Scholar]
- 18.Arslanian S, Suprasongsin C. Differences in the in vivo insulin sectretion and sensitivity in healthy black versus white adolescents. J Pediatr. 1996;229:440–3. doi: 10.1016/s0022-3476(96)70078-1. [DOI] [PubMed] [Google Scholar]
- 19.Arslanian S, Suprasongsin C, Janosky JE. Insulin secretion and sensitivity in black versus white prepubertal healthy children. J Clin Endocrinol Metab. 1997;82:1923–7. doi: 10.1210/jcem.82.6.4002. [DOI] [PubMed] [Google Scholar]
- 20.Cook JS, Hoffman RP, Stene MA, Hansen JR. Effects of maturational stage on insulin sensitivity during puberty. J Clin Endocrinol Metab. 1993;77:725–30. doi: 10.1210/jcem.77.3.7690363. [DOI] [PubMed] [Google Scholar]
- 21.Uehata A, Lieberman EH, Gerhard MD, Anderson TJ, et al. Non invasive assessment of endothelium dependent flow-mediated dilation in the brachial artery. Vasc Med. 1997;2:87–92. doi: 10.1177/1358863X9700200203. [DOI] [PubMed] [Google Scholar]
- 22.Higashi Y, Yoshizumi M. New methods to evaluate endothelial function: method for assessing endothelial function in humans using a strain-gauge plethysmography: nitric oxide-dependent and -independent vasodilation. Journal Pharmacol Sci. 2003;93:399–404. doi: 10.1254/jphs.93.399. [DOI] [PubMed] [Google Scholar]
- 23.Singhal A, Jamison N, Fewtrell M, Deanfield J, Lucas A, Sattar N. Adiponectin predicts insulin resistance but not endothelial function inhealthy young adults. J Clin Endocrinol Metab. 2005;90:4615–21. doi: 10.1210/jc.2005-0131. [DOI] [PubMed] [Google Scholar]
- 24.Dagre A, Lekakis J, Mihas C, Protgerou A, Thalassinou L, Tryhonopoulus D, Douridas G, Papamichael C, Alevizaki M. Association of dehydroepiandrosterone-sulfate with endothelial function in young women with polycystic ovary syndrome. Eur J Endocrinol. 2006;154:883–890. doi: 10.1530/eje.1.02153. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman RP, Armstrong PT. Glucose effectiveness, peripheral and hepatic insulin sensitivity in obese and lean prepubertal children. Int J Obes Relat Metab Disord. 1996;20:521–5. [PubMed] [Google Scholar]
- 26.Hoffman RP, Vicini P, Sivitz WI, Cobelli C. Pubertal adolescent male-female differences in insulin sensitivity and glucose effectiveness determined by the one compartment minimal model. Pediatr Res. 2000;48:384–8. doi: 10.1203/00006450-200009000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Steele R. Use of C14-glucose to measure hepatic glucose production following an I.V. glucose load or after injection of insulin. Metabolism. 1959;8:512–519. [PubMed] [Google Scholar]
- 28.Nekummet KM, Goble MM, Young RB, Kaplowitz PB, Schiekin RM. Altered blood pressure reactivity in adolescent diabetics. Pediatrics. 1994;93:616–21. [PubMed] [Google Scholar]
- 29.Serne EH, Gans ROB, ter Maaten JC, ter Wee PM, Donker AJM, Stehouwer CDA. Capillary recruitment is impaired in essential hypertension and relates to insulin’s metabolic and vascular actions. Cardiovascular Res. 2001;49:161–168. doi: 10.1016/s0008-6363(00)00198-x. [DOI] [PubMed] [Google Scholar]
- 30.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese men. J Clin Invest. 1990;85:1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. J Clin Invest. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svec F, Nastasi K, Hilton C, Bao W, Srinivasan S, Berenson GS. Black-white contrasts in insulin levels during pubertal development: the Bogalusa heart Study. Diabetes. 1992;41:313–7. doi: 10.2337/diab.41.3.313. [DOI] [PubMed] [Google Scholar]
- 34.Schuster DP, Kien CL, Osei K. Differential impact of obesity on glucose metabolism in black and white American adolescents. Am J Med Sci. 1998;316:361–7. doi: 10.1097/00000441-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51:3014–19. doi: 10.2337/diabetes.51.10.3014. [DOI] [PubMed] [Google Scholar]
- 36.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic Children. Diabetes Care. 2002;25:2184–90. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 37.Preeyasombat C, Bacchetti P, Lazar AA, Lustig RH. Racial and etiopathologic dichotomies in insulin hypersecretion and resistance in obese children. J Pediatr. 2005;146:474–81. doi: 10.1016/j.jpeds.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Arslanian SA, Hiel BV, Becker DJ, Drash AL. Sexual dimorphism in insulin sensitivity in adolescents with insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1991;72:920–926. doi: 10.1210/jcem-72-4-920. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto M, Akishita M, Eto M, Ishikawa M, et al. Modulation of endothelium-dependent flow mediated dilation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–3435. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]


