Abstract
We describe a straightforward method that accomplishes both the labeling of projecting neurons and the identification of apoptosis in those neurons. A single dye, 4′, 6-diamidino-2-phenylindole (DAPI), is both retrogradely transported and binds DNA. When delivered to the sites of neuronal projections, DAPI travels via retrograde transport from neuronal projections to the soma and stain nuclei with little or no cytoplasmic labeling. The staining of the nuclei allows for visualization of their morphological characteristics; DAPI-stained living cells appear markedly different from DAPI-stained apoptotic cells, due to the nuclear changes that apoptotic cells undergo. This technique has been successfully employed with retinal ganglion cells in the retinocollicular pathway. The use of a single dye not only eliminates the need for secondary staining for apoptosis, but also allows for the use of a wider variety of non-overlapping fluorescent dyes for other studies.
Introduction
The ability to identify neuronal projections by retrograde labeling is a widely used technique, basic to studies of a variety of structures such as the facial motor nucleus, motor cortex, or retina. To this end, a wide variety of tracers, and in particular, fluorescent dyes, have been used to label projecting neurons (Kuypers et al., 1979). These dyes are delivered to the projection sites of these neurons and are transported in retrograde to the soma. Researchers can then identify the neurons of interest within a mixed cell population.
In many cases studies of projecting neurons involve identification of cell death after ischemia, trauma, or other injuries. Since central nervous system axons do not normally regenerate after injury, there has been much effort in developing therapies for axotomy using techniques of neuroprotection. A common model is optic nerve injury, which results in apoptosis of retinal ganglion cells (RGCs), the projecting neurons from the retina (Berkelaar et al., 1994; Garcia-Valenzuela et al., 1994; Quigley et al., 1995; Levin and Louhab, 1996).
Our laboratory investigates the sequence of events signaling apoptosis of axotomized RGCs. To study RGCs in mixed retinal cultures, it is necessary to label them so that they can be distinguish from other retinal neurons and glia. To do this, we have used the fluorescent tracer 4′, 6-diamidino-2-phenylindole (DAPI) for this purpose. In performing these studies, we also realized that the DNA-binding properties of DAPI meant that it could be used to identify apoptotic cells through the changes in nuclear morphology and the demonstration of chromatin condensation. We present here a method for using a single dye that can both retrograde label RGCs and be used to visualize apoptosis.
Methods
Animals
These methods were designed for use with retinal ganglion cells (RGCs) from Long-Evans rats. All experiments were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO), institutional, federal, and state guidelines regarding animal research.
Materials
Cell culture reagents were obtained from Gibco (Grand Island, NY). The fluorescent tracers 4′, 6-diamidino-2-phenylindole (DAPI), 1, 1′-dioctadecyl-3, 3, 3′, 3′-tetramethylindocarbocyanine (DiI), and 4-(4-(didecylamino)styryl)-N-methylpyridinium iodide (4-Di-10-ASP) were obtained from Molecular Probes (Eugene, OR). Hank’s balanced salt solution (HBSS) was obtained from BioWhittaker, Inc. (Walkersville, MD). Chambered #1.0 German borosilicate coverglasses were obtained from Fisher Scientific (Pittsburgh, PA). Papain was obtained from Worthington Biochemical (Freehold, NJ). Staurosporine was obtained from Alexis Biochemical (San Diego, CA). Unless noted, all other reagents were obtained from Sigma (St. Louis, MO).
Retinal ganglion cell labeling and culture
Retinal ganglion cells were labeled and cultured using modifications of previously described methods (Levin et al., 1996). RGCs were retrogradely labeled by stereotactic injection of the fluorescent tracer DAPI (5 mM) dissolved in dimethylformamide into the superior colliculi of anesthetized postnatal day 2–4 Long-Evans rats. To do this, incisions were made across the top of their heads to reveal bregma. The two points of injection were 2 mm rostral and 2 mm lateral from the center of bregma. With a 10 μL Hamilton syringe, 4 μL of tracer solution was delivered at each injection site at a depth of 4 mm. The wound was sealed with cyanoacrylate adhesive and bandaged with a small strip of paper towel and cyanoacrylate.
To study labeling efficiency, DiI was coinjected with DAPI. Tracer solutions consisted of 5.4 mM DiI and 2.5 mM DAPI in dimethylformamide, and 4 μL of solution was delivered to each injection site. During the 3–4 days after injection, the dyes travel by retrograde transport from RGC projection sites in the superior colliculi to the RGC somas in the retina. DiI uniformly labels neurons via lateral diffusion in the plasma membrane, while DAPI stains nuclei specifically with little or no cytoplasmic labeling.
At postnatal day 7–8, the animals were sacrificed by decapitation, the eyes were enucleated, and the retinas were dissected free in HBSS. After two incubations in HBSS containing papain (12.5 U/ml), each for 7 minutes at 37°C, the retinas were gently triturated with a serological pipette and plated on poly-L-lysine-coated chambered coverglasses at a density of approximately 2000 cells/mm2. The cells were cultured for 24 hours in Neurobasal A (Gibco) with 0.7% methyl cellulose, 2% B27 serum-free supplement (Gibco), and gentamicin (5 μg/ml) at 37°C and 5% CO2.
Additional studies of labeling efficiency were performed by injecting 6 μL of a tracer solution containing 2.5 mM DAPI and 10 mg/ml 4-Di-10-ASP in 50% DMSO/50% H2O in the manner described above. Animals were sacrificed one week later, and the eyes enucleated in HBSS. Retinas were removed, wholemounted, and imaged immediately under appropriate filters.
Epifluorescence microscopy for visual assessment
DAPI-positive cells were identified using a Zeiss Axiovert 135 inverted microscope and filters for DAPI (330 nm excitation, 450 nm emission). DiI positive cells were identified with appropriate filters (540 nm excitation, 610 nm emission). Images of the cells were acquired with a cooled CCD camera (Roper Scientific, Trenton, NJ) mounted on the microscope, using Metafluor software at a binning of 1, an exposure time of 200 ms, and 1X gain. For the DAPI-DiI dual injection condition, cells were scored as DAPI−/DiI−, DAPI+/DiI−, DAPI−/DiI+, or DAPI+/DiI+.
Retinal wholemounts were imaged with a digital camera (Axiocam HRc) attached to a fluorescence microscope (Axiophot; Carl Zeiss Meditec, Inc., Dublin, CA) at several levels of magnification. Cells were identified in the respective images as being DAPI+ and 4-Di-10-ASP+ before merging of the pictures to check for colocalization of staining with the two tracers. In separate experiments (n = 4), staurosporine was added to the culture medium to induces apoptosis by inhibiting protein kinases and prevent ATP binding. Following cell plating, staurosporine prepared in DMSO was added to a final concentration of 1 μM (0.1% DMSO). Images of DAPI positive cells were acquired 24 hours later.
Results
DAPI is a retrograde tracer for retinal ganglion cells
To verify that DAPI is indeed detectable in RGC somas with our injection and culture protocols, we injected DAPI into the superior colliculi of postnatal day 2–4 Long-Evans rats, cultured the retinas at postnatal day 7–9, and imaged the cells using DAPI appropriate filters. Images of DAPI positive cells were acquired with a cooled CCD digital camera. We found that DAPI stained a subpopulation of cells in culture (Figure), consistent with it being a retrograde tracer for identifying RGCs in the retina.
Figure.
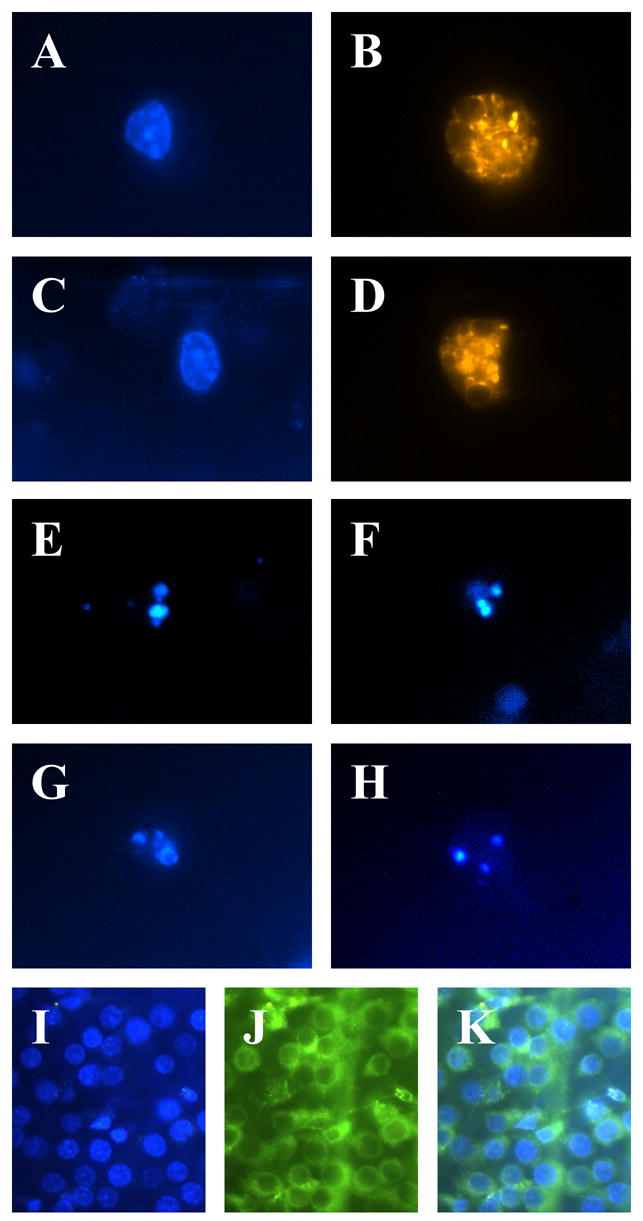
A–H: Images of RGCs taken 4–5 days after dye injection. Staurosporine treatment, where applicable, was at 1 mM for 24 hours. A: RGC labeled with DAPI. B: Same cell as A labeled with DiI. C: RGC labeled with Hoechst 33258. D: Same cell as C labeled with DiI. E, F: Two staurosporine-treated RGCs labeled with DAPI. G, H: Two staurosporine-treated RGCs labeled with Hoechst 33258. I–K: Images of wholemount retinas taken 7 days after dye injection. I: DAPI labeling of double-labeled retina. J: 4-Di-10-ASP labeling of the same retina. K: Overlay of I and J. All cells were imaged with dye-appropriate filters (330 nm excitation, 450 nm emission for DAPI and Hoechst 33258; 540 nm excitation, 610 nm emission for DiI; 470 nm excitation, 540 emission for 4-Di-10-ASP).
It is possible that DAPI, after having been transported to the RGC somas, could have either diffused to other cells in the retina or been phagocytosed by resident microglia. In order to ascertain the specificity of DAPI staining, we injected rats with a solution composed of both DAPI and the carbocyanine tracer dye DiI, and then assessed dye content at 4–5 days. Cells labeled with a single dye would be indicative of either incomplete retrograde labeling or reuptake of dye from one neuron to another. Since DAPI and DiI have different excitation/emission spectra, each dye should be distinctly discernable with their respective filter sets (Figure). Of the cells scored, 6.5% were only DAPI+, 2.4% were only DiI+, and 91.1% were both DAPI+ and DiI+ (n = 168). These data suggest that DAPI has a relatively low rate of transfer from one RGC soma to another, and therefore is unlikely to stain other cells; i.e. DAPI is a viable retrograde tracer of RGCs for up to 4–5 days. None of the cells scored had microglial morphology, making it unlikely that microglial phagocytosis contributes to misidentification of RGCs to a significant extent.
As an additional test of labeling specificity, rats co-labeled with DAPI and 4-Di-10-ASP were sacrificed 7–8 days after dye injection, and the retinas removed and wholemounted (Figure). The retinas were imaged under appropriate filters, and examined for coincident staining. We found that 92.8 ± 3.0% of 4-Di-10-ASP-labeled cells also stained brightly for DAPI, while 81.5 ± 2.3% of DAPI-labeled cells were also positive for 4-Di-10-ASP (n = 20 fields). This apparent discrepancy in co-labeling rates was likely caused in part by the depth of the ganglion cell layer and the greater cytoplasmic staining area of 4-Di-10-ASP, which obscures labeled cells below the first layer of RGCs, while the nuclear-labeled DAPI cells are more easily seen at greater depth.
DAPI for use in the indication of apoptosis
The other goal of this study was to establish whether DAPI could be used as an indicator of apoptosis after retrograde transport. Staining with Hoechst 33258 (bisbenzimide) and similar dyes have often been used to reveal chromatin condensation, a nuclear morphological feature consistent with apoptotic cell death (Wiesner and Dawson, 1996; Ohgoh et al., 2000). DAPI and Hoechst 33258 are both nuclear stains with similar excitation/emission spectra. To test if DAPI staining could reveal the presence of chromatin condensation, the potent apoptosis inducer staurosporine was added to RGC cultures. The concentration of staurosporine (1 μM) used insured that virtually all of the cells in the cultures would be apoptotic. Compared to age-matched control cultures (Figure), the appearance of nearly all DAPI+ cells was markedly apoptotic (Figure). The multiple irregular or variably sized globular appearance of DAPI staining was similar to that which is seen with Hoechst 33258 in apoptotic cells, and suggests the condensation of chromatin. DAPI therefore appears to be a suitable dye for the visual assessment of apoptosis, even after it is retrogradely transported.
Discussion
These results show that the fluorescent tracer DAPI can be used both for the retrograde labeling of retinal ganglion cells and in the identification of apoptosis. DAPI is transported to RGC somas when injected at the RGC projection sites within the superior colliculus, with relatively low levels of nonspecific staining. DAPI-stained nuclei of RGCs treated with staurosporine had the classic appearance of apoptosis.
This technique may be applicable to other neuronal systems. Provided that DAPI can be delivered to the projection sites of the neurons under study, it should be possible to distinguish those neurons in a mixed cell culture or in situ, and to identify whether the neurons are apoptotic. Indeed, this method should be appropriate for any projecting neuron where retrograde transport can be achieved. Additionally, the technique for retrograde labeling is adaptable to other nuclear tracers, such as bisbenzamide (data not shown), though such a change would require further optimization. It should also be noted that there is a risk of toxicity when using any fluorescent nuclear probe. While no toxicity was seen in our culture studies, adaptation of this technique to a different fluorescent tracer would require further optimization and the ruling out of possible toxic effects.
There do not appear to be significant levels of DAPI uptake into cells other than RGCs in the retina 4–7 days after injection. However, this may not be true in general, as DAPI presumably could leach out over longer periods of time. If given sufficient time in vivo, nuclear dyes like bisbenzimide may migrate to neighboring glial cells, yielding a false positive result (Bentivoglio et al., 1980a; Bentivoglio et al., 1980b).
There are two main advantages to using this technique. First, it is efficient. Because only one step (the injection in the neuronal projection area) is involved, less time and effort is needed to both label neurons and assay for apoptosis. Second, this technique allows for the use of a wider variety of dyes for multiple fluorescence experiments. If two dyes are used for labeling and assaying for apoptosis, then there is more potential for overlap of excitation or emission spectra, and thus fewer available dyes to use. Instead, if a single dye is used for both labeling and detection of apoptosis, then a greater number of fluorescent dyes can be used to study other cellular events (Lieven et al., 2006).
Acknowledgments
Supported by NIH R01 EY12492, P30 EY016665, the Glaucoma Foundation, the Retina Research Foundation, and an unrestricted departmental grant from Research to Prevent Blindness, Inc. LAL is a Research to Prevent Blindness Dolly Green Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bentivoglio M, Kuypers HG, Catsman-Berrevoets CE. Retrograde neuronal labeling by means of Bisbenzimide and Nuclear Yellow (Hoechst S 769121). Measures to prevent diffusion of the tracers out of retrogradely labeled neurons. Neurosci Lett. 1980a;18:19–24. doi: 10.1016/0304-3940(80)90207-4. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Kuypers HG, Catsman-Berrevoets CE, Loewe H, Dann O. Two new fluorescent retrograde neuronal tracers which are transported over long distances. Neurosci Lett. 1980b;18:25–30. doi: 10.1016/0304-3940(80)90208-6. [DOI] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Valenzuela E, Gorczyca W, Darzynkiewicz Z, Sharma SC. Apoptosis in adult retinal ganglion cells after axotomy. J Neurobiol. 1994;25:431–438. doi: 10.1002/neu.480250408. [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Bentivoglio M, van der Kooy D, Catsman-Berrevoets CE. Retrograde transport of bisbenzimide and propidium iodide through axons to their parent cell bodies. Neurosci Lett. 1979;12:1–7. doi: 10.1016/0304-3940(79)91471-x. [DOI] [PubMed] [Google Scholar]
- Levin LA, Louhab A. Apoptosis of retinal ganglion cells in anterior ischemic optic neuropathy. Arch Ophthalmol. 1996;114:488–491. doi: 10.1001/archopht.1996.01100130484027. [DOI] [PubMed] [Google Scholar]
- Levin LA, Clark JA, Johns LK. Effect of lipid peroxidation inhibition on retinal ganglion cell death. Invest Ophthalmol Vis Sci. 1996;37:2744–2749. [PubMed] [Google Scholar]
- Lieven CJ, Schlieve CR, Hoegger MJ, Levin LA. Retinal ganglion cell axotomy induces an increase in intracellular superoxide anion. Invest Ophthalmol Vis Sci. 2006;47:1477–1485. doi: 10.1167/iovs.05-0921. [DOI] [PubMed] [Google Scholar]
- Ohgoh M, Shimizu H, Ogura H, Nishizawa Y. Astroglial trophic support and neuronal cell death: influence of cellular energy level on type of cell death induced by mitochondrial toxin in cultured rat cortical neurons. J Neurochem. 2000;75:925–933. doi: 10.1046/j.1471-4159.2000.0750925.x. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- Wiesner DA, Dawson G. Staurosporine induces programmed cell death in embryonic neurons and activation of the ceramide pathway. J Neurochem. 1996;66:1418–1425. doi: 10.1046/j.1471-4159.1996.66041418.x. [DOI] [PubMed] [Google Scholar]


