Abstract
The bleeding disorder associated with factor XI (fXI) deficiency is typically inherited as an autosomal recessive trait. However, some fXI mutations may be associated with dominant disease transmission. FXI is a homodimer, a feature that could allow certain mutations to exert a dominant-negative effect on wild-type fXI secretion through heterodimer formation. We describe 2 novel fXI mutations (Ser225Phe and Cys398Tyr) that form intracellular dimers, are secreted poorly, and exhibit dominant-negative effects on wild-type fXI secretion in cotransfection experiments. Available data now suggest that mutations associated with crossreactive material-negative fXI deficiency fall into 1 of 3 mechanistic categories: (1) mutations that reduce or prevent polypeptide synthesis, (2) polypeptides that fail to form intracellular dimers and are retained in cells as monomers, and (3) polypeptides that form dimers that are not secreted. The latter category likely accounts for many cases of dominant disease transmission. (Blood. 2005;105: 4671-4673)
Introduction
The homodimeric plasma protein factor XI (fXI) is the precursor of the coagulation protease fXIa.1,2 FXI dimer formation, which is unique among coagulation proteases,1-3 is a prerequisite for fXI secretion from cells.4,5 The noncatalytic portion of the fXI polypeptide contains 4 repeats called apple domains (A1-A4 from the N-terminus).2 The A4 domain is the primary mediator of dimer formation,6 and mutations in A4 that interfere with dimerization are associated with retention of fXI monomer within cells and low plasma fXI levels.4,5 The dimeric structure has implications for inheritance patterns in congenital fXI deficiency. This disorder is prevalent in Ashkenazi Jews, in whom 2 point mutations, Glu117Stop and Phe283Leu, predominate.7,8 These mutations are associated with an apparently autosomal recessive bleeding disorder, as heterozygotes are generally asymptomatic.7,9 The situation is not as clear for fXI-deficient patients not of Jewish ancestry, in whom no single mutation predominates. There are reports of heterozygotes for fXI mutations with plasma fXI levels of less than 0.2 U/mL, a range commonly seen in homozygous or compound heterozygous fXI-deficient Jewish patients.5,10-12 In 2 cases, mutations were identified (Gly400Val and Trp569Ser) that exert dominant-negative effects on fXI secretion in transfection experiments, probably through formation of nonsecretable heterodimers.5 It is not clear whether these cases are unusual or represent a common mechanism in fXI deficiency. Prompted by these data, and personal observations of patients with fXI levels in an intermediate range (20%-30% of normal) between those typically seen in homozygous and heterozygous Jewish patients, we analyzed the effects of 2 novel fXI mutations on wild-type fXI secretion. The results, in conjunction with prior work, suggest a simple classification system for cross-reactive material-negative (CRM-) fXI deficiency.
Study design
Patients
Patient 1 is a 34-year-old man with a history of left knee hemarthrosis and frequent epistaxis. His fXI levels range from 22% to 30% of normal. His 2 children have fXI levels of 25% to 37%. All 3 individuals are heterozygous for a C>T change in fXI exon 7, resulting in a Ser225 to Phe substitution. Patient 2 is a 38-year-old woman with heavy postpartum bleeding, idiopathic hematuria, and low fXI activity (25%-39%). She is heterozygous for a G>A change in exon 11, resulting in a Cys398 to Tyr substitution. A homozygote and 2 heterozygotes for Cys398Tyr were previously reported in unrelated families.13 Plasma fXI antigen levels for all patients match plasma fXI activity. Procedures for obtaining blood for DNA extraction and factor XI gene analysis were approved by the Institutional Review Boards of Vanderbilt University and the University of North Carolina. Informed consent was obtained according to the Declaration of Helsinki.
Transient transfections
Point mutations for Ser225Phe or Cys398Tyr were introduced into the wild-type fXI (fXI-WT) cDNA in pJVCMV as described.14 Transfections of 293 fibroblasts, growing in Dulbecco modified Eagle medium (DMEM) with sodium pyruvate, L-glutamine, and 5% fetal bovine serum, were performed in 6-well culture plates using SuperFect reagent (Qiagen, Valencia, CA).5 Single transfections used 80 ng, and cotransfections 160 ng, of fXI/pJVCMV construct. All transfections included 40 ng Renilla luciferase (pRL-CMV) vector (Promega, Madison, WI) to control for transfection efficiency, and empty pJVCMV to bring total DNA to 2 μg/transfection. Media were collected 67 hours after transfection, and cell lysates were prepared using a Dual Luciferase Reporter kit (Promega). Luciferase activity was measured on a Monolight 2010 luminometer (Analytical Luminescence, San Diego, CA). FXI in media and lysates was measured by enzyme-linked immunosorbent assay (ELISA) using goat polyclonal anti-human fXI immunoglobulin G (IgG; Affinity Biologicals, Hamilton, Ontario).5 Results were corrected for variation in transfection efficiency using the luciferase signals and reported as relative fXI levels. The mean for fXI-WT was assigned a value of 100%.
Western blots of intracellular fXI
Baby hamster kidney (BHK) fibroblasts were transfected with pLg-fXI-IN constructs,15 and stably transfected clones were selected by addition of G418 (500 μg/mL) to media. pLg-fXI-IN constructs use a cytomegalovirus promoter to generate a single mRNA encoding both fXI and a neomycin resistance marker (N) separated by an internal ribosomal entry site (I). Supernatants from cells lysed by sonication were size fractionated on 7.5% polyacrylamide-sodium dodecyl sulfate (SDS) gels, followed by Western blotting using goat anti-human fXI polyclonal antibodies (described under “Transient transfections”) and chemiluminescence.
Results and discussion
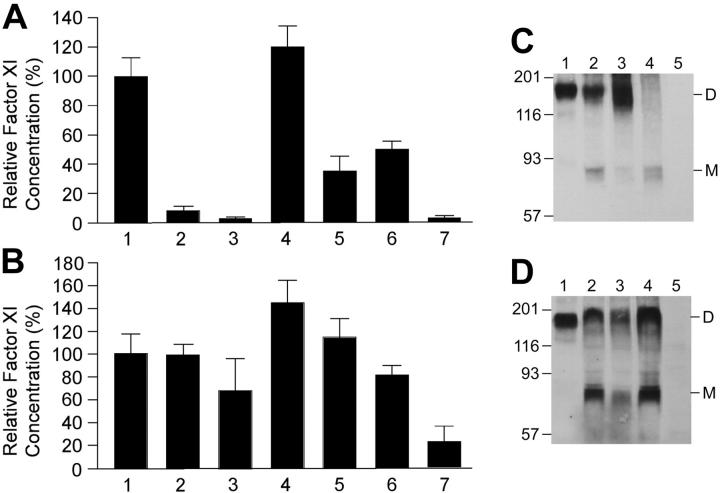
In single transient transfections, fXI-Phe225 and fXI-Tyr398 levels in media are low (8.8% ± 2.8% and 1.4% ± 1.8%, respectively) compared with fXI-WT (100% ± 11.7%) (Figure 1A), with results for fXI-Tyr398 similar to negative control (1.9% ± 1.7%). This appears to be due to reduced secretion, as fXI-Phe225 and fXI-Tyr398 levels in lysates (99.4% ± 10.3% and 65.7% ± 29.9%) are comparable to fXI-WT (100% ± 18.3%) (Figure 1B). Cotransfection of fXI-WT with fXI-Phe225 or fXI-Tyr398 results in reduced fXI in media (36.6% ± 8.5% and 50.2% ± 6.5%, respectively) compared with fXI-WT control, with little change in intracellular protein (115.3% ± 18.0% and 81.1% ± 8.7%). In comparison, cotransfection of fXI-WT with an additional equal amount of fXI-WT construct results in modest increases in secreted and intracellular antigen (121.1% ± 12.8% and 144.8% ± 18.2%). Stably transfected BHK lines were prepared for fXI-WT, fXI-Phe225, and fXI-Tyr398, as well as the previously reported dominant-negative mutant fXI-Ser569 and the dimerization-defective mutant fXI-Glu350.5 Only fXI-WT was secreted to a significant extent (data not shown). Western blots of intracellular protein (Figure 1C-D) show that fXI-Phe225, fXI-Tyr398, and fXI-Ser569 form intracellular dimers similar to fXI-WT, indicating that poor secretion of these mutants is not due to a failure to form dimers, as in the case of fXI-Glu350.
Figure 1.
Transfections of 293 cells with fXI/pJVCMV expression constructs. FXI antigen levels in media (A) and cell lysates (B) of transient transfections are shown. 293 fibroblasts were transfected with 80 ng expression construct for individual transfections (columns 1-3) and 80 ng fXI-WT expression construct plus 80 ng construct to be tested for cotransfections (columns 4-6). The negative control in column 7 contains empty pJVCMV vector. Shown are means and standard deviations for 6 separate transfections, each measured in triplicate by ELISA and corrected for transfection efficiency by Renilla luciferase assay. The mean for single fXI-WT transfections was assigned a value of 100%. Lane (1) fXI-WT, (2) fXI-Phe225, (3) fXI-Tyr398, (4) fXI-WT + fXI-WT, (5) fXI-WT + fXI-Phe225, (6) fXI-WT + fXI-Tyr398, (7) pJVCMV without cDNA. Western blots for fXI in lysates of BHK cells transfected with pLg-fXI-IN constructs. Samples from stably transfected cell lines were run on a 7.5% polyacrylamide-SDS gel, followed by Western blotting with goat anti-human fXI polyclonal antibody. (C) Samples are (1) conditioned media from fXI-WT cells and lysates from cells transfected with (2) fXI-WT, (3) fXI-Phe225, (4) fXI-Glu350, and (5) untransfected BHK cells. (D) Samples are (1) conditioned media from fXI-WT cells and lysates from cells transfected with (2) fXI-WT, (3) fXI-Tyr398, (4) fXI-Ser569, and (5) untransfected BHK cells. Positions of dimeric (D) and monomeric (M) fXI are indicated to the right of each panel, and positions of molecular mass standards in kilodaltons are shown on the left. The ratio of dimer to monomer varied between experiments. Note that, with the exception of fXI-Glu350, which does not dimerize,5 patterns for mutants match the wild-type pattern.
Most fXI-deficient patients have proportional decreases in plasma activity and antigen (CRM- deficiency).9,10,16 Observations over the past 40 years suggest that fXI deficiency in Jewish patients is inherited primarily as a recessive condition,9,10,17 and our understanding of the mutations involved is consistent with this interpretation (Figure 2).5,18 However, the multimeric structure of fXI, like those of von Willebrand factor19 and fibrinogen,20 is conducive to CRM- dominant forms of disease through formation of nonsecretable heteromultimers. Hypothetically, any fXI mutation that interferes with protein secretion, but not dimer formation, could inhibit normal fXI polypeptide secretion. If the mutant polypeptide is expressed at a high enough level, the effect on plasma fXI level may be great enough to compromise hemostasis. The data herein indicate that this mechanism, previously proposed for Gly400Val and Trp569Ser,5 applies to Ser225Phe and Cys398Tyr and is, therefore, probably relatively common.
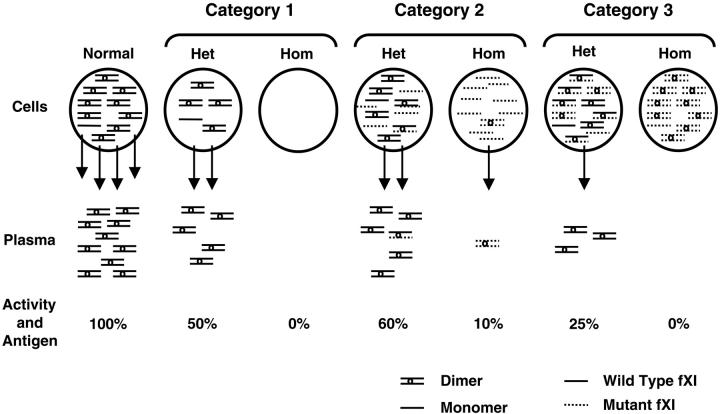
Figure 2.
Proposed mechanisms for CRM-negative fXI deficiency. FXI dimers and monomers within circles at the top of the figure represent intracellular protein, arrows indicate secretion, and FXI dimers below the arrows represent plasma fXI. The percentage of activity and antigen values shown are what would be expected, on average, for different mechanisms. Mutations in category 1 prevent or reduce synthesis of fXI polypeptide, and heterozygosity for such a mutation would not affect the product of the normal allele. Glu117Stop is the most common example from this group. Category 2 contains polypeptides that are synthesized but dimerize poorly, resulting in intracellular retention of monomers. Heterozygosity for this type of mutant would have little effect on secretion of product from the normal allele, because the mutant would dimerize poorly with wild-type polypeptide, and heterodimers that do form are secreted. The diagram shows the expected result for the most common mutation in this group, Phe283Leu, which has a partial defect in dimerization. Category 3 includes polypeptides that are synthesized and form dimers, but that are secreted poorly in homodimeric or heterodimeric forms. This category includes Ser225Phe and Cys398Tyr. The mutant polypeptide reduces wild-type fXI polypeptide secretion through formation of nonsecretable heterodimers (dominant-negative effect). Het indicates heterozygote; and Hom, homozygote for fXI gene mutation.
These observations suggest a 3-part mechanistic classification system for CRM- fXI deficiency (Figure 2). The first category contains mutations that inhibit fXI polypeptide synthesis and include nonsense mutations such as Glu117Stop,18 frame shifts, deletions, splicing defects, and possibly amino acid substitutions that cause severe polypeptide instability. A second category, represented by the A4 domain substitutions Phe283Leu and Gly350Glu,4,5,18 includes mutations that interfere with dimer formation, resulting in intracellular retention of fXI as monomer. Mutations in categories 1 and 2 are expected to cause little or no interference with the product of the normal fXI allele (Figure 2). The third category, which includes Gly400Val and Trp569Ser, and now Ser225Phe and Cys398Tyr, contains mutations that form nonsecretable homodimers and probably nonsecretable heterodimers with wild-type fXI. Saito et al16 noted that fXI levels tend to be lower in non-Jewish patients heterozygous for fXI gene mutations compared with their Jewish counterparts. The dominant-negative effect proposed for category 3 explains this observation and would account for some families with apparent autosomal-dominant fXI deficiency.5,10,11,21
Acknowledgments
We thank Jean McClure for her expertise in preparing figures.
Prepublished online as Blood First Edition Paper, February 22, 2005; DOI 10.1182/blood-2004-05-1864.
Supported by the National Heart, Lung, and Blood Institute (grant HL58 837) and by a Grant-in-Aid from the American Heart Association, Southeastern Region.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Davie E, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance and regulation. Biochemistry. 1991;30: 10363-10370. [DOI] [PubMed] [Google Scholar]
- 2.McMullen BA, Fujikawa K, Davie EW. Location of the disulfide bonds in human coagulation factor XI: the presence of tandem apple domains. Biochemistry. 1991;30: 2056-2060. [DOI] [PubMed] [Google Scholar]
- 3.Bouma B, Griffin J. Human blood coagulation factor XI, purification, properties, and mechanism of activation by activated factor XII. J Biol Chem. 1977;252: 6432-6437. [PubMed] [Google Scholar]
- 4.Meijers J, Davie E, Chung D. Expression of human blood coagulation factor XI: characterization of the defect in factor XI type III deficiency. Blood. 1992;79: 1435-1440. [PubMed] [Google Scholar]
- 5.Kravtsov DV, Wu W, Meijers JCM, et al. Dominant factor XI deficiency caused by mutations in the factor XI catalytic domain. Blood. 2004;104: 128-134. [DOI] [PubMed] [Google Scholar]
- 6.Meijers J, Mulvihill E, Davie E, Chung D. Apple 4 in human blood coagulation factor XI mediates dimer formation. Biochemistry. 1992;31: 4680-4684. [DOI] [PubMed] [Google Scholar]
- 7.Asakai R, Chung D, Davie E, Seligsohn U. Factor XI deficiency in Ashkenazi Jews in Israel. N Engl J Med. 1991;325: 153-158. [DOI] [PubMed] [Google Scholar]
- 8.Peretz H, Mulai A, Usher S, et al. The two common mutations causing factor XI deficiency in Jews stem from distinct founders: one of ancient Middle Eastern origin and another of more recent European origin. Blood. 1997;90: 2654-2659. [PubMed] [Google Scholar]
- 9.Seligsohn U, Griffin, JH. Contact activation and factor XI. In Scriver CR, Beaudet AL, Sly WS, Valle D, eds. Metabolic and Molecular Basis of Inherited Disease. 7th ed. New York, NY: McGraw-Hill; 1995: 3285-3311.
- 10.Bolton-Maggs P, Wan-Yin B, McGraw A, Slack J, Kernoff P. Inheritance and bleeding in factor XI deficiency. Br J Haematol. 1988;69: 521-528. [DOI] [PubMed] [Google Scholar]
- 11.Litz C, Swaim W, Dalmasso A. Factor XI deficiency: genetic and clinical studies of a single kindred. Am J Hematol. 1988;28: 8-12. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell M, Cutler J, Thompson S, et al. Heterozygous factor XI deficiency associated with three novel mutations. Br J Haematol. 1999;107: 763-765. [DOI] [PubMed] [Google Scholar]
- 13.Gailani D, Bolton-Maggs PHB, Blinder M, et al. Amino acid substitutions in the factor XI catalytic domain associated with factor XI deficiency [abstract P1112]. Supplement to the journal Thromb Haemost. 2001;(suppl).
- 14.Sun M-F, Zhao M, Gailani D. Identification of amino acids on the factor XI apple 3 domain required for activation of factor IX. J Biol Chem. 1999;274: 36373-36378. [DOI] [PubMed] [Google Scholar]
- 15.Zaboikin M, Schuening F. Poor expression of MDR1 transgene in HeLa cells by bicistronic Moloney murine leukemia virus-based vector. Hum Gene Ther. 1998;9: 2263-2275. [DOI] [PubMed] [Google Scholar]
- 16.Saito H, Ratnoff O, Bouma B, Seligsohn U. Failure to detect variant (CRM+) plasma thromboplastin antecedent (factor XI) molecules in hereditary plasma thromboplastin antecedent deficiency: a study of 125 patients of several ethnic backgrounds. J Lab Clin Med. 1985;106: 718-722. [PubMed] [Google Scholar]
- 17.Rapaport S, Proctor R, Patch M, Yettra M. The mode of inheritance of PTA deficiency: evidence for the existence of major PTA deficiency and minor PTA deficiency. Blood. 1961;18: 149-165. [PubMed] [Google Scholar]
- 18.Asakai R, Chung D, Ratnoff O, Davie E. Factor XI (plasma thromboplastin antecedent) deficiency in Ashkenazi Jews is a bleeding disorder that can result from three types of point mutations. Proc Natl Acad Sci U S A. 1989;86: 7667-7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodo I, Katsumi A, Tuley E, Eikenboom J, Dong Z, Sadler J. Type I von Willebrand disease mutation Cys1149Arg causes intracellular retention and degradation of heterodimers: a possible mechanism for dominant mutations in oligomeric proteins. Blood. 2001;98: 2973-2979. [DOI] [PubMed] [Google Scholar]
- 20.Duga S, Asselta R, Santagostino E, et al. Missense mutations in the human beta fibrinogen gene cause congenital afibrinogenemia by impairing fibrinogen secretion. Blood. 2000;95: 1336-1341. [PubMed] [Google Scholar]
- 21.Campbell E, Mednicoff I, Dameshek M. Plasma thromboplastin antecedent (PTA) deficiency. Arch Int Med. 1957;100: 232-240. [DOI] [PubMed] [Google Scholar]