Abstract
CXCR4 is a chemokine receptor required for hematopoietic stem cell engraftment and B-cell development. This study found that a small fraction of primitive CD34+/CD19+ B-cell progenitors do not express CXCR4. These CD34+/CD19+/CXCR4- cells were also remarkable for the relative lack of primitive myeloid or lymphoid surface markers. When placed in B-lymphocyte culture conditions these cells matured to express CXCR4 and other surface antigens characteristic of B cells. Surprisingly, when placed in a myeloid culture environment, the CXCR4- B-cell progenitors could differentiate into granulocyte, macrophage, and erythroid cells at a high frequency. These data define a novel B-cell/myeloid common progenitor (termed the BMP) and imply a less restrictive pathway of myeloid versus lymphoid development than previously postulated.
Introduction
The first developmental decision a hematopoietic stem cell makes is whether to differentiate into a common lymphoid progenitor or a common myeloid progenitor.1,2 In general, it is thought that there is normally infrequent cross-lineage differentiation once the stem cell differentiates into a lymphoid or myeloid progenitor.3-7 Thus, a common lymphoid progenitor in normal situations is considered to rarely form myeloid lineages. However, artificially enforcing myeloid gene expression in the murine common lymphoid progenitor can induce it to differentiate down myeloid lineages.3,4 For example, transducing the myeloid transcription factors C/EBP (CCAAT/enhancer binding protein)–α or -β into B cells reprograms them into macrophages in a PU.1-dependent manner.
However, enforcing myeloid gene expression is a circumstance not normally occurring in human hematopoiesis.3 Recently, 2 reports have indicated that some normal human B-cell progenitors can rarely differentiate into macrophages or T/natural killer (NK) cells.8,9 Although the frequency of this translineage plasticity was rare, these studies called into question not only the lineage commitment of the differentiating stem cell but also the pathways available to the stem cell.
CXCR4 is the receptor for the chemokine CXCL12 (stromal cell-derived factor 1 [SDF-1])10-12 and is required for hematopoietic stem cell engraftment and subsequent B-cell development.11-17 It is almost universally expressed on hematopoietic progenitors, including B-cell progenitors and their more mature progeny.12,13 The CXCL12/CXCR4 axis not only regulates proper localization of B-cell progenitors but also provides a proliferation and maturation signal to developing B cells.14-17
We sought to demonstrate whether relative expression of the B-cell chemokine receptor CXCR4 could define a distinct stage of B-cell progenitor development. We found that there was a subset of primitive B-cell progenitors that did not express CXCR4, but gained CXCR4 expression as they matured in lymphocyte culture. Significantly, these CXCR4- B-cell progenitors were flexible in their lymphoid lineage commitment and could differentiate to multiple myeloid lineages at a significantly higher frequency than previously reported for any other B-cell progenitor type.8,9 These data imply a new, less restrictive pathway of hematopoietic stem cell differentiation.
Materials and methods
B-cell progenitor isolation
Approval was obtained from the Indiana University Institutional Review Board (IRB) for these studies. Informed consent was provided according to the Declaration of Helsinki. Bone marrow (BM) samples were collected from healthy adult volunteer donors under an IRB protocol and B-cell progenitors flow sorted as we described.18,19 B-cell progenitors were defined as CD34+/CD19+ BM mononuclear cells as previously described,20,21 and then subdivided by CXCR4 expression. CD34+/CD19+/CXCR4+ and CD34+/CD19+/CXCR4- cells were isolated using a FACS-Vantage (BD Immunocytometry Systems, San Jose, CA) flow sorter equipped with 3 lasers, an argon laser, a HeNe laser, and a tunable Innova 70C spectrum laser. All antibodies were purchased from BD PharMingen (San Diego, CA) including fluorescein isothiocyanate (FITC)–conjugated mouse anti–human CD34, FITC-conjugated anti–human IgD, FITC-conjugated anti–human immunoglobulin light chain κ/λ monoclonal antibody, cyanin-chrome–conjugated mouse anti–human CD19 monoclonal antibody, allophycocyanin (APC)–conjugated mouse anti–human CXCR4 monoclonal antibody, and streptavidin-phycoerythrin (PE). Matched isotype controls were used according to the manufacturer's recommendations.
In brief, isolated BM mononuclear cells were resuspended at 3 × 107/mL in the final volume of 1 mL RPMI 1640 sterile medium supplemented with 10% fetal calf serum. The cells were incubated with 2 μg of the desired antibodies for 1 hour in the dark on ice. Cells were then washed in medium twice and resuspended at 1 × 107/mL in a 5-mL polystyrene round-bottom tube for sorting. A lymphocyte gate was established for both forward and side scatter, and sorting for desired fractions was performed using stringent fluorescence gates to obtain pure fractions. All sorted cells were reanalyzed to ensure purity of the selected populations and discarded if not 99% pure or better. All experiments were performed at least 3 times, using 3 distinct BM samples, with the data averaged and SEs indicated.
Flow analysis
Surface phenotypic characterization of the CD34+/CD19+/CXCR4- cells was accomplished using 6-color immunofluorescence analysis on a FAC-SAria (BD Immunocytometry Systems). Low-density nonadherent BM mononuclear cells were stained with the following antibodies: APC-conjugated anti–human CD34, PE-TR–conjugated anti–human CD19, and biotin-conjugated anti–human CXCR4 (CD184). These cells were also stained with 3 of the following 6 antibodies: PE-cyanin 5.5 (Cy5.5)–conjugated anti–human CD90 or CD117, FITC-conjugated anti–human CD16 or CD33, and PE-conjugated anti–human CD127 or CD133. All antibodies were obtained from BD PharMingen except PE-TR–conjugated CD19 (Serotec, Raleigh NC), PE-conjugated CD127 (Beckman Coulter, Fullerton, CA), and PE-conjugated CD133 (Miltenyi Biotec, Auburn, CA). APC-Cy7–conjugated streptavidin (BD PharMingen) was used as a secondary fluorochrome for biotin-conjugated anti-CXCR4. All flow cytometric analyses were performed at least 3 times using 3 distinct BM samples, with the data averaged and SDs indicated.
B-cell progenitor culture
Sorted CD34+/CD19+/CXCR4- cells were cultured in 10% phytohemagglutinin (PHA)–stimulated human peripheral blood lymphocyte-conditioned medium, SDF-1 (400 ng/mL), and interleukin 6 (IL-6; 10 ng/mL), and then incubated at 37°C in a 5% CO2 incubator as we described.22 All experiments were performed at least 3 times in triplicate with 3 distinct BM samples, and the data averaged with SEs indicated.
Colony formation assays
Erythroid burst-forming unit (BFU-E), granulocyte-macrophage (GM), and granulocyte-erythroid-macrophage-megakaryocyte (GEMM) colony formation assays were conducted as we previously reported.18,19 All experiments were performed at least 3 times in triplicate with 3 distinct BM samples, and the results analyzed for differences using the Student t test.
Results
The fraction of CD34+/CD19+ B-cell progenitors was found here to be stable between individual donors, averaging 2.7% plus or minus 0.1% of total BM low-density nonadherent mononuclear cells within the lymphoid scatter gate (Figure 1A). In this CD34+/CD19+ population, 77.5% plus or minus 4.7% of the cells expressed CXCR4, whereas surprisingly 22.5% did not express CXCR4. Not surprisingly, CCR7, a chemokine receptor on more mature B cells, is more highly expressed in the CD34- population, and poorly expressed in the CD34+/CD19+ population as compared to CXCR4. Thus, CD34+/CD19+/CXCR4- cells represented an average of 0.6% of BM low-density nonadherent mononuclear cells within the lymphoid gate. This would represent 0.3% of all low-density BM mononuclear cells because the lymphoid gate contained an average of 53.9% of the total low-density BM mononuclear cells in our samples. The percentage of CD34+/CD19+/CXCR4- cells among all total BM low-density mononuclear cells averages 0.072%.
Figure 1.
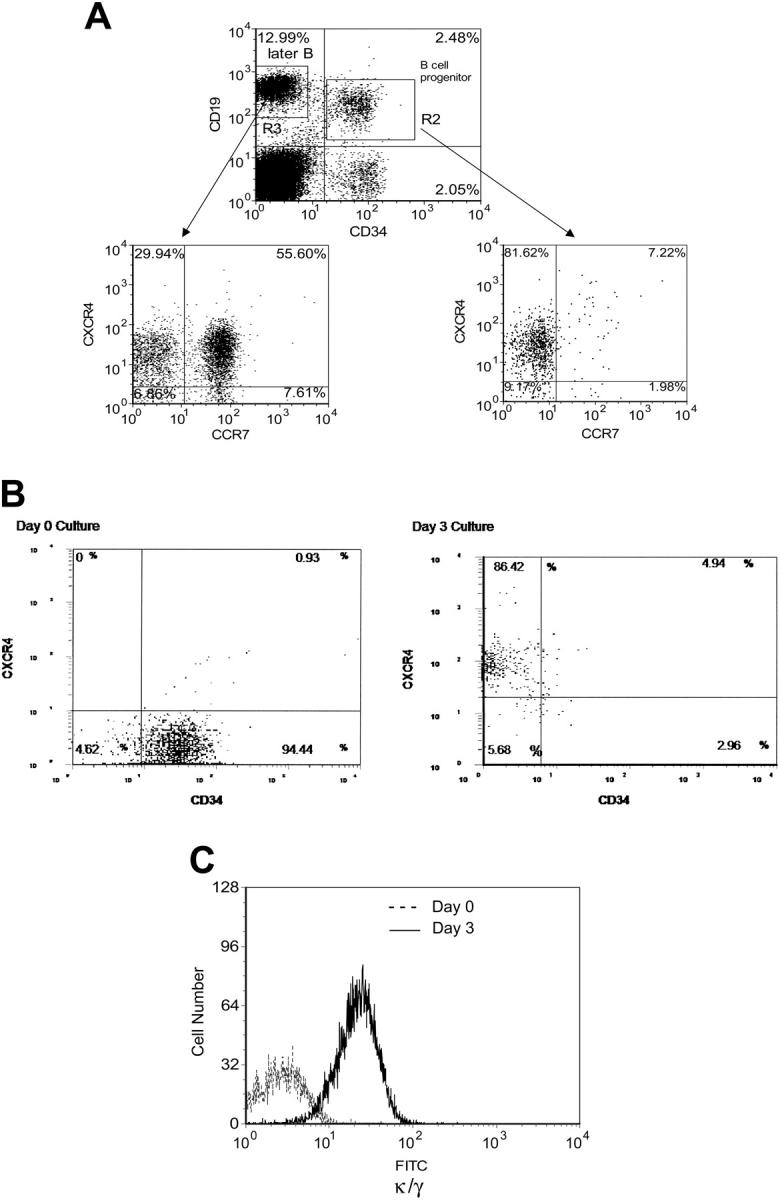
Flow cytometric analysis of CXCR4 expression in B-cell progenitor maturation. (A) Representative flow histograms of CD34+/CD19+ B-cell progenitor (gate R2) and CD34-/CXCR4+ later B cells (gate R3) sorted and analyzed for CCXCR4 and CCR7 expression. There was a surprisingly large fraction of CD34+/CD19+ B-cell progenitors that did not express the chemokine receptor CXCR4. More mature CD34-/CD19+ BM B cells express CXCR4 at a high level. The percentage of each subpopulation relative to the total number of cells in gates R2 or R3 is shown. (B) Representative flow histograms of CD34+/CD19+/CXCR4- cells cultured for 3 days in B-lymphoid conditions showing that the majority of cells lose expression of CD34 and a gain expression of CXCR4. The percentage of each subpopulation relative to the total number of cells is shown. (C) The majority of CD34+/CD19+/CXCR4- cells cultured in B-lymphoid conditions gain expression of κ/λ light chains. Text data are the average of 3 distinct experiments; this figure shows a representative histogram.
In B-lymphoid culture conditions CD34+/CD19+/CXCR4- cells quickly lost expression of CD34 and gained expression of CXCR4 (Figure 1B). After 3 days of culture in B-lymphoid conditions an average of only 7.3% plus or minus 0.5% cells remained CD34+ (Figure 1B), whereas 99.2% remained CD19+ (data not shown). After 3 days of lymphoid culture an average of 87.2% plus or minus 6.9% of the CD34+/CD19+/CXCR4- cells expressed CXCR4 (Figure 1B). An average of 25.2% plus or minus 1.4% expressed surface κ/λ, a marker of B-cell maturity (Figure 1C). This indicated that the B-cell progenitors that did not express CXCR4 gave rise to more mature B cells that did express CXCR4 at a high frequency.
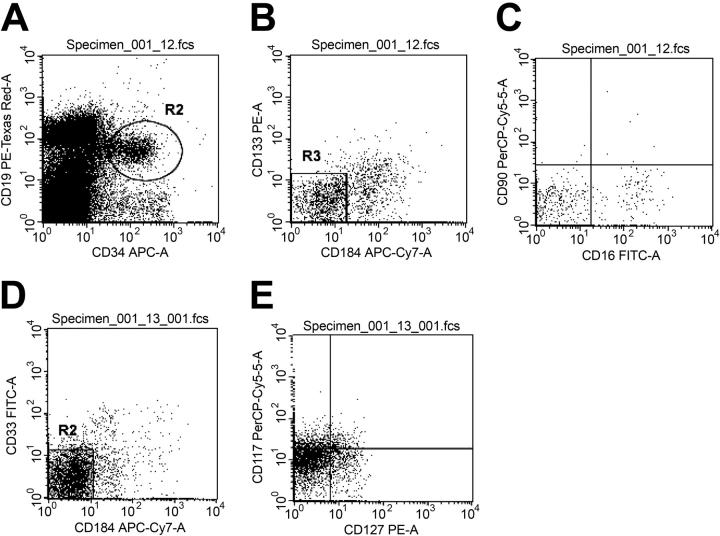
To better define the phenotypic profile of these CD34+/CD19+/CXCR4- cells, this group of cells was further analyzed for the expression of 6 additional primitive hematopoietic surface markers. Data collected from these analyses are shown in Figure 2. The most prominent antigen expressed on CD34+/CD19+/CXCR4- cells was CD117. CD117 (c-Kit) was expressed on an average of 16.0% plus or minus 1.3% of these cells. CD16, CD33, CD90, CD127, and CD133 were all less well expressed than CD117 (Table 1). It should be noted that none of these markers were expressed on any appreciable fraction of these cells. The CD34+/CD19+/CXCR4- cells are therefore more remarkable for their lack of other primitive myeloid or lymphoid markers than the expression of any other hematopoietic marker.
Figure 2.
Flow cytometric characterization of the expression of other primitive hematopoietic surface markers on CD34+/CD19+/CXCR4- cells. Low-density BM cells were stained for 6-color immunofluorescence as described in “Materials and methods.” Representative histograms of the flow cytometric phenotypic characterization of the CD34+/CD19+/CXCR4- cells are shown in dot plots A-E. Refer to Table 1 for the averaged fraction of the CD34+/CD19+/CXCR4- cells expressing combinations of primitive hematopoietic surface antigen. Dot plot A depicts CD34+CD19+ cells within R2. Cells contained in R2 were then analyzed for the expression of CXCR4 (CD184 in the figure) and CD133 (VEGFr2) in dot plot B. Essentially no CD34+/CD19+/CXCR4- cells were CD133+. CD34+/CD19+/CXCR4- cells were also analyzed for the expression of CD16 and CD90 (dot plot C). Whereas a small fraction of these cells expressed CD16, very few expressed CD90. Dot plot D depicts the expression of CD33 fraction of CD34+/CD19+/CXCR4- cells. Dot plot E shows the expression of CD127 and CD117 in the CD34+/CD19+/CXCR4- population. CD117 analysis found a significant increase in the mean fluorescence intensity, but the gated fraction of cells expressing CD177 is still small, as shown in Table 1.
Table 1.
Percent of total CD34+/CD19+/CXCR4- cells analyzed
| Cells | Percentage |
|---|---|
| CD16+/CD133- | 10.5 ± 4.3 |
| CD16-/CD133+ | 4.6 ± 4.5 |
| CD16+/CD90- | 9.3 ± 4.4 |
| CD33+/CD127- | 4.5 ± 2.3 |
| CD33-/CD127+ | 12.0 ± 1.8 |
| CD33-/CD117+ | 16.0 ± 1.3 |
| CD127+/CD117- | 7.0 ± 1.6 |
| CD127-/CD117+ | 15.6 ± 3.5 |
Values reported for each phenotype are the mean ± SD from 3 independent measurements collected from 3 different BM samples from healthy volunteer donors. All other phenotypic combinations obtained with these analyses were less than 3% of total CD34+/CD19+/CXCR4- cells.
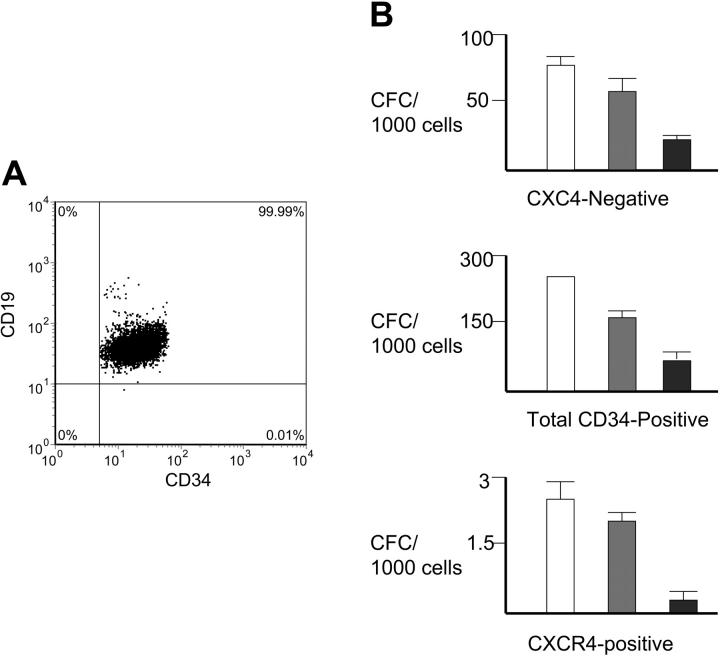
Because these CD34+/CD19+/CXCR4- B-cell progenitors were more primitive than B-cell progenitors that were CXCR4+, we hypothesized that they might not be fully committed to the B-lymphoid lineage.8,9,12,19,20,23 The CD34+/CD19+/CXCR4- cells were isolated by flow cytometric sorting and placed in myeloid differentiation colony formation assays. Re-examining the sorted cells showed that 100% were CD34+/CD19+, indicating that it was unlikely that CD34+/CD19- cells contaminated the myeloid cultures (Figure 3A).
Figure 3.
B-cell progenitors in myeloid colony formation assays. (A) Reanalyzing the sorted CD34+/CD19+ cells in each experiment for the presence of any CD34+/CD19- cells that might form myeloid colonies showed that 100% of sorted cells expressed CD19. The percentage of each subpopulation relative to the total number of cells is shown. (B) Myeloid colony formation per 1000 cells. A significant fraction of CD34+/CD19+/CXCR4- BM cells was able to form myeloid colonies containing granulocytes, macrophages, or erythrocytes (top) as compared to CD34+/CD19+/CXCR4+ cells (middle) and total CD34+ cells (bottom). □ indicates granulocyte-macrophage colony-forming units (CFU-GMs);  , BFU-E; and ▪, CFU-GEMM. Note the different scales showing the colony numbers between the graphs; n = 3 each performed in triplicate. Error bars indicate standard error (SE).
, BFU-E; and ▪, CFU-GEMM. Note the different scales showing the colony numbers between the graphs; n = 3 each performed in triplicate. Error bars indicate standard error (SE).
The CD34+/CD19+/CXCR4- BM B-cell progenitors produced myeloid colonies at a high rate in methylcellulose myeloid colony formation assays (Figure 3B). One thousand CD34+/CD19+/CXCR4- cells formed an average of 77 plus or minus 3.6 GM colony-forming units (CFU-GMs), 38 plus or minus 1.6 BFU-Es, and 13 plus or minus 0.5 CFU-GEMMs. Thus, a total of 12% of all CD34+/CD19+/CXCR4- cells represented progenitors that could differentiate into one of the major myeloid lineages. In sharp contrast, CD34+/CD19+/CXCR4+ BM B-cell progenitors produced only 2 plus or minus 0.5 CFU-GMs, 1.7 plus or minus 0.3 BFU-Es, and 0.3 plus or minus 0.3 CFU-GEMMs per 1000 cells. Thus, only 0.4% of all CD34+/CD19+/CXCR4+ cells could form myeloid colonies.
As a control, normal human BM CD34+ total sorted cells formed 280 plus or minus 10 CFU-GMs, 165 plus or minus 20 BFU-Es, and 110 plus or minus 35 CFU-GEMMs per 1000 cells (scored at 200 cells/plate, normalized to 100018,19). Remarkably, the CD34+/CD19+/CXCR4- cells formed myeloid colonies at a 22% efficiency of total CD34+ cells in the BM. Thus, the CXCR4- B-cell progenitors had fully one fifth of the capability of total CD34+ hematopoietic progenitors to differentiate down myeloid lineages.
Discussion
The finding that CXCR4- B-cell progenitors had the ability to form myeloid colonies at high frequency implies that this progenitor represents a unique unrestricted stage of early B-cell development. These cells are remarkable for their relative lack of expression of other primitive hematopoietic markers. CD117 was expressed on approximately one seventh of the cells, a relatively small fraction, whereas other primitive myeloid or lymphoid markers, such as CD16 (Fcγ receptor III [FcγRIII]), CD33 (sialic acid receptor 3), CD90 (Thy-1), CD127 (IL-7 receptor α [IL-7Rα]), and CD133 (vascular endothelial growth factor receptor 2 [VEGFr2]), were all expressed at even lower levels. Interestingly, both primitive myeloid (CD33) and lymphoid markers (IL-7Rα) were poorly expressed. Thus, at least by flow cytometric surface phenotype analysis, these cells do not fit well within any previously characterized category of primitive hematopoietic progenitors.1-4
It is possible that the CD34+/CD19+/CXCR4- cells may represent a storage pool of uncommitted hematopoietic progenitors that could be induced down either a myeloid or B-lymphoid developmental path, depending on the organism's requirements and subsequent BM microenvironmental signals. These data are consistent with the requirement of CXCR4/CXCL12 for B-cell proliferation and maturation.14-17 If CXCR4 is not expressed, then these cells will not be able to respond the BM environmental CCL12 signals to proliferate and differentiate down B-cell pathways. This is also similar to the finding that CXCR4- hematopoietic stem cells can be induced to express CXCR4 and engraft nonobese diabetic/severe combined immunodeficiency mice.23 This report found that the CXCR4- stem cells became CXCR4+ as they were stimulated to proliferate to engraft the recipient mouse.
It has been reported that FcγRIII-expressing murine fetal liver hematopoietic cells could differentiate into both B cells and macrophages.24 There are also 2 seminal reports that certain subsets of normal adult human B-cell progenitors, CD79a+/IL-7Rα+ or CD45R-/CD19+, can form macrophages, T cells, and NK cells.8,9 However, those B-cell progenitors differentiated into other lineages at a frequency many fold less than that seen here for the CXCR4- B-cell progenitors forming myeloid colonies. In addition, the CD34+/CD19+/CXCR4- B-cell/myeloid common progenitor defined here formed progenitors representing all myeloid lineages, which is in contrast to the more limited B-cell progenitor lineage plasticity described previously. It should also be noted that the progenitor defined here lacked the expression of IL-7Rα, and therefore may be a precursor of this previously described plastic B-cell progenitor type.8 Therefore, the B-cell progenitor described here has greater lineage plasticity, able to truly function as a common myeloid progenitor besides giving rise to mature B cells.
The paradigm of lymphoid development involves a committed decision by the stem cell to form a common lymphoid progenitor,1-4 which in turn differentiates into the B and T lineages. It is possible that this may not be completely applicable to all normal in vivo lymphoid development. The data here imply that the CXCR4- B-cell progenitor may not have developed from a common progenitor committed to lymphoid lineages. This B-cell progenitor may be ontologically closer to a stem cell, and to the fundamental lymphoid-myeloid lineage decision, than a common lymphoid progenitor.
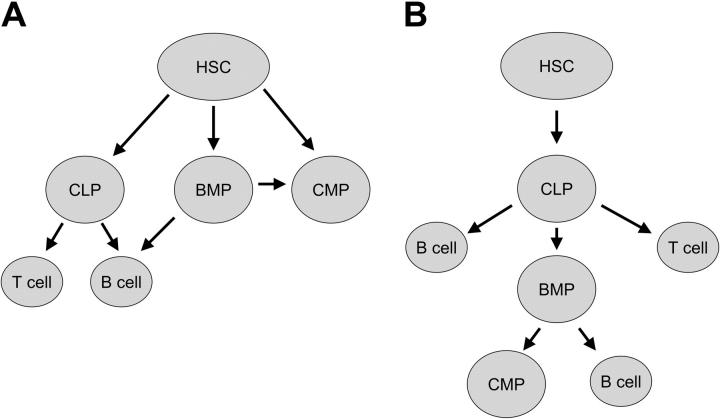
Two possible models explaining this finding are shown in Figure 4. In model A, the hematopoietic stem cell could form 3 common progenitors, the common myeloid progenitor (CMP), the common lymphoid progenitor (CLP), and the B-cell/myeloid common progenitor (BMP) described here. In model B, the stem cell differentiates to only the CLP, which in turn differentiates to the BMP, and the CMP. Although we favor model A, because it is more consistent with murine data,1-7 one provocative possibility implied by these data is that the stem cell does not directly give rise to the CMP but has a single first differentiation step to a CLP.
Figure 4.
Two models of hematopoietic stem cell development consistent with the data presented here. In stem cell differentiation model A, the hematopoietic stem cell could form 3 common progenitors, the common myeloid progenitor, the common lymphoid progenitor, and the B-cell/myeloid common progenitor described here. In stem cell differentiation model B, the stem cell differentiates to only the common lymphoid progenitor; however, along with differentiating into Band T cells, the CLP can give rise to a BMP cell, which can differentiate into B cells and myeloid cells. HSC indicates hematopoietic stem cell; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; BMP, B-cell/myeloid progenitor.
Another finding that also lends weight to this hypothesis is the phenotype of the PU.1 knockout mouse.25,26 When PU.1 is homozygously deleted in a murine model, B cells, macrophages, and granulocytes are missing, and the mice are anemic. One explanation of this is that PU.1 is required in these distinct lineages at a similar primitive stage, but perhaps a simpler explanation is that there is a common progenitor to these lineages that requires PU.1. This early hematopoietic progenitor in which PU.1 is required may be similar to the BMP defined here.
In addition, the Pax5-/- B-cell progenitor can be induced to differentiate to macrophages simply by withdrawing lymphoid cytokines, indicating that Pax5 may be important in maintaining B-cell lineage restriction.27 Finally, it has been noted that human BM cells that are CXCR4- can engraft immunodeficient mice with a similar efficiency and similar lineages as CXCR4+ cells, indicating that there is primitive hematopoietic function in the CXCR4- population.28 It is tempting to speculate that the BMP described here might be the engrafting cell in that report. Although that report conflicts with previous studies that describe the expression of CXCR4 as essential for stem cell engraftment in immunodeficient mice,17 that report28 is also more consistent with the data here.
The data presented here imply that the first developmental decision a stem cell makes, to differentiate down the lymphoid versus myeloid pathways, is not as final as was generally thought. Thus, though the CLP certainly exists,1-3 lymphoid development may not be that simple, and there may be other undefined hematopoietic stem cell differentiation pathways.
Prepublished online as Blood First Edition Paper, January 13, 2005; DOI 10.1182/blood-2004-07-2839.
Supported by National Institutions of Health grants RO1 HL66308, RO1 CA102283, and RO1 HL075783 (R.H.) and R01 HL5416, R01 DK53674, and R01 HL67384 (H.E.B.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Bonnet D. Haematopoietic stem cells. J Pathol. 2002;197: 430-440. [DOI] [PubMed] [Google Scholar]
- 2.Akashi K, Reya T, Dalma-Weiszhausz D, Weissman IL. Lymphoid precursors. Curr Opin Immunol. 2000;12: 144-150. [DOI] [PubMed] [Google Scholar]
- 3.Kondo M, Scherer DC, Miyamoto T, et al. Cellfate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407: 383-386. [DOI] [PubMed] [Google Scholar]
- 4.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117: 663-676. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki-Arai J, Iwasaki H, Miyamoto T, Watanabe S, Akashi K. Enforced granulocyte/macrophage colony-stimulating factor signals do not support lymphopoiesis, but instruct lymphoid to myelomonocytic lineage conversion. J Exp Med. 2003;197: 1311-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne KJ, Crooks GM. Human hematopoietic lineage commitment. Immunol Rev. 2002;187: 48-64. [DOI] [PubMed] [Google Scholar]
- 7.Katsura Y, Kawamoto H. Stepwise lineage restriction of progenitors in lympho-myelopoiesis. Int Rev Immunol. 2001;20: 1-20. [DOI] [PubMed] [Google Scholar]
- 8.Reynaud D, Lefort N, Manie E, Coulombel L, Levy Y. In vitro identification of human pro-B cells that give rise to macrophages, natural killer cells, and T cells. Blood. 2003;101: 4313-4321. [DOI] [PubMed] [Google Scholar]
- 9.Montecino-Rodriguez E, Dorshkind K. Identification of B/macrophage progenitors in adult bone marrow. Semin Immunol. 2002;14: 371-376. [DOI] [PubMed] [Google Scholar]
- 10.IUIS/WHO Subcommittee on Chemokine Nomenclature. Chemokine/chemokine receptor nomenclature. Cytokine. 2003;21: 48-49.12668160 [Google Scholar]
- 11.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994;91: 2305-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aiuti A, Tavian M, Cipponi A, et al. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999;29: 1823-1831. [DOI] [PubMed] [Google Scholar]
- 13.McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213: 442-456. [DOI] [PubMed] [Google Scholar]
- 14.Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95: 9448-9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393: 595-599. [DOI] [PubMed] [Google Scholar]
- 16.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10: 463-471. [DOI] [PubMed] [Google Scholar]
- 17.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283: 845-848. [DOI] [PubMed] [Google Scholar]
- 18.Hromas R, Cripe L, Hangoc G, Cooper S, Broxmeyer HE. The Exodus subfamily of CC chemokines inhibits the proliferation of chronic myelogenous leukemia progenitors. Blood. 2000;95: 1506-1508. [PubMed] [Google Scholar]
- 19.Lu L, Xiao M, Shen RN, Grigsby S, Broxmeyer HE. Enrichment, characterization, and responsiveness of single primitive CD34 human umbilical cord blood hematopoietic progenitors with high proliferative and replating potential. Blood. 1993;81: 41-48. [PubMed] [Google Scholar]
- 20.Loken MR, Shah VO, Dattilio KL, Civin CI. Flow cytometric analysis of human bone marrow, II: normal B lymphocyte development. Blood. 1987;70: 1316-1324. [PubMed] [Google Scholar]
- 21.Honczarenko M, Douglas RS, Mathias C, et al. SDF-1 responsiveness does not correlate with CXCR4 expression levels of developing human bone marrow B cells. Blood. 1999;94: 2990-2998. [PubMed] [Google Scholar]
- 22.Robertson MJ, Williams BT, Christopherson K 2nd, Brahmi Z, Hromas R. Regulation of human natural killer cell migration and proliferation by the exodus subfamily of CC chemokines. Cell Immunol. 2000;199: 8-14. [DOI] [PubMed] [Google Scholar]
- 23.llet O, Petit I, Kahn J, et al. Human CD34(+)CXCR4(-) sorted cells harbor intracellular CXCR4, which can be functionally expressed and provide NOD/SCID repopulation. Blood. 2002;100: 2778-2786. [DOI] [PubMed] [Google Scholar]
- 24.Carlsson L, Candeias S, Staerz U, Keller G. Expression of Fc gamma RIII defines distinct subpopulations of fetal liver B cell and myeloid precursors. Eur J Immunol. 1995;25: 2308-2317. [DOI] [PubMed] [Google Scholar]
- 25.Singh H. Gene targeting reveals a hierarchy of transcription factors regulating specification of lymphoid cell fates. Curr Opin Immunol. 1996;8: 160-165. [DOI] [PubMed] [Google Scholar]
- 26.McKercher SR, Torbett BE, Anderson KL, et al. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15: 5647-5658. [PMC free article] [PubMed] [Google Scholar]
- 27.Rolink AG, Schaniel C, Busslinger M, Nutt SL, Melchers F. Fidelity and infidelity in commitment to B-lymphocyte lineage development. Immunol Rev. 2000;175: 104-111. [PubMed] [Google Scholar]
- 28.Rosu-Myles M, Gallacher L, Murdoch B, et al. The human hematopoietic stem cell compartment is heterogeneous for CXCR4 expression. Proc Natl Acad Sci U S A. 2000;97: 14626-14631. [DOI] [PMC free article] [PubMed] [Google Scholar]