Abstract
Thrombin-catalyzed proteolysis at Arg372 of factor VIII is essential for procofactor activation. However, hemophilia A patients with the missense mutation Arg372 to His possess a mild to moderate phenotype yet show no detectable cleavage at this bond. To evaluate this discrepancy, we prepared and stably expressed a recombinant, B-domainless factor VIII mutant (R372H) that possessed approximately 1% the specific activity of wild type. Cleavage at R372H by thrombin occurred with an approximately 80-fold decreased rate compared with wild type. N-terminal sequence analysis of the derived A2 subunit confirmed that cleavage occurred at the His372-Ser373 bond. Factor VIII R372H was activated slowly, attained lower activity levels, and exhibited an apparent reduced inactivation rate compared with factor VIII wild type. These observations were attributed to a reduced cleavage rate at His372. Factor Xa generation assays showed similar Michaelis-Menten constant (Km, apparent) values for thrombin-catalyzed activation for either factor VIII form, but suggested an approximately 70-fold reduced maximum velocity (Vmax) for factor VIII R372H. However, prolonged reaction with thrombin yielded similar activity and stability values for the mutant and wild-type factor VIIIa forms. These results indicate a markedly reduced rate of cleavage following substitution at the P1Arg, and this property likely reflects the severity of the hemophilia A phenotype.
Introduction
Factor VIII, a plasma protein deficient or defective in individuals with hemophilia A, functions as a cofactor for the serine protease, factor IXa, in the anionic phospholipid surface-dependent conversion of factor X to Xa.1 Factor VIII is synthesized as a multidomain, single-chain molecule (A1-A2-B-A3-C1-C2) consisting of 2332 amino acid residues with a molecular mass of approximately 300 kDa.2,3 Factor VIII is processed to a series of divalent metal ion–linked heterodimers by cleavage at the B-A3 junction, generating a variable heavy chain (90-210 kDa) consisting of the A1, A2, and heterogeneous fragments of partially proteolyzed B domains, together with a light chain (80 kDa) consisting of the A3, C1, and C2 domains.2-4
Activation of factor VIII proceeds by limited proteolysis catalyzed by thrombin or factor Xa, with the former likely representing the physiologic activator.5 Both proteases convert factor VIII into the active cofactor form, factor VIIIa, following cleavage of 3 P1 positions at Arg372 between the A1-A2 domainal junction and at Arg740 between the A2-B domainal junction to generate the 50-kDa A1 and 40-kDa A2 subunits, and at Arg1689 to release a 40 acidic residue-rich peptide from the 80-kDa light chain to generate the 70-kDa A3-C1-C2 fragment.6 Proteolysis at Arg372 and Arg1689 appears essential for generating factor VIIIa cofactor activity (see Fay7 for review). Cleavage at the former site exposes a functional factor IXa–interactive site within the A2 domain that is cryptic in the unactivated molecule.8 Cleavage at the latter site liberates the cofactor from its carrier protein, von Willebrand factor,9 as well as contributes to overall specific activity of the cofactor.10,11
Approximately 5% of hemophilia A patients have circulating dysfunctional factor VIII molecules, detectable by immunoradiometric or enzyme-linked immunosorbent assays.12,13 These individuals are termed cross-reacting material-positive (CRM+),14 and the dysfunctional molecules generated have been useful in elucidating structure/function relationships of factor VIII. Examination of the hemophilia A database reveals a number of point mutations at position 372.15 These mutant factor VIII molecules, obtained from either patient plasma or a recombinant protein, are resistant to cleavage at the mutated site by thrombin,16,17 consistent with blocking conversion to the active cofactor molecule. The missense substitutions identified at these sites include His, Cys, and Pro.15 The Cys and Pro substitutions invariably yield moderate to severe hemophilia A phenotypes, while the substitution of His for Arg appears to possess a milder phenotype. However, the underlying reasons for the correlation between P1 residue and phenotype are unknown.
In the present study, we investigated thrombin-catalyzed activation of factor VIII using a recombinant factor VIII mutant with His substitution of the P1 372 position following site-directed mutagenesis. We demonstrate that the His372 residue can be accommodated within the active-site pocket of thrombin, although thrombin-catalyzed cleavage at His was much slower relative to Arg, likely reflecting the underlying severity of the hemophilia A phenotype. Furthermore, His substitution at the P1 site did not appear to affect formation of the thrombin–factor VIII complex, suggesting that other mechanisms such as exosite binding predominate relative to active-site docking at the P1 site in procofactor activation.
Materials and methods
Reagents
The anti–factor VIII monoclonal antibody C518 recognizing the C-terminal end of the factor VIIIa A1 subunit was a gift from Dr Zaverio Ruggeri. The anti–factor VIII monoclonal antibodies R8B1219 recognizing the A2 domain, 10104 recognizing the acidic region preceding the A3 domain, and ESH-820 recognizing the C2 domain were obtained from Green Mountain Antibodies (Burlington, VT), QED Bioscience (San Diego, CA), and American Diagnostica (Burlington, VT), respectively. The reagents human α-thrombin, factor IXa, factor X, and factor Xa (Enzyme Research Laboratories, South Bend, IN); chromogenic factor Xa substrate S-2765 (N-α-benzyloxycarbonyl-D-arginyl-glycyl-L-arginyl-P-nitroanilide dihydrochloride; DiaPharm Group, Westchester, OH); and hirudin (Calbiochem, San Diego, CA) were purchased from the indicated vendors. Factor VIII–deficient plasma was prepared as previously described.21 Phospholipid vesicles containing 20% phosphatidylserine, 40% phosphatidylcholine, and 40% phosphatidylethanolamine (Sigma, St Louis, MO) were prepared using N-octylglucoside as described previously.22 The B-domainless factor VIII expression construct HSQ-MSAB-NotI-RENeo factor VIII was a gift kindly provided by Dr Pete Lollar and John Healey.
Construction, expression, and purification of recombinant factor VIII
B-domainless factor VIII cDNA was restricted from the factor VIII expression construct HSQ-MSAB-NotI-RENeo using the endonucleases XhoI and NotI, and cloned into the Bluescript II K/S-vector. The Arg372→His (CGC to CAC as noted for hemophilia patients) and Gln (from CGC to CAA) mutations were introduced into the shuttle constructs using the Stratagene QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) as described.23 The presence of only the desired mutation was confirmed by dideoxy sequencing. The mutated factor VIII cDNA was then restricted and ligated back into the factor VIII expression construct, and subjected to a second round of dideoxy sequencing (PE Applied Biosystems, Foster, CA) to confirm that only the desired mutation was present. The factor VIII expression vector constructs were transfected in baby hamster kidney (BHK) cells by liposome-mediated transfection. The selection, subcloning, and cloning of stable transfectants were performed by standard methods, and the cloned cells were cultured in roller bottles.23 The conditioned media were collected daily, and the expressed proteins were purified following chromatography using SP-Sepharose as previously described.24 Resultant factor VIII was more than 85% pure as judged by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and staining with GelCode Blue (Pierce, Rockford, IL). The primary contaminant was albumin. Active fractions were detected using a one-stage clotting assay. A sandwich enzyme-linked immunosorbent assay (ELISA) using 2 anti–factor VIII monoclonal antibodies, ESH8 (anti-C2) and R8B12 (anti-A2), was performed to measure the concentration of the purified factor VIII proteins as previously described.24 Factor VIII samples were quick frozen and stored at - 80 °C.
Activation of factor VIII by thrombin
Factor VIII (50 nM) was incubated with 20 nM thrombin in 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.2), 0.1 M NaCl, 5 mM CaCl2, 0.01% Tween 20 at 22°C. At timed intervals, samples were taken from the mixture, and the activation was terminated by hirudin (2 units/mL) and diluted appropriately for activity determinations. Each sample was tested for factor VIIIa activity in a one-stage clotting assay. The presence of thrombin and hirudin in the diluted samples did not affect the factor VIII activity in the coagulation assay. Furthermore, preincubation of factor VIII in the absence of thrombin over an extended reaction time yielded equivalent results to factor VIII assayed immediately, indicating no detectable endogenous proteases in the preparation.
Factor Xa generation assay
The rate of conversion of factor X to factor Xa was monitored in a purified system.25 Various concentrations of factor VIII were activated by the addition of limiting thrombin (0.04 nM) for 1 minute, after which thrombin activity was inhibited by the addition of hirudin (0.1 U/mL). The generated factor VIIIa was reacted with factor IXa (20 nM) in the presence of phospholipid vesicles (10 μM), and reactions were initiated with the addition of factor X (300 nM). Aliquots were removed at appropriate times to assess initial rates of product formation and added to tubes containing EDTA (ethylenediaminetetraacetic acid, 50 mM final concentration) to stop the reaction. Rates of factor Xa generation were determined by the addition of the chromogenic substrate, S-2765 (0.46 mM final concentration). Reactions were read at 405 nm using a Vmax microtiter plate reader (Molecular Devices, Sunnyvale, CA). For the experiment assessing the stability of thrombin-activated factor VIIIa, factor VIII (300 nM) was reacted with thrombin (50 nM) for 18 hours at 37°C. After the addition of hirudin (20 U/mL) and a 30-fold dilution, aliquots were removed at the indicated times. Each sample was reacted with factor IXa (20 nM) and phospholipid vesicles (10 μM), and reactions were initiated with the addition of factor X (300 nM). The Km (apparent) and relative Vmax values for thrombin-catalyzed activation of factor VIII, dissociation constant of an inhibitor (Ki, apparent) value for R372H obtained by competitive assay, and rate constants for decay of factor Xa activity were calculated from the Michaelis-Menten, noncompetitive inhibition, and single exponential decay equations, respectively, using Kaleidagraph (Synergy, Reading, PA).
Cleavage of factor VIII by thrombin
Factor VIII (100 nM) was reacted with 2.5 nM thrombin in a buffer containing 20 mM HEPES (pH 7.2), 0.1 M NaCl, 5 mM CaCl2, and 0.01% Tween 20, and the reactions were run at 22°C. Samples were taken at indicated times, and the reactions were immediately terminated and prepared for SDS-PAGE by adding SDS and boiling for 3 minutes.
Electrophoresis and Western blotting
SDS-PAGE was performed on 8% gels using the procedure of Laemmli.26 Electrophoresis was carried out using a Bio-Rad mini gel apparatus (Hercules, CA) at 50 V for 2 hours. The protein was transferred to a polyvinylidenefluoride membrane and probed using the indicated anti–factor VIII monoclonal antibody followed by goat antimouse alkaline phosphatase–linked secondary antibody. The signal was detected using the enhanced chemifluorescence (ECF) system (Amersham Biosciences, Freiburg, Germany), and the blots were scanned at 570 nm using Storm 860 (Molecular Devices, Sunnyvale, CA). Densitometry scans were quantitated using ImageQuant software (Molecular Devices).
NH2-terminal sequence analysis
NH2-terminal sequence analysis of the A2 subunit following thrombin cleavage of recombinant factor VIII R372H was performed using a PE Applied Biosystems Model 494 Sequencer by the Protein Chemistry Core Facility at Columbia University. Thrombin-cleaved factor VIII was blotted onto a polyvinylidenefluoride membrane and stained with Ponceau S, and the band corresponding to the A2 subunit was excised. The sample was subjected to 5 cycles of automated sequencing.
Results
Characterization of recombinant factor VIII proteins
Thrombin cleavage at Arg372 is required to expose functional factor IXa–interactive sites necessary for generating factor VIIIa cofactor activity,8 and this contention is supported by the absence of detected cleavage at this site in hemophilia A patients possessing missense mutations at Arg372.16,17 However, the basis for the observed mild to moderate phenotype in some patients possessing an Arg372His mutation is unclear. Limitations in the patient plasma-derived or transiently expressed factor VIII proteins have precluded detailed studies of the interactions with thrombin and visualization of reaction products. In this study, we examine thrombin-catalyzed activation of a recombinant factor VIII R372H. This reagent was constructed following site-directed mutagenesis and stably expressed in BHK cells as B-domainless factor VIII form. Approximately 200 μg wild-type and R372H factor VIII were purified from approximately 1 liter of conditioned medium, while a similar amount of medium yielded approximately 25 μg purified R372Q factor VIII, suggesting a lower level of expression for the latter factor VIII form. All factor VIII forms were more than 85% pure.
Specific activity values for the expressed recombinant proteins and activity and antigen values for database entries are summarized in Table 1. Wild-type factor VIII showed a specific activity (3.53 units/μg) similar to a previously reported value.24 Data from the hemophilia A database15 for the R372H mutation indicated an activity-to-antigen ratio of 0.04, or 4% the activity value of native factor VIII. The recombinant R372H mutant exhibited a similar reduction in specific activity corresponding to approximately 1% of the wild-type value. The reduction in activity of the mutant factor VIII forms may include a fraction of misfolded species.
Table 1.
Specific activity values for factor VIII wild type and R372H
|
Database
|
Recombinant factor VIII
|
||||
|---|---|---|---|---|---|
| Factor VIII | Ac, unit/dL | Ag, unit/dL | Ac/Ag ratio | Specific activity, unit/μg | % of wild type |
| Wild type | NA | NA | NA | 3.53 ± 0.54 | 100 |
| R372H | 4.2 ± 1.0 | 122 ± 51 | 0.04 ± 0.03 | 0.024 ± 0.003 | 0.69 |
NA, not applicable.
Database values were obtained from the hemophilia A mutation database (HAMSTeRs database; europium.csc.mrc.ac.uk). Ac and Ag refer to factor VIII activity and antigen, respectively. Factor VIII activity was determined by a one-stage clotting assay as described in “Materials and methods.” Factor VIII antigen was determined using ELISA as described in “Materials and methods.” Values represent the mean ± standard deviation from 4 separate determinations.
Cleavage of the A1-A2 domainal junction of factor VIII R372H by thrombin
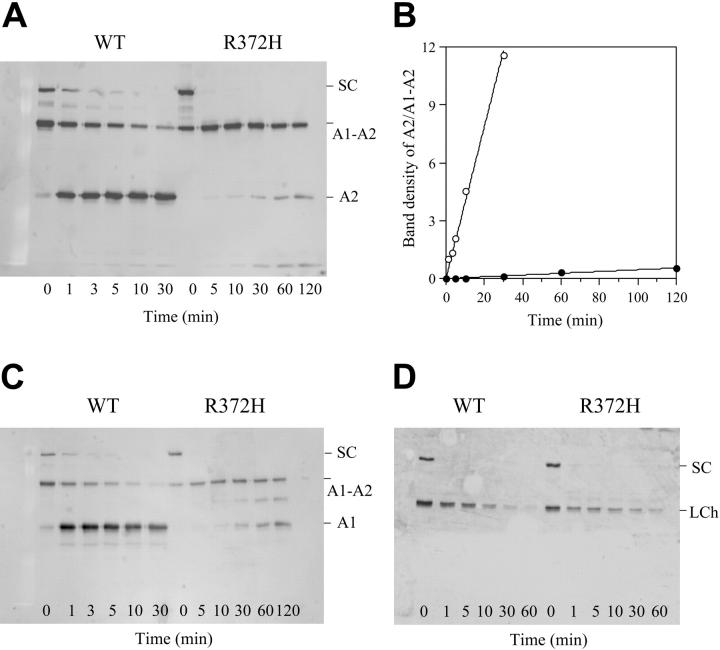
An earlier report demonstrated that factor VIII containing the R372H mutation obtained from patient's plasma was resistant to thrombin-catalyzed cleavage at the mutated site,16 leading these authors to suggest that the mutation abrogated cleavage at this site. However, the mild to moderate phenotype observed for this mutation may reflect reduced cleavage rates at the A1-A2 domainal junction that were not detected in the earlier study. This alternative was examined following cleavage of factor VIII (100 nM) by thrombin (2.5 nM) using SDS-PAGE analysis and Western blotting. Figure 1A shows the results of the cleavage time course using an anti-A2 monoclonal antibody, R8B12.19 In both samples, low levels of single-chain factor VIII were observed. The presence of this factor VIII species and potential differences with plasma-derived factor VIII are likely inconsequential due to its rapid cleavage by thrombin, presumably at Arg740/Arg1649 to yield the heavy and light chains of factor VIII. That this cleavage occurs in the mutant at an equivalent rate as observed for the wild type indicated that recognition of other scissile bonds within the mutant substrate are not affected by the R372H point mutation. The contiguous A1-A2 heavy chain of wild-type factor VIII was rapidly proteolyzed by thrombin to yield the A1 and A2 subunits, showing more than 50% conversion of substrate by the initial (1 minute) time point. In contrast, the cleavage at the A1-A2 junction of the R372H mutant was significantly retarded with little detectable product formed at 10 minutes after thrombin reaction, and this reaction remained largely incomplete at the 2-hour time point. Examination of the ratio of A2 product/A1-A2 substrate by scanning densitometry of the bands (Figure 1B) suggested that the rate of cleavage at the A1-A2 junction of R372H was reduced approximately 80-fold compared with the wild-type value. This reduction in relative rate of cleavage was similar to the reduction in specific activity of the R372H mutant, suggesting this ratio likely reflects the slow conversion of heavy chain to activation products. The appearance of A2 subunit observed in Figure 1A was paralleled by the generation of A1 subunit, as detected by the anti-A1 antibody (Figure 1C). Figure 1C also shows an additional, minor band that is somewhat smaller than the contiguous A1-A2 chain. Based upon its size and the observation that it blots with the anti-A1 but not anti-A2 antibody, this fragment derives from cleavage within the A2 domain and represents a fragment comprised of the entire A1 and a portion of the A2 domain. Thus it appears to be a secondary cleavage fragment generated by thrombin when cleavage at the 372 site is inhibited. Furthermore, conversion of the factor VIII light chain in both wild type and mutant, as judged by loss of staining intensity due to cleavage at Arg1689 liberating the N-terminal segment containing the antibody-reactive epitope, appeared to occur at similar rates (Figure 1D). The latter result again confirms that the susceptibility of the factor VIII R372H mutant to thrombin-catalyzed proteolysis at other cleavage sites was unaffected by the mutation at Arg372. Assessment of factor Xa cleavage of R372H using similar blotting experiments revealed no proteolysis at position 372 (data not shown), although the Arg372-Ser373 bond of wild-type factor VIII was readily cleaved by factor Xa under similar reaction conditions. Furthermore, factor Xa did cleave the R372H factor VIII heavy chain at a site consistent with cleavage at Arg336 (data not shown), a known factor Xa site resulting in cofactor inactivation.
Figure 1.
Time course of cleavage of recombinant factor VIII by thrombin. Recombinant factor VIII wild type (WT) and R372H (100 nM) were reacted with thrombin (2.5 nM) for the indicated times as described in “Materials and methods.” Samples were run on 8% gel followed by Western blotting using an anti-A2 (A), anti-A1 (C), or anti–light chain (D) monoclonal antibody. Panel B shows quantitative densitometry of the ratio of A2 subunit/A1-A2 subunit from blotting data obtained from panel A. ○ indicates wild type; •, R372H. The data were fitted to a straight line. SC and LCh represent a single chain and the intact light chain, respectively.
Identification of the N-terminal sequence of factor VIIIa R372H A2 subunit
From the blotting data (Figure 1), the apparent molecular masses of A1 or A2 subunits produced by thrombin cleavage of R372H mutant appeared to be equivalent to those of wild type, suggesting that cleavage indeed occurred at the authentic site. To confirm the site of proteolysis, the A2 subunit derived from thrombin cleavage of the R372H mutant was subjected to 5 cycles of automated N-terminal sequence analysis (Table 2). Results from this analysis indicated that the A2 subunit was derived from the cleavage at the His372-Ser373 bond, confirming His372 as the P1 residue.
Table 2.
Amino-terminal sequence analysis of the A2 subunit of thrombin-cleaved factor VIII R372H mutant
|
Cycle no.
|
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Residues 373-377 | S | V | A | K | K |
| A2 subunit of R372H | S | V | A | K | K |
| pmol | 27.9 | 10.5 | 11.9 | 2.3 | 3.1 |
The A2 subunit of thrombin-cleaved factor VIII was subjected to 5 cycles of sequencing. Sequences were determined as described in “Materials and methods” and were aligned using the published factor VIII sequences.2 Technical problems with the quantitation of Lys residues render the picomole yield values for this residue unreliable.
Thrombin activation of factor VIII R372H mutant
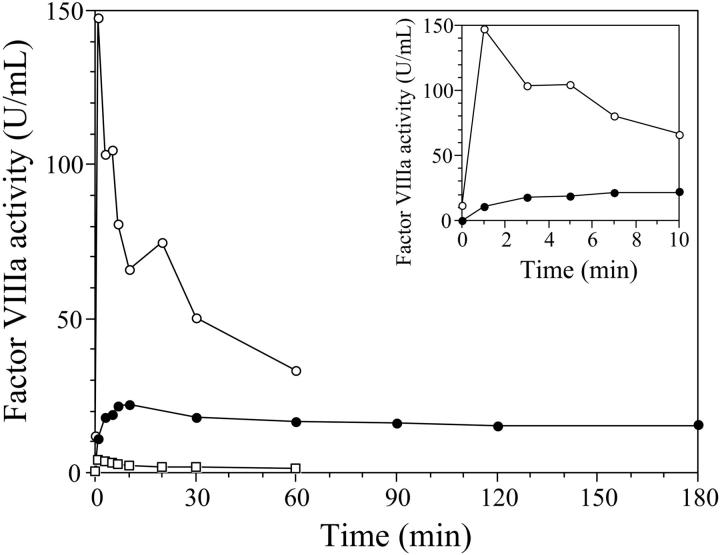
In order to further investigate the action of thrombin on the factor VIII R372H mutant, a time course of factor VIII activation was performed using a one-stage clotting assay as described in “Materials and methods” (Figure 2). Typical of factor VIII activation, the activity of wild-type factor VIII increased approximately 15-fold at 1 minute following thrombin addition, and this activity peak was followed by a sharp decline to approximately 20% peak activity at 60 minutes. This latter effect results from the dissociation of the A2 subunit from the A1/A3-C1-C2 dimer. In contrast, factor VIII R372H showed a significantly lower initial activity level. Upon addition of thrombin, cofactor activity increased slowly (Figure 2 inset), reaching a peak activity that was 10% to 15% that observed for wild type. Furthermore, the characteristic “spike” of factor VIIIa activity was replaced by an apparently stable, broad activation plateau retaining approximately 80% of peak activity after 3 hours. Since the activity of factor VIIIa visualized at any point in the time course likely represents contributions from unactivated molecules, activated molecules, and activated molecules that have decayed following subunit dissociation (Fay7), the apparent enhanced stability of the factor VIIIa R372H at low activity levels likely reflects a similarly reduced rate of cofactor activation.
Figure 2.
Time course of thrombin activation of recombinant factor VIII. Recombinant factor VIII wild type, R372H, and R372Q (50 nM) were reacted with thrombin (20 nM) for the indicated times, after which activation was terminated by hirudin and each sample was tested immediately for factor VIIIa activity in a one-stage clotting assay. The inset shows an expanded activation time course during the initial 10 minutes of the reaction. ○ indicates wild type; •, R372H; and □, R372Q. The zero point was taken prior to addition of thrombin.
This hypothesis was supported by an experiment using the factor VIII mutant (R372Q) in which arginine is replaced by glutamine at residue 372. Treatment of this factor VIII with thrombin yielded no detectable cleavage (< 5 ng/mL cleaved protein) at position 372 over an extended reaction time course (data not shown). Furthermore, evaluation of factor VIII activity showed essentially no thrombin-catalyzed activation (< 0.01 units/mL) compared with activity values obtained for the R372H factor VIII, supporting a requirement for cleavage at the A1-A2 junction in the generation of cofactor activity.
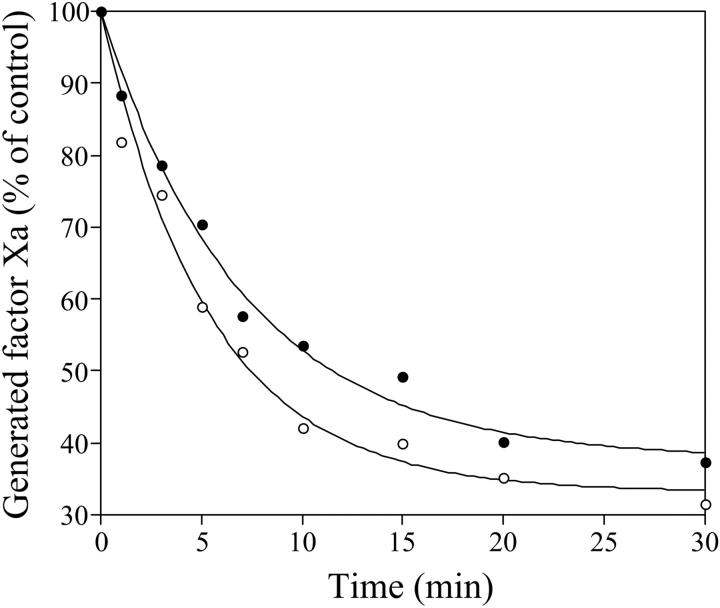
Further evidence that the limited activity attributed to factor VIIIa R372H was derived from a reduced rate in His372-Ser373 bond cleavage rather than a partially activated species possessing an intact heavy chain was obtained following activity and stability of the fully cleaved form. The mutant and wild-type factor VIII proteins (300 nM) were treated for an extended time period (37°C, 18 hours) with thrombin (50 nM) to maximize generation of factor VIIIa in the mutant. This reaction resulted in more than 80% cleavage of the R372H mutant heavy chain and complete cleavage of wild-type heavy chain (data not shown). Following this reaction, factor VIIIa samples were diluted 30-fold and cofactor activity and stability were assessed in a factor Xa generation assay (Figure 3). Activity values observed for the wild-type and mutant factor VIIIa forms were similar at the initiation of the factor Xa generation reaction, yielding approximately 13.0 and approximately 6.1 nM factor Xa generated/min, respectively. These results suggest that factor VIII R372H possesses wild-type–like cofactor activity following cleavage at the His372 site. Overall, these activity values were reduced compared with those observed for wild type following a conventional activation protocol (Figure 4) and likely reflected the more harsh conditions used to activate the proteins. Examination of the reduction in rates of factor Xa generating activity with time, attributed to inactivation of factor VIIIa due to A2 subunit dissociation,27 indicated that both cofactors decayed at near equivalent rates (0.185 ± 0.019 minute-1 and 0.141 ± 0.014 minute-1 for wild type and R372H, respectively). This result suggested that the dissociation of A2 subunit from either factor VIIIa form was not impaired by the mutation at P1 site and also supports the previous contention that the appearance of enhanced stability of the factor VIIIa R372H at low activity levels directly reflected a reduced rate of cofactor activation.
Figure 3.
Time-dependent decay of factor Xa generating activity by factor Xase composed of R372H and wild-type factor VIIIa forms. Factor VIII (300 nM) was reacted with thrombin (50 nM) for 18 hours at 37°C. After the addition of hirudin and a 30-fold dilution of reactant, aliquots were removed at the indicated times and reacted with factor IXa (20 nM) and phospholipid vesicles (10 μM), and factor Xa generation was initiated with the addition of factor X (300 nM) as described in “Materials and methods.” ○ indicates wild type; •, R372H. The initial activity of factor Xa generated (100% level) for factor VIII wild type and R372H was 13.0 and 6.1 nM/min, respectively. Initial rates of factor Xa generation were plotted as a function of incubation time and fitted to a single exponential decay curve. Experiments were performed at least 3 separate times and average values are shown.
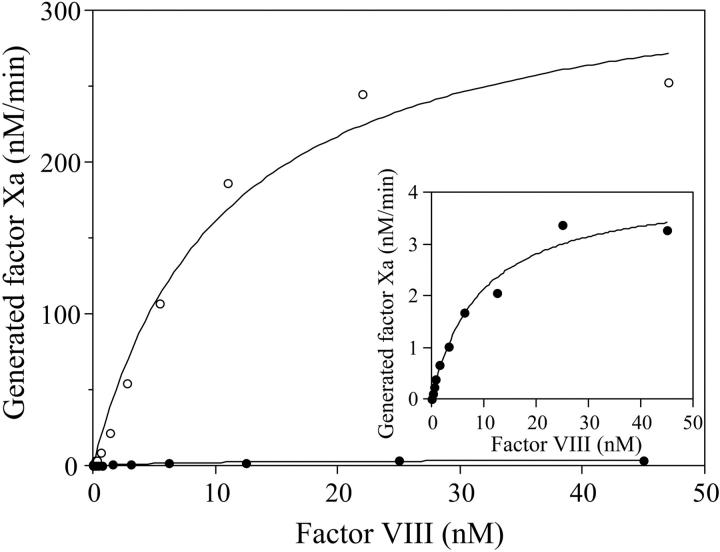
Figure 4.
Kinetics of recombinant factor VIII activation by thrombin. Variable amounts of factor VIII were reacted with thrombin (0.04 nM) for 1 minute. Thrombin was inactivated by addition of hirudin, and factor VIIIa was reacted with factor IXa (20 nM) in the presence of phospholipid vesicles (10 μM). Factor Xa generation was initiated by addition of factor X (300 nM) as described in “Materials and methods.” ○ indicates wild type; •, R372H. Initial rates of factor Xa generation are plotted as a function of factor VIII concentration and fitted to the Michaelis-Menten equation by nonlinear least squares regression. The inset shows the rate of factor Xa generation using factor VIII R372H activated by thrombin. All experiments were performed at least 3 separate times and average values are shown.
Evaluation of the activation kinetics for factor VIII R372H relative to wild type
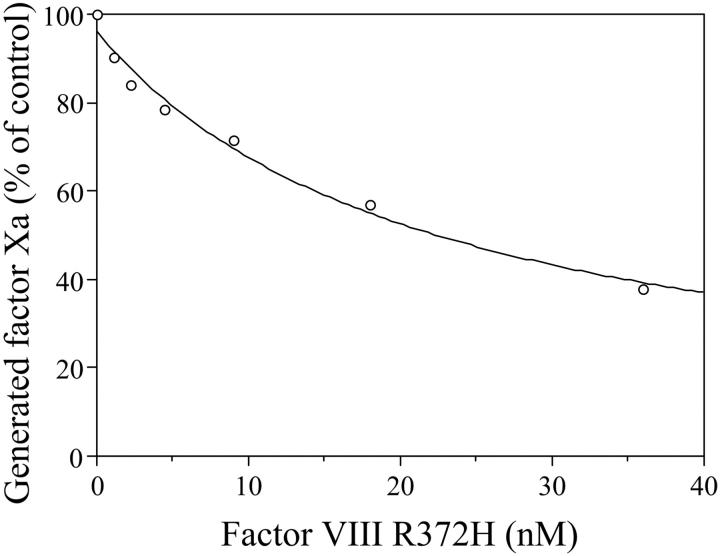
To further evaluate the kinetics of cleavage yielding cofactor activation, an experiment was performed in which the concentrations of factor VIII wild type and R372H were varied, and thrombin-catalyzed cofactor activation was measured indirectly using a factor Xa generation assay as described in “Materials and methods.” Factor VIII forms activated by limiting thrombin (0.04 nM) were subsequently reacted with a high concentration of factor IXa (20 nM) in the presence of phospholipid vesicles in order to drive factor VIIIa generated into intrinsic factor Xase complex. Reactions were then initiated with factor X (300 nM). Using these reaction conditions, the factor Xa generation rate is directly related to the concentration of factor VIIIa. Thus, this rate can be used to assess the Km for thrombin activation of the procofactor. Furthermore, comparison of maximal rates for factor Xa generation allows for estimation of the (relative) maximal concentrations of factor VIIIa wild type and R372H generated following the limiting reaction with thrombin. Results from these studies are shown in Figure 4. The Vmax value for factor Xa generated obtained from the fitted curve for wild-type factor VIII (337 ± 23 nM minute-1) was approximately 80-fold greater than that for factor VIII R372H (4.1 ± 0.3 nM minute-1). Examination of the factor VIIIa concentrations at half-maximal rate of factor Xa generation indicated near equivalent concentrations of the wild type (10.7 ± 1.9 nM) and mutant (9.5 ± 1.8 nM), which represent Km (apparent) values for factor VIII forms. The observation that these values were similar, whereas the Vmax value for the mutant was markedly reduced for factor Xase comprised of factor VIIIa R372H, suggested that kcat for the thrombin-catalyzed conversion of the procofactor to active cofactor was the kinetic parameter primarily affected. The magnitude reduction in this parameter agreed well with the relative cleavage rates of the A1-A2 domainal junctions for the wild-type and mutant proteins (Figure 1). Furthermore, the near equivalence of the fold reduction in the Vmax values with the specific activity values suggested that the defect in the latter parameter resulted from the reduced catalytic rate constant for the activation of factor VIII R372H.
This reduction in factor VIII activation rate did not result from a defect in the binding of thrombin to the factor VIII R372H substrate. Figure 5 shows results from an experiment in which the effects of increasing concentrations of the mutant factor VIII on the activation of wild-type factor VIII were determined. Wild type of factor VIII (10 nM) was reacted with thrombin (0.04 nM) for 1 minute in the absence or presence of variable concentrations of factor VIII R372H. After the addition of hirudin, the factor VIIIa sample was reacted with factor IXa (20 nM) and factor X (300 nM) in the presence of phospholipid vesicles. Inclusion of the mutant factor VIII inhibited thrombin-catalyzed activation of factor VIII wild type in a dose-dependent manner with more than 60% inhibition observed at a 4-fold excess of mutant, indicating that the mutant was an effective competitor of wild-type factor VIII for interaction with thrombin. The apparent Ki value for the factor VIII R372H (12.6 ± 4.0 nM) was similar to the Km value determined for factor VIII wild type (Figure 4), indicating that thrombin binding to factor VIII R372H mutant was not impaired by mutation at the P1 site.
Figure 5.
Competition of thrombin activation of factor VIII wild type by factor VIII R372H. Mixtures of factor VIII wild type (10 nM) and variable concentrations of R372H were reacted with thrombin (0.04 nM) for 1 minute. After the addition of hirudin, factor VIIIa was reacted with factor IXa (20 nM) in the presence of phospholipid vesicles (10 μM). Factor Xa generation was initiated by addition of factor X (300 nM). Factor Xa generated in the absence of R372H (100% level) was approximately 160 nM/min. Data were corrected for the amount of factor Xa generated from factor VIII R372H. Initial rates of factor Xa generation were plotted as a function of R372H and fitted to a noncompetitive inhibition equation by nonlinear least squares regression. Experiments were performed at least 3 separate times and average values are shown.
Discussion
Several lines of evidence support the essential role for cleavage of the Arg372-Ser373 bond separating the A1 and A2 domains of factor VIII in the generation of cofactor activity. Analysis of a site-directed recombinant factor VIII mutant, R372L,28 or naturally occurring factor VIII point mutations, R372H16 and R372C,17,29 have consistently failed to detect thrombin-catalyzed cleavage at this site. Activities attributed to these molecules were consistent with a hemophilia A phenotype. Interestingly, while Cys, Leu, and Pro15 substitutions yield moderate to severe phenotypes, His substitution appeared to yield a mild hemophilia A.16 Mechanistically, cleavage at this site has been suggested to expose functional factor IXa–interactive site(s) in the A2 subunit that is (are) masked in the unactivated molecule where the A2 domain is contiguous with A1.8 Thus, the variability in phenotype observed with point mutations at Arg372 suggested the potential that an uncleaved heavy chain may contribute some low level of activity dependent upon the residue at this position. Results from this study support an alternate model where rates of the requisite cleavage at position 372 define the severity of the phenotype. Thus, we speculate that the more severe phenotype observed with the Cys or Pro substitution represents a more impaired interaction for active site engagement of the P1 residue and/or cleavage of the scissile bond.
The apparent rate for thrombin action on the wild-type substrate was markedly enhanced compared with the R372H substrate. The magnitude of this differential (∼80-fold), based upon generation of factor VIIIa cofactor activity as evaluated in a factor Xa generation assay, paralleled the magnitude decrease observed for the apparent cleavage rate at Arg372 in the mutant protein. These observations suggested that cleavage at the A1-A2 domain represented the rate-limiting step in the generation of cofactor activity. This observation is consistent with the ordered cleavage of the A1-A2-B domain-containing heavy chain initially at Arg740 to generate the contiguous A1-A2 fragment followed by cleavage at Arg372 to separate these domains, coupled with the rapid cleavage of light chain at Arg1689.4,6 While the absolute Vmax value for the thrombin-catalyzed activation of the 2 factor VIII forms was not determined, the Km (apparent) values for these reactions were equivalent (∼10 nM). These values were substantially lower than Km values reported for the thrombin-catalyzed cleavage atArg372 in porcine factor VIII (Km ∼ 150 nM30). The reason(s) for this difference is not clear but may reflect differences in the experimental methods, which in the earlier study monitored rates of thrombin cleavage of 125I-labeled porcine factor VIII using SDS-PAGE and scanning densitometry.
Although, the rate of cofactor activation was markedly reduced in factor VIII R372H, the activity obtained gave the impression of enhanced stability due to the absence of the rapid decay phase typically observed following factor VIII activation. This result was also observed in the low activation rates for a factor VIII R372C mutant17 and the P1′ S373L mutant31 in which no proteolysis at residue 372 was detected. This plateau of activity may derive from a partially activated form of factor VIIIa wherein light chain is cleaved and heavy chain is not, thus the basis for inherent cofactor instability, dissociation of A2 subunit,19,32 is not relevant. Thus there may be significant residual activity in the absence of detectable cleavage. This alternative is not supported by the data based upon the observation that factor VIII R372Q was not cleaved at the 372 site and consequently demonstrated no appreciable activation. Furthermore, activity of the near fully cleaved R372H factor VIIIa was within a factor of 2 of the value observed for wild-type factor VIII, and the dissociation rate of A2 subunit from factor VIIIa R372H was similar with that of the wild type. Alternatively, the slow rate of cleavage at the mutant scissile bond gives the appearance of a stable factor VIIIa, since molecules are continuously activated throughout the time course. This latter explanation is likely correct since earlier studies showing activation of native factor VIII at diminishing thrombin concentrations yielded reduced peak activity heights coupled with a broadening of the activity level over the activation time course.33 Thus limiting enzyme in these reactions would yield the same observed activation profile as a situation in which higher relative enzyme is present but kcat is reduced due to mutation in the substrate.
Results presented in this study parallel the earlier observations of the P1 Arg16His mutation in the Aα chain of a variant fibrinogen.34-36 Analysis of the thrombin-catalyzed cleavage at the His16-Gly17 bond revealed an approximately 80-fold reduced rate of the release of fibrinopeptide A,36 a value similar to the reduced kcat for cleavage at His372 in factor VIII. The similarity of these results suggests that other sites involved in catalysis (eg, other residues within the P3-P3′ sequences and more distal, exosite interactions) are not differentially perturbed by the His substitution at the P1 position in the 2 scissile bonds. Thus, results using the 2 substrates suggest that His is marginally tolerated at the P1 site and yields a 60- to 80-fold reduction in kcat compared with the native P1Arg.
Based upon the crystal structure of fibrinopeptide A bound to thrombin,37 the P1Arg shows a complex interaction with thrombin that includes formation of a primary salt bridge with the S1Asp189 and a hydrogen bond to Gly219, as well as additional interactions with Ser214, Gly193, Ser195, and His57. Southan et al36 reported on the interaction between the fibrinopeptide A substituted to His at the P1 site and thrombin using computer modeling. These authors speculated that although the imidazole ring of His engaged the specificity binding pocket, interactive distances were too great for direct hydrogen bonding, and the presence of an additional water molecule was required to facilitate these interactions. Thus the histidyl side chain would be less rigid within the pocket, leading to an altered orientation of the scissile bond relative to the active site.
The equivalence of Km values for both the wild-type and mutant factor VIII substrates indicates that the P1His does not appreciably perturb this parameter. Indeed, factor VIII R372H effectively inhibited the activation of wild-type factor VIII by thrombin, consistent with the mutant protein sequestering the enzyme. This observation underscores the importance of exosite tethering during thrombin-catalyzed factor VIII activation, and studies have implicated both anion-binding exosite 1 and exosite 238,39 in contributing to this reaction mechanism. Thus our results also emphasize the importance of exosite binding relative to active-site docking for association with factor VIII and thrombin. In conclusion, the results from this study support a model whereby mutation of Arg372 to His results in a defect in engagement of His at the S1 site in the exosite site–docked thrombin-factor VIII complex and/or impaired cleavage at the P1-P1′ site, reflecting a marked reduction in cleavage rate of the scissile bond. We suggest that the observed low levels of cofactor activity generated are derived from this slow rate of proteolysis, which defines the severity of the CRM+ hemophilia A phenotype.
Acknowledgments
We thank Zaverio Ruggeri for the gift of C5 antibody, John Healey and Pete Lollar for providing the factor VIII cloning and expression vectors, and Jan Freas for excellent technical assistance.
Prepublished online as Blood First Edition Paper, February 10, 2005; DOI 10.1182/blood-2004-10-3939.
Supported by National Institutes of Health grants HL 38199 and HL 76213.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy S. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76: 1-16. [PubMed] [Google Scholar]
- 2.Wood WI, Capon DJ, Simonsen CC, et al. Expression of active human factor VIII from recombinant DNA clones. Nature. 1984;312: 330-337. [DOI] [PubMed] [Google Scholar]
- 3.Vehar GA, Keyt B, Eaton D, et al. Structure of human factor VIII. Nature. 1984;312: 337-342. [DOI] [PubMed] [Google Scholar]
- 4.Fay PJ, Anderson MT, Chavin SI, Marder VJ. The size of human factor VIII heterodimers and the effects produced by thrombin. Biochim Biophys Acta. 1986;871: 268-278. [DOI] [PubMed] [Google Scholar]
- 5.Pieters J, Lindhout T, Hemker HC. In situ-generated thrombin is the only enzyme that effectively activates factor VIII and factor V in thromboplastin-activated plasma. Blood. 1989;74: 1021-1024. [PubMed] [Google Scholar]
- 6.Eaton D, Rodriguez H, Vehar GA. Proteolytic processing of human factor VIII: correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986;25: 505-512. [DOI] [PubMed] [Google Scholar]
- 7.Fay PJ. Activation of factor VIII and mechanisms of cofactor action. Blood Rev. 2004;18: 1-15. [DOI] [PubMed] [Google Scholar]
- 8.Fay PJ, Mastri M, Koszelak ME, Wakabayashi H. Cleavage of factor VIII heavy chain is required for the functional interaction of A2 subunit with factor IXa. J Biol Chem. 2001;276: 12434-12439. [DOI] [PubMed] [Google Scholar]
- 9.Lollar P, Hill-Eubanks DC, Parker CG. Association of the factor VIII light chain with von Willebrand factor. J Biol Chem. 1988;263: 10451-10455. [PubMed] [Google Scholar]
- 10.Regan LM, Fay PJ. Cleavage of factor VIII light chain is required for maximal generation of factor VIIIa activity. J Biol Chem. 1995;270: 8546-8552. [DOI] [PubMed] [Google Scholar]
- 11.Donath MS, Lenting PJ, van Mourik JA, Mertens K. The role of cleavage of the light chain at positions Arg1689 or Arg1721 in subunit interaction and activation of human blood coagulation factor VIII. J Biol Chem. 1995;270: 3648-3655. [DOI] [PubMed] [Google Scholar]
- 12.Lazarchick J, Hoyer LW. Immunoradiometric measurement of factor VIII procoagulant antigen. J Clin Invest. 1978;62: 1048-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peake IR, Bloom AL, Giddings JC, Ludlum CA. An immunoradiometric assay for procoagulant factor VIII antigen: results in haemophilia A. Br J Haematol. 1979;42: 269-281. [DOI] [PubMed] [Google Scholar]
- 14.Hoyer LW, Breckenridge RT. Immunologic studies of antihemophilic factor: crossreacting material in a genetic variant of hemophilia A. Blood. 1968;32: 962-971. [PubMed] [Google Scholar]
- 15.Kemball-Cook G, Tuddenham EGD, Wacey AI. The factor VIII structure and mutation resource site: HAMSTeRS version 4. Nucleic Acids Res. 1998;26: 216-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arai M, Inaba H, Higuchi M, et al. Direct characterization of factor VIII in plasma: detection of a mutation altering a thrombin cleavage site (arginine-372 to histidine). Proc Natl Acad Sci U S A. 1989;86: 4277-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien DP, Pattinson JK, Tuddenham EGD. Purification and characterization of factor VIII 372-Cys: a hypofunctional cofactor from a patient with moderately severe hemophilia A. Blood. 1990;75: 1664-1672. [PubMed] [Google Scholar]
- 18.Foster PA, Fulcher CA, Houghten RA, de Graaf Mahoney S, Zimmerman TS. Localization of the binding regions of a murine monoclonal anti-factor VIII antibody and a human anti-factor VIII alloantibody, both of which inhibit factor VIII procoagulant activity, to amino acid residues threonine351-serine365 of the factor VIII heavy chain. J Clin Invest. 1988;82: 123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fay PJ, Haidaris PJ, Smudzin TM. Human factor VIIIa subunit structure: reconstitution of factor VIIIa from the isolated A1/A3-C1-C2 dimer and A2 subunit. J Biol Chem. 1991;266: 8957-8962. [PubMed] [Google Scholar]
- 20.Saenko EL, Shima M, Gilbert GE, Scandella D. Slowed release of thrombin-cleaved factor VIII from von Willebrand factor by a monoclonal and a human antibody is a novel mechanism for factor VIII inhibition. J Biol Chem. 1996;271: 27424-27431. [DOI] [PubMed] [Google Scholar]
- 21.Casillas G, Simonetti C, Pavlovsky A. Artificial substrate for the assay of factor V and VIII. Coagulation. 1971;4: 107-111. [Google Scholar]
- 22.Mimms LT, Zampighi G, Nozaki Y, Tanford C, Reynolds JA. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry. 1981;20: 833-840. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins PV, Freas J, Schmidt KM, Zhou Q, Fay PJ. Mutations associated with hemophilia A in the 558-565 loop of the factor VIIIa A2 subunit alter the catalytic activity of the factor Xase complex. Blood. 2002;100: 501-508. [DOI] [PubMed] [Google Scholar]
- 24.Wakabayashi H, Freas J, Zhou Q, Fay PJ. Residues 110-126 in the A1 domain of factor VIII contain a Ca2+ binding site required for cofactor activity. J Biol Chem. 2004;279: 12677-12684. [DOI] [PubMed] [Google Scholar]
- 25.Lollar P, Fay PJ, Fass DN. Factor VIII and factor VIIIa. Methods Enzymol. 1993;222: 128-143. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227: 680-685. [DOI] [PubMed] [Google Scholar]
- 27.Fay PJ, Beattie TL, Regan LM, O'Brien LM, Kaufman RJ. Model for the factor VIIIa-dependent decay of the intrinsic factor Xase: role of subunit dissociation and factor IXa-catalyzed proteolysis. J Biol Chem. 1996;271: 6027-6032. [DOI] [PubMed] [Google Scholar]
- 28.Pittman DD, Kaufman RJ. Proteolytic requirements for thrombin activation of anti-hemophilic factor (factor VIII). Proc Natl Acad Sci U S A. 1988;85: 2429-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shima M, Ware J, Yoshioka A, Fukui H, Fulcher CA. An arginine to cysteine amino acid substitution at a critical thrombin cleavage site in a dysfunctional factor VIII molecule. Blood. 1989;74: 1612-1617. [PubMed] [Google Scholar]
- 30.Hill-Eubanks DC, Lollar P. von Willebrand factor is a cofactor for thrombin-catalyzed cleavage of the factor VIII light chain. J Biol Chem. 1990;265: 17854-17858. [PubMed] [Google Scholar]
- 31.Johnson DJ, Pemberton S, Acquila M, Mori PG, Tuddenham EG, O'Brien DP. Factor VIII S373L: mutation at P1' site confers thrombin cleavage resistance, causing mild haemophilia A. Thromb Haemostasis. 1994;71: 428-433. [PubMed] [Google Scholar]
- 32.Lollar P, Parker ET. Structural basis for the decreased procoagulant activity of human factor VIII compared to the porcine homolog. J Biol Chem. 1991;266: 12481-12486. [PubMed] [Google Scholar]
- 33.Hoyer LW, Trabold NC. The effect of thrombin on human factor VIII: cleavage of the factor VIII procoagulant protein during activation. J Lab Clin Med. 1981;97: 50-64. [PubMed] [Google Scholar]
- 34.Higgins DL, Shafer J. Fibrinogen Petoskey, a dysfibrinogenemia characterized by replacement of Arg-Aα16 by a histidyl residue. J Biol Chem. 1981;256: 12013-12017. [PubMed] [Google Scholar]
- 35.Lane DA, Southan C, Ireland H, Thompson E, Kehl M, Henschen A. Delayed release of an abnormal fibrinopeptide A from fibrinogen Manchester: effect of the A alpha 16 Arg leads to His substitution upon fibrin monomer polymerization and the immunological crossreactivity of the peptide. Br J Haematol. 1983;53: 587-597. [DOI] [PubMed] [Google Scholar]
- 36.Southan C, Lane DA, Bode W, Henschen A. Thrombin-induced fibrinopeptide release from a fibrinogen variant (fibrinogen Sydney I) with an Aα Arg-16 to his substitution. Eur J Biochem. 1985;147: 593-600. [DOI] [PubMed] [Google Scholar]
- 37.Martin PD, Robertson W, Turl D, Huber R, Bode W, Edwards BFP. The structure of residues 7-16 of the Aα-chain of human fibrinogen bound to bovine thrombin at 2.3-A resolution. J Biol Chem. 1992;267: 7911-7920. [PubMed] [Google Scholar]
- 38.Esmon CT, Lollar P. Involvement of thrombin anion-binding exosites 1 and 2 in the activation of factor V and factor VIII. J Biol Chem. 1996;271: 13882-13887. [DOI] [PubMed] [Google Scholar]
- 39.Myles T, Yun TH, Leung LL. Structural requirements for the activation of human factor VIII by thrombin. Blood. 2002;100: 2820-2826. [DOI] [PubMed] [Google Scholar]