Abstract
The peripheral benzodiazepine receptor (pBR) ligand, PK11195, promotes mitochondrial apoptosis and blocks P-glycoprotein (Pgp)-mediated drug efflux to chemosensitize cancer cells at least as well or better than the Pgp modulator, cyclosporine A (CSA). We now show that PK11195 broadly inhibits adenosine triphosphate (ATP)-binding cassette (ABC) transporters in hematologic cancer cell lines and primary leukemia-cell samples, including multidrug resistance protein (MRP), breast cancer resistance protein (BCRP), and/or Pgp. Ectopic expression models confirmed that pBR can directly mediate chemosensitizing by PK11195, presumably via mitochondrial activities, but showed that pBR expression is unnecessary to PK11195-mediated efflux inhibition. PK11195 binds plasma-membrane sites in Pgp-expressing cells, stimulates Pgp-associated adenosine triphosphatase (ATPase) activity, and causes conformational changes in Pgp, suggesting that PK11195 modulates Pgp-mediated efflux by direct transporter interaction(s). PK11195 and CSA bind noncompetitively in Pgp-expressing cells, indicating that PK11195 interacts with Pgp at sites that are distinct from CSA-binding sites. Importantly, PK11195 concentrations that were effective in these in vitro assays can be safely achieved in patients. Because PK11195 promotes chemotherapy-induced apoptosis by a pBR-dependent mitochondrial mechanism and broadly blocks drug efflux by an apparently pBR-independent, ABC transporter-dependent mechanism, PK11195 may be a useful clinical chemosensitizer in cancer patients.
Introduction
Clinical multidrug resistance (MDR) is frequently associated in tumors with expression of adenosine triphosphate (ATP)-binding cassette (ABC) transporter proteins that actively efflux a variety of drugs to reduce intracellular drug concentrations (reviewed by Endicott and Ling1). For example, in acute myeloid leukemia (AML), the ABC transporter, P-glycoprotein (Pgp), is commonly expressed at high levels in elderly patients with AML and in patients with secondary AMLs. Several Pgp substrate drugs, including daunomycin (DNR), mitoxantrone (MIT), and etoposide (VP-16), are used in standard AML therapies, and Pgp expression is independently associated with lower complete remission (CR) rates and shorter remission durations.2,3 Related proteins of the ABC transporter family, including the multidrug resistance protein (MRP) and the breast cancer resistance protein (BCRP), are also expressed in AML and have been associated with MDR and poor clinical outcomes.4,5 Consequently, MDR modulators are being clinically tested in AML. For example, a phase 3 Southwest Oncology Group (SWOG) trial tested infusional cyclosporine A (CSA) with cytarabine plus DNR.6 CSA significantly reduced refractory disease and improved overall and relapse-free survival but only clearly benefited patients with Pgp-expressing leukemias. Other clinical studies of Pgp inhibitors have been less encouraging, particularly because CSA and similarly acting agents often increased regimen-related toxicities, necessitating drug dose reductions (reviewed by Gottesman et al7 and Chauncey8).
CSA not only efficiently inhibits Pgp-mediated efflux but also weakly inhibits MRP-mediated and BCRP-mediated transport,9-11 as does the investigational agent, VX-710 (Biricodar; Vertex Pharmaceuticals, Cambridge, MA), for example.12 More selective and potent Pgp modulators, such as LY335979 (Zosuquidar, Eli Lilly, Indianapolis, IN), are also being developed.13,14 However, MDR AMLs often express more than one ABC transporter and express Bcl-2-related antiapoptotic protein(s), particularly at relapse.15-17 Therefore, clinical chemosensitizers that impact multiple mechanisms of chemoresistance may be desirable if they do not unacceptably increase regimen-related toxicities.
We have recently discovered that peripheral benzodiazepine receptor (pBR) ligands, including PK11195, are such multifunctional chemosensitizers.18 PK11195 promotes mitochondrial apoptosis, as others have also shown (eg, Shimizu et al19 and Hirsch et al20), and blocks Pgp-mediated drug efflux at least as potently as CSA. PK11195 effectively blocked efflux of the Pgp substrate dye, DiOC23, and chemosensitized most AML samples to DNR. PK11195 also increased gemtuzumab ozogamicin (Mylotarg; Wyeth Pharmaceuticals, Philadelphia, PA) cytotoxicity in AML cells expressing Pgp, MRP, and/or antiapoptotic proteins and safely improved gemtuzumab ozogamicin efficacy in a human AML xenograft model.21 Importantly, PK11195 concentrations that were effective in these preclinical studies have been safely achieved in patients.22
We have now addressed the mechanism by which PK11195 blocks efflux. First, we asked how broadly PK11195 blocks efflux and chemosensitizes hematologic cancer cells. Second, we asked whether PK11195's efflux-blocking activities involve pBR interactions. We show herein that nontoxic doses of PK11195 block efflux in Pgp-expressing, MRP-expressing, and BCRP-expressing leukemia and multiple myeloma (MM) cell lines and primary AML cells. PK11195 broadly increases the cytotoxicity of the efflux substrate, MIT, as we previously showed for DNR and gemtuzumab ozogamicin. PK11195 is apparently not an efflux substrate but modulates Pgp-mediated efflux by a pBR-independent mechanism that involves direct interaction with a Pgp site(s) to which CSA does not bind. Distinct from its efflux-blocking activity, PK11195 increases MIT-induced apoptosis by an efflux-independent mechanism that is enhanced by pBR overexpression. Because PK11195 can chemosensitize cancer cells that are abnormally dependent on one or more of these common drug resistance mechanisms, it may be an effective MDR modulator in cancer patients who would not benefit from CSA-based regimens, for example. Our data warrant additional preclinical studies and clinical tests of PK11195 as a chemosensitizer.
Materials and methods
Chemical reagents
All reagents were purchased from Sigma (St Louis, MO) except DiOC63, Rhodamine 123, Hoechst 33342, and BODIPY-prazosin (Molecular Probes, Eugene, OR), MK-571 (Biomol, Plymouth Meeting, PA), GF120918 (kindly provided by GlaxoSmithKline, Research Triangle, NC), and Ko143 (kindly provided by Dr A. H. Schinkel, The Netherlands Cancer Institute, Amsterdam).
Primary-cell samples
Cryopreserved cell samples from patients with AML were obtained from SWOG with all appropriate consent, and CD34-selected cadaveric bone marrow samples were obtained from the Cell Repository of the Fred Hutchinson Cancer Research Center (FHCRC) Large-Scale Cell Processing Core, as approved by the Institutional Review Boards of the FHCRC and other SWOG institutions. Samples were thawed (with 60% to 98% viability) and cultured as previously described.17,18,21 Descriptive data were obtained from the SWOG clinical and cytogenetics databases. Characteristics of the 29 patients are summarized in Tables 1 and 2. All 29 patients had previously untreated AML. Cytogenetic data are based on centrally reviewed studies by SWOG-approved laboratories.
Table 1.
Characteristics of 29 previously untreated AML patients from whom primary-cell samples were obtained for efflux studies
| No. | % | |
|---|---|---|
| Sex | ||
| Female | 16 | 55 |
| Male | 13 | 45 |
| Race | ||
| Asian, non-Hispanic | 1 | 3 |
| Black, non-Hispanic | 3 | 10 |
| White, non-Hispanic | 25 | 86 |
| FAB class | ||
| M0 | 3 | 10 |
| M1 | 4 | 14 |
| M2 | 13 | 45 |
| M4 | 6 | 21 |
| M5 | 3 | 10 |
| AML onset | ||
| De novo | 16 | 55 |
| Secondary | 5 | 17 |
| Unknown | 8 | 27 |
| Evaluable cytogenetics | 24 | 83 |
| Normal karyotype | ||
| Yes | 10 | 42 |
| No | 14 | 58 |
| Specific abnormalities | ||
| 5-/5q- | 1 | 4 |
| inv(16) | 3 | 13 |
| t(8;21) | 1 | 4 |
| 8+ | 2 | 8 |
| Other abnormality | 7 | 29 |
Table 2.
Additional characteristics of AML patients from whom primary-cell samples were obtained for efflux studies
| No. | Median | Range | |
|---|---|---|---|
| Age, y | 29 | 63 | 21-86 |
| WBC count, × 109/L | 29 | 37.4 | 1.9-176 |
| Marrow blasts, % | 27 | 73 | 19-95 |
| Peripheral blasts, % | 29 | 54 | 0-94 |
WBC indicates white blood cell.
Cell culture
Cell lines were obtained from the American Type Culture Collection (Manassas, VA) except RPMI 8226-derived MM and HL60-derived AML cell lines that were provided by Drs W. Dalton and K.N. Bhalla (both of the University of South Florida, Tampa). Jurkat and KG1a derivatives were generated as described in “Ectopic protein expression.” Stable BCRP-expressing ML1 and HL60 sublines and GFP control lines were previously produced by lentiviral transduction.23 Cells were cultured in RPMI 1640 supplemented with 5% heat-inactivated bovine calf serum (BCS; Hyclone, Logan UT), except for HL60 cells that were cultured in Iscove modified Dulbecco medium (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% cosmic calf serum (Hyclone).
Efflux assays
MIT is a fluorescent substrate for Pgp, MRP, and BCRP.24 Cells were incubated in MIT (20 μM, 60 minutes, 37°C) in the presence or absence of CSA (1 μM), MK-571 (25 μM), GF120918 (1 μM), or Ko143 (200 nM) as Pgp, MRP, or BCRP modulators.1-8,18,21,25-29 BCRP function was also measured as Hoechst 33342 (2 μM) efflux reduced by Ko143,30 and Pgp function was measured as DiOC23 (40 nM) efflux inhibited by CSA2 or as Hoechst 33342, Rhodamine 123 (5 μg/mL), or BODIPY-prazosin (100 nM) efflux31,32 after exposures without inhibitor (30 minutes, 37°C) and release with or without inhibitor (30 minutes, 37°C). Data were collected on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) and analyzed with Multiplus software (Phoenix Flow Systems, San Diego, CA). All assays were repeated at least 3 times and expressed as mean ratios (mean fluorescence intensity [MFI] in the presence of inhibitor/MFI in the absence of inhibitor) ± standard errors of the mean (SEMs).
Chemosensitivity assays
Parallel cell cultures were incubated with drugs at concentrations described in the text. Cytotoxicity was determined by flow cytometry after DiOC63 plus propidium iodide (PI) staining was performed or by scintillation counting after 24 hours of 3H-thymidine (3H-Tdr; Amersham Biosciences, Piscataway, NJ) incorporation, as previously described.18,21 All assays were repeated at least 3 times in duplicate and expressed as means relative to untreated viabilities ± SEM.
pBR RNA assays
RNAs were prepared using TRIzol (Invitrogen), and oligo(dT)-primed cDNA was synthesized using Superscript II and the manufacturer's recommended protocols (Invitrogen). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) polymerase chain reactions (PCRs) were performed with standard techniques using 45 cycles (95°C denaturation, 70°C annealing, 72°C extension), AmpliTaq Polymerase (Roche Molecular Systems, Pleasanton, CA), and intron-spanning primers (forward 5′GTCTTCACCACCATGGAGAAG3′ and reverse 5′GCTTCACCACCTTCT-TGATGTCATC3′) to confirm mRNA integrity. pBR PCRs were performed with forward (5′CTCCCCTGAACAGCAGCTGC3′) and reverse (5′GCCAAGGCCCTGACAGACTAGC3′) primers, and PCR products were separated on 2% agarose gels and extracted for reamplification and dideoxy sequencing to confirm pBR identity.33
3H-PK11195 binding assays
Assays were performed in the linear range, as we previously reported,18 and repeated at least 3 times in triplicate. Relative values are presented (PK11195 counts per minute bound in the presence - counts per minute in the absence of 1000-fold molar excess competitors) as means plus or minus SEM.
Ectopic protein expression
We obtained full-length pBR sequence by PCR using cDNA from KG1a cells with forward (5′GGGGACAAGTTTGTACAAAAAAGCAGGCTTCGAAGGAGATAGAACTAGAACCATGGCCCCGCCCTGGGTGCCCGCCATGGGCTTCACG3′) and reverse (5′GGGGACCACTTTGTACAAGAAAGCTGGGTTCACTCTGGCAGCCGCCGTCCCCCATGCCA3′) primers. We confirmed wild-type pBR sequence,33 produced lentivirus in 293T cells in the FHCRC Vector Production and Tracking Core, and transduced recipient cells.34 Pgp and control bicistronic expression vectors35 were kindly provided by Dr S. Kane (City of Hope National Medical Center, Duarte, CA) and introduced by electroporation using the Nucleofector I apparatus and the manufacturer's recommended protocols (Amaxa Biosystems; Gaithersburg, MD). After 24 hours, and before functional analyses, Pgp-transfected cells were cultured in 2 ng/mL colchicine for 7 days.35
Flow cytometry assays of MDR protein expression
Immunolabeling and flow cytometry were used to confirm the expression of specific drug transporters, as we previously described.21,23 Specific protein expression levels are presented (MFI of cells stained with isotype control antibody - MFI of cells stained with specific antibody).
Microsome and plasma-membrane (PM) preparation and characterization
Cells were washed in Ca/Mg-free phosphate-buffered saline (PBS), resuspended in homogenization buffer (HB; 0.25 M sucrose, 1 mM EGTA [ethylene glycol tetraacetic acid], 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]/NaOH pH 7.4, 0.5% bovine serum albumin [BSA]), incubated on ice (10 minutes), and homogenized. Homogenates were centrifuged at low speed (10 minutes, 600g, 4°C) and at higher speed (12 minutes, 12 000g) to recover “microsome” pellets that were resuspended (10 mM Tris [tris(hydroxymethyl)aminomethane] HCl pH 7.5, 1 mM EGTA, 0.25 M sucrose). Microsome supernatants were centrifuged twice (12 minutes, 12 000g; 1 hour, 100 000g) to recover “PMs” that were resuspended in HB. PM purity was assessed in Western blot assays using antibodies recognizing mitochondria-specific proteins, voltage-dependent anion channel (VDAC; Calbiochem, San Diego, CA), and cytochrome c oxidase III (COXIII; Molecular Probes). C219 antibody recognizes both human MDR-1 (Pgp) and MDR-3 gene products (CHEMICON International, Temecula, CA), and we used it to recognize Pgp.
ATPase assays
Pgp-transfected insect-cell microsomes were obtained from Gentest/BD Biosciences (Woburn, MA). Adenosine triphosphatase (ATPase) assays were performed with phosphate standard curves used as interassay controls,36 and 6 mM ouabain was used to block Na/K ATPase activity. Negative controls contained 170 μM sodium orthovanadate to inhibit all ATPase activities, and 20 μM verapamil (VPL) was used as a positive treatment control. ATPase activity was measured as the liberation of inorganic phosphate, detected by absorbance at 800 nM, and quantitated (nanomoles of phosphate relative to the standard curve). For every test treatment, t = 0, and negative control values were subtracted, and relative ATPase activities were calculated (vanadate-sensitive activity in treated divided by untreated samples). All assays were repeated at least 3 times in triplicate, and means plus or minus SEM are shown.
UIC2 antibody-binding assay
Conformation-specific UIC2 and conformation-insensitive 15D3 phycoerythrin (PE)-labeled anti-Pgp monoclonal antibodies (Beckman Coulter, Miami, FL; Becton Dickinson) were used to analyze PK11195 effects on Pgp.37,38 Live cells were incubated with or without test reagents in PBS (20 minutes, 37°C) before antibody incubation (30 minutes), washing, and resuspension in ice-cold PBS. Flow cytometry data collection and analysis were as described in “Efflux assays.” Data are presented as mean ratios (MFI in the presence of a test reagent divided by MFI in the absence of that reagent) plus or minus SEM.
Statistics
Due to the relatively small sample numbers that were analyzed in the current studies, nonparametric analyses with 2-tailed Mann-Whitney tests, Wilcoxon matched pairs tests, and Spearman rank correlations were calculated using Instat3 software (GraphPad, San Diego, CA). P values of less than .05 were considered statistically significant.
Results
PK11195 can block efflux by Pgp, MRP, and BCRP drug transporters
We previously showed that PK11195 blocks Pgp-mediated efflux, often more effectively than the established Pgp modulator, CSA, and sensitizes Pgp-expressing and MRP-expressing AML and MM cells to efflux substrate drugs, DNR and gemtuzumab ozogamicin.18,21 Like DNR, MIT is used in standard cancer therapies, including AML regimens. Both DNR and MIT are ABC transporter substrates, but MIT can be efficiently effluxed by MRP-expressing and BCRP-expressing as well as Pgp-expressing cells. Because Pgp, MRP, and BCRP expression have all been associated with clinical chemoresistance in AML and other cancers,1-5 and MIT fluorescence can be easily measured in standard flow cytometers,29 we used MIT to further characterize settings in which PK11195 blocks drug efflux and chemosensitizes cancer cells.
For these analyses, we used AML and MM cell lines as ABC transporter-expressing cancer models, as in our published studies,18,21,23,39 and used nontoxic doses of efflux modulators to model safe clinical applications. Per our previous reports, 75 μM PK11195 was not measurably toxic in Pgp-expressing 8226-DOX40 MM cells (DOX40), while CSA was toxic at doses above 1 μM (Figure 1A); 25 μM MK-571 (MRP modulator25) and 1 μM GF120918 (BCRP modulator26) were also nontoxic (data not shown). Therefore, we used these doses in MIT efflux assays in which ratios greater than 1 represent efflux inhibition; 1 μM CSA dosing blocked MIT efflux in DOX40 cells (Figure 1A), consistent with our previous findings using DiOC23 as the Pgp substrate dye.18 Higher, toxic CSA doses were not more effective, but 75 μM PK11195 was more effective than 1 μM CSA (P < .05). PK11195 also significantly blocked Pgp-mediated MIT efflux more effectively than CSA in 8226-DOX6 (DOX6) MM (P < .01), KG1a (P < .05), and HL60-VCR (VCR) AML (P < .05) cells (Figure 1B).
Figure 1.

Nontoxic PK11195 doses can block efflux by Pgp, MRP, and BCRP drug transporters. (A) To identify nontoxic Pgp-modulator doses, DOX40 cells were incubated across micromolar dose ranges with PK11195 (PK; 5 to 150 μM) and the established Pgp-modulator, CSA (0.2 to 5 μM). After 48-hour treatments, flow cytometry was performed in which “live” cell fractions contained cells with high DiOC63 staining (full mitochondrial membrane potential) and low PI fluorescence (high integrity), as illustrated in the top left panel. After other 48-hour treatments, 3H-Tdr was added for an additional 24 hours, and “live” cell fractions were measured as 3H-Tdr counts per minute relative to counts per minute in untreated wells. Treatment-specific live-cell fractions were calculated by subtracting untreated from treated fractions, and summary data from at least 3 assays are shown in the top right panel as means ± SEM. CSA (up to 1 μM) and PK11195 (up to 75 μM) did not significantly reduce live-cell fractions. Effects of CSA and PK11195 dose ranges were also measured in flow cytometry efflux assays after 1-hour MIT (20 μM) exposures. Representative histograms are shown in the bottom left panel. Efflux-blocking ratios were calculated as the MFI in the presence of test agent/MFI in the absence of test agent: “1” represents no efflux blocking, as denoted with a bold, horizontal line in the summary data from at least 3 assays that are shown in the bottom right panel. Also, 75 μM PK11195 blocked MIT efflux significantly more than any CSA dose in DOX40 cells. (B) Nontoxic doses of CSA (1 μM), MK-571 (MK; 25 μM), and GF120918 (GF; 1 μM) were used as Pgp, MRP, and BCRP modulators, respectively, along with dose ranges of PK11195, in MIT efflux assays of 7 additional AML and MM cell lines. Seventy-five μM PK11195 blocked MIT efflux significantly more than CSA in all cell lines tested and blocked MIT efflux significantly more than GF in BCRP-transduced HL60 cells. In all cases, efflux rations were calculated as described in panel A, and summary data from at least 3 assays are shown as means plus or minus SEM.
As expected for established efflux inhibitors, MK-571 tended to increase MIT retention in MRP-expressing HL60-AR (AR) AML cells (P = .05), and GF120918 significantly increased MIT retention in BCRP-transduced HL60 and ML1 AML cells (P < .05 for both HL60-BCRP and ML1-BCRP; Figure 1B). Both MK-571 and GF120918 significantly blocked MIT efflux in 8226-MR20 (MR20) MM cells that express both MRP and BCRP (P < .05 and P < .005, respectively). CSA did not significantly block MIT efflux in MRP-expressing AR or MR20 cells, but PK11195 significantly blocked efflux in MR20 cells (P < .005) and tended to increase MIT retention in AR cells (P = .06). PK11195 also blocked or tended to block efflux in HL60-BCRP and ML1-BCRP AML cells (P < .05 and P = .08, respectively) and blocked MIT efflux in HL60-BCRP cells significantly more than GF120918 (P < .05). Neither PK11195 nor any of the established efflux modulators had any effect on MIT retention in efflux-deficient RPMI 8226 (8226), ML1, or HL60 cells (data not shown).
Thus, nontoxic doses of PK11195 blocked efflux in cancer cells expressing one or more clinically relevant drug efflux proteins. PK11195 blocked efflux more effectively than nontoxic CSA doses in Pgp-expressing, MRP-expressing, and BCRP-expressing cells; blocked efflux as well as the established MRP modulator, MK-571, in MRP-expressing cells; and inhibited as well or better than the established BCRP modulator, GF120918, in BCRP-expressing cells.
PK11195 efficiently sensitizes cancer cells to MIT, a clinically relevant drug that is an efflux substrate for Pgp, MRP, and BCRP
Using flow cytometry assays to measure apoptosis and cell death, we next asked whether PK11195 chemosensitizes hematologic cancer cells in which PK11195 blocks efflux. As expected, Pgp, MRP, and BCRP overexpression were all associated with relative MIT resistance in these assays (Figure 2). For example, Pgp-overexpressing DOX40 cells showed larger live-cell fractions than parental 8226 cells after 24-hour MIT treatments (eg, P < .05 at 5 μM MIT). PK11195 alone did not significantly reduce live-cell fractions (Figure 1A), but PK11195 cotreatments significantly increased MIT cytotoxicity in 24-hour cotreatments of DOX40 cells (eg, P < .001 at 5 μM MIT).
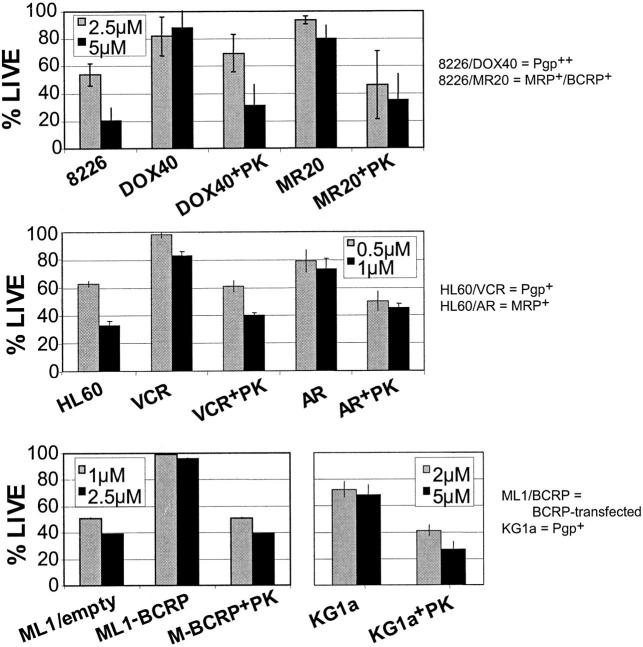
Figure 2.
PK11195 can MIT-sensitize MDR cancer cells expressing Pgp, MRP, and/or BCRP. To determine the effects of nontoxic PK11195 (PK) doses on MIT cytotoxicity, DiOC63/PI flow cytometry assays were performed, as described for Figure 1. Cells were incubated with MIT doses that killed substantial fractions of isogenic parental, efflux-deficient cells, including RPMI 8226 (8226), HL60, and ML1 cells, as detailed in individual panel legends. Larger fractions of DOX40 (Pgp-expressing), MR20 (MR20; MRP- and BCRP-expressing), VCR (Pgp-expressing), AR (MRP-expressing), and ML1-BCRP (BCRP-transduced ML1) cells survived 24-hour MIT treatments relative to isogenic parental cells. KG1a (Pgp-expressing) cells were also relatively MIT resistant; 75 μM PK11195 cotreatments substantially decreased surviving fractions in ABC transporter-expressing cells. Summary data from at least 3 assays are shown as means plus or minus SEM.
Other Pgp-expressing cells, including KG1a and VCR cells (Figure 2) and DOX6 cells (data not shown), were MIT-sensitized by PK11195, as were MRP-expressing AR cells, ML1-BCRP cells, and MR20 cells that express both MRP and BCRP. All of these efflux-proficient cells also express one or more antiapoptotic proteins of the Bcl-2 family, as do their efflux-deficient parental cells (Banker et al,18 Walter et al,21 and data not shown). Thus, nontoxic doses of PK11195 were chemosensitized hematologic cancer cells expressing relatively high levels of one or more clinically relevant drug efflux proteins even when antiapoptotic proteins were also expressed. Moreover, parental 8226, ML1, and HL60 cells that do not overexpress any ABC transporter were also MIT-sensitized by PK11195 cotreatments but to a smaller degree than were efflux-proficient derivative cells (data not shown), consistent with PK11195's established ability to overcome the protective effects of Bcl-2 family proteins.19-21
PK11195 blocks MIT efflux in primary AML cells in association with relatively high levels of specific PK11195 binding
To expand upon our cell line data, we performed efflux assays with primary AML cell samples. We previously reported18 that PK11195 blocks efflux of the Pgp-selective dye, DiOC23. Upon further analysis of these data, we found that efflux blockade by PK11195 and CSA was correlated (R = 0.78, P = .005), although PK11195 increased DiOC23 retention in 3 samples in which CSA had no effect (Figure 3A). When we performed MIT efflux assays in other primary AML cell samples, we found that PK11195 and/or CSA measurably increased MIT retention in 12 of 13 samples. CSA and PK11195 effects were again significantly correlated (R = 0.78, P = .001). Eight samples were of sufficient size to also test MK-571 and Ko143 as specific MRP and BCRP modulators, respectively.18,21,25,27 We previously showed that these modulators identify MRP-expressing and BCRP-expressing AML samples, in concordance with immunostaining by ABC transporter-specific monoclonal antibodies.22,24 In this study, 4 AML samples were determined to be BCRP-expressing samples, because Ko143 measurably blocked MIT efflux in these (Figure 3B, nos. 158736, 155976, 163086, 157389); one sample also functionally expressed Pgp (Figure 3B, no. 158736), because MIT retention was increased substantially more by PK11195 and CSA than by Ko143, and one sample (Figure 3B, no. 155976) also expressed MRP, because MK-571 was effective. Four other samples apparently only expressed Pgp, because only PK11195 and CSA blocked MIT efflux in these samples. PK11195 was substantially more effective than any other efflux-blocking agent in 3 of 8 samples (38%), including BCRP-expressing and MRP-expressing cells.
Figure 3.
PK11195 blocks MIT efflux in primary AML cells. To further assess the clinical relevance of our cell-line data, we performed efflux assays in primary AML cell samples. (A) Efflux blockade in Pgp-selective DiOC23 and ABC transporter-general MIT efflux assays was measured as increased dye retention. Efflux-blocking effects are presented as ratios, as in Figure 1. PK11195 and CSA effects were correlated in both assays, as noted in the figure. (B) In additional MIT efflux assays of 8 efflux-competent AML samples, MK-571 and Ko143 were used as specific MRP and BCRP modulators along with PK11195 and CSA. PK11195 again substantially blocked MIT efflux in all of these, and Ko143 measurably blocked MIT efflux in 4 samples, suggesting that these functionally expressed BCRP. MK-571 measurably blocked MIT efflux one, apparently MRP-expressing sample. PK11195 blocked efflux substantially more effectively than CSA in 3 samples. PK11195, CSA, and Ko143 also significantly increased MIT retention in repeated assays of normal CD34+CD38- hematopoietic progenitor cells (P = .01, P < .005, and P < .05, respectively), and PK11195 and CSA significantly increased MIT retention in more mature CD34+CD38+ normal cells (P = .05 in each case). Three immunolabeled NBM samples were analyzed, and summary data are shown as means ± SEM. KG1a cells were assayed along with primary cells in every assay; summary data from at least 5 assays are shown as means ± SEM. (C) 3H-PK11195 binding assays were performed in additional aliquots of 11 primary AML cell samples. Specific 3H-PK11195 binding was determined by competition with 1000-fold excess unlabeled PK11195, and competed counts are shown with means plus or minus SEMs for 7 samples (Pgp+ AMLs) in which both CSA and PK11195 increased DiOC23 retention and for 4 samples without efflux capacity (Pgp- AMLs). 3H-PK11195 binding was significantly higher in Pgp-positive AMLs than in Pgp-negative AMLs (P < .05), and Pgp-expressing DOX6 and DOX40 MM cells also bound very high levels of 3H-PK11195, but Pgp-expressing VCR and KG1a cells bound relatively low levels of PK11195 (means ± SEM are shown from at least 3 assays for each cell line). 3H-PK11195 binding was competed by excess of another pBR ligand, FGIN-1-27 (FGIN), but excess unlabeled CSA did not compete for 3H-PK11195 binding in any assay of Pgp-expressing cells.
Efflux is not specific to malignant hematopoietic cells. For example, CD34+CD38- subpopulations of normal bone marrow (NBM) samples contain efflux-competent hematopoietic progenitor cells, including Pgp-expressing and BCRP-expressing cells, while CD34+CD38+ subpopulations contain more mature cells with less efflux competence (eg, Raaijmakers et al,40 Smeets et al,41 and Chute et al42). Consistent with these findings, PK11195, CSA, and Ko143 all significantly increased MIT retention in normal CD34+CD38- cells (P = .01, P < .005, and P < .05, respectively; Figure 3B), while only PK11195 and CSA tended to increase MIT retention in normal CD34+CD38+ cells (P = .05 in each case). Pgp-expressing KG1a AML cells were used as interassay controls in all primary-cell assays. As before, MIT retention was reproducibly increased by PK11195 more than by CSA, and neither MK-571 nor Ko143 affected efflux in KG1a cells. Thus, as in our cell line analyses, we found that PK11195 can effectively block efflux in primary cells expressing one or more ABC drug transporters.
Because Pgp-mediated efflux and efflux modulation have been well-studied in tumor models, including AML and MM, and Pgp modulators, including CSA, are being tested as clinical chemosensitizers,1-8 we focused on Pgp-expressing cells to further characterize the mechanism(s) by which PK11195 blocks efflux. CSA and many other efflux modulators bind transporters directly.31,32,43-51 First, we asked whether PK11195 binds Pgp, first by comparing PK11195 binding in 9 primary AML samples for which additional aliquots were available. CSA and PK11195 had increased dye retention in 5 of these samples (Pgp-positive AMLs), whereas 4 samples showed no efflux capacity (Pgp-negative AMLs). Specific 3H-PK11195 binding was determined by competition with 1000-fold excess unlabeled PK11195 (Figure 3C) and was significantly higher in Pgp-positive AMLs than in Pgp-negative AMLs (P < .05), suggesting that PK11195 might bind Pgp directly. However, Pgp-expressing VCR and KG1a AML cells bound relatively low levels of PK11195, and Pgp-expressing DOX6 and DOX40 MM Pgp-expressing VCR and KG1a AML cells bound very high levels of 3H-PK11195 that were similar to PK11195 binding levels in Pgp-negative 8226 parental cells (data not shown). In no case did excess unlabeled CSA significantly compete for 3H-PK11195 binding in Pgp-expressing cells. Even 15 000-fold excess unlabeled CSA did not reduce 3H-PK11195 binding (data not shown), suggesting that, whichever site(s) PK11195 binds, PK11195 and CSA do not bind the same site(s).
pBR expression is neither sufficient nor necessary for efflux inhibition by PK11195
We next asked whether PK11195 effects on efflux are pBR dependent, using ectopic protein expression models. First, we used Jurkat lymphoid leukemia cells as lentivirus transduction recipients because these cells are pBR deficient,52 as we confirmed in reverse transcriptase (RT)-PCR and ligand-binding assays (Figure 4A), and efflux deficient (Figure 4B and data not shown). Like DOX40 cells, stably GFP/pBR-transduced Jurkat cells (J-pBR) specifically bound high levels of 3H-PK11195 that were efficiently competed by excess unlabeled PK11195 (Figure 4A). Specific PK11195 binding could also be measured in mitochondrial fractions produced from J-pBR and DOX40 cells, as expected for mitochondrial pBR binding.
Figure 4.
pBR expression is not sufficient to produce efflux inhibition by PK11195. (A) Jurkat lymphocytic leukemia cells were confirmed as pBR RNA-deficient in pBR-specific RT-PCR assays in which AML and MM cell lines served as positive controls (inset) and in ligand-binding assays in which DOX40 MM cells served as positive controls. Specific 3H-PK11195 binding was calculated by subtracting 3H-PK11195 counts per minute in the presence of 1000-fold excess unlabeled PK11195 from counts per minute in the absence of competitor. Lentiviral pBR transduction and flow sorting for GFP expression were used to produce J-pBR cells that stably expressed high levels of pBR as measured in 3H-PK11195 binding assays of whole-cell and mitochondrial lysates and as compared with positive control cells (DOX40) and negative controls (Jurkat, J-GFP, J-Neo). (B) Like parental Jurkat cells (white bars) and control J-GFP cells (gray bars), J-pBR cells (black bars) showed no MIT efflux that could be blocked by PK11195 (PK) or by known efflux inhibitors CSA, MK-571 (MK), or Ko143 (KO). Seventy-five micromolar PK11195 sensitized J-pBR cells to MIT significantly more than control J-GFP cells were affected, demonstrating functional pBR expression in J-pBR cells. (C) Lentiviral transduction was used to create K-pBR cells from Pgp-expressing, efflux-competent KG1a parental cells, as demonstrated in whole-cell PK11195 binding assays. K-pBR cells showed no more MIT efflux than control K-GFP cells when PK and CSA were used as efflux inhibitors. Cytotoxicity and efflux data and 3H-PK11195 binding data are presented as described for Figures 1 and 2, with summary data from 3 to 5 assays shown as means plus or minus SEM.
J-pBR cells were as sensitive to MIT cytotoxicity as GFP-transduced control cells (J-GFP) but were significantly more sensitized to MIT by PK11195 cotreatments (eg, P < .05 at 0.04 μM MIT; Figure 4B), supporting pBR-specific chemosensitization by PK11195. Because parental Jurkat cells and all Jurkat-derived sublines expressed relatively high levels of Bcl-2 (data not shown), these data are also consistent with previous demonstrations of PK11195's chemosensitizing activity in cells expressing antiapoptotic proteins.18,21 As with parental Jurkat cells, we were unable to measure MIT efflux in isogenic J-GFP and J-pBR cells, using CSA, MK-571, and Ko143 as Pgp, MRP, and BCRP efflux modulators, respectively. PK11195 also had no effect. Thus, pBR expression alone was not sufficient to convey measurable efflux capacity to leukemia cells.
To address the possibility that cells must coexpress pBR and Pgp for pBR to affect efflux, we next used KG1a AML cells as transduction recipients. Like parental KG1a cells (Figures 1B and 3C), control GFP-transduced KG1a cells (K-GFP) bound relatively low levels of PK11195, expressed Pgp, and were efflux competent (Figure 4C). KG1a cells stably expressing the pBR/GFP expression vector (K-pBR) showed substantially increased pBR expression, but neither PK11195 nor CSA had greater efflux-blocking effects in K-pBR cells than in K-GFP cells. Thus, pBR apparently does not directly mediate efflux.
To ask whether pBR expression is required for PK11195's efflux-blocking activity, we produced Jurkat-cell derivatives that functionally overexpress Pgp (J-MDR) or BCRP (J-BCRP), as demonstrated in specific antibody-binding and efflux assays (Figure 5A-B). In addition to MIT, we used DiOC23 and Hoechst 33342 as established Pgp and BCRP efflux substrates, respectively, and used CSA and Ko143 as established Pgp and BCRP modulators. Like parental cells and control J-GFP cells, J-MDR and J-BCRP cells did not show significant levels of specific 3H-PK11195 binding. However, PK11195 significantly increased DiOC23 and MIT accumulation in J-MDR cells (P < .01 each), as did CSA (P < .005, P < .01). PK11195 significantly increased MIT accumulation in J-BCRP cells (P < .005), as did CSA (P < .05), blocking MIT efflux significantly more than CSA or Ko143 (both P < .05). PK11195 tended to increase Hoechst accumulation in J-BCRP cells (P = .06), while CSA and Ko143 had no significant effect. Dye retention was not affected by any compound in parental Jurkat or control J-GFP cells. These data substantiate PK11195's Pgp-inhibiting and BCRP-inhibiting activities and again show that nontoxic doses of PK11195 can block efflux more effectively than nontoxic doses of CSA. Together, data from ectopic protein expression models strongly suggest that pBR expression is not required for PK11195's efflux-blocking activities.
Figure 5.
pBR expression is not necessary for efflux inhibition by PK11195 in MDR cells. (A) pBR-deficient Jurkat-cell derivatives were produced that overexpress Pgp (J-MDR) as demonstrated with MRK16 anti-Pgp antibody staining. Representative flow cytometry data are shown as histograms, and summary data from at least 3 assays are shown as mean arbitrary fluorescence units (a.f.u.). Both CSA ( ) and PK11195 (▪) blocked DiOC23 and MIT efflux in J-MDR cells but not in parental Jurkat cells or control J-Neo cells, demonstrating specific efflux capacity in J-MDR cells and demonstrating PK11195's ability to block Pgp-mediated efflux in pBR-deficient cells. (B) Derivative Jurkat cells that overexpress BCRP (J-BCRP) were produced and characterized with BXP-34 anti-BCRP antibody staining. CSA (
) and PK11195 (▪) blocked DiOC23 and MIT efflux in J-MDR cells but not in parental Jurkat cells or control J-Neo cells, demonstrating specific efflux capacity in J-MDR cells and demonstrating PK11195's ability to block Pgp-mediated efflux in pBR-deficient cells. (B) Derivative Jurkat cells that overexpress BCRP (J-BCRP) were produced and characterized with BXP-34 anti-BCRP antibody staining. CSA ( ) and Ko143 (KO-143) (□) both increased MIT dye retention in J-BCRP cells, and PK11195 (▪) significantly blocked both MIT and Hoechst efflux in J-BCRP cells. Neither Hoechst 33342 nor MIT efflux was measured in control transporter-deficient cells, demonstrating specific efflux capacity in J-BCRP cells and demonstrating PK11195's ability to block BCRP-mediated efflux in pBR-deficient cells. Efflux data from at least 3 assays are presented, as described for Figure 1.
) and Ko143 (KO-143) (□) both increased MIT dye retention in J-BCRP cells, and PK11195 (▪) significantly blocked both MIT and Hoechst efflux in J-BCRP cells. Neither Hoechst 33342 nor MIT efflux was measured in control transporter-deficient cells, demonstrating specific efflux capacity in J-BCRP cells and demonstrating PK11195's ability to block BCRP-mediated efflux in pBR-deficient cells. Efflux data from at least 3 assays are presented, as described for Figure 1.
PK11195 binds Pgp at binding sites distinct from those bound by CSA
Because we had shown that Pgp-expressing MM and AML cell lines and primary AML cell samples also express pBR (Figures 3C and 4C), we wondered whether PK11195 binding to more abundant and/or higher affinity mitochondrial pBR reduced our ability to measure Pgp-specific 3H-PK11195 binding and/or CSA competition. Functional Pgp is primarily localized to PM sites. Therefore, we prepared PMs from DOX40 cells to further characterize PK11195 binding in Pgp-expressing cells and documented that PM fractions contained Pgp but were depleted of mitochondrial inner and outer mitochondrial membrane markers (Figure 6A). DOX40 PM fractions showed specific 3H-CSA binding, consistent with documented Pgp binding by CSA,31,32,43-51 and specific 3H-PK11195 binding was also reproducibly measured in DOX40 PMs. Specific PK11195 binding that was measured in 8226 PM assays (data not shown) suggests that pBR can be PM-localized, consistent with other reports.53,54 In any case, CSA and PK11195 did not cross-compete for binding in PMs from DOX40 or 8226 cells, supporting the idea that CSA and PK11195 bind different sites.
Figure 6.
PK11195 apparently binds Pgp at PM sites distinct from CSA-binding sites. (A) Mitochondrial (MITO) and PM fractions were prepared from DOX40 MM cells by high-speed centrifugation. Fraction purities were confirmed in Western blot assays using antibodies recognizing mitochondrial-specific proteins (VDAC, COXIII), and the C219 antibody was used to recognize Pgp in PM fractions. 3H-PK11195 and 3H-CSA binding was measured in PM fractions and competed by PK11195 and CSA, respectively, but PK11195 and CSA did not cross-compete in these assays, further demonstrating that PK11195 binding is CSA independent. (B) PK11195 (▪) and CSA ( ) increased Rhodamine (RHO), Hoechst 33342 (Hoechst), and BODIPY-prazosin (PRAZ) retention in DOX40 cells, suggesting that both modulate multiple Pgp substrate-binding sites. PK11195 blocked RHO efflux significantly more than CSA, suggesting that PK11195 binds particular Pgp-binding sites more efficiently than CSA. Efflux data from at least 3 assays are presented, as in Figure 1.
) increased Rhodamine (RHO), Hoechst 33342 (Hoechst), and BODIPY-prazosin (PRAZ) retention in DOX40 cells, suggesting that both modulate multiple Pgp substrate-binding sites. PK11195 blocked RHO efflux significantly more than CSA, suggesting that PK11195 binds particular Pgp-binding sites more efficiently than CSA. Efflux data from at least 3 assays are presented, as in Figure 1.
Others have reported that Pgp contains multiple substrate-binding sites, including CSA-binding sites and other sites that do not bind CSA. For example, Shapiro and his colleagues reported that Hoechst 33342, prazosin, and Rhodamine 123 (RHO) bind different substrate-binding sites on Pgp.31,32 Because RHO is a fluorescent molecule that can be used as a substrate in flow cytometry assays of Pgp-mediated efflux, as are Hoechst 33342 and BODIPY-prazosin (PRAZ),30 we performed efflux assays with these dyes. Both PK11195 and CSA increased the retention of RHO, Hoechst 33342, and Bodipy-PRAZ in Pgp-expressing DOX40 cells (Figure 6B) but not in Pgp-negative 8226 cells (data not shown). RHO efflux, like MIT efflux (Figures 1 and 3), was blocked significantly more by PK11195 than by CSA (P < .005). These data again show that PK11195 can block Pgp-mediated efflux more potently than CSA and suggest that both PK11195 and CSA interact with multiple substrate-binding sites on Pgp. Moreover, PK11195 blocked RHO efflux significantly more in DOX40 cells than PK11195 blocked Hoechst or PRAZ efflux (P < .05 for both), supporting the idea that PK11195 can bind more effectively to particular Pgp sites.
PK11195 activates Pgp-associated ATPase activity and alters Pgp conformation
Although PK11195 broadly and efficiently inhibited Pgp-mediated efflux and bound PM sites in Pgp-expressing cells, our data did not support that idea that PK11195 is a Pgp efflux substrate. As noted, specific PK11195 binding levels in Pgp-expressing DOX6 and DOX40 cells were similar to levels in Pgp-negative 8226 parental cells, and DOX40 cells did not consistently bind more PK11195 when held at 4°C, rather than 37°C, to prevent energy-dependent efflux (data not shown). In addition, PK11195 binding was not reproducibly competed by known Pgp substrates, including CSA (Figures 3 and 6), RHO, Hoechst 33342, and PRAZ (data not shown).
Therefore, our data suggested that PK11195 might be a nonsubstrate Pgp modulator. Pgp inhibitors include “substrates” that bind Pgp at transport sites and translocate across the lipid bilayer in an ATP-dependent process and “modulators” that bind Pgp at regulatory sites to affect substrate recognition and/or ATP hydrolysis.49 Both transported and nontransported modulators can induce Pgp-associated ATPase activity.50 Therefore, we assayed PK11195 effects on Pgp-associated ATPase activities to ask whether PK11195 directly impacts Pgp function. First, we used commercially available Pgp-containing microsomes produced from Pgp-transfected insect cells. Using verapamil (VPL) as a positive control treatment,49 we found that nontoxic doses of CSA (1 μM) and PK11195 (75 μM) substantially increased Pgp-associated ATPase activity in this model (Figure 7A). Next, we produced microsomal (mitochondria plus PM) fractions from parental 8226 cells and Pgp-expressing DOX40 cells and found that PK11195 and VPL substantially stimulated ATPase activity in DOX40 cells, but not in 8226 cells, and that CSA had no effect (Figure 7B). Finally, we prepared PM fractions from DOX40 cells and tested a wider dose range of CSA and PK11195. Once again, VPL substantially increased ATPase activity (Figure 7C). Nontoxic PK11195 doses (5 to 75 μM) also stimulated ATPase activity, but a toxic dose of CSA (5 μM) was needed to substantially increase ATPase activity. One micromolar CSA had no consistent effect. Others have shown that CSA can have no effect, stimulate, or even inhibit Pgp-associated ATPase activity, depending on the model used for analyses.47-50,54 Our data showing differential CSA effects in different models are consistent with these reports and suggest that PK11195 can directly modulate Pgp-associated ATPase activity.
Figure 7.
PK11195 can activate Pgp-associated ATPase activity and alter Pgp conformation. To show that a nontoxic dose (75 μM) of PK11195 (PK) can specifically stimulate Pgp-associated ATPase activity, we performed assays in which treatment-specific (relative) ATPase activities were calculated (vanadate-sensitive ATPase activities in treated/untreated samples). Assays were performed with microsomal membranes (containing PMs and mitochondria) commercially prepared from Pgp-expressing SF9 insect cells (A), microsomes from Pgp-negative 8226 (gray bars) and Pgp-expressing DOX40 cells (black bars) (B), or PMs from DOX40 cells (C). Like VPL, PK specifically induced ATPase activity in all Pgp-positive models, with dose dependence in DOX40 PMs. Nontoxic doses of CSA (0.1, 1 μM) were ineffective in DOX40 models, but 1 μM CSA induced ATPase activity in Pgp-positive SF9 microsomes and 5 μM CSA induced activity in DOX40 PMs. Data from at least 3 assays are expressed as means plus or minus SEMs. (D) Specific effects of PK11195 on Pgp protein conformation were measured in 3 flow cytometry assays using the Pgp conformation-specific antibody, UIC2 (black bars), and the conformation-insensitive antibody, 15D3 (gray bars). PK11195 increased UIC2 immunoreactivity, but not 15D3 immunoreactivity, in live, Pgp-expressing DOX6, DOX40, KG1a, and VCR cells but not in Pgp-negative 8226 or HL60 parental cells.
Mechetner and colleagues previously showed that the UIC2 monoclonal anti-Pgp antibody preferentially binds substrate-activated Pgp,37 and others have used UIC2 to demonstrate that nonsubstrate Pgp modulators can also induce Pgp conformational changes.46 Consistent with these reports, VPL and CSA increased UIC2 immunoreactivity in Pgp-expressing cells (data not shown). PK11195 also increased UIC2 binding in KG1a and DOX40 cells (both P < .05) and tended to increase UIC2 immunostaining in VCR and DOX6 cells (P = .07, P = .09, respectively) but did not change UIC2 immunostaining in Pgp-negative HL60 or 8226 cells (Figure 7D). Immunostaining with a conformation-insensitive anti-Pgp antibody (15D3) was unchanged by PK11195, VPL, or CSA treatments (Figure 7D and data not shown). Thus, efflux-blocking doses of PK11195 did not reduce Pgp expression or cell-surface localization during periods in which efflux effects were measured but did apparently modulate Pgp conformation, consistent with PK11195's ATPase-activating activity.
Discussion
We previously showed that PK11195 is relatively nontoxic as a single agent when used at doses that can be safely achieved in patients but that it consistently chemosensitizes AML and MM cells, including cells expressing Pgp, MRP, and/or antiapoptotic proteins.18,21 We now confirm these findings in leukemia and MM cell lines and primary AML cells, and show that nontoxic PK11195 doses can also block BCRP-mediated efflux and reverse chemoresistance in cancer cells overexpressing any of these efflux proteins. As in cancer cells that are responsible for refractory disease and relapse, chemotherapy resistance is associated with the expression of various ABC transporters in normal hematopoietic stem cells (eg, Raaijmakers et al,40 Smeets et al,41 and Chute et al42). Consistent with these findings, we found that PK11195, CSA, and Ko143 all block efflux in normal CD34+CD38- progenitor cells, as in malignant cells. Nonetheless, PK11195 had no significant chemosensitizing effect in NBM cell samples in our previous study18 and does not exacerbate regimen-related toxicities in xenograft studies, including our AML model21 and a cholangiocarcinoma model.55 Consistent with the idea that MDR modulators can be safely used in cancer patients, CSA did not prolong neutropenia in patients with AML when added to a DNR plus cytarabine regimen, despite improving outcomes for patients with Pgp-expressing AMLs.6
Like PK11195, CSA is a broad-spectrum efflux inhibitor,9-11,43 but PK11195 is frequently more effective than CSA in efflux-competent hematologic cancer cells. PK11195 is also frequently more effective than the MRP-selective modulator, MK-571, or the BCRP modulators, GF120918 and Ko143.25-29 It remains unclear whether high-affinity, highly selective Pgp modulators might be clinically more useful than broadly effective MDR modulators, and agents of both types are being tested in chemotherapy regimens. For example, LY335979 is a potent and specific inhibitor of Pgp, with no measured effect on MRP or BCRP,13,14 whereas VX-710 potently modulates Pgp, MRP, and BCRP.12 However, neither of these agents has been reported to have any activity against antiapoptotic mechanisms of chemoresistance, and cancer cells commonly overexpress antiapoptotic proteins as well as ABC transporters. Therefore, PK11195 still offers advantages as a chemoresistance modulator in its ability to both broadly block drug efflux and promote apoptosis, if it is sufficiently bioavailable and does not produce unacceptable toxicities.
Addressing these points, we note that high micromolar doses are needed for full PK11195 chemosensitizing efficacy in vitro, but these doses can be safely achieved in patients in whom PK11195's plasma stability is 3 to 12 hours, with oral administration yielding longer half-lives.22 As noted, PK11195 can chemosensitize in vivo, suggesting that its bioavailability is adequate for safe and effective therapeutic applications. Nonetheless, PK11195's clinical utility might be reduced by other pharmacokinetic limitations. For example, CSA interacts with drug-metabolizing cytochrome P450s (CYPs) and can thereby dramatically increase plasma levels of CYP substrate drugs, producing unacceptable toxicities that necessitate drug dosereduction (reviewed by Wacher et al56). On the other hand, intestinal CYP metabolism of CSA can reduce MDR modulation. Two studies suggested that PK11195 might induce CYP3A4,57,58 but our preliminary data suggest that PK11195 instead inhibits CYP3A4 activity (data not shown). Clearly, additional studies are needed to determine whether PK11195 alters the metabolism of any coadministered therapeutic and/or whether PK11195 reduces normal stem-cell viability. However, if true, these limitations could be practically addressed in regimen design. For example, continuous infusion rather than bolus drug administration reduces CSA-related toxicities including mucositis (eg, List et al6 and Chauncey8). In addition, drug-drug interactions and normal cell toxicities are likely to be less problematic when PK11195 is combined with targeted therapies. We have shown that PK11195 effectively sensitizes AML cells to the antibody-directed toxin gemtuzumab ozogamicin,21 and others have shown that PK11195 sensitizes MM cells to the proteasome inhibitor, bortezomib.59
Importantly, we have discovered that PK11195 promotes apoptosis and modulates efflux by distinct mechanisms. Previously, we found that PK11195 cotreatments increased the cytotoxicity of a noneffluxed drug, cytarabine, in 3 of 12 primary AML cell samples.18 Seven of 12 AML samples were sensitized to the efflux substrate drug, DNR. PK11195 can bind mitochondrial sites in both ABC transporter-expressing cells and transporter-deficient cells, as expected for PK11195 binding to mitochondrial pBR. And increased pBR expression enhances PK11195's promotion of mitochondrial apoptosis in efflux-deficient cells, as others have also shown.20,52 In whole-cell assays, specific PK11195 binding can vary more than 20-fold in primary AML cell samples and is frequently higher in AMLs than in normal bone marrow cells (Walter et al21 and data not shown). Cells from ovary, liver, colon, breast, and brain tumors also express dramatically higher levels of pBR relative to normal cells of the same tissue origin (eg, Galiegue et al60). Because we have shown that increased pBR expression can increase the degree to which PK11195 chemosensitizes transporter-deficient cancer cells, PK11195 binding data may have prognostic value for PK11195 clinical applications aimed at promoting mitochondrial-mediated apoptosis.
Having confirmed PK11195's promotion of MIT-induced apoptosis by a pBR-dependent, mitochondrial mechanism, we were interested to find that AML and MM cell lines that express Bcl-2 family proteins are sensitized to MIT by PK11195 cotreatments but that parental, efflux-deficient cells are chemosensitized to a smaller degree than efflux-competent derivatives. These data suggested to us that PK11195's apoptosis-promoting and efflux-blocking chemosensitizing effects are distinct. In fact, we have now determined that PK11195 blocks Pgp efflux by a pBR-independent, PM-localized mechanism. PM-localized pBRs have been found in liver cells and lymphocytes,53,54 and we have now shown that PK11195 binds PM sites in Pgp-positive DOX40 MM cells. However, pBR expression is not required for PK11195's efflux-inhibiting activity in MDR-transduced Jurkat leukemia models. PK11195 can alter Pgp conformation and directly stimulate Pgp-associated ATPase activity in PM fractions, but PK11195 binding levels are not temperature dependent. Therefore, we hypothesize that PK11195 is a nontransported efflux modulator that directly binds Pgp (and presumably other ABC transporters). A small survey of primary AML samples showed higher PK11195 binding in Pgp-expressing samples, but we have been unable to unequivocally document Pgp binding by PK11195, perhaps due to very low-affinity binding. Consistent with this idea is the fact that micromolar concentrations of PK11195 are needed to block efflux. In addition, higher affinity and/or more abundant pBR binding may obscure PK11195 binding to Pgp in pBR-expressing cells. Future studies will address these points.
To our knowledge, PK11195 is the first MDR modulator shown to both broadly modulate ABC transporter-related and inhibit mitochondrial pore-related chemoresistance mechanisms. Because drug resistance limits cures in many cancers and is commonly associated in cancer cells with overexpression of one or more drug transporters and one or more antiapoptotic proteins, PK11195 might be a uniquely effective adjunct to standard chemotherapies, useful in settings in which CSA and other agents that incapacitate only one MDR mechanism might be ineffective. Our data warrant additional preclinical studies and clinical tests of PK11195 as a chemosensitizer.
Acknowledgments
We recognize the critical help of the FHCRC Large-Scale Cell Processing Core (Dr Shelley Heimfeld, principal investigator) and the Vector Production and Tracking Core (Dr Hans-Peter Kiem, principal investigator), which are supported by NIH DK 56465, a Core Center of Excellence in Molecular Hematology grant (Dr Beverly Torok-Storb, principal investigator), and Dr Kenneth Kopecky in deriving patient data from the SWOG database.
Prepublished online as Blood First Edition Paper, July 28, 2005; DOI 10.1182/blood-2005-02-0711.
Supported in part by a grant from the National Cancer Institute (NCI; CA 89491, D.E.B., principal investigator). R.B.W. is the recipient of an American Society of Hematology “Clinical/Translational Research Fellow” Scholar award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Endicott JA, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58: 137-171. [DOI] [PubMed] [Google Scholar]
- 2.Leith CP, Kopecky KJ, Godwin J, et al Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89: 3323-3329. [PubMed] [Google Scholar]
- 3.Guerci A, Merlin JL, Missoum N, et al. Predictive value for treatment outcome in acute myeloid leukemia of cellular daunorubicin accumulation and P-glycoprotein expression simultaneously determined by flow cytomery. Blood. 1995;85: 2147-2153. [PubMed] [Google Scholar]
- 4.Ross DD. Novel mechanisms of drug resistance in leukemia. Leukemia. 2000;14: 467-473. [DOI] [PubMed] [Google Scholar]
- 5.Laupeze B, Amiot L, Drenou B, et al. High multidrug resistance protein activity in acute myeloid leukaemias is associated with poor response to chemotherapy and reduced patient survival. Br J Haematol. 2002;116: 834-838. [DOI] [PubMed] [Google Scholar]
- 6.List AF, Kopecky KJ, Willman CL, et al. Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood. 2001;98: 3212-3220. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2: 48-58. [DOI] [PubMed] [Google Scholar]
- 8.Chauncey TR. Drug resistance mechanisms in acute leukemia. Curr Opin Oncol. 2001;13: 21-26. [DOI] [PubMed] [Google Scholar]
- 9.Kamisako T, Leier I, Cui Y, et al. Transport of monoglucuronosyl and bisglucuronosyl bilirubin by recombinant human and rat multidrug resistance protein 2. Hepatology. 1999;30: 485-490. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZS, Kawabe T, Ono M, et al. Effect of multidrug resistance-reversing agents on transporting activity of human canalicular multispecific organic anion transporter. Mol Pharmacol. 1999;56: 1219-1228. [DOI] [PubMed] [Google Scholar]
- 11.Qadir M, O'Loughlin KL, Fricke SM, et al. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11: 2320-2326. [DOI] [PubMed] [Google Scholar]
- 12.Minderman H, O'Loughlin KL, Pendyala L, Baer MR. VX-710 (biricodar) increases drug retention and enhances chemosensitivity in resistant cells overexpressing P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Clin Cancer Res. 2004;10: 1826-1834. [DOI] [PubMed] [Google Scholar]
- 13.Sandler A, Gordon M, De Alwis DP, et al. A Phase I trial of a potent P-glycoprotein inhibitor, zosuquidar trihydrochloride (LY335979), administered intravenously in combination with doxorubicin in patients with advanced malignancy. Clin Cancer Res. 2004;10: 3265-3272. [DOI] [PubMed] [Google Scholar]
- 14.Gerrard G, Payne E, Baker RJ, et al. Clinical effects and P-glycoprotein inhibition in patients with acute myeloid leukemia treated with zosuquidar trihydrochloride, daunorubicin and cytarabine. Haematologica. 2004;89: 782-790. [PubMed] [Google Scholar]
- 15.Maung ZT, MacLean FR, Reid MM, et al. The relationship between bcl-2 expression and response to chemotherapy in acute leukaemia. Br J Haematol. 1994;88: 105-109. [DOI] [PubMed] [Google Scholar]
- 16.Lauria F, Raspadori D, Rondelli D, et al. High bcl-2 expression in acute myeloid leukemia cells correlates with CD34 positivity and complete remission rate. Leukemia. 1997;11: 2075-2078. [DOI] [PubMed] [Google Scholar]
- 17.Banker DE, Groudine M, Norwood T, Appelbaum FR. Measurement of spontaneous and therapeutic agent-induced apoptosis with Bcl-2 protein expression in acute myeloid leukemia. Blood. 1997;89: 243-255. [PubMed] [Google Scholar]
- 18.Banker DE, Cooper JJ, Fennell DA, Willman C, Appelbaum FR, Cotter FE. PK11195, a peripheral benzodiazepine receptor ligand, chemosensitizes acute myeloid leukemia cells to relevant therapeutic agents, by mitochondrial and non-mitochondrial mechanisms. Leuk Res. 2002;26: 91-106. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399: 483-487. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch T, Decaudin D, Susin SA, et al. PK11195, a ligand of the mitochondrial benzodiazepine receptor, facilitates the induction of apoptosis and reverses Bcl-2-mediated cytoprotection. Exp Cell Res. 1998;241: 426-434. [DOI] [PubMed] [Google Scholar]
- 21.Walter RB, Raden BW, Cronk M, Appelbaum FR, Bernstein ID, Banker DE. The peripheral benzodiazepine receptor ligand PK11195 overcomes different resistance mechanisms to sensitize acute myeloid leukemia cells to gemtuzumab ozogamicin. Blood. 2004;103: 4276-4284. [DOI] [PubMed] [Google Scholar]
- 22.Ferry A, Jaillon P, Lecocq B, Lecocq V, Jozefczak C. Pharmacokinetics and effects on exercise heart rate of PK 11195 (52028 RP), an antagonist of peripheral benzodiazepine receptors, in healthy volunteers. Fundam Clin Pharmacol. 1989;3: 383-392. [DOI] [PubMed] [Google Scholar]
- 23.Walter RB, Raden BW, Thompson J, et al. Breast cancer resistance protein (BCRP/ABCG2) does not confer resistance to gemtuzumab ozogamicin and calicheamicin-gamma1 in acute myeloid leukemia cells. Leukemia. 2004;18: 1914-1917. [DOI] [PubMed] [Google Scholar]
- 24.van der Graaf WT, de Vries EG, Timmer-Bosscha H, et al. Effects of amiodarone, cyclosporin A, and PSC 833 on the cytotoxicity of mitoxantrone, doxorubicin, and vincristine in non-P-glycoprotein human small cell lung cancer cell lines. Cancer Res. 1994;54: 5368-5373. [PubMed] [Google Scholar]
- 25.Gekeler V, Ise W, Sanders KH, Ulrich WR, Beck J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem Biophys Res Commun. 1995;208: 345-352. [DOI] [PubMed] [Google Scholar]
- 26.de Bruin M, Miyake K, Litman T, Robey R, Bates SE. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999;146: 117-126. [DOI] [PubMed] [Google Scholar]
- 27.Allen JD, van Loevezijn A, Lakhai JM, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1: 417-425. [PubMed] [Google Scholar]
- 28.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285: L97-L104. [DOI] [PubMed] [Google Scholar]
- 29.Robey RW, Honjo Y, van de Laar A, et al. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2). Biochim Biophys Acta. 2001;1512: 171-182. [DOI] [PubMed] [Google Scholar]
- 30.Lahmy S, Lautier D, Canitrot Y, Laurent G, Salmon JM. Staining with Hoechst 33342 and rhodamine 123: an attempt to detect multidrug resistant phenotype cells in leukemia. Leuk Res. 1993;17: 1021-1029. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro AB, Fox K, Lam P, Ling V. Stimulation of P-glycoprotein-mediated drug transport by prazosin and progesterone. Evidence for a third drug-binding site. Eur J Biochem. 1999;259: 841-850. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro AB, Ling V. Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur J Biochem. 1997;250: 130-137. [DOI] [PubMed] [Google Scholar]
- 33.Hardwick M, Fertikh D, Culty M, Li H, Vidic B, Papadopoulos V. Peripheral-type benzodiazepine receptor (PBR) in human breast cancer: correlation of breast cancer cell aggressive phenotype with PBR expression, nuclear localization, and PBR-mediated cell proliferation and nuclear transport of cholesterol. Cancer Res. 1999;59: 831-842. [PubMed] [Google Scholar]
- 34.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15: 871-875. [DOI] [PubMed] [Google Scholar]
- 35.Kane SE, Matsumoto L, Metz MZ, et al. MDR1 bicistronic vectors: analysis of selection stringency, amplified gene expression, and vector stability in cell lines. Biochem Pharmacol. 2001;62: 693-704. [DOI] [PubMed] [Google Scholar]
- 36.Drueckes P, Schinzel R, Palm D. Photometric microtiter assay of inorganic phosphate in the presence of acid-labile organic phosphates. Anal Biochem. 1995;230: 173-177 [DOI] [PubMed] [Google Scholar]
- 37.Park SW, Lomri N, Simeoni LA, Fruehauf JP, Mechetner E. Analysis of P-glycoprotein-mediated membrane transport in human peripheral blood lymphocytes using the UIC2 shift assay. Cytometry. 2003;53A: 67-78. [DOI] [PubMed] [Google Scholar]
- 38.Chan HSL, Gaddad G, Zheng L, Bradley G, Dalton WS, Ling V. Sensitive immunofluorescence detection of the expression of P-glycoprotein in malignant cells. Cytometry. 1999;29: 65-75. [DOI] [PubMed] [Google Scholar]
- 39.Walter RB, Raden BW, Hong TC, Flowers DA, Bernstein ID, Linenberger ML. Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood. 2003;102: 1466-1473. [DOI] [PubMed] [Google Scholar]
- 40.Raaijmakers HG, Van Den Bosch G, Boezeman J, De Witte T, Raymakers RA. Single-cell image analysis to assess ABC-transporter-mediated efflux in highly purified hematopoietic progenitors. Cytometry. 2002;49: 135-142. [DOI] [PubMed] [Google Scholar]
- 41.Smeets M, Raymakers R, Vierwinden G, et al. A low but functionally significant MDR1 expression protects primitive haemopoietic progenitor cells from anthracycline toxicity. Br J Haematol. 1997;96: 346-355. [DOI] [PubMed] [Google Scholar]
- 42.Chute JP, Muramoto GG, Fung J, Oxford C. Soluble factors elaborated by human brain endothelial cells induce the concomitant expansion of purified human BM CD34+CD38- cells and SCID-repopulating cells. Blood. 2005;105: 576-583. [DOI] [PubMed] [Google Scholar]
- 43.Maki N, Hafkemeyer P, Dey S. Inhibition of transport by preventing substrate translocation and dissociation. J Biol Chem. 2003;278: 18132-18139. [DOI] [PubMed] [Google Scholar]
- 44.Litman T, Skovsgaard T, Stein WD. Pumping of drugs by P-glycoprotein: a two-step process? J Pharmacol Exp Ther. 2003;307: 846-853. [DOI] [PubMed] [Google Scholar]
- 45.Loor F, Tiberghien F, Wenandy T, Didier A, Traber R. Cyclosporins: structure-activity relationships for the inhibition of the human MDR1 P-glycoprotein ABC transporter. J Med Chem. 2002;45: 4598-4612. [DOI] [PubMed] [Google Scholar]
- 46.Nagy H, Goda K, Fenyvesi F, et al. Distinct groups of multidrug resistance modulating agents are distinguished by competition of P-glycoprotein-specific antibodies. Biochem Biophys Res Commun. 2004;315: 942-949. [DOI] [PubMed] [Google Scholar]
- 47.Sharom FJ, DiDiodato G, Yu X, Ashbourne KJ. Interaction of the P-glycoprotein multidrug transporter with peptides and ionophores. J Biol Chem. 1995;270: 10334-10341. [DOI] [PubMed] [Google Scholar]
- 48.Ayesh S, Shao YM, Stein WD. Co-operative, competitive and non-competitive interactions between modulators of P-glycoprotein. Biochim Biophys Acta. 1996;1316: 8-18. [DOI] [PubMed] [Google Scholar]
- 49.DiDiodato G, Sharom FJ. Interaction of combinations of drugs, chemosensitizers, and peptides with the P-glycoprotein multidrug transporter. Biochem Pharmacol. 1997;53: 1789-1797. [DOI] [PubMed] [Google Scholar]
- 50.Dey S, Ramachandra M, Pastan I, Gottesman MM, Ambudkar SV. Evidence for two nonidentical drug-interaction sites in the human P-glycoprotein. Proc Natl Acad Sci U S A. 1997;94: 10594-10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scala S, Akhmed N, Rao US, et al. P-glycoprotein substrates and antagonists cluster into two distinct groups. Mol Pharmacol. 1997;51: 1024-1033. [DOI] [PubMed] [Google Scholar]
- 52.Carayon P, Portier M, Dussossoy D, et al. Involvement of peripheral benzodiazepine receptors in the protection of hematopoietic cells against oxygen radical damage. Blood. 1996;87: 3170-3178. [PubMed] [Google Scholar]
- 53.Woods MJ, Zisterer DM, Williams DC. Two cellular and subcellular locations for the peripheral-type benzodiazepine receptor in rat liver. Biochem Pharmacol. 1996;51: 1283-1292. [DOI] [PubMed] [Google Scholar]
- 54.Berkovich A, Ferrarese C, Cavaletti G, et al. Topology of two DBI receptors in human lymphocytes. Life Sci. 1993;52: 1265-1277. [PubMed] [Google Scholar]
- 55.Okaro AC, Fennell DA, Corbo M, Davidson BR, Cotter FE. PK11195, a mitochondrial benzodiazepine receptor antagonist, reduces apoptosis threshold in Bcl-X(L) and Mcl-1 expressing human cholangiocarcinoma cells. Gut. 2002;51: 556-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wacher VJ, Wu C-Y, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13: 129-134. [DOI] [PubMed] [Google Scholar]
- 57.Freche JP, Decolin D, Siest JP, Batt AM, Panis-Rouzier R, Siest G. Variation in the urinary excretion of 6-beta-hydroxycortisol in humans after administration of the new isoquinoline derivative, PK-11195 (52028RP). Therapie. 1989;44: 327-330. [PubMed] [Google Scholar]
- 58.Yamada H, Matsuki Y, Yamaguchi T, Oguri K. Effect of a ligand selective for peripheral benzodiazepine receptors on the expression of rat hepatic P-450 cytochromes: assessment of the effect in vivo and in a hepatocyte culture system. Drug Metab Dispos. 1999;27: 1242-1247. [PubMed] [Google Scholar]
- 59.Chauhan D, Li G, Podar K, et al. Targeting mitochondria to overcome conventional and bortezomib/proteasome inhibitor PS-341 resistance in multiple myeloma (MM) cells. Blood. 2004;104: 2458-2466. [DOI] [PubMed] [Google Scholar]
- 60.Galiegue S, Casellas P, Kramar A, Tinel N, Simony-Lafontaine J. Immunohistochemical assessment of the peripheral benzodiazepine receptor in breast cancer and its relationship with survival. Clin Cancer Res. 2004;10: 2058-2064. [DOI] [PubMed] [Google Scholar]