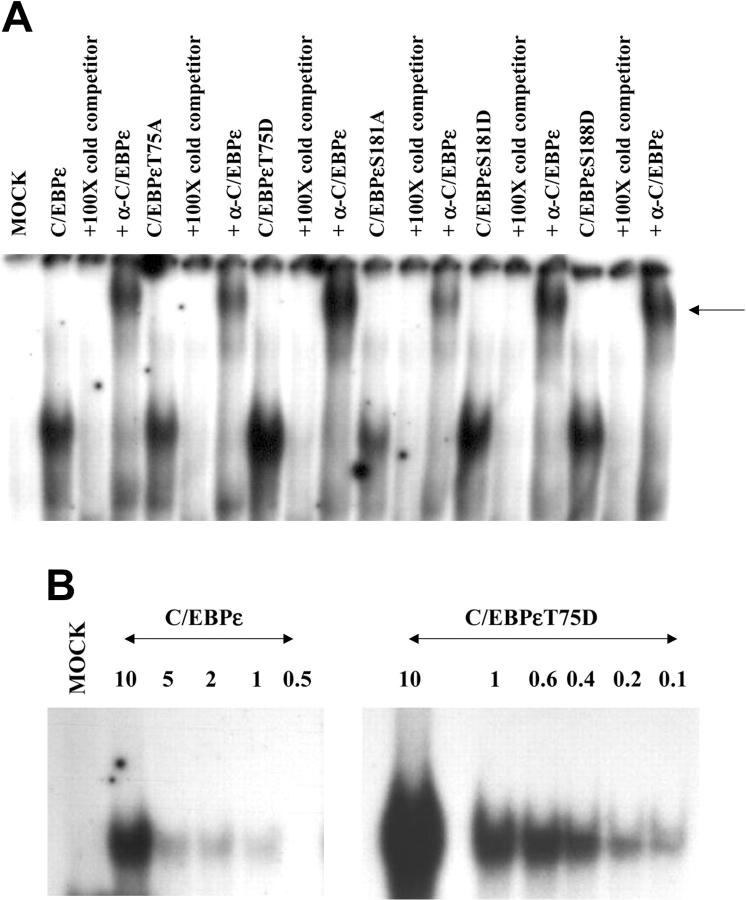
Figure 5.
DNA binding affinity of C/EBPε is altered by phosphorylation. Gel shift analysis was carried out on nuclear extracts derived from Jurkat cells transfected with wild-type and mutant C/EBPε expression plasmids and an oligonucleotide containing the C/EBP site from the mim promoter. (A) Nuclear extracts from transfected Jurkat cells give a slow migrating band with this oligonucleotide. This retarded band can be competed by 100-fold excess of cold double-stranded (ds) oligonucleotide and supershifted by C/EBPε-specific antisera. (B) EMSA was carried out using the same amount of nuclear extract with decreasing concentrations of hot oligonucleotides. The gel shifts were analyzed on a Phosphoimager (Molecular Dynamics, Piscataway, NJ) to obtain the radioactive counts of bound and free probe. Scatchard analysis determined the affinity of these C/EBPε proteins for the C/EBP site in the mim promoter as follows: C/EBPε 7.69 nM; C/EBPεT75D 0.48 nM.