Abstract
The anionic phospholipid, phosphatidyl-l-serine (PS), is sequestered in the inner layer of the plasma membrane in normal cells. Upon injury, activation, and apoptosis, PS becomes exposed on the surfaces of cells and sheds microparticles, which are procoagulant. Coagulation is initiated by formation of a tissue factor/factor VIIa complex on PS-exposed membranes and propagated through the assembly of intrinsic tenase (factor VIIIa/factor IXa), prothrombinase (factor Va/factor Xa), and factor XIa complexes on PS-exposed activated platelets. We constructed a novel series of recombinant anticoagulant fusion proteins by linking annexin V (ANV), a PS-binding protein, to the Kunitz-type protease inhibitor (KPI) domain of tick anticoagulant protein, an aprotinin mutant (6L15), amyloid β-protein precursor, or tissue factor pathway inhibitor. The resulting ANV-KPI fusion proteins were 6- to 86-fold more active than recombinant tissue factor pathway inhibitor and tick anticoagulant protein in an in vitro tissue factor–initiated clotting assay. The in vivo antithrombotic activities of the most active constructs were 3- to 10-fold higher than that of ANV in a mouse arterial thrombosis model. ANV-KPI fusion proteins represent a new class of anticoagulants that specifically target the anionic membrane-associated coagulation enzyme complexes present at sites of thrombogenesis and are potentially useful as antithrombotic agents.
Introduction
Following injury to the endothelium and vessel wall, a series of molecular and cellular events occur leading to the formation of a blood clot. Platelets adhere within seconds to the subendothelial matrix, become activated, and form the primary hemostatic plug. Concomitantly, plasma factor VII/VIIa binds to the vessel wall tissue factor (TF) and initiates a sequence of coagulation reactions leading to the formation of fibrin. The fibrin network stabilizes the aggregated platelets, resulting in the formation of a fibrin-platelet thrombus. A cell-based model of blood coagulation has been proposed based on in vitro studies in whole blood and reconstituted systems.1-3 Upon exposure to blood, the membrane-anchored TF binds circulating factor VII/VIIa. The resulting TF/VIIa complex initiates coagulation by activating factor IX and factor X to generate small amounts of IXa and Xa. Following activation of factor V to Va by Xa on the membrane surfaces of TF-bearing cells/microparticles, a prothrombinase (Va/Xa) complex is assembled, leading to the formation of a small amount of thrombin that amplifies the generation of Va, VIIIa, and XIa on the membrane surfaces of activated platelets. The activated platelets provide the requisite anionic membrane surfaces for the assembly and catalysis of intrinsic tenase (VIIIa/IXa), prothrombinase (Va/Xa), and XIa complexes, leading to explosive thrombin generation and consolidation of the fibrin-platelet plug. Furie and colleagues have recently developed an intravital confocal and widefield microscopy system that allowed real-time in vivo imaging of platelet deposition, TF accumulation, and fibrin generation during thrombus formation following vascular injury in mice.4,5 Their results were consistent with the above cell-based model but further showed that TF and fibrin were initially localized to the vessel wall–thrombus interface and subsequently propagated through the thrombus by accumulation of blood-borne TF-bearing microparticles derived from leukocytes.4,5
The plasma membrane phospholipids (PLs) of mammalian cells are normally asymmetrically distributed: The amino-PLs, phosphatidyl-l-serine (PS), and phosphatidylethanolamine (PE) are segregated to the internal leaflet, whereas the choline-PLs, phosphatidylcholine, and sphingomyelin reside on the outer leaflet.6,7 Extensive plasma membrane remodeling occurs in response to injury, activation, and apoptotic stimuli, resulting in the loss of membrane asymmetry, egress of PS/PE to the cell surface, and shedding of PS/PE-exposed microparticles.6-8 PS/PE exposure greatly enhances the catalytic efficiency of the membrane-anchored TF/VIIa 9 and promotes the assembly and catalysis of the intrinsic tenase, prothrombinase, and XIa complexes on the membrane surfaces of activated platelets and microparticles.10,11 Thus, PS/PE-exposed membranes are the key thrombogenic stimuli that drive the initiation and propagation of the clotting cascade and can be considered as the hallmark of thrombogenic sites. Unlike solutionphase coagulation enzymes, the membrane-associated cofactor/enzyme complexes appear to be protected from circulating inactivators. For example, prothrombinase is protected from inactivation by tissue factor pathway inhibitor (TFPI)12,13 and antithrombin.14,15 Va on the activated platelet membrane surface is resistant to proteolytic inactivation by activated protein C 16; intrinsic tenase is not appreciably inhibited by plasma protease inhibitors during clotting17; and platelet-bound XIa is resistant to platelet-derived protease nexin II/amyloid precursor protein that inhibits solutionphase XIa with high affinity.18
Annexin V (ANV) is a Ca++-dependent PL-binding protein with strong affinities for PS, PE, and other anionic PLs.19 It possesses anticoagulant activity in in vitro plasma-based coagulation assays20,21 and antithrombotic activity in in vivo animal models.22-25 ANV's mechanism of action is different from that of other known anticoagulants in that it targets the anionic PLs rather than the protein factors of the clotting machinery. ANV forms 2-dimensional arrays on anionic membrane surfaces, thus making the anionic PLs unavailable for assembly of coagulation enzyme complexes.19 In this study, we have constructed several 2-domain fusion proteins consisting of an ANV moiety and a Kunitz protease inhibitor (KPI) domain that binds to various coagulation factors with high affinity and specificity. We hypothesize that such ANV-KPI fusion proteins may possess the ability to target themselves to PS/PE-rich thrombogenic sites and potently suppress thrombogenesis by facilitating the inhibition of the membrane-associated coagulation complexes.
Materials and methods
Reagents
Dade Innovin was from Baxter Diagnostics (Deerfield, IL). APTT-SP was from Instrumentation Laboratory (Lexington, MA). Bovine factor Xa was supplied by American Diagnostica (Greenwich, CT). Trypsin and p-nitrophenyl p′-guanidinobenzoate HCl were from Sigma (St Louis, MO). S2444 and S2765 were obtained from DiaPharma (West Chester, OH). Mammalian C127 cell-derived recombinant TFPI was prepared as previously described.26 Escherichia coli–derived recombinant TFPI1-160 (residues 1 to 160 containing Kunitz domains 1 and 2) and yeast-derived recombinant tick anticoagulant protein (TAP) were gifts from Drs George Broze and Dana Abendschein, respectively (Washington University, St Louis, MO).
Cloning of cDNA for annexin V
ANV cDNA, lacking a stop codon for the purpose of creating fusion molecules, was generated from human placental mRNA by polymerase chain reaction (PCR) using ANV reverse primer 1 and forward primer 2 (Table 1). ANV cDNA mutation of Cys316 to Ala was created by PCR using oligonucleotide-directed mutagenesis. Control recombinant ANV with its natural stop codon was expressed without mutation of Cys316. For all other ANV-KPI fusions, ANV cDNA with the Cys316Ala mutation was used.
Table 1.
PCR primers
| ANV reverse primer 1 | 5′-ATCAAGCTTATGCATGTCATCTTCTCCACAGAG-3′ |
| ANV forward primer 2 | 5′-GATCGGATCCAGTCTGGTCCTGCTTCACCTT-3′ |
| ANV-NdeI | 5′-GGAATTCCATATGGCACAGGTTCTCAGAGG-3′ |
| ANV-NsiI | 5′-CCAATGCATGTCATCTTCTCCAGC-3′ |
| 6L15-NsiI | 5′-CCAATGCATCCGGACTTCTGCCTG-3′ |
| KKTFPI-NsiI | 5′-CCAATGCATTCATTTTGTGCATTC-3′ |
| 6L15-SalI | 5′-ACGCGTCGACTTAAGCACCACCGCAAG-3′ |
| KKTFPI-SalI | 5′-ACGCGTCGACTTAGGTTCCATAATTATCC-3′ |
| TAP-NdeI | 5′-GGAATTCCATATGGCTTACAACCGTCTGTG-3′ |
| TAP-BamHI | 5′-CGGGATCCGATGCAAGCGTTGAAGCAG-3′ |
| ANV-BamHI | 5′-CGGGATCCGCACAGGTTCTCAGAGGC-3′ |
| ANV-SalI | 5′-ACGCGTCGACTTAGTCATCTTCTCCAGCG-3′ |
| ANV-XhoI | 5′-CCGCTCGAGAAAAGAGCACAGGTTCTCAGAG-3′ |
| KAPP-NotI | 5′-ATAAGAATGCGGCCGCTTAAATAGCAGAACCAC-3′ |
| ANV-EcoRV | 5′-CGCGATATCATCTTCTCCAGCGAG-3′ |
| 5′-KAPP | 5′-GAGGTTTGTTCTGAGCAAGC-3′ |
Restriction enzyme cleavage sites are underlined.
Construction of 6L15, TAP, and KAPP genes
The synthetic genes encoding 6L15, TAP, and Kunitz domain of amyloid β-protein precursor (KAPP) genes were each constructed by annealing and ligation of 3 pairs of overlapping oligonucleotides. The translated amino acid sequences of these synthetic genes match the published sequence of 6L15 27 and those of TAP and KAPP listed in the GenBank database.
Construction of plasmids and expression of ANV-6L15, ANV-KKTFPI, TAP-ANV, ANV, and 6L15 in E coli
Restriction sites were introduced into the coding sequences of ANV, 6L15, KKTFPI (residues 22 to 161 of TFPI), and TAP using the PCR primers listed in Table 1. The gene fragment of ANV generated with primers ANV-NdeI and ANV-NsiI was digested with NdeI and NsiI and linked to the NsiI- and SalI-digested 6L15 or KKTFPI PCR fragment. The ANV-6L15 or ANV-KKTFPI fusion gene was ligated into the expression vector pET20b(+) (Novagen, Madison, WI) linearized with NdeI and SalI. The gene fragment of ANV generated with primers ANV-BamHI and ANV-SalI was digested with BamHI and SalI and linked to the digested TAP PCR fragment. The TAP-ANV fusion gene was digested with NdeI and BamHI and linked to the BamHI- and SalI-digested ANV gene fragment. The fusion gene was inserted into the expression vector pET20b(+) linearized with NdeI and SalI. The PCR-generated gene fragments of ANV and 6L15 were inserted into pET20b(+) using the same strategy.
Recombinant plasmids were transfected into E coli DH5α for cloning and selection. E coli BL21(DE3)pLysS (Novagen) was used for expression of recombinant proteins. A total of 10 mL of an overnight culture was inoculated into 1 L medium and maintained at 37°C until the optical density at 600 nm (OD600) of the culture reached 0.5. The culture was induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG) (Promega, Madison, WI) to a final concentration of 1 mM and continuously shaking at 37°C for 4 hours. The cells were harvested by centrifugation, and the cell pellet was frozen at -80°C for further use.
Construction of plasmid and expression of ANV-KAPP in Pichia pastoris
To generate the ANV-KAPP fusion gene, we designed primer ANV-EcoRV, which creates an EcoRV site at the 3′-end of the ANV gene fragment without changing the last encoded amino acid (Asp). The 5′-KAPP primer is a forward sequence of the KAPP gene from the initiation codon Glu (GAG). The KAPP gene fragment amplified by primers 5′-KAPP and KAPP-NotI was blunt-end ligated to the ANV gene amplified by primers ANV-XhoI and ANV-EcoRV and digested by EcoRV to generate the fusion gene ANV-KAPP. The fusion gene was digested by XhoI and NotI and ligated to pPIC9 (Invitrogen, Carlsbad, CA), which was linearized using the same enzymes. The ligation mixture was transformed into E coli DH5α, and the desired clone was screened by PCR and confirmed by DNA sequence analysis. Integration was targeted by digesting the expression plasmid with SacI restriction enzyme prior to transformation. The α-factor fused gene cassette, including His4 as the selection marker, was inserted into the genome of Pichia pastoris GS115 (his4) at the AOX1 locus via electroporation.28 The recombinant strains were selected by growing from the minimal dextrose (MD) plate (1.34% yeast nitrogen base, 4 × 10-5% biotin, 2% dextrose, 1.5% bacto-agar; Invitrogen) through His4 compensation.
For expression of ANV-KAPP in P pastoris a single colony of P pastoris GS115 recombinant strain from the fresh MD plate was inoculated into 25 mL buffered methanol complex medium (BMMY; 1% yeast extract; 2% peptone; 100 mM potassium phosphate, pH 6.0; 1.34% yeast nitrogen base; 4 × 10-5% biotin, 0.5% methanol) medium for growth and induction of protein synthesis according to Invitrogen's protocol. The cells were removed by centrifugation, and the supernatant was frozen at -80°C.
Isolation of inclusion bodies, sulfonation, Q-Sepharose chromatography, and refolding of E coli–expressed proteins
Most of the E coli expressed fusion proteins and KPIs were in the inclusion bodies. The isolation of the inclusion bodies, sulfonation, Q-Sepharose chromatography, and refolding of these proteins were carried out by methods described previously for production of recombinant E coli–derived TFPI.29
Q-Sepharose purification of refolded TAP-ANV, ANV-6L15, ANV-KKTFPI, and 6L15
A1 × 8 cm Q-Sepharose Fast-Flow (FF) column was used for purification of refolded TAP-ANV, ANV-6L15, and ANV-KKTFPI. The column was preequilibrated in 20 mM Tris (tris(hydroxymethyl)aminomethane), pH 8.0. After loading of refold mixture and washing with the buffer, the column was eluted with an NaCl gradient. TAP-ANV, ANV-6L15, and ANV-KKTFPI elute at about 0.25 to 0.33 M NaCl.
Purification of 6L15 from the refold mixture was carried out on a 1 × 8 cm Q-Sepharose FF column preequilibrated in 20 mM Na citrate, pH 3.0, and eluted with an NaCl gradient. 6L15 eluted as a symmetrical peak around 0.5 M NaCl.
Purification of TAP-ANV, ANV-6L15, ANV, and ANV-KAPP by binding and elution from anionic liposomes
The Q-Sepharose–purified TAP-ANV and ANV-6L15 were further purified by adsorption to PS-containing liposomes in the presence of 5 mM Ca++ followed by elution with 5 mM EDTA (ethylenediaminetetraacetic acid) as described by Thiagarajan and Benedict.25 Recombinant ANV and ANV-KAPP were directly isolated from the E coli lysate and P pastoris culture medium by binding to the PS-containing liposomes25 without prior refold and purification steps. The protein solutions were filtered through a CentriPlus YM-100 membrane (Millipore, Bedford, MA) to remove residual vesicles.
Protein determination
The concentrations of proteins were determined by absorbance at 280 nm using theoretical extinction coefficients calculated from amino acid sequence data as described by Gill and von Hippel.30 The following molar extinction coefficients were used: ANV (21 050), TAP-ANV (39 550), ANV-6L15 (28 170), ANV-KAPP (31 300), ANV-KKTFPI (30 170), TAP (18 500), 6L15 (7120), C127 full-length TFPI (20 650), TFPI1-160 (7840).
Determination of stoichiometries of inhibitor-protease interactions
Bovine Xa and porcine trypsin were titrated with p-nitrophenyl p′-guanidinobenzoate according to Smith31 and Chase and Shaw,32 respectively, to determine the concentrations of active factor Xa and trypsin. Inhibitory activities of TAP, TAP-ANV, and ANV-KKTFPI against factor Xa were assayed by amidolysis of S2765 as described previously.26 Inhibitory activities of 6L15, ANV-6L15, and ANV-KAPP against trypsin were assayed by amidolysis of S2444. Stoichiometries of inhibition were determined as follows: 10 μL of 50 nM bovine Xa was mixed with 10 μL inhibitor in 10 mM Tris, pH 7.5; 0.15 M NaCl; 1 mg/mL bovine serum albumin (BSA); 0.002% Tween 20; 0.02% NaN3. After incubation at room temperature for 30 minutes, 10 μL of the reaction mixture was taken into a 96-well plate and mixed with 85 μL Tris-buffered saline (TBS) (50 mM Tris, pH 7.5; 0.15 M NaCl; 0.02% NaN3) containing 5 mM CaCl2 and 5 μL of 10 mM S2765. The absorbance change at 405 nm was recorded on a SPECTRAmax PLUS384 (Molecular Devices, Sunnyvale, CA) at room temperature for 60 seconds. A diluted trypsin solution (23 nM) was freshly prepared from the stock in a buffer containing TBS, 0.1 mg/mL BSA, 20 mM CaCl2. Ten microliters of the trypsin dilution was mixed with 10 μL of inhibitor diluted in the same buffer. After incubation at room temperature for 10 minutes, 75 μL TBS containing 20 mM CaCl2 and 5 μL of 10 mM S2444 was added, and the absorbance change at 405 nm was recorded at room temperature for 2 minutes.
Plasma clotting time assays
TF-induced plasma clotting and activated partial thromboplastin time (aPTT) assays were carried out on an ACL 200 coagulation analyzer (Instrumentation Laboratory) or a fibrometer (BBL, Cockeysville, MD).
Photochemically induced carotid artery thrombosis in mice
The protocol of Eitzman et al33 was followed with slight modification. Male C57BL/6 mice (10 to 12 weeks old) were anesthetized with pentobarbital, secured in the supine position, and placed under a dissecting microscope. Following a midline cervical incision, the right common carotid artery was isolated and a Doppler flow probe (model 0.5 VB; Transonic Systems, Ithaca, NY) was applied. Five minutes before induction of thrombosis, animals were injected in the tail vein with various doses of purified recombinant ANV, TAP-ANV, or saline (control). Thrombosis was induced by injection of rose bengal dye (Fisher Scientific, Pittsburgh, PA) into the tail vein in a volume of 0.12 mL at a concentration of 50 mg/kg using a 29-gauge needle. Just before injection, a 1.5 mW, 540 nm green light laser (Melles Griot, Carlsbad, CA) was applied to the desired site of injury from a distance of 6 cm. The laser remained on until thrombosis occurred. Flow in the vessel was monitored with the Doppler probe for 150 minutes from the onset of injury, at which time the animal was humanely killed.
Results
Construction and expression of recombinant ANV and ANV-KPI fusion proteins
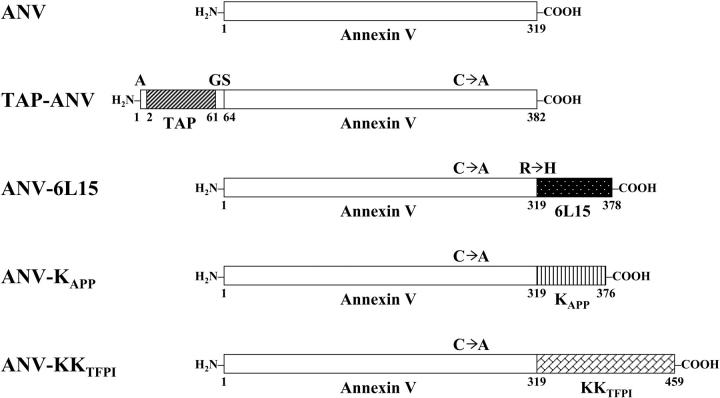
Plasmid vectors were constructed by PCR using oligonucleotide primers (Table 1) and used for expression of recombinant ANV and its fusion proteins with TAP (inhibitor of Xa),34 6L15 (aprotinin mutant; inhibitor of TF/VIIa),27 KAPP (inhibitor of XIa, Xa, IXa, and TF/VIIa),35-38 and KKTFPI (inhibitor of Xa and TF/VIIa).39 Figure 1 depicts the structures of these proteins. ANV was expressed as a full-length unmutated molecule of 319 amino acid residues in E coli. For other ANV-KPI fusions, Cys316 of ANV was mutated to Ala to avoid forming disulfide bonds with the cysteines within the Kunitz domains during protein refolding. TAP-ANV contains 382 amino acid residues starting with an Ala residue followed by 60 amino acids of TAP (Tyr1-Ile60), a dipeptide (Gly-Ser), and full-length ANV. ANV-6L15 contains 378 amino acid residues, with full-length ANV followed by 59 amino acids of 6L15 (Met1-Ala59). To create the NsiI restriction site for ligation, the second amino acid of 6L15 in the fusion protein was changed from Arg to His. ANV-KAPP contains 376 amino acids, with full-length ANV followed by 57 amino acids of KAPP (Asp1-Ile57). ANV-KKTFPI contains 459 amino acid residues, with full-length ANV followed by 140 amino acids of TFPI (Met22-Thr161), which includes Kunitz domains 1 and 2.
Figure 1.
Schematic diagram of annexin V and its fusion products with various Kunitz-type inhibitors. ANV indicates annexin V; TAP-ANV, Ala-tick anticoagulant peptide linked to annexin V by Gly-Ser dipeptide; ANV-6L15, annexin V linked to 6L15 (a Kunitz inhibitor derived from aprotinin with high affinity for TF/VIIa); ANV-KAPP, annexin V linked to KAPP (Kunitz inhibitory domain of amyloid β-protein precursor); ANV-KKTFPI, annexin V linked to KKTFPI (TFPI residues 22 to 161 containing Kunitz-1 and Kunitz-2 domains); C → A, Cys316Ala mutation in ANV; R → H, Arg2His mutation in 6L15.
Purification of ANV and ANV-KPI fusion proteins
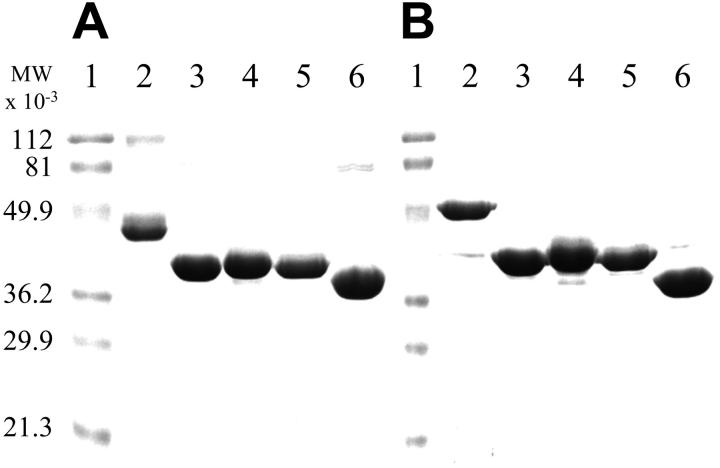
Recombinant ANV, TAP-ANV, ANV-6L15, and ANV-KKTFPI were expressed intracellularly in E coli. Essentially all the ANV molecules present in the E coli lysate were capable of binding to PS-containing liposomes in the presence of Ca++ when analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), suggesting that the expressed protein spontaneously folded itself into active forms. For other E coli–expressed ANV-KPI fusions, most of the expressed proteins occurred in inclusion bodies and required refolding to obtain active molecules. Using a sulfonation refold process developed previously for TFPI,29 we were able to achieve refolding of ANV-KPI fusion proteins as evidenced from the increase in inhibitory activity against trypsin or factor Xa during refolding. One-step Q-Sepharose chromatography of a refold mixture achieved a high degree of purification, as a single major band with the expected apparent molecular mass was observed in SDS-PAGE. Further purification was carried out by binding to PS-containing liposomes in the presence of Ca++ followed by elution with EDTA. Recombinant ANV-KAPP was expressed and secreted into the culture medium of P pastoris in active form. Active ANV-KAPP was purified from concentrated medium by binding to PS-containing liposomes in the presence of Ca++ and elution with EDTA. SDS-PAGE analysis of the final purified products is shown in Figure 2. Under nonreducing conditions (Figure 2A), a major band was seen in each preparation. ANV-KKTFPI (Figure 2A, lane 2) and ANV (Figure 2A, lane 6) both contained traces of dimers. Under reducing conditions (Figure 2B), the dimers disappeared and the fusion protein bands migrated slightly more slowly, possibly because of disruption of disulfide bonds and unfolding of the Kunitz domains.
Figure 2.
SDS-PAGE analysis of purified ANV and its fusion products with various Kunitz-type inhibitors. Samples were analyzed by 12% SDS-PAGE followed by Coomassie blue staining. All samples were boiled for 3 minutes without (A) or with (B) 50 mM dithiothreitol. Approximately 5 μg protein was loaded on each lane. (A-B) Lane 1, molecular weight (MW) markers; lane 2, ANV-KKTFPI; lane 3, ANV-6L15; lane 4, TAP-ANV; lane 5 ANV-KAPP; lane 6, ANV.
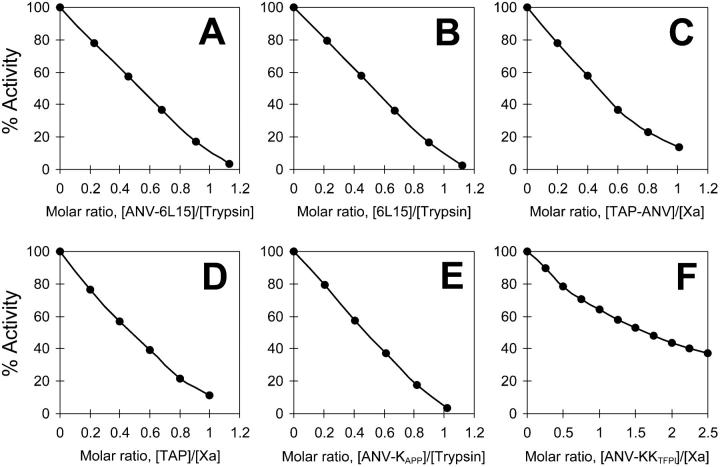
Stoichiometries of the interaction of the purified inhibitors with trypsin or factor Xa
Figure 3 shows the titrations of trypsin or factor Xa activities by the purified inhibitors. Except for ANV-KKTFPI, the purified fusion inhibitors (ANV-6L15, TAP-ANV, and ANV-KAPP) and the Kunitz inhibitors (6L15 and TAP) all inhibited trypsin or factor Xa with apparent stoichiometries of 1:1. These results indicated that all the purified inhibitors containing a single Kunitz domain were substantially pure and fully active. Titration of factor Xa (final concentration 2.5 nM) with ANV-KKTFPI (0.5 to 2.5 nM) showed deviation from 1:1 stoichiometry under the experimental conditions (Figure 3F), possibly because the separate Kunitz-2 domain of TFPI inhibits factor Xa with low affinity (inhibition constant [Ki] 90 nM)39 while the Kunitz-1 domain does not inhibit factor Xa. Alternatively, the purified ANV-KKTFPI may have contained inactive misfolded species.
Figure 3.
Stoichiometries of inhibition of bovine Xa and porcine trypsin by purified inhibitors. (A) Inhibition of trypsin by ANV-6L15. (B) Inhibition of trypsin by 6L15. (C) Inhibition of Xa by TAP-ANV. (D) Inhibition of Xa by TAP. (E) Inhibition of trypsin by ANV-KAPP. (F) Inhibition of Xa by ANV-KKTFPI.
Prolongation of the TF-initiated clotting time in plasma
Purified inhibitors were added to pooled human plasma at different concentrations, and plasma clotting was initiated by adding a thromboplastin reagent (1:100 dilution of Dade Innovin) that contains both TF and anionic PLs. With increasing concentrations of inhibitors, the clotting time was progressively prolonged. Table 2 shows the concentrations of the various inhibitors required to prolong the clotting time 1.5-fold and their relative potencies. Because TFPI is the most important physiological regulator of the TF pathway of coagulation, we have chosen recombinant full-length TFPI as a reference standard for comparison. TAP-ANV, ANV-6L15, ANV-KAPP, and ANV-KKTFPI were 6- to 86-fold more potent than TFPI, whereas ANV alone was 2.4-fold more potent than TFPI. TAP had the same potency as TFPI, but the other isolated Kunitz inhibitors (TFPI1-160 and 6L15) were 40- to 59-fold less active than TFPI.
Table 2.
Effects of various inhibitors on the TF-induced clotting time in human plasma
| Inhibitor | [Inhibitor]1.5 × CT,*nM | Relative potency† |
|---|---|---|
| TAP-ANV | 0.80 | 86 |
| ANV 6L15 | 6.0 | 12 |
| ANV-KAPP | 9.4 | 7.3 |
| ANV-KKTFPI | 11 | 6.3 |
| ANV | 29 | 2.4 |
| TAP | 68 | 1 |
| TFPI | 69 | 1 |
| E. coli TFPI1-160 | 2750 | 0.025 |
| 6L15 | 5900 | 0.012 |
Pooled human plasma (100 μL) was mixed with an equal volume of an inhibitor dissolved in buffer (10 mM Tris, pH 7.4; 0.15 M NaCl; 1 mg/mL BSA; 0.02% NaN3) at varying concentrations. Dade Innovin was diluted 1:100 with buffer (75 mM NaCl, 12.5 mM CaCl2, 0.5 mg/mL BSA, 0.02% NaN3) for the assay. Clotting times were determined with an ACL 200 coagulation analyzer.
Concentration of inhibitor that prolongs the TF-induced clotting time 1.5-fold relative to control (ie, from 40.7 to 61.1 seconds)
Calculated by dividing [Inhibitor]1.5 × CT for TFPI by the value for the other specified inhibitor
Effects of various inhibitors on the aPTT
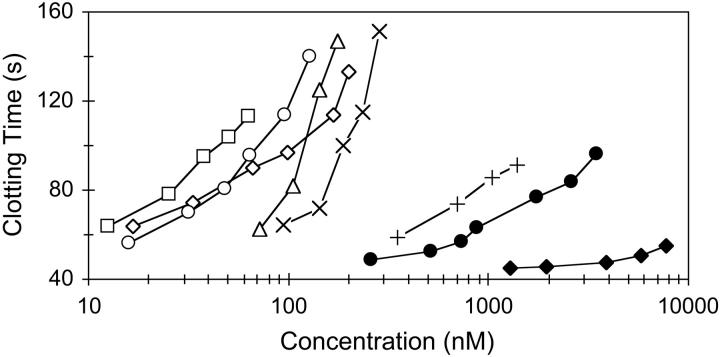
The aPTT measures intrinsic pathway activity. The effects of various inhibitors in prolonging the aPTT are shown in Figure 4. The most potent molecule, TAP-ANV, was about an order of magnitude more potent than ANV. ANV-KAPP, ANV-KKTFPI, and ANV-6L15 were several-fold more potent than ANV. The Kunitz inhibitors alone (6L15, TAP, and TFPI1-160) were about 2 orders of magnitude less potent than their fusions with ANV.
Figure 4.
Effects of various inhibitors in the aPTT assay. Pooled human plasma (180 μL) was mixed with 20 μL of various inhibitors to attain the indicated final concentrations. Clotting times were determined with an ACL 200 coagulation analyzer. The plasma with control buffer had an aPTT of 40.7 seconds. □ indicates TAP-ANV; ○, ANV-KAPP; ⋄, ANV-KKTFPI; ▵, ANV-6L15; ×, ANV; +, 6L15; •, TAP; ♦, TFPI1-160.
Comparison of the antithrombotic effects of ANV and TAP-ANV in a mouse model of arterial thrombosis
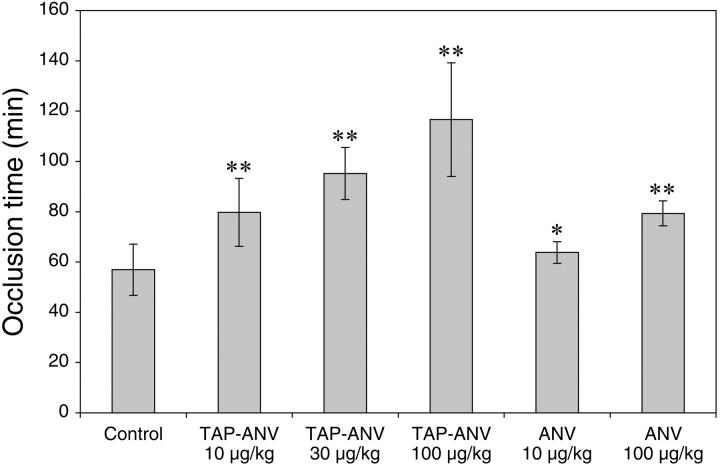
In this mouse thrombosis model, rose bengal dye in the lumen of the carotid artery was excited by transillumination with a green light laser to generate singlet oxygen. This treatment causes membrane damage, resulting in detachment of the endothelial cells from a localized segment of the vessel wall followed by formation of a thrombus that is rich in platelets and fibrin.40 The abilities of ANV and TAP-ANV to prolong the time to occlusion are shown in Figure 5. The time to occlusion in the control group was 57 ± 10 minutes (mean ± SD, n = 5). Following bolus injection of ANV at doses of 10 and 100 μg/kg body weight 5 minutes before induction of thrombosis, the time to occlusion was prolonged to 64 ± 4 (n = 3) and 79 ± 5 (n = 5) minutes, respectively. TAP-ANV at bolus doses of 10, 30, and 100 μg/kg body weight gave prolonged occlusion times of 80 ± 14 (n = 6), 95 ± 10 (n = 5), and 117 ± 23 (n = 5) minutes, respectively. With the exception of ANV at 10 μg/kg, the occlusion times of all treatment groups were significantly prolonged in comparison with the control group, and there were clear dose-response effects with both TAP-ANV and ANV. These data clearly show that TAP-ANV has greater antithrombotic potency than ANV. This order paralleled the relative potencies observed in the in vitro TF-induced plasma clotting and aPTT assays. TAP-ANV at a dose of 10 μg/kg body weight had antithrombotic efficacy comparable to that of ANV at a dose of 100 μg/kg body weight.
Figure 5.
Prolongation of time to occlusion by ANV and TAP-ANV in a photochemically induced carotid artery thrombosis model in mice. Experimental conditions are described in “Materials and methods.” *P = .32 versus control; **P = .013 versus control (2-tailed t test). Error bars indicate mean ± standard deviation.
Comparison of in vitro and in vivo activities of TAP-ANV and a combination of TAP and ANV
The anticoagulant activities of TAP-ANV and an equimolar mixture of TAP plus ANV were compared using in vitro and in vivo assays. The clotting times in the TF-initiated plasma clotting and aPTT assays and the occlusion time in the mouse thrombosis model were significantly prolonged by TAP-ANV versus equimolar amounts of TAP plus ANV (Table 3). These data indicate that conjugation of TAP and ANV results in enhanced anticoagulant potency versus a combination of TAP and ANV as separate entities.
Table 3.
Comparison of TAP-ANV and a combination of TAP and ANV in TF-initiated plasma clotting, aPTT, and a mouse thrombosis model
| TF-initiated clotting time,*s | aPTT,†s | Occlusion time,‡min | |
|---|---|---|---|
| Control | 42.3 ± 1.3 (n = 7) | 33.2 ± 1.0 (n = 4) | 57 ± 10.2 (n = 5) |
| TAP-ANV | 60.7 ± 1.2 (n = 7)§ | 52.8 ± 2.1 (n = 4)§ | 80 ± 13.5 (n = 6)§ |
| TAP + ANV | 42.5 ± 0.6 (n = 7) | 36.9 ± 0.9 (n = 4)§ | 49 ± 2.4 (n = 5) |
Values are mean ± SD.
Plasma was supplemented with buffer (control), 2.5 nM fusion protein (TAP-ANV), or 2.5 nM TAP and 2.5 nM ANV (TAP + ANV), and the TF-initiated clotting was determined using a fibrometer
Plasma was supplemented with buffer (control), 50 nM fusion protein (TAP-ANV), or 50 nM TAP and 50 nM ANV (TAP + ANV), and the aPTT was determined using a fibrometer
Mice were injected with PBS (control), 0.23 nmol/kg (10 μg/kg) fusion protein (TAP-ANV), or a mixture of 0.23 nmol/kg TAP and 0.23 nmol/kg ANV (TAP + ANV), and the occlusion time was determined as described in “Materials and methods”
P ≤ .01 versus control (2-tailed t test)
Discussion
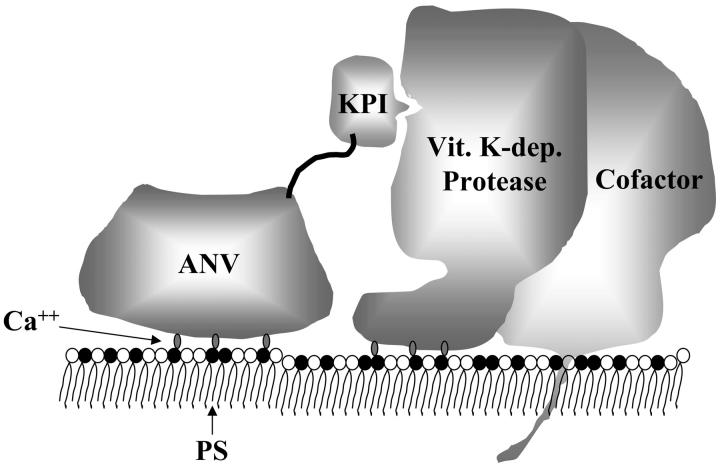
In this study, we have used recombinant DNA technology to create 4 fusion proteins that share a common ANV domain linked to different KPI domains (TAP, 6L15, KAPP, and KKTFPI). ANV has high Ca++-dependent affinity (dissociation constant [Kd] less than 0.1 nM) for PS-containing membranes.19 Our ANV-KPI fusion proteins apparently preserved this affinity, because they were all purified by Ca++-dependent binding to PS-containing liposomes and subsequent elution with EDTA. The 4 KPIs chosen for this study had been reported to have the following specificities and inhibition constants (Ki) for various coagulation serine proteases: TAP, 0.2 nM for Xa 34; 6L15, 0.2 nM for TF/VIIa and 13 nM for XIa 27; KAPP, 0.08 nM for IXa, 0.29 nM for XIa, 10 nM for Xa, and 17 nM for TF/VIIa 35-38; and KKTFPI, 90 nM for Xa and 240 nM for TF/VIIa.39 Assuming that the KPI domains in the ANV-KPI fusion proteins conserved their inhibitory specificities, we might expect that these fusion proteins would inhibit various membrane-associated coagulation complexes as follows: (a) TAP-ANV specifically inhibited prothrombinase (Va/Xa); (b) ANV-6L15 inhibited TF/VIIa and XIa complexes; (c) ANV-KAPP inhibited intrinsic tenase (VIIIa/IXa), XIa, prothrombinase (Va/Xa), and TF/VIIa complexes; and (d) ANV-KKTFPI inhibited TF/VIIa, prothrombinase, and TF/VIIa/Xa complexes. Based on results of this study and other published structural information,41 we propose the following working model for the inhibition of membrane-associated coagulation enzyme complexes by the ANV-KPI fusion proteins (Figure 6). The protein core of ANV consists of 4 homologous domains forming a highly α-helical and tightly packed disk with 2 principal sides.41 The convex side contains Ca++-binding sites and faces the membrane when binding to it. The concave surface points away from the membrane and harbors both the amino- and carboxy-terminal tails in proximity. Either the amino- or the carboxy-terminal tail of ANV can be linked to a KPI domain to create an ANV-KPI fusion protein. Ca++-dependent binding of the ANV domain to PS on the membrane surface greatly facilitates the binding of the KPI domain to the active site of the membrane-associated enzyme/cofactor complexes.
Figure 6.
Model of the inhibition of a membrane-associated coagulation complex by the ANV-KPI fusion protein. The fusion protein binds to PS on the membrane surface in a Ca++-dependent manner via the ANV domain. This facilitates the binding of the KPI domain to the active site of the coagulation complex. Vit. K-dep. indicates vitamin K–dependent.
TF-initiated plasma clotting and aPTT assays are useful in in vitro global tests for assessing the activities of the classical extrinsic, intrinsic, and common pathways of coagulation and for monitoring anticoagulant therapy. In these plasma-based assays, the anticoagulant activities of ANV-KPI fusion proteins were far more potent than those of the Kunitz-type protease inhibitors tested, including recombinant TFPI, TAP, and 6L15 (Table 2 and Figure 4). Thermodynamically, full-length rTFPI exerts a stronger inhibition of prothrombinase (Ki = 3 pM)13 than of solution-phase Xa (Ki = 85 pM).42 The assembly of Xa and Va into prothrombinase also enhances the association rate of the reaction between Xa and full-length rTFPI by approximately 20-fold.13 However, a physiological concentration of prothrombin (1.4 μM) effectively competes with rTFPI for prothrombinase.12,13 As a consequence, rTFPI inhibits prothrombinase weakly in the presence of prothrombin and requires supraphysiological concentrations to achieve an anticoagulant effect.12 The relatively low in vitro anticoagulant potency of rTFPI correlated with results from several in vivo animal studies where maintenance of rather high plasma levels of rTFPI (about 50 to 125 nM) was needed to achieve therapeutic effects in an E coli sepsis model in baboons43 and restenosis after angioplasty models in rabbits44 and minipigs.45 Such rTFPI levels were approximately 20- to 50-fold that of the endogenous TFPI in normal plasma. A likely explanation for the unremarkable potency of exogenous rTFPI may be related to its failure to bind to cell surfaces,46 in sharp contrast to the endogenous TFPI, which is predominantly localized to the membrane surfaces of endothelium and monocytes via a glycosylphosphatidylinositol (GPI) anchor.47,48 GPI-mediated membrane anchorage of TFPI has been shown to enhance the inhibition of membrane-associated TF/VIIa compared with that of nonmembrane-anchored TFPI.49
TAP is a reversible, slow, tight-binding inhibitor of solution-phase Xa with a Ki of 0.18 nM.34 Assembly of Xa into the prothrombinase (Va/Xa) complex on an anionic membrane surface results in a 30-fold enhanced binding of TAP (Ki = 5.3 pM) and a modest 3-fold increase in the rate of inhibition.50 Despite an extremely high affinity for prothrombinase, TAP possesses only moderate anticoagulant activities in both TF-initiated coagulation and aPTT assays (Table 2 and Figure 4). The modest anticoagulant activity of TAP was thought to be due to its relatively slow rate of prothrombinase inhibition.51 The aprotinin mutant 6L15 binds membrane-associated TF/VIIa with very high affinity (Ki = 0.2 nM),27 yet, consistent with a previous report,52 it is a very poor inhibitor of TF-initiated coagulation (Table 2). In comparison, TAP-ANV and ANV-6L15 are close to 2 and 3 orders of magnitude, respectively, more potent than their Kunitz domains alone in TF-initiated plasma clotting. The greatly enhanced potencies of the fusion proteins cannot be attributed to ANV alone, because TAP-ANV and ANV-6L15 are 36- and 5-fold, respectively, more potent than ANV. The results taken together suggest that binding of TAP-ANV and ANV-6L15 to PS greatly facilitates the inhibition of prothrombinase and TF/VIIa by the fusion proteins on the 2-dimensional membrane surface. In addition, the fusion proteins may have overcome the protective effect of the anionic membrane on the enzyme complexes against their inhibitors.
We chose a mouse model for initial testing of the in vivo antithrombotic potency of TAP-ANV in comparison with ANV. The results showed that the in vivo antithrombotic potencies paralleled their in vitro anticoagulant activities in TF-initiated plasma clotting and aPTT assays: TAP-ANV greater than ANV approximately equal to TAP plus ANV. The circulating half-lives of ANV-KPIs are likely similar to that of ANV, which was reported to have a rather short half-life of 4.2 minutes in mice.53 It is of great interest that the test molecules were administered at very low doses (10 to 100 μg/kg) by bolus injection 5 minutes before induction of vessel damage, yet the occlusion times were prolonged from 57 minutes for the control to up to about 2 hours for the treated animals. This suggested that ANV and ANV-KPIs might exert a passivating effect on the thrombogenic sites and maintain vascular patency after their clearance from the circulation. The results are consistent with a previous study of the antithrombotic effect of ANV in a rabbit jugular vein injury model.23 In the latter study, it was shown that bolus injection of ANV reduced fibrin deposition onto the injured vessel wall segment for more than 3 hours after its anticoagulant effect had disappeared from the circulation. In addition, 4-fold more ANV bound to the injured jugular vein segments compared with the noninjured jugular vein, suggesting the selective accumulation of ANV at the site of vascular injury and local inhibition of thrombin generation without inducing a generalized state of hypocoagulation.23
Anticoagulants in current use and under development generally aim at inhibiting coagulation protease activity or suppressing the level of certain clotting factors. Maintenance of therapeutic concentrations of these drugs in the circulating blood is generally required. Because normal hemostasis and pathological thrombosis involve basically the same clotting components, the risk of systemic bleeding increases when a high concentration of an anticoagulant drug is maintained in the systemic circulation. A strategy to avoid systemic anticoagulation is targeted delivery. Indeed, topical applications of TFPI and active site–inactivated factor VIIa have been shown to prevent thrombosis in animal models of surgery without systemic anticoagulation.54,55 However, many prothrombotic vascular lesions are not easily accessible for topical application. Because ANV and ANV-KPI fusions possess the ability to target the PS-exposed membranes of developing thrombi at sites of vascular lesions, it might be possible to inhibit thrombogenesis in situ by bolus injection or prolonged infusion at very low doses without intense systemic anticoagulation, thus minimizing the risk of bleeding.
Asymmetric distribution of plasma membrane phospholipids was initially discovered in platelets and red blood cells. Subsequent investigations of more cell types led to the concept that asymmetry is a general rule for normal mammalian cells.6,7 Loss of asymmetry, especially the appearance of PS at the membrane surface due to cell injury, activation, and apoptosis, has 2 major biologic consequences: First, the PS-exposed cells and membrane vesicles are recognized by the reticuloendothelial system and destined for phagocytic clearance56; second, the PS-exposed membranes stimulate thrombogenesis via efficient assembly and catalysis of the coagulation enzyme complexes. Both biologic functions are important for removing unwanted cells and for healing. However, uncontrolled PS exposure on apoptotic, activated, and injured cells can lead to thromboembolism and other pathological consequences. Apoptosis occurs in human atherosclerotic plaques. Endothelial apoptosis is thought to be a critical step in the transition from a stable endothelialized plaque to plaque erosion and thrombosis.57 Rupture of vulnerable plaques leads to the exposure of the lipid core, which is highly enriched with thrombogenic TF- and PS-exposed apoptotic microparticles, resulting in acute coronary syndromes and acute cerebrovascular events.58 Thromboses are common complications in cancer. Tumor cell production of TF59 and increased exposure of PS in the tumor vasculature60 are 2 key players contributing to the prothrombotic state of cancer. Increased apoptosis occurs during cancer therapy, further enhancing the risk of thrombosis via increased availability of TF and PS. Increased TF-positive, PS-exposed cells and microparticles derived from platelets, leukocytes, and endothelial cells as a result of cell activation and apopotosis has also been reported in many other pathological states associated with thrombosis, including heparin-induced thrombocytopenia, complicated diabetes mellitus, severe hypertension, end-stage renal disease,61 antiphospholipid syndromes,62 systemic lupus erythematosus,63 sickle cell disease,64 paroxysmal nocturnal hemoglobinuria,65 and biomaterial-associated thrombosis.66 It would be of considerable interest to explore the therapeutic use of ANV-KPI fusion proteins in the prevention and treatment of thrombosis associated with these disorders.
Prepublished online as Blood First Edition Paper, January 27, 2005; DOI 10.1182/blood-2004-11-4435.
Supported by National Institutes of Health grant RO1 HL55520 and EVAS Therapeutics.
One of the authors (T.-C.W.) has declared a financial interest in a company (EVAS Therapeutics) whose potential products were studied in the present work.
An Inside Blood analysis of this article appears at the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Butenas S, Mann KG. Blood coagulation. Biochemistry (Mosc). 2002;67: 5-15. [DOI] [PubMed] [Google Scholar]
- 2.Rand MD, Lock JB, van't Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88: 3432-3445. [PubMed] [Google Scholar]
- 3.Hoffman M, Monroe DM III. A cell-based model of hemostasis. Thromb Haemost. 2001;85: 958-960. [PubMed] [Google Scholar]
- 4.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8: 1175-1180. [DOI] [PubMed] [Google Scholar]
- 5.Falati S, Liu Q, Gross P, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197: 1585-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sims PJ, Wiedmer T. Unraveling the mysteries of phospholipid scrambling. Thromb Haemost. 2001;86: 266-275. [PubMed] [Google Scholar]
- 7.Zwaal RFA, Schroit AJ. Pathophysiological implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89: 1121-1132. [PubMed] [Google Scholar]
- 8.Emoto K, Toyama-Sorimachi N, Karasuyama H, Inoue K, Umeda M. Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp Cell Res. 1997;232: 430-434. [DOI] [PubMed] [Google Scholar]
- 9.Morrissey JH, Neuenschwander PF, Huang Q, McCallum CD, Su B, Johnsom AE. Factor VIIa-tissue factor: functional importance of protein membrane interaction. Thromb Haemost. 1997;78: 112-116. [PubMed] [Google Scholar]
- 10.Zwaal RFA, Comfurius P, Bevers EM. Lipid-protein interactions in blood coagulation. Biochim Biophys Acta. 1998;1376: 433-453. [DOI] [PubMed] [Google Scholar]
- 11.Dachary-Prigent J, Toti F, Satta N, Pasquet J-M, Uzan A, Freyssinet J-M. Physiopathological significance of catalytic phospholipids in the generation of thrombin. Semin Thromb Hemost. 1996;22: 157-164. [DOI] [PubMed] [Google Scholar]
- 12.Mast A, Broze GJ Jr. Physiological concentrations of tissue factor pathway inhibitor do not inhibit prothrombinase. Blood. 1996;87: 1845-1850. [PubMed] [Google Scholar]
- 13.Franssen J, Salemink I, Willems GM, Wun TC, Hemker C, Lindhout T. Prothrombinase is protected from inactivation by tissue factor pathway inhibitor: competition between prothrombin and inhibitor. Biochem J. 1997;323: 33-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miletich JP, Jackson CM, Majerus PW. Properties of the factor Xa binding site on human platelets. J Biol Chem. 1978;253: 6908-6916. [PubMed] [Google Scholar]
- 15.Ellis V, Scully MF, Kakkar VV. Inhibition of prothrombinase complex by plasma proteinase inhibitors. Biochemistry. 1984;23: 5882-5887. [DOI] [PubMed] [Google Scholar]
- 16.Oliver JA, Monroe DM, Church FC, Roberts HR, Hoffman M. Activated protein C cleaves factor Va more efficiently on endothelium than on platelet surfaces. Blood. 2002;100: 539-545. [DOI] [PubMed] [Google Scholar]
- 17.Broze GJ Jr. Tissue factor pathway inhibitor and the current concept of blood coagulation. Blood Coagul Fibrinolysis. 1995;6(suppl 1): S7-S13. [DOI] [PubMed] [Google Scholar]
- 18.Walsh PN. Roles of platelets and factor XI in the initiation of blood coagulation by thrombin. Thromb Haemost. 2001;86: 75-82. [PubMed] [Google Scholar]
- 19.van Heerde WL, de Groot PG, Reutelingsperger CPM. The complexity of the phospholipid binding protein annexin V. Thromb Haemost. 1995;73: 172-179. [PubMed] [Google Scholar]
- 20.Reutelingsperger CPM, Hornstra G, Hemker HC. Isolation and partial purification of a novel anticoagulant from arteries of human umbilical cord. Eur J BioChem. 1985;151: 625-629. [DOI] [PubMed] [Google Scholar]
- 21.Funakoshi T, Heimark RL, Hendrickson LE, McMullen BA, Fujikawa K. Human placental antico-agulant protein: isolation and characterization. Biochemistry. 1987;26: 5572-5578. [DOI] [PubMed] [Google Scholar]
- 22.Romisch J, Seiffge D, Reiner G, Paques EP, Heimburger N. In vivo antithrombotic potency of placenta protein 4 (annexin V). Thromb Res. 1991;61: 93-104. [DOI] [PubMed] [Google Scholar]
- 23.Van Ryn McKenna J, Merk H, Muller TH, Buchanan MR, Eisert WG. The effect of heparin and annexin V on fibrin accretion after injury in the jugular vein of rabbit. Thromb Haemost. 1993;69: 227-230. [PubMed] [Google Scholar]
- 24.Tait JF, Cerqueira MD, Dewhurst TA, Fujikawa K, Ritchie JL, Stratton JR. Evaluation of annexin V as a platelet-directed thrombus targeting agent. Thromb Res. 1994;75: 491-501. [DOI] [PubMed] [Google Scholar]
- 25.Thiagarajan P, Benedict CR. Inhibition of arterial thrombosis by recombinant annexin V in a rabbit carotid artery injury model. Circulation. 1997;96: 2339-2347. [DOI] [PubMed] [Google Scholar]
- 26.Wun TC, Kretzmer KK, Palmier MO, et al. Comparison of recombinant tissue factor pathway inhibitors expressed in human SK hepatoma, mouse C127, baby hamster kidney, and Chinese hamster ovary cells. Thromb Haemost. 1992;68: 54-59. [PubMed] [Google Scholar]
- 27.Stassen JM, Lambeir AM, Matthyssens G, et al. Characterization of a novel series of aprotinin-derived anticoagulants. I. In vitro and pharmacological properties. Thromb Haemost. 1995;74: 646-654. [PubMed] [Google Scholar]
- 28.Scorer CA, Clare JJ, McCombie WR, Romanos MA, Sreekrishna K. Rapid selection using G418 of high copy number transformants of Pichia pastoris for high-level foreign gene expression. Biotechnology. 1994;12: 181-184. [DOI] [PubMed] [Google Scholar]
- 29.Diaz-Collier JA, Palmier MO, Kretzmer KK, et al. Refold and characterization of recombinant tissue factor pathway inhibitor expressed in E. coli. Thromb Haemost. 1994;71: 339-346. [PubMed] [Google Scholar]
- 30.Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal BioChem. 1989;182: 319-326. [DOI] [PubMed] [Google Scholar]
- 31.Smith RL. Titration of activated bovine factor X. J Biol Chem. 1973;248: 2418-2423. [PubMed] [Google Scholar]
- 32.Chase T Jr, Shaw E. Titration of trypsin, plasmin, and thrombin with p-nitrophenyl p'-guanidinobenzoate HCl. Methods Enzymol. 1970;19: 20-27. [Google Scholar]
- 33.Eitzman DT, Westrick RJ, Nabel EG, Ginsburg D. Plasminogen activator inhibitor-1 and vitronectin promote vascular thrombosis in mice. Blood. 2000;95: 577-580. [PubMed] [Google Scholar]
- 34.Jordan SP, Waxman L, Smith DE, Vlasuk GP. Tick anticoagulant peptide: kinetic analysis of the recombinant inhibitor with blood coagulation factor Xa. Biochemistry. 1990;29: 11095-11100. [DOI] [PubMed] [Google Scholar]
- 35.Mahdi F, Rehemtulla A, Van Nostrand WE, Bajaj SP, Schmaier AH. Protease nexin-2/amyloid β-protein precursor regulates factor VIIa and the factor VIIa-tissue factor complex. Thromb Res. 2000;99: 267-276. [DOI] [PubMed] [Google Scholar]
- 36.Van Nostrand WE, Wagner SL, Farrow JS, Cunningham DD. Immunopurification and protease inhibitory properties of protease nexin-2/amyloid beta-protein precursor. J Biol Chem. 1990;265: 9591-9594. [PubMed] [Google Scholar]
- 37.Mahdi F, Van Nostrand WE, Schmaier AH. Protease nexin-2/amyloid beta-protein precursor inhibits factor Xa in the prothrombinase complex. J Biol Chem. 1995;270: 23468-23474. [DOI] [PubMed] [Google Scholar]
- 38.Schmaier AH, Dahl LD, Rozemuller AJM, et al. Protease nexin-2/amyloid β protein precursor. A tight-binding inhibitor of coagulation factor IXa. J Clin Invest. 1993;92: 2540-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen LC, Bjorn SE, Olsen OH, Nordfang O, Norris F, Norris K. Inhibitory properties of separate recombinant Kunitz-type-protease-inhibitor domains from tissue factor pathway inhibitor. Eur J BioChem. 1996;235; 310-316. [DOI] [PubMed] [Google Scholar]
- 40.Saniabadi AR, Umemura K, Matsumoto N, Sakuma S, Nakashima M. Vessel wall injury and arterial thrombosis induced by photochemical reaction. Thromb Haemost. 1995;73: 868-872. [PubMed] [Google Scholar]
- 41.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82: 331-371. [DOI] [PubMed] [Google Scholar]
- 42.Huang Z-F, Wun T-C, Broze GJ Jr. Kinetics of factor Xa inhibition by tissue factor pathway inhibitor. J Biol Chem. 1993;268: 26950-26955. [PubMed] [Google Scholar]
- 43.Creasey AA, Chang ACK, Feigen L, Wun T-C, Taylor FB Jr, Hinshaw LB. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J Clin Invest. 1993;91: 2850-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jang Y, Guzman LA, Lincoff AM, et al. Influence of blockade at specific levels of the coagulation cascade on restenosis in a rabbit atherosclerotic femoral artery injury model. Circulation. 1995;92: 3041-3050. [DOI] [PubMed] [Google Scholar]
- 45.Oltrona L, Speidel CM, Recchia D, Wickline SA, Eisenberg PR, Abendschein DR. Inhibition of tissue factor-mediated coagulation markedly attenuates stenosis after balloon-induced arterial injury in minipigs. Circulation. 1997;96: 646-652. [DOI] [PubMed] [Google Scholar]
- 46.Ho G, Narita M, Broze GJ Jr. Schwartz AL. Recombinant full-length tissue factor pathway inhibitor fails to bind to the cell surface: implication for catabolism in vitro and in vivo. Blood. 2000;95: 1973-1978. [PubMed] [Google Scholar]
- 47.Ott I, Miyagi Y, Miyazaki K, et al. Reversible regulation of tissue factor-induced coagulation by glycosyl phosphatidylinositol-anchored tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2000;20: 874-882. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Piro O, Lu L, Broze GJ Jr. Glycosyl phosphatidylinositol anchorage of tissue factor pathway inhibitor. Circulation. 2003;108: 623-627. [DOI] [PubMed] [Google Scholar]
- 49.Ott I, Vukovich R, Schomig A, Neumann FJ. Overexpression of glycosyl phosphatidylinositol-anchored tissue factor pathway inhibitor-1 inhibits tissue factor activity. Blood Coagul Fibrinolysis. 2003;14: 539-544. [DOI] [PubMed] [Google Scholar]
- 50.Krishnaswamy S, Vlasuk GP, Bergum PW. Assembly of the prothrombinase complex enhances the inhibition of bovine factor Xa by tick anticoagulant peptide. Biochemistry. 1994;33: 7897-7907. [DOI] [PubMed] [Google Scholar]
- 51.Dunwiddie CT, Smith DE, Nutt EM, Vlasuk GP. Anticoagulant effects of the selective factor XA inhibitors tick anticoagulant peptide and antistasin in the APTT assay are determined by the relative rate of prothrombinase inhibition. Thromb Res. 1991;64: 787-794. [DOI] [PubMed] [Google Scholar]
- 52.Stassen JM, Lambeir AM, Vreys I, et al. Characterization of a novel series of aprotinin-derived anticoagulants. II. Comparative antithrombotic effects on primary thrombus formation in vivo. Thromb Haemost. 1995;74: 655-659. [PubMed] [Google Scholar]
- 53.Wen X-X, Wu Q-P, Ke S. Improved radiolabeling of PEGylated protein: PEGylated annexin V for noninvasive imaging of tumor apoptosis. Cancer Biother Radiopharm. 2003;18: 819-827. [DOI] [PubMed] [Google Scholar]
- 54.Ozbeck MR, Brown DM, Deune EG, et al. Topical tissue factor pathway inhibitor improves free flap survival in a model simulating free flap errors. J Reconstr Microsurg. 1995;11: 185-188. [DOI] [PubMed] [Google Scholar]
- 55.Hedner U, Erhardtsen E. Future possibility in the regulation of the extrinsic pathway: rFVIIa and TFPI. Ann Med. 2000;32(suppl 1): 68-72. [PubMed] [Google Scholar]
- 56.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405: 85-90. [DOI] [PubMed] [Google Scholar]
- 57.Tedgui A. Mallat Z. Apoptosis as a determinant of atherothrombosis. Thromb Haemost. 2001;86: 420-426. [PubMed] [Google Scholar]
- 58.Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15: 439-446. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez PM, Patierno SR, Rickles FR. Tissue factor and fibrin in tumor angiogenesis. Semin Thromb Hemost. 2004;30: 31-44. [DOI] [PubMed] [Google Scholar]
- 60.Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62: 6132-6140. [PubMed] [Google Scholar]
- 61.Diamant M, Tushuizen ME, Sturk A, Nieuwland R. Cellular microparticles: new players in the field of vascular diseases? Eur J Clin Invest. 2004;34: 392-340. [DOI] [PubMed] [Google Scholar]
- 62.Pierangeli SS, Harris EN. Probing antiphospholipid-mediated thrombosis: the interplay between anticardiolipin antibodies and endothelial cells. Lupus. 2003;12: 539-545. [DOI] [PubMed] [Google Scholar]
- 63.Rajagopalan S, Somers EC, Brook RD, et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103: 3677-3683. [DOI] [PubMed] [Google Scholar]
- 64.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102: 2678-2683. [DOI] [PubMed] [Google Scholar]
- 65.Liebman HA, Feinstein DI. Thrombosis in patients with paroxysmal nocturnal hemoglobinuria is associated with markedly elevated plasma levels of leukocyte-derived tissue factor. Thromb Res. 2003;111: 235-238. [DOI] [PubMed] [Google Scholar]
- 66.Gorbet MB, Sefton MV. Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials. 2004;25: 5681-5703. [DOI] [PubMed] [Google Scholar]