Abstract
The c-Myb transcription factor controls differentiation and proliferation in hematopoietic and other cell types and has latent transforming activity, but little is known about its regulation during the cell cycle. Here, c-Myb was identified as part of a protein complex from human T cells containing the cyclin-dependent kinase (CDK) CDK6. Assays using model reporter constructs as well as endogenous target genes showed that the activity of c-Myb was inhibited by cyclin D1 plus CDK4 or CDK6 but stimulated by expression of the CDK inhibitors p16 Ink4a, p21 Cip1, or p27 Kip1. Mapping experiments identified a highly conserved region in c-Myb which, when transferred to the related A-Myb transcription factor, also rendered it responsive to CDKs and p27. The results suggest that c-Myb activity is directly regulated by cyclin D1 and CDKs and imply that c-Myb activity is regulated during the cell cycle in hematopoietic cells.
Introduction
The c-Myb protein is a DNA-binding transcription factor that regulates the expression of specific genes in different cell types during development and during cellular differentiation.1-4 Expression of c-Myb is required for normal hematopoiesis5 and for the proliferation of hematopoietic cells in tissue culture,6-8 and c-Myb has been implicated in the regulation of proliferation of other cell types such as colon, mammary, and endothelial cells.9-14 As the product of the c-myb protooncogene, the c-Myb protein has latent transforming activity that can be unleashed through point mutations and C-terminal deletions.15-18 Thus, relatively minor changes in c-Myb can convert it from a docile regulator of normal proliferation and differentiation to a potent transforming protein that induces leukemias in birds and rodents.19-25
Since c-Myb protein is linked to the regulation of proliferation, it is likely to play a role in regulating the cell cycle. Although c-Myb protein levels rise when T lymphocytes enter the cell cycle,26,27 several types of evidence suggest that c-Myb protein activity is regulated by posttranslational mechanisms.17,22,28-33 Interestingly, the related transcription factor B-Myb (MYBL2) regulates genes during S phase and G2 and its activity is regulated by cyclin A/cyclin-dependent kinase 2 (CDK2) phosphorylation.34 Thus, it seems likely that c-Myb activity could be regulated by cell-cycle–specific protein interactions or modifications that focus the changes in its activity to the G1/S transition. The most important regulators of this transition are cyclin D1, which interacts with and regulates the cyclin-dependent kinases CDK4 and CDK6, and cyclin E, which interacts with and regulates CDK2. In vertebrates, the activities of the cyclin/CDK complexes are further regulated by the cyclin-dependent kinase inhibitors, especially p16Ink4a, p21 Cip1, and p27 Kip1, which are in turn subject to their own regulation via phosphorylation and subcellular localization.35,36 Although c-Myb has been shown to interact with cyclin D1,37 previous reports suggested that c-Myb activity was not affected by the interaction. Thus, the relationship between cell-cycle regulation and changes in c-Myb activity has remained obscure.
Here, the relationship between cell-cycle regulators and c-Myb activity was investigated by testing whether c-Myb interacts with important regulators of the cell cycle in hematopoietic cells. We found that c-Myb exists in a stable complex with the cyclin D1–regulated kinase CDK6, suggesting that c-Myb is directly regulated by a cell-cycle–dependent mechanism in the G1 phase of the cell cycle. The results link c-Myb to cell-cycle control and outline a regulatory pathway from the CDK inhibitors p16 Ink4a, p21 Cip1, and p27 Kip1 to c-Myb and downstream target genes that are likely to affect the proliferation or differentiation of hematopoietic cells.
Materials and methods
Plasmids, expression vectors, and reporter assays
The c-Myb, A-Myb, and B-Myb expression vectors, the Myb-responsive reporter plasmid, and the transfection assays have been described,38 as has the plasmid expressing NF-M.39 The pCMXp27 (mouse p27) expression plasmid was provided by Tony Hunter (Toyoshima and Hunter40). Plasmids expressing human p21, p16, and p19 from cytomegalovirus promoters were obtained from Richard Pestell (Ashton et al41). The A-Myb/c-Myb recombinants were constructed by swapping cDNA fragments at the conserved EcoRI and HincII sites, as described in the legend to Figure 2. QT6 and HD-11 cells were transfected essentially as described previously38 using Lipofectamine reagent as suggested by the manufacturer (GIBCO BRL, Gaithersburg, MD). Luciferase reporter gene activities were analyzed using a Turner Designs TD-20/20 luminometer (Turner BioSystems, Sunnyvale, CA). The error bars in Figures 4 and 5 represent the range of duplicate assays performed in parallel.
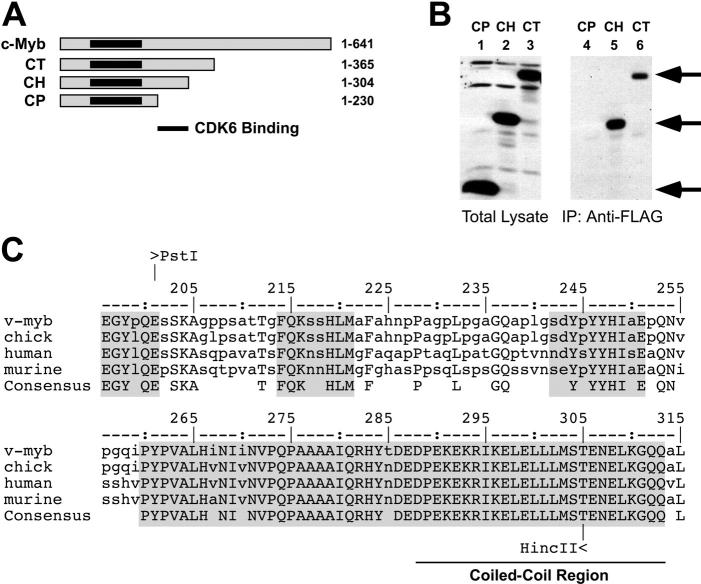
Figure 2.
A CDK interaction domain in c-Myb. (A) Deletion constructs. C-terminal deletion constructs of c-Myb were prepared using various restriction sites. The amino acid residues retained in each construct are shown at the right. The highly conserved DNA-binding domain of c-Myb is shaded black. (B) Mapping the interaction domains. The deletion constructs CP (lanes 1, 4), CH (lanes 2, 5), or CT (lanes 3, 6) were coexpressed with FLAG epitope-tagged CDK6 and the resulting complexes were isolated by immunoprecipitation with anti-FLAG beads then analyzed by Western blotting using anti-Myb antibodies, as described for Figure 1. The left panel (lanes 1-3) shows the total extracts; the immunoprecipitated samples are shown at the right (lanes 4-6). Arrows indicate the migration of the c-Myb protein derivatives. (C) Conservation of the CDK interaction domain. The amino acid sequences of v-Myb from avian myeloblastosis virus or c-Myb proteins from chicken, human, or mouse were aligned using ClustalW.43 The bottom line shows the identical amino acids, shaded boxes show blocks of highest conservation. The numbering scheme comes from the chicken c-Myb protein.44 The coiled-coil region predicted using the SMART analysis package45,46 is indicated at bottom. The coiled-coil region has been shown to interact with the transcriptional coactivator CBP.48
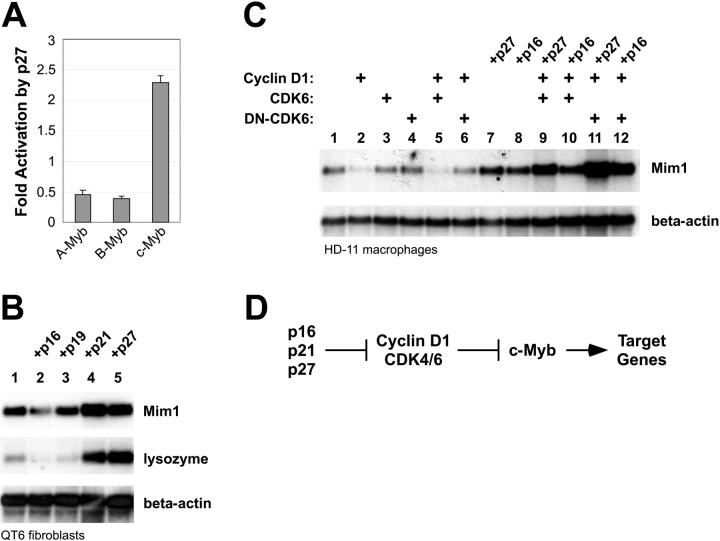
Figure 4.
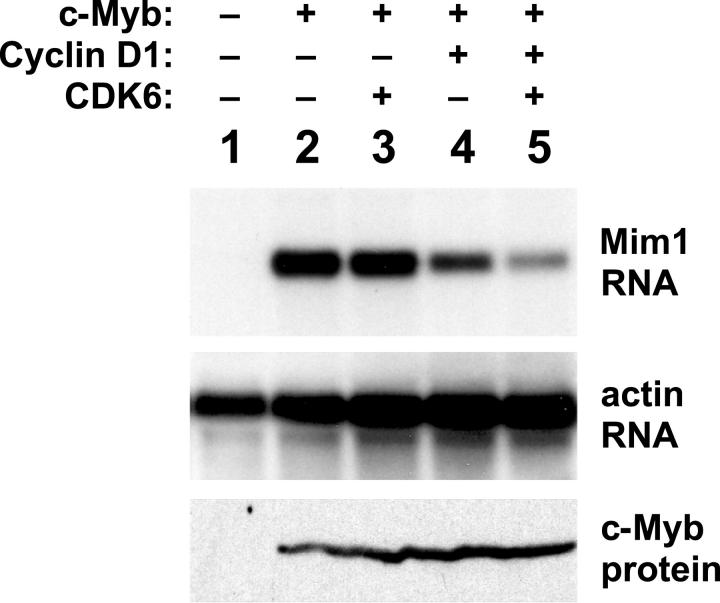
CDK inhibitors affect c-Myb activity. (A) Reporter-gene assays. A Myb-responsive reporter plasmid was transfected into QT6 cells along with plasmids expressing A-Myb, B-Myb, or c-Myb with or without a plasmid expressing p27 Kip1. Data are plotted as mean fold stimulation relative to the samples lacking p27. Error bars illustrate the range of replicate assays performed in parallel. (B) Endogenous gene activation assay. QT6 cells were transfected with combinations of plasmids expressing NF-M plus c-Myb and various CDK inhibitors as indicated at the top. After 2 days, cells were harvested and analyzed by Northern blotting using a probe specific for Mim1 then stripped and sequentially rehybridized with probes for lysozyme and beta-actin (as an RNA loading control). Western blotting showed that equivalent levels of c-Myb were expressed in all the samples (not shown). (C) Effects of cell-cycle regulators. HD-11 cells were transfected with plasmids expressing c-Myb alone (lane 1) or together with plasmids expressing the indicated combinations of cell-cycle regulators. After 2 days, RNA was harvested and analyzed by Northern blotting for the expression of the endogenous Mim1 or beta-actin genes. (D) Regulation summary. The data suggest a regulatory pathway in which increased cyclin D1/CDK inhibits the ability of c-Myb to activate specific target genes. Expression of CDK inhibitors p16, p21, or p27 stimulates c-Myb by inhibiting the activity of the cyclin D1/CDK complex.
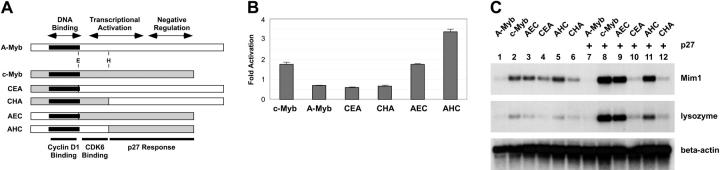
Figure 5.
Transferring the p27-responsive domain. (A) Myb protein structures. The diagrams summarize the structures of A-Myb (□) and c-Myb ( ) and illustrate the conserved DNA binding, transcriptional activation, and negative regulatory domains. The highly conserved DNA-binding domain is labeled and shaded black. The diagrammed recombinant proteins were constructed by swapping cDNA fragments at the conserved EcoRI (E) and HincII (H) sites, as indicated. Regions of c-Myb involved in interactions with cyclin D1 and CDK6 or that confer p27 responsiveness are indicated at the bottom. (B) Activation of A-Myb/c-Myb recombinants. QT6 cells were transfected with a Myb-responsive reporter plasmid as described in Figure 4 along with plasmids expressing c-Myb, A-Myb, or the recombinant Myb proteins CEA, CHA, AEC,or AHC, either alone or along with a plasmid expressing p27. The data are plotted as fold activation by p27, relative to the activity observed by the various Myb proteins alone. (C) Target gene activation. QT6 cells were transfected with combinations of plasmids expressing NF-M and various Myb proteins or swap constructs either with or without p27, as indicated at the top. After 2 days, the samples were harvested and analyzed by Northern blotting for Mim1 then stripped and reprobed for lysozyme and beta-actin. Not shown: Western blotting showed that p27 expression had little or no effect on the expression or stability of the Myb proteins.
) and illustrate the conserved DNA binding, transcriptional activation, and negative regulatory domains. The highly conserved DNA-binding domain is labeled and shaded black. The diagrammed recombinant proteins were constructed by swapping cDNA fragments at the conserved EcoRI (E) and HincII (H) sites, as indicated. Regions of c-Myb involved in interactions with cyclin D1 and CDK6 or that confer p27 responsiveness are indicated at the bottom. (B) Activation of A-Myb/c-Myb recombinants. QT6 cells were transfected with a Myb-responsive reporter plasmid as described in Figure 4 along with plasmids expressing c-Myb, A-Myb, or the recombinant Myb proteins CEA, CHA, AEC,or AHC, either alone or along with a plasmid expressing p27. The data are plotted as fold activation by p27, relative to the activity observed by the various Myb proteins alone. (C) Target gene activation. QT6 cells were transfected with combinations of plasmids expressing NF-M and various Myb proteins or swap constructs either with or without p27, as indicated at the top. After 2 days, the samples were harvested and analyzed by Northern blotting for Mim1 then stripped and reprobed for lysozyme and beta-actin. Not shown: Western blotting showed that p27 expression had little or no effect on the expression or stability of the Myb proteins.
Northern and Western blots and immunoprecipitations
QT6 or HD-11 cells were seeded into 6-well plates (3.75 × 105 cells/well) 24 hours in advance and were transfected using 1.0 μg or 0.2 μg of each expression plasmid and a total of 3.0 μg or 0.6 μg of plasmid DNA (adjusted with empty expression plasmid), respectively. Forty-eight hours later, 10% of the recovered cells were used for Western blotting to detect the expressed proteins. Remaining cells were used for total RNA extraction with the RNeasy Mini Kit (Qiagen, Valencia, CA). Five micrograms of the resulting total RNA was separated in a 1% formaldehyde agarose gel and transferred to Highbond-N membrane (Amersham Pharmacia Biotech, Piscataway, NJ). The blots were hybridized with radiolabeled probes specific for the Mim1, lysozyme, and β-actin genes, as described.39
Western blots were performed as described previously18 using rabbit antibodies specific to the Myb DNA-binding domain.29 Antibody-protein complexes were detected using enhanced chemiluminescence (ECL) reagents as described by the manufacturer (Amersham Life Science, Arlington Heights, IL). Coimmunoprecipitations using anti-FLAG epitope beads (Sigma, St Louis, MO) were performed as described previously.31,32
For Jurkat cell extract coimmunoprecipitations, approximately 1 × 108 cells were lysed by sonication in lysis buffer (50 mM Tris-Cl, pH 7.4; 1% nonidet P–40; 0.5% deoxycholate; 0.1% sodium dodecyl sulfate; 150 mM NaCl) supplemented with protease inhibitors (1 μM each chymostatin, leupeptin, antipain, pepstatin-A; 1 mM each phenylmethylsulfonyl fluoride and benzamidine). The extract was cleared by centrifugation (20 minutes at 15 000g) and pretreated for 1 hour with protein-G beads at 4°C (Amersham Biosciences, Sunnyvale, CA). After centrifugation the supernatant was mixed with 10 μL of nonimmune rabbit serum or anti–c-Myb antiserum (SC7874; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour, then collected by addition of protein-G beads for another hour. The complexes were collected by centrifugation, washed 3 times with lysis buffer, then analyzed by Western blotting.
Results
CDK4 and CDK6 interact with c-Myb in human cells
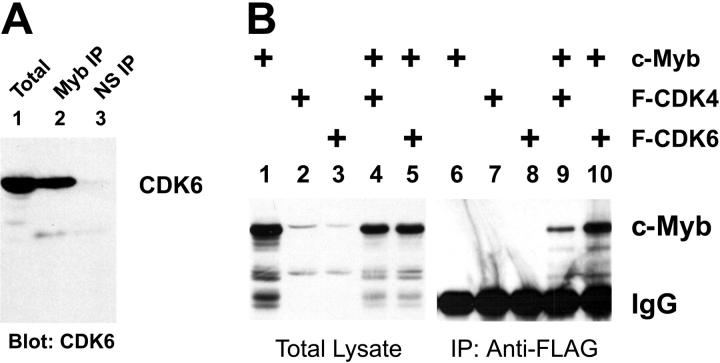
The c-Myb protein has been linked to the regulation of proliferation7,8 and can interact with cyclin D1.37 We used coimmunoprecipitation assays to test whether c-Myb also formed stable complexes with cyclin-dependent kinases (CDKs) in animal cells. As a first approach, we used a coimmunoprecipitation assay to test whether c-Myb and CDK6 were present in a stable complex in Jurkat cells, a human T-cell line that expresses high levels of c-Myb and CDK6. Briefly, cell extracts from Jurkat cells were subjected to immunoprecipitation using several different anti–c-Myb antibodies. After extensive washing, the resulting complexes were analyzed by Western blotting using anti-CDK6 antibodies. As shown in Figure 1A, CDK6 was readily detected both in the total extract used as a starting point in the coimmunoprecipitation assay (lane 1) and in the anti-Myb immunoprecipitate (lane 2) but was not detected in the sample immunoprecipitated with a control, nonspecific antiserum (lane 3). Since 10-fold more extract was used for the immunoprecipitation than for the analysis of the total sample, we estimate that approximately 10% of the endogenous CDK6 protein in Jurkat cells exists in a complex with c-Myb. The interactions were not affected by addition of ethidium bromide (not shown), suggesting that they were independent of DNA and occurred through protein-protein interactions.42 These results show that c-Myb and CDK6 exist in a stable complex in human T cells and suggest that CDK6 is likely to be an important regulator of c-Myb transcriptional activity. The c-Myb protein has been shown to interact directly with cyclin D1,37 a result that we have confirmed in cotransfection/immunoprecipitation assays (data not shown). However, repeated attempts were unable to detect the presence of cyclin D1 in immunoprecipitates from untransfected Jurkat cells, probably because the endogenous cyclin D1 protein is very unstable and present at very low steady-state levels. Although c-Myb has been shown to be required for T-cell proliferation, this is the first time that c-Myb has been shown to be associated with components of the cell-cycle machinery in human hematopoietic or lymphoid cells, without overexpression or transfection, and when both proteins are expressed at their normal, physiologically relevant levels.
Figure 1.
CDKs interact with c-Myb. (A) CDK6 and c-Myb are in a complex in human cells. Extracts from Jurkat T cells were prepared and analyzed for CDK6 expression using a total extract (lane 1) or samples that were first immunoprecipitated (IP) using anti-Myb antibodies (lane 2) or nonspecific antiserum (NS; lane 3). The samples were analyzed by Western blotting using anti-CDK6 antibodies for detection. The amount of sample analyzed in lane 1 is 10% of the amount used for the immunoprecipitations in lanes 2 and 3. (B) CDK coimmunoprecipitation. QT6 fibroblast cells were transfected with plasmids expressing c-Myb (lanes 1, 4-6, 9, 10), FLAG epitope-tagged CDK4 (lanes 2, 4, 7, 9), and FLAG epitope-tagged CDK6 (lanes 3, 5, 8, 10) as indicated at top. After 2 days, extracts were prepared and a small sample (10% of total) was saved for total extract analysis (lanes 1-5) while the remainder of each was subjected to immunoprecipitation using anti-FLAG beads (lanes 6-10). All the samples were analyzed by Western blotting using anti-Myb antibodies. The migration of the c-Myb protein is indicated at the right as is the immunoglobulin (IgG) band from the immunoprecipitation that cross-reacts with the second antibody used in the Western blot analysis.
To characterize the interactions between c-Myb and CDK inhibitors in more detail we constructed expression vectors for CDK4 and CDK6 that expressed the cell-cycle regulators as fusion proteins containing N-terminal FLAG epitope tags, then expressed the modified CDKs either alone or in combination with c-Myb. We performed the experiment in fibroblast cells, which express little or no endogenous c-Myb protein, in order to avoid any interference by endogenous proteins. Anti-FLAG beads were used to harvest the CDK-containing protein complexes, the immunoprecipitates were washed under stringent conditions, and the recovered complexes were analyzed by Western blotting using anti-Myb antibodies. The left side of Figure 1B (lanes 1-5) shows the Western blot of the starting lysates. The right side (Figure 1B lanes 6-10) shows the coimmunoprecipitated samples. The anti-FLAG beads did not precipitate any detectable c-Myb in the absence of the CDKs (Figure 1B lane 6) or when the CDKs were expressed without c-Myb (Figure 1B lanes 7-8), but c-Myb coprecipitated with both CDK4 and CDK6 when the proteins were coexpressed (Figure 1B lanes 9-10). Similar results were obtained using chicken, mouse, or human c-Myb or CDK proteins (data not shown), suggesting that the domains required for interactions have been conserved in vertebrate evolution. Judging from the intensities of the bands and the fact that 10 times more extract was used for the immunoprecipitations than for the initial Western blot analysis, we estimate that approximately 10% of the c-Myb protein coimmunoprecipitated with the FLAG-CDK6, somewhat less with the FLAG-CDK4. The finding that only a fraction of the c-Myb coprecipitated could indicate that its association with CDK4 or CDK6 is transient or cell-cycle dependent or could indicate that not all cells were equally transfected by both expression plasmids. Nevertheless, based on the results from the Jurkat cells shown in Figure 1A and these overexpression experiments, we conclude that c-Myb can form a stable complex with either CDK4 or CDK6 in human or animal cells.
The c-Myb transcriptional activation domain is required for CDK6 complex formation
The results described in Figure 1 showed that full-length c-Myb and CDK proteins could form a stable complex. Next, we used several c-Myb deletion constructs (Figure 2A) to map the region of the c-Myb protein that was required for the interaction with CDK6. Plasmids expressing the various deletion constructs were cotransfected along with the FLAG-CDK6 expression vector in fibroblasts, then coimmunoprecipitation assays were used to detect CDK6-Myb complexes, as described above. As shown in Figure 2B, FLAG-CDK6 associated with the deletion constructs CT and CH, which were truncated at residues 365 and 304, respectively (Figure 2B lanes 5-6), but did not interact with the shorter construct, CP, which was truncated at residue 230 (Figure 2B lane 4). Thus, the region of c-Myb between residues 230 and 304 was required for interaction with CDK6 and, as shown in similar experiments, CDK4 (data not shown). The structures of the CDK-binding regions from human, murine, and chicken c-Myb proteins are compared in Figure 2C, where conserved residues are shown in upper case and the most conserved blocks of amino acids are shaded. The domain contains a 54-residue block that is almost totally conserved between the c-Myb proteins of humans, rodents, and birds and that is predicted45,46 to contain a coiled-coil region that could mediate protein interactions. This coiled-coil region is also conserved in the related protein A-Myb. The conserved block is highly charged, containing numerous acidic and basic residues, and constitutes part of the minimal transcriptional activation domain of c-Myb.48-50 Part of the conserved block has been shown to bind transcriptional coactivators such as CBP (cyclic adenosine monophosphate [cAMP]–response element–binding protein [CREB]–binding protein).47,51-53 In glutathione-S–transferase (GST) pull-down experiments, bacterially expressed fragments of c-Myb were unable to form a stable complex with in vitro–translated CDK6, suggesting that either the complex formation requires CBP or another as-yet-unidentified protein that was not present in the in vitro binding assay or that complex formation required one or both of the components to be posttranslationally modified. For example, activation of cyclin-dependent kinases depends on specific activating kinases54-56 that could affect their ability to interact with c-Myb. Although our results show that c-Myb forms a stable complex with CDK4 and CDK6 in animal cells, the data do not rule out the possibility that an unidentified third protein might act as an adaptor to mediate the interactions between c-Myb and CDKs.
Cyclin/CDK expression affects c-Myb activity
The results described so far showed that c-Myb could interact with CDKs. Next, we set out to test whether coexpression of cyclins and CDKs could influence c-Myb transcriptional activity. Several reports have shown that reporter gene assays using model promoters can give misleading results about the activities of various Myb proteins.29,38,50,57,58 To avoid such complications, we started by testing whether cyclins and CDKs could influence the ability of c-Myb to activate an endogenous target gene. Unfortunately, very few Myb-regulated genes have been well characterized from human cells. However, the chicken Mim1 gene is one of the best-characterized natural target genes known to be regulated by Myb proteins in normal and transformed cells.59 The Mim1 gene promoter contains binding sites for c-Myb as well as NF-M, the chicken version of CCAAT/enhancer-binding protein β (C/EBPβ).60 Furthermore, ectopic expression of c-Myb is sufficient to activate transcription of the endogenous Mim1 gene in cells that already express NF-M, such as chicken HD-11 macrophage cells.39 Coexpression of c-Myb plus NF-M can activate the endogenous Mim1 gene in other cells, such as QT6 fibroblasts.39,50 Activation of Mim1 gene expression has been used in several previous studies to follow the regulation of c-Myb transcriptional activity.39,50,57,58 Here, chicken HD-11 cells were transfected with plasmids expressing c-Myb alone or in combination with cyclin D1 and CDK6. After 2 days, RNA was purified and assayed by Northern blotting to monitor the activation of the endogenous Mim1 gene.39,59 As shown in Figure 3, no Mim1 RNA was detectable in the control sample (lane 1), but Mim1 was strongly expressed in the cells that were transfected with the c-Myb expression vector (lanes 2-5). Coexpression of CDK6 by itself had little, if any, effect on the ability of c-Myb to activate the Mim1 gene (Figure 3 lane 3). However, coexpression of cyclin D1 led to partial inhibition of c-Myb activity (Figure 3 lane 4), which was more dramatic when cyclin D1 and CDK6 were expressed together (Figure 3 lane 5). Western blot analysis (Figure 3 bottom panel) showed that the coexpression of cyclin D1 and/or CDK6 had no effect on the expression of c-Myb, suggesting that the decrease in Mim1 gene expression was due to decreased c-Myb activity rather than a reduction in c-Myb protein levels. The inability of cyclin D1 plus CDK6 to completely block c-Myb activity may reflect the fact that only a fraction of the c-Myb formed a complex with CDK6 in cotransfection experiments (Figure 1). These results, which are representative of many similar experiments, suggest that increased activity of cyclin D1/CDK leads to an inhibition of c-Myb transcriptional activity and affects the ability of c-Myb to activate target genes in hematopoietic cells.
Figure 3.
Cell-cycle regulators affect c-Myb activity. Chicken HD-11 macrophage cells were transfected with plasmids expressing c-Myb plus combinations of cyclin D1 and CDK6, as indicated at the top. After 2 days, RNA was prepared and analyzed by Northern blotting for expression of the Myb-inducible Mim1 gene (top) or beta-actin as an RNA loading control (middle). Aliquots of the samples were also analyzed for c-Myb protein expression by Western blotting using anti-Myb antibodies (bottom).
Activation of c-Myb activity by p27 Kip1
The results described so far showed that c-Myb interacted with CDKs and that overexpression of cyclin D1 plus CDK6 could inhibit the ability of c-Myb to activate endogenous target genes. However, it was possible that overexpression of the cell-cycle regulators affected c-Myb activity through some indirect mechanism. The p27 Kip1 protein is a tumor suppressor and inhibitor of CDKs. We reasoned that testing the effect of p27 and other CDK inhibitors that affect cyclin/CDK activity would help verify that c-Myb activity is regulated by cyclins and CDKs by showing that c-Myb activity could be influenced without overexpressing the cyclin or CDK molecules themselves. Besides c-Myb, vertebrates also express 2 related proteins, A-Myb and B-Myb, with nearly identical DNA-binding domains but unique C-terminal domains and biologic activities. All 3 Myb proteins bind the same DNA sequences and activate the same reporter genes in transfection assays.38,58 However, as shown in Figure 4A, when the 3 proteins were tested in a reporter gene assay with or without coexpressed p27, the CDK inhibitor stimulated the activity of c-Myb about 2.5-fold, whereas the relative activities of A-Myb and B-Myb were inhibited about 2-fold in the presence of p27. Thus, expression of the CDK inhibitor p27 specifically stimulated the activity of c-Myb but not the related proteins A-Myb or B-Myb, suggesting that the effect of p27 was a specific effect on c-Myb activity and not a general effect on the reporter gene assay or on transcription in general.
We next tested the ability of p27 to influence c-Myb activity in endogenous gene activation assays like the one described in Figure 3. In addition to the Mim1 gene, the lysozyme gene is also Myb-regulated in some cell types.61 We used these Myb-responsive genes to test whether p27 would also affect the ability of c-Myb to activate endogenous target genes. Briefly, QT6 fibroblasts, which usually do not express Mim1 or lysozyme, were transfected with plasmids expressing c-Myb and NF-M along with plasmids expressing various CDK inhibitors. After 2 days, RNA was harvested and analyzed by Northern blotting using probes specific for Mim1 or lysozyme (or beta-actin as an RNA loading control). As shown in Figure 4B, expression of c-Myb plus NF-M led to modest expression of Mim1 and minimally detectable expression of the lysozyme gene (lane 1). Neither gene was expressed when the c-Myb expression plasmid was omitted (data not shown). In these transfected QT6 cells, coexpression of CDK inhibitors p16 (Ink4a) or p19 (Arf) had no effect, but coexpression of p21 (Cip1) or p27 (Kip1) led to dramatically increased induction of the Mim1 and lysozyme genes. Control experiments showed that the CDK inhibitors did not activate the Mim1 or lysozyme genes in the absence of Myb proteins (data not shown; Figure 5), suggesting that the response depended on c-Myb and not the coexpressed NF-M or other transcription factors that might also bind the promoters. None of the transfected plasmids affected the expression of beta-actin, which was used as an RNA loading control.
The results described showed that p21 and p27 could stimulate c-Myb activity in transfected fibroblasts. A similar approach was used to test whether CDK inhibitors could also affect c-Myb activity in myeloid cells. Chicken HD-11 macrophage cells were transfected with plasmids expressing c-Myb either alone (Figure 4C lane 1) or together with combinations of plasmids expressing cyclin D1, wild-type, or dominant-negative (DN) kinase-dead CDK6 and the CDK inhibitors p27 Kip1 or p16 Ink4a. As shown in Figure 4C, the ability of c-Myb to activate the endogenous Mim1 gene was inhibited by cyclin D1 (lane 2) or cyclin D1 plus CDK6 (lane 5) in the absence of CDK inhibitors. However, that inhibition was blocked by expression of the DN-CDK6 (Figure 4C lanes 4, 6), suggesting that CDK6 kinase activity was required for c-Myb inhibition. In contrast to the results obtained using fibroblasts, expression of either p16 Ink4a or p27 Kip1 had a strong stimulatory effect on c-Myb transcriptional activity in the myeloid cells (Figure 4C lanes 7-12). The most dramatic effect was observed when p16 or p21 was expressed along with the DN-CDK6, again suggesting that inhibition of CDK6 kinase activity led to stimulation of c-Myb transcriptional activity. Thus, overexpression of CDK inhibitors stimulated c-Myb activity, consistent with a model in which p16, p21, or p27 inhibited the cyclin/CDK complexes that in turn inhibited c-Myb (Figure 4D). The effects of CDK inhibitors have been shown to vary dramatically in different cell types,62-64 perhaps because of differences in the expression of specific cyclins.65 The finding that p16 expression was able to activate c-Myb activity in hematopoietic cells but not fibroblasts suggests that the cyclins or cyclin-dependent regulatory cascades differ substantially in the 2 cell types.
Mapping the p27 response domain in c-Myb
Since p27 was able to stimulate the activity of c-Myb but not A-Myb, we took advantage of conserved restriction sites and constructed several hybrids between these 2 related transcription factors in order to map the p27-responsive region in c-Myb. As shown in Figure 5A, the A-Myb and c-Myb proteins share a highly conserved DNA-binding domain located near the N-terminus that was shown previously to bind to cyclin D1,37 a result that we have confirmed (data not shown). The 2 proteins also share some conserved regions throughout the transcriptional activation and negative regulatory domains,4 and the cDNAs share conserved EcoRI and HincII sites. We used these sites to construct recombinants containing the N-terminus and DNA-binding domain of c-Myb fused to the remainder of A-Myb at the EcoRI (CEA) or HincII (CHA) sites or the reciprocal constructs (AEC and AHC) with the N-terminus and DNA-binding domain of A-Myb fused to the C-terminal domains of c-Myb. All of the hybrids were verified by nucleotide sequencing and each expressed proteins of the expected sizes (data not shown) and activated reporter genes, suggesting that the DNA binding and transcriptional activation functions were intact and functional.
The ability of coexpressed p27 to stimulate the activity of the various swap constructs was first tested in a reporter gene assay. As shown in Figure 5B, p27 stimulated the activity of c-Myb about 2-fold but not A-Myb or the hybrid CEA and CHA proteins containing the C-terminal domains of A-Myb, all of which were inhibited somewhat by p27 expression. However, p27 did stimulate the complementary proteins AEC and AHC containing the N-terminus and DNA-binding domain of A-Myb fused to the C-terminal regions of c-Myb. The AHC protein contains residues 1 to 285 of A-Myb fused to residues 307 to 641 of c-Myb. Thus, even in the transfected cells in which c-Myb was dramatically overexpressed, coexpression of p27 stimulated c-Myb activity 1.5- to 3.5-fold. These results confirm that p27 expression can stimulate the activity of c-Myb and show that the stimulation is both specific and dependent on the C-terminal domain of c-Myb. The results also show that the ability of c-Myb to respond to p27 can be transferred to a heterologous transcription factor, in this case A-Myb, by transferring the p27-responsive domain.
The swap constructs were also tested for their ability to activate endogenous Myb-dependent target genes. As shown in Figure 5C, A-Myb was unable to cooperate with NF-M to activate the endogenous Mim1 or lysozyme genes, regardless of p27 expression (lanes 1, 7). However, c-Myb did activate the Mim1 and lysozyme genes (Figure 5C lane 2), and the activation increased dramatically in the presence of p27 (Figure 5C lane 8). Although the recombinants containing the c-Myb DNA-binding domain fused to the A-Myb transcriptional activation domain (CEA and CHA) were able to induce weak expression of Mim1 and lysozyme (Figure 5C lanes 4, 6), they were unaffected by p27 (Figure 5C lanes 10, 12). In contrast, the swap proteins containing the c-Myb transcriptional activation domain fused to the A-Myb DNA-binding domain (AEC and AHC) activated Mim1 and lysozyme expression and were strongly stimulated by coexpression of p27 (Figure 5C lanes 3, 5, 9, 11). Thus, p27 expression stimulated the transcriptional activity of c-Myb, whether measured on plasmid-based reporter genes or endogenous Myb-regulated target genes, and the ability to respond to p27 mapped to the unique C-terminal regulatory domains of c-Myb.
Discussion
The c-Myb transcription factor has been implicated in the regulation of hematopoietic cell proliferation and differentiation,4,39 and oncogenic versions of c-Myb induce leukemias and lymphomas in birds and mammals. Although c-Myb has been shown to interact with cyclin D1,37 little is known about the regulation of c-Myb transcriptional activity during the cell cycle or by cell-cycle regulators such as CDKs or CDK inhibitors. The results described here show that c-Myb forms a stable complex with CDKs in human T cells and that expression of cell-cycle regulators affects the transcriptional activity of c-Myb, whether measured with plasmid-based reporter genes containing model promoters or by following well-characterized target genes that are known to be directly regulated by c-Myb in hematopoietic cells. Interestingly, the CDK6-binding domain in c-Myb contains a coiled-coil region that is also highly conserved in A-Myb. This may explain why transferring the C-terminal domain of c-Myb to A-Myb renders fusion constructs like the AHC protein p27 responsive (Figure 5).
One of the most important conclusions from our results is that a relatively large fraction of c-Myb and CDK6 exists in a stable complex in human T cells. The complex was detected using coimmunoprecipitation from Jurkat cells and also formed when c-Myb was ectopically coexpressed with CDK4 or CDK6 in other cell types. The stable interaction between c-Myb and CDKs detected here suggests that c-Myb is under direct control of the cell-cycle machinery and that its transcriptional activity changes during the G1/S phase transition, when cyclin D1–directed kinases become active. This model is analogous to the findings for the related transcription factor B-Myb, which is subject to regulation by cyclin A/CDK2 phosphorylation in S phase and G2.34,66-68 However, our results indicated that the effects of cyclin D1/CDK6 or p27 Kip1 were specific for c-Myb and did not affect the activities of B-Myb or A-Myb, the other Myb-related protein expressed in vertebrates. Thus, c-Myb appears to be regulated by a specific cell-cycle–dependent regulatory mechanism that distinguishes it from A-Myb or B-Myb.
Our results showed that c-Myb transcriptional activity could be inhibited by the combination of cyclin D1 plus CDK4 or CDK6 or stimulated by expression of the CDK inhibitors p16 Ink4a, p21 Cip1, or p27 Kip1. Based on these results, we predict that c-Myb transcriptional activity should be inhibited during the early part of the G1 phase of the cell cycle, when cyclin D1–dependent CDK activity is high, and should increase in late G1 or at the G1/S phase transition, when p21 and p27 levels rise and cyclin D1/CDK activity is inhibited, and provide a mechanism for how c-Myb activity is regulated during the cell cycle. The combined results place c-Myb in a pathway downstream of p21 Cip1, p27 Kip1, cyclin D1, and CDK6 that regulates important genes required for the initiation of S phase in human T cells (Figure 4D). Our recent results have shown that small changes in the transcriptional activation domain of c-Myb can have profound effects on its transcriptional activity,50 suggesting that point mutations or posttranslational modifications are likely to affect the specificity of c-Myb and determine which genes it is able to regulate in each cellular context.69 A combination of proteomic and genomic approaches will likely be required to fully investigate the flexible activities of Myb transcription factors during the cell cycle in different cell types and in normal and transformed cells.
The complex formation with CDK6 depended on a highly conserved domain within the minimal transcriptional activation domain of c-Myb that includes a coiled-coil domain that binds the transcriptional coactivator CBP47 and that is also conserved in the related protein A-Myb. It is possible that the cell-cycle regulators cyclin D1/CDK6 regulate c-Myb activity indirectly by modifying the activity of or the interaction with CBP. Alternatively, a third as-yet-unidentified protein may bind c-Myb and serve as the target for cell-cycle regulation. Interestingly, response to the expression of the CDK inhibitor p27 Kip1 was mapped to the C-terminal regulatory domains of c-Myb that contain a previously identified phosphorylation site,28,70 which could be a target of CDKs and that is involved in intramolecular interactions within the c-Myb protein.29 Thus, CDK phosphorylation of c-Myb could influence the intramolecular interactions and affect the transcriptional activity of c-Myb. One of the most interesting results was the cell type–specific activity of some CDK inhibitors, especially p16, which activated c-Myb in hematopoietic cells but not in fibroblasts. Although cell type–specific effects of p16 have been reported previously,62-64 this result is unexpected, and comparing the activities of p16 in different cell types may provide a means of identifying the mechanisms involved in CDK regulation of c-Myb activity. The c-Myb/CDK6 complex from T cells will likely be a fruitful starting place for future biochemical or proteomic studies that can dissect the interactions and the nature of the cell-cycle–regulated complex in more detail.
Acknowledgments
The authors thank J. P. O'Rourke and J. J. Rushton in the Ness laboratory for helpful advice, discussions, and reagents; J. D. Leverson who performed the initial experiments with p27 and c-Myb; and D. Beach, R. G. Pestell, and C. J. Sherr for providing expression vectors and cDNAs.
Prepublished online as Blood First Edition Paper, February 1, 2005; DOI 10.1182/blood-2004-08-3342.
Supported by grants to S.A.N. from the United States Public Health Service (USPHS)/National Cancer Institute (no. RO1 CA58443) and The Human Frontiers Science Program (no. RG0358/1999M) and by institutional support from the University of New Mexico (UNM) Health Sciences Center and the UNM Cancer Research and Treatment Center.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Graf T. Myb: a transcriptional activator linking proliferation and differentiation in hematopoietic cells. Curr Opin Genet Dev. 1992;2: 249-255. [DOI] [PubMed] [Google Scholar]
- 2.Weston K. Myb proteins in life, death and differentiation. Curr Opin Genet Dev. 1998;8: 76-81. [DOI] [PubMed] [Google Scholar]
- 3.Ness SA. Myb binding proteins: regulators and cohorts in transformation. Oncogene. 1999;18: 3039-3046. [DOI] [PubMed] [Google Scholar]
- 4.Ness SA. The myb oncoprotein: regulating a regulator. Biochim Biophys Acta. 1996;1288: F123-F139. [DOI] [PubMed] [Google Scholar]
- 5.Mucenski ML, McLain K, Kier AB, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65: 677-689. [DOI] [PubMed] [Google Scholar]
- 6.Gewirtz AM, Calabretta B. A c-myb antisense oligodeoxynucleotide inhibits normal human hematopoiesis in vitro. Science. 1988;242: 1303-1306. [DOI] [PubMed] [Google Scholar]
- 7.Gewirtz AM, Anfossi G, Venturelli D, Valpreda S, Sims R, Calabretta B. G1/S transition in normal human T-lymphocytes requires the nuclear protein encoded by c-myb. Science. 1989;245: 180-183. [DOI] [PubMed] [Google Scholar]
- 8.Anfossi G, Gewirtz AM, Calabretta B. An oligomer complementary to c-myb-encoded mRNA inhibits proliferation of human myeloid leukemia cell lines. Proc Natl Acad Sci U S A. 1989;86: 3379-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melani C, Rivoltini L, Parmiani G, Calabretta B, Colombo MP. Inhibition of proliferation by c-myb antisense oligodeoxynucleotides in colon adenocarcinoma cell lines that express c-myb. Cancer Res. 1991;51: 2897-2901. [PubMed] [Google Scholar]
- 10.Zorbas M, Sicurella C, Bertoncello I, et al. c-Myb is critical for murine colon development. Oncogene. 1999;18: 5821-5830. [DOI] [PubMed] [Google Scholar]
- 11.Hodges LC, Cook JD, Lobenhofer EK, et al. Tamoxifen functions as a molecular agonist inducing cell cycle-associated genes in breast cancer cells. Mol Cancer Res. 2003;1: 300-311. [PubMed] [Google Scholar]
- 12.Kauraniemi P, Hedenfalk I, Persson K, et al. MYB oncogene amplification in hereditary BRCA1 breast cancer. Cancer Res. 2000;60: 5323-5328. [PubMed] [Google Scholar]
- 13.Villa AE, Guzman LA, Poptic EJ, et al. Effects of antisense c-myb oligonucleotides on vascular smooth muscle cell proliferation and response to vessel wall injury. Circ Res. 1995;76: 505-513. [DOI] [PubMed] [Google Scholar]
- 14.Husain M, Bein K, Jiang L, Alper SL, Simons M, Rosenberg RD. c-Myb-dependent cell cycle progression and Ca2+ storage in cultured vascular smooth muscle cells. Circ Res. 1997;80: 617-626. [DOI] [PubMed] [Google Scholar]
- 15.Dubendorff JW, Whittaker LJ, Eltman JT, Lipsick JS. Carboxy-terminal elements of c-Myb negatively regulate transcriptional activation in cis and in trans. Genes Dev. 1992;6: 2524-2535. [DOI] [PubMed] [Google Scholar]
- 16.Miglarese MR, Richardson AF, Aziz N, Bender T. Differential regulation of c-Myb-induced transcription activation by a phosphorylation site in the negative regulatory domain. J Biol Chem. 1996; 271: 22697-22705. [DOI] [PubMed] [Google Scholar]
- 17.Andersson KB, Kowenz-Leutz E, Brendeford EM, Tygsett AH, Leutz A, Gabrielsen OS. Phosphorylation dependent down-regulation of c-Myb DNA-binding is abrogated by a point mutation in the v-myb oncogene. J Biol Chem. 2002;278: 3816-3824. [DOI] [PubMed] [Google Scholar]
- 18.Leverson JD, Ness SA. Point mutations in v-Myb disrupt a cyclophilin-catalyzed negative regulatory mechanism. Mol Cell. 1998;1: 203-211. [DOI] [PubMed] [Google Scholar]
- 19.Badiani PA, Kioussis D, Swirsky DM, Lampert IA, Weston K. T-cell lymphomas in v-Myb transgenic mice. Oncogene. 1996;13: 2205-2212. [PubMed] [Google Scholar]
- 20.Davies J, Badiani P, Weston K. Cooperation of Myb and Myc proteins in T cell lymphomagenesis. Oncogene. 1999;18: 3643-3647. [DOI] [PubMed] [Google Scholar]
- 21.Gonda TJ, Cory S, Sobieszsczuk P, Holtzman D, Adams J. Generation of altered transcripts by retroviral insertion within the c-myb gene in two murine monocytic leukemias. J Virol. 1987;61: 2754-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y, Ramsay RG, Kanei-Ishii C, Ishii S, Gonda TJ. Transformation by carboxyl-deleted Myb reflects increased transactivating capacity and disruption of a negative regulatory domain. Oncogene. 1991;6: 1549-1551. [PubMed] [Google Scholar]
- 23.Gonda TJ, Ramsay RG, Johnson GR. Murine myeloid cell lines derived by in vitro infection with recombinant c-myb retroviruses express myb from rearranged vector proviruses. EMBO J. 1989;8: 1767-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grässer FA, Graf T, Lipsick JS. Protein truncation is required for the activation of the c-myb protooncogene. Mol Cell Biol. 1991;11: 3987-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dini PW, Eltman JT, Lipsick JS. Mutations in the DNA-binding and transcriptional activation domains of v-Myb cooperate in transformation. J Virol. 1995;69: 2515-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torelli G, Selleri L, Donelli A, et al. Activation of c-myb expression by phytohemagglutinin stimulation in normal human T lymphocytes. Mol Cell Biol. 1985;5: 2874-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipsick JS, Boyle WJ. c-myb protein expression is a late event during T-lymphocyte activation. Mol Cell Biol. 1987;7: 3358-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aziz N, Wu J, Dubendorff JW, Lipsick JS, Sturgill TW, Bender TP. c-Myb and v-Myb are differentially phosphorylated by p42mapk in vitro. Oncogene. 1993;8: 2259-2265. [PubMed] [Google Scholar]
- 29.Dash AB, Orrico FC, Ness SA. The EVES motif mediates both intermolecular and intramolecular regulation of c-Myb. Genes Dev. 1996;10: 1858-1869. [DOI] [PubMed] [Google Scholar]
- 30.Kanei-Ishii C, MacMillan EM, Nomura T, et al. Transactivation and transformation by Myb are negatively regulated by a leucine-zipper structure. Proc Natl Acad Sci U S A. 1992;89: 3088-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leverson JD, Koskinen PJ, Orrico FC, et al. Pim-1 kinase and p100 cooperate to enhance c-Myb activity. Mol Cell. 1998;2: 417-425. [DOI] [PubMed] [Google Scholar]
- 32.Winn LM, Lei W, Ness SA. Pim-1 phosphorylates the DNA binding domain of c-Myb. Cell Cycle. 2003;2: 258-262. [PubMed] [Google Scholar]
- 33.Bies J, Markus J, Wolff L. Covalent attachment of the SUMO-1 protein to the negative regulatory domain of the c-Myb transcription factor modifies its stability and transactivation capacity. J Biol Chem. 2002;277: 8999-9009. [DOI] [PubMed] [Google Scholar]
- 34.Saville MK, Watson RJ. The cell-cycle regulated transcription factor B-Myb is phosphorylated by cyclin A/Cdk2 at sites that enhance its transactivation properties. Oncogene. 1998;17: 2679-2689. [DOI] [PubMed] [Google Scholar]
- 35.Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116: 221-234. [DOI] [PubMed] [Google Scholar]
- 36.Sherr CJ. Principles of tumor suppression. Cell. 2004;116: 235-246. [DOI] [PubMed] [Google Scholar]
- 37.Ganter B, Fu S, Lipsick JS. D-type cyclins repress transcriptional activation by the v-Myb but not the c-Myb DNA-binding domain. EMBO J. 1998;17: 255-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rushton JJ, Ness SA. The conserved DNA binding domain mediates similar regulatory interactions for A-Myb, B-Myb, and c-Myb transcription factors. Blood Cells Mol Dis. 2001;27: 459-463. [DOI] [PubMed] [Google Scholar]
- 39.Ness SA, Kowenz-Leutz E, Casini T, Graf T, Leutz A. Myb and NF-M: combinatorial activators of myeloid genes in heterologous cell types. Genes Dev. 1993;7: 749-759. [DOI] [PubMed] [Google Scholar]
- 40.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78: 67-74. [DOI] [PubMed] [Google Scholar]
- 41.Ashton AW, Watanabe G, Albanese C, Harrington EO, Ware JA, Pestell RG. Protein kinase Cdelta inhibition of S-phase transition in capillary endothelial cells involves the cyclin-dependent kinase inhibitor p27(Kip1). J Biol Chem. 1999;274: 20805-20811. [DOI] [PubMed] [Google Scholar]
- 42.Lai JS, Herr W. Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc Natl Acad Sci U S A. 1992;89: 6958-6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chenna R, Sugawara H, Koike T, et al. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31: 3497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerondakis S, Bishop JM. Structure of the protein encoded by the chicken proto-oncogene c-myb. Mol Cell Biol. 1986;6: 3677-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letunic I, Copley RR, Schmidt S, et al. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 2004;32(database issue): D142-D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci U S A. 1998;95: 5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zor T, De Guzman RN, Dyson HJ, Wright PE. Solution structure of the KIX domain of CBP bound to the transactivation domain of c-Myb. J Mol Biol. 2004;337: 521-534. [DOI] [PubMed] [Google Scholar]
- 48.Weston K, Bishop JM. Transcriptional activation by the v-myb oncogene and its cellular progenitor, c-myb. Cell. 1989;58: 85-93. [DOI] [PubMed] [Google Scholar]
- 49.Wang DM, Lipsick JS. Mutational analysis of the transcriptional activation domains of v-Myb. Oncogene. 2002;21: 1611-1615. [DOI] [PubMed] [Google Scholar]
- 50.Lei W, Rushton JJ, Davis LM, Liu F, Ness SA. Positive and negative determinants of target gene specificity in Myb transcription factors. J Biol Chem. 2004;279: 29519-29527. [DOI] [PubMed] [Google Scholar]
- 51.Dai P, Akimaru H, Tanaka Y, et al. CBP as a transcriptional coactivator of c-Myb. Genes Dev. 1996;10: 528-540. [DOI] [PubMed] [Google Scholar]
- 52.Oelgeschläger M, Janknecht R, Krieg J, Schreek S, Lüscher B. Interaction of the co-activator CBP with Myb proteins: effects on Myb-specific transcription and on the cooperativity with NF-M. EMBO J. 1996;15: 2771-2780. [PMC free article] [PubMed] [Google Scholar]
- 53.Kiewitz A, Wolfes H. Mapping of protein-protein interactions between c-myb and its coactivator CBP by a new phage display technique. FEBS Lett. 1997;415: 258-262. [DOI] [PubMed] [Google Scholar]
- 54.Blain SW, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J Biol Chem. 1997;272: 25863-25872. [DOI] [PubMed] [Google Scholar]
- 55.Kaldis P, Russo AA, Chou HS, Pavletich NP, Solomon MJ. Human and yeast cdk-activating kinases (CAKs) display distinct substrate specificities. Mol Biol Cell. 1998;9: 2545-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaldis P, Solomon MJ. Analysis of CAK activities from human cells. Eur J BioChem. 2000;267: 4213-4221. [DOI] [PubMed] [Google Scholar]
- 57.Introna M, Golay J, Frampton J, Nakano T, Ness SA, Graf T. Mutations in v-myb alter the differentiation of myelomonocytic cells transformed by the oncogene. Cell. 1990;63: 1287-1297. [DOI] [PubMed] [Google Scholar]
- 58.Rushton JJ, Davis LM, Lei W, Mo X, Leutz A, Ness SA. Distinct changes in gene expression induced by A-Myb, B-Myb and c-Myb proteins. Oncogene. 2003;22: 308-313. [DOI] [PubMed] [Google Scholar]
- 59.Ness SA, Marknell Å, Graf T. The v-myb oncogene product binds to and activates the promyelocyte-specific mim-1 gene. Cell. 1989;59: 1115-1125. [DOI] [PubMed] [Google Scholar]
- 60.Sterneck E, Müller C, Katz S, Leutz A. Autocrine growth induced by kinase-type oncogenes in myeloid cells requires AP-1 and NF-M, a myeloid-specific, C/EBP-like factor. EMBO J. 1992;11: 115-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klempnauer KH, Arnold H, Biedenkapp H. Activation of transcription by v-myb: evidence for two different mechanisms. Genes Dev. 1989;3: 1582-1589. [DOI] [PubMed] [Google Scholar]
- 62.Faast R, White J, Cartwright P, Crocker L, Sarcevic B, Dalton S. Cdk6-cyclin D3 activity in murine ES cells is resistant to inhibition by p16(INK4a). Oncogene. 2004;23: 491-502. [DOI] [PubMed] [Google Scholar]
- 63.Lee YK, Park JY, Kang HJ, Cho HC. Overexpression of p16Ink4a and p14ARF in haematological malignancies. Clin Lab Haematol. 2003;25: 233-237. [DOI] [PubMed] [Google Scholar]
- 64.Easton J, Wei T, Lahti JM, Kidd VJ. Disruption of the cyclin D/cyclin-dependent kinase/INK4/retinoblastoma protein regulatory pathway in human neuroblastoma. Cancer Res. 1998;58: 2624-2632. [PubMed] [Google Scholar]
- 65.Mettus RV, Rane SG. Characterization of the abnormal pancreatic development, reduced growth and infertility in Cdk4 mutant mice. Oncogene. 2003;22: 8413-8421. [DOI] [PubMed] [Google Scholar]
- 66.Lam EW, Bennett JD, Watson RJ. Cell-cycle regulation of human B-myb transcription. Gene. 1995;160: 277-281. [DOI] [PubMed] [Google Scholar]
- 67.Ziebold U, Bartsch O, Marais R, Ferrari S, Klempnauer KH. Phosphorylation and activation of B-Myb by cyclin A-Cdk2. Curr Biol. 1997;7: 253-260. [DOI] [PubMed] [Google Scholar]
- 68.Muller-Tidow C, Wang W, Idos GE, et al. Cyclin A1 directly interacts with B-myb and cyclin A1/cdk2 phosphorylate B-myb at functionally important serine and threonine residues: tissue-specific regulation of B-myb function. Blood. 2001;97: 2091-2097. [DOI] [PubMed] [Google Scholar]
- 69.Ness SA. Myb protein specificity: evidence of a context-specific transcription factor code. Blood Cells Mol Dis. 2003;31: 192-200. [DOI] [PubMed] [Google Scholar]
- 70.Aziz N, Miglarese MR, Hendrickson RC, et al. Modulation of c-Myb-induced transcription activation by a phosphorylation site near the negative regulatory domain. Proc Natl Acad Sci U S A. 1995;92: 6429-6433. [DOI] [PMC free article] [PubMed] [Google Scholar]