Abstract
Megakaryocytes (MKs) undergo successive rounds of endomitosis during differentiation, resulting in polyploidy (typically, 16-64N). Previous studies have demonstrated that this occurs through an interruption of normal cell cycle progression during anaphase. However, the molecular mechanism(s) controlling this unique process is undefined. In the present report, we examine the effect of an Src kinase inhibitor, SU6656, on thrombopoietin (TPO)-induced growth and differentiation. Remarkably, when SU6656 (2.5 μM) was added to a megakaryocytic cell line, UT-7/TPO, the cells ceased cell division but continued to accumulate DNA by endomitosis. During this interval, CD41 and CD61 expression on the cell surface increased. Similar effects on polyploidization and MK differentiation were seen with expanded primary MKs, bone marrow from 2 patients with myelodysplastic syndrome, and other cell lines with MK potential. Our data suggest that SU6656 might be useful as a differentiation-inducing agent for MKs and is an important tool for understanding the molecular basis of MK endomitosis.
Introduction
Thrombopoiesis is a complex process of megakaryocyte (MK) differentiation and fragmentation that can be divided into 4 distinct stages: (1) commitment of pluripotent stem cell to the MK lineage; (2) proliferation of MK progenitors (ie, cell division without differentiation); (3) terminal differentiation of MKs, characterized by endomitosis and cellular expansion; and (4) platelet shedding through fragmentation (reviewed in Vainchenker et al1 and Italiano and Shivdasani2). Among hematopoietic cells, polyploidization through endomitosis is unique to MKs. However, it is also seen in other cell types, including salivary glands, trophoblast, and urinary bladder epithelium.3,4
The progression of cell cycle through chromosome duplication, assembly of nuclear spindles, dissolution of the nuclear envelope, and partial separation of homologous chromosomes has been well documented in MKs.5,6 However, the process is interrupted prior to cell division, resulting in cells with twice the number of chromosomes. Our prior studies demonstrate that activation of the Src family kinases (SFKs) Lyn and Fyn may partially block MK development.7,8 We have shown that SFK inhibitors PP1 and PP2 as well as a dominant-negative form of Lyn lead to increased proliferation, higher ploidy classes, and increased extracellular signal-related kinase 1/2 (Erk1/2) activity.8 These data led us to hypothesize that SU6656, a small molecule reported to be a more specific SFK inhibitor, might be useful in evaluating MK cell cycle control during endoreduplication.9
The results presented in this paper add to the growing application of protein kinase inhibitors as tools in treating cancer and inflammatory, neurodegenerative, and cardiovascular diseases.10-13 Recently, it was demonstrated that Src family kinase (SFK) inhibitors are capable of blocking growth of leukemic cells, suggesting that targeted inhibition of SFKs may have a therapeutic role in human disease.10 Currently, a number of studies are using SU6656 to examine molecular aspects of signal transduction pathways.11,14,15 In this study we demonstrate that SU6656 may also induce polyploidization and maturation of human leukemic cell lines and primary human bone marrow progenitors.
Study design
Cells and cell culture
Myelodysplastic bone marrow cells and cadaveric organ donor marrow were used after obtaining approval from the University of Washington institutional review board. No personal identifying data were provided to the investigators, and bone marrow cells from living individuals were leftover diagnostic specimens. K562 and HEL cell lines were cultured in Iscoves modified Dulbecco medium (IMDM; Sigma, St Louis, MO) supplemented with 10% fetal bovine serum (BioWhittaker, Walkersville, MD), 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine (BioWhittaker). UT-7/thromobopoietin (TPO; kindly provided by N. Komatsu16) was maintained in IMDM with 10% fetal calf serum and 5 ng/mL human TPO (PeproTech, Rocky Hill, NJ). SU6656 (CalBiochem, La Jolla, CA) was dissolved in dimethyl sulfoxide (DMSO) and added to cells at a final concentration of 2.5 μM and 0.1% DMSO (dosage was determined based on titration vs phenotypic effect). Primary human CD34+/CD38lo were isolated and maintained as previously described.7 After 10 days in culture, cytokines were removed by washing the cells 3 times, and the pellet was resuspended in serum-free media containing recombinant human TPO (rhTPO, 35 ng/mL).
Flow cytometry
Cells were labeled with propidium iodide and nuclear ploidy was determined by flow cytometry as previously described.8 Flow cytometric analysis after immunostaining for surface expression of CD41, CD61, or an isotype-matched control antibody was performed as previously described.7 Analysis was performed using a FACScan analyzer using CELLQuest software (Becton Dickinson, San Diego, CA).
Cell viability and histologic analysis
Cells were observed by inverted light microscopy; aliquots were stained with trypan blue and counted for total viable cells by a hemocytometer. Each cell count was performed 4 times, and results were counted in triplicate. Cytospins were prepared and stained as previously described.7
Western blotting and kinase assays
Whole cell lysates were analyzed on 10% polyacrylamide gels. Transfer to nitrocellulose, blocking, probing with antibodies, and chemiluminescence were performed as previously described.8 Kinase reactions were performed at 30°C for 10 minutes in kinase buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.6], 5 mM EGTA [ethylene glycol tetraacetic acid], 1 mM dithiothreitol, 25 mM β-glycerophosphate, 7.5 mM magnesium chloride, 200 μM adenosine triphosphate (ATP), 1 μg Histone H3 [Upstate Biotechnology, Lake Placid, NY]) and 1 μg active Aurora B kinases (Upstate Biotechnology) in a total volume of 15 μL. Kinase reactions were terminated by the addition of 7 μL 3x sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer, separated by SDS-PAGE, blotted to nitrocellulose, and probed with anti-phosphohistone H3 (Upstate Biotechnology). Immunocomplex kinase assays using anti-Aurora B (Cell Signaling Technology, Beverly, MA) were performed as previously described.8
Results and discussion
SFK inhibitor SU6656 induces polyploidization of transformed human MK cell lines
UT-7/TPO is a human cell line, derived from the leukemic UT-7 line, that has characteristics of both megakaryocyte and erythroid cells. These cells proliferate but do not differentiate significantly in response to exogenous rhTPO.16 We have found that the addition of the SFK inhibitor, SU6656, at a concentration of 2.5 μM, results in rapid terminal differentiation of UT-7/TPO cells. In as early as 12 hours, a dramatic shift in cell cycle was apparent, with the majority of the cells shifting to 4N. After 24 hours 8N cells appear, and by 48 hours, 16N are readily seen (Figure 1A). Continued growth in Src kinase inhibitor yields higher ploidy states (32 and 64N; data not shown). In contrast, cells grown in the presence of 0.1% DMSO showed no change in ploidy (Figure 1A). We found a similar effect on polyploidization when other cells with megakaryocytic potential (ie, HEL and K562) were cultured in the presence of SU6656 (Figure 1B). Of note, HEL and K562 cells do not require exogenous cytokines for proliferation.
Figure 1.
SU6656 induces polyploidization. (A) UT-7/TPO cells were grown in the presence of 10 ng/mL rhTPO + 0.1% DMSO or 10 ng/mL rhTPO + 2.5 μM SU6656 for 48 hours. Cell were partially permeabilized, incubated with RNase and propidium iodide (10 ng/mL), and analyzed by flow cytometry to detect changes in nuclear ploidy under each condition: T = 0, 12, 24, and 48 hours. (B) HEL cells (upper panels) and K562 cells (lower panels) were each cultured for 72 hours under normal growth conditions (+ 0.1% DMSO) or in the presence of 2.5 μM SU6656 (+ 0.1% DMSO). Nuclear ploidy was determined by flow cytometry. Bars mark the DNA contents of 2N, 4N, 8N, 16N, and 32N. (C) Cells were cultured for 48 hours with 10 ng/mL rhTPO in the absence or presence of 2.5 μM SU6656. Morphologic changes were examined by staining cytocentrifugation preparations with Wright-Giemsa (magnification × 100). (D) Cytoplasmic protrusions are seen in the presence of SU6656 as show by Wright-Giemsastained UT-7/TPO cells at higher magnification (× 400) after 72 hours. (E) UT-7/TPO cells were cultured from an initial density of 1 × 105/mL in media containing rhTPO ± SU6656 (2.5 μM). Each day, the cell number was counted using a hemocytometer and methylene blue to exclude nonviable cells. (F) Flow cytometric analysis of indirect immunofluorescence staining of SU6656-treated and untreated cells with anti-CD41-APC, or CD61-APC, and an isotype-matched irrelevant control APC antibody. The percentage of CD41/CD61 cells is an average of 4 independent experiments. Error bars indicate standard deviation. Mean values are significantly different from untreated cells (CD41, P < .05; CD61, P < .01).
UT-7/TPO cells undergo terminal differentiation in the presence of SU6656
In addition to polyploidization, we recognized morphologic changes indicative of megakaryocytic maturation after prolonged exposure to TPO and the SFK inhibitor SU6656. Cells cultured longer than 48 hours displayed cellular enlargement and the appearance of proplatelet processes and platelet-sized particles (Figure 1C-D, and data not shown). Interestingly, cell division ceased in SU6656, but there was no significant change in the apoptotic rate as measured by annexin V staining (Figure 1E; data not shown). The expression of MK-specific markers was then evaluated using flow cytometry. The percentage of cells expressing glycoprotein IIb (CD41) and glycoprotein IIIa (CD61) increased significantly after 48 hours in the presence of SU6656 (Figure 1F). In contrast, there was no change in the expression of erythroid antigen glycophorin A (data not shown). These results suggest that SU6656 induces MK differentiation, as assessed by cell morphology and expression of specific differentiation markers. Furthermore, this represents a powerful in vitro model system to study polyploidization and cell cycle regulation.
SU6656 induces polyploidization of expanded human bone marrow progenitors
To test the effects of SU6656 on primary cells, human undifferentiated hematopoietic progenitors (CD34+/CD38lo) were isolated by flow cytometry from whole marrow. Purified CD34+/CD38lo cells were cultured in serum-free media supplemented with interleukin-3 (IL-3; 50 pg/mL), IL-6 (10 ng/mL), stem cell factor (SCF, 10 ng/mL), and TPO (50 ng/mL) (PeproTech) to expand MKs as previously described.7 After 10 days in culture, cytokines were removed by washing the cells 3 times, and cells were then resuspended in serum-free media. Half of the cells received TPO (35 ng/mL) + 0.1% DMSO, and half were treated with TPO (35 ng/mL) + SU6656 (2.5 μM). Similar to our previous studies using cell lines, primary cells demonstrated an increase in the number of higher ploidy megakaryocytes as well as an increase in cell size when SU6656 was present (Figure 2A).
Figure 2.
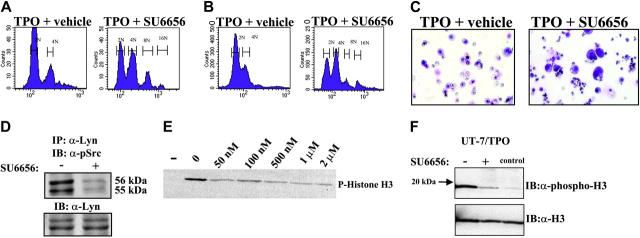
MK maturation of normal and MDS marrow in response to SU6656. (A) Primary human CD34 +/CD38lo cells were cultured in a 4-cytokine media for 10 days as previously described.7 After 10 days, cultures were washed and resuspended in serum-free media containing 35 ng/mL rhTPO. Cells were then cultured in the absence (vehicle) or the presence of 2.5 μM SU6656. After 72 hours, the samples were labeled with propidium iodide and analyzed by flow cytometry to detect ploidy classes. (B) Mononuclear cells from an individual with myelodysplastic syndrome were cultured in the presence of rhTPO (35 ng/mL) for 5 days in the absence or presence of SU6656 (2.5 μM). Cells were treated with 0.1% SDS, RNase, and propidium iodide. Nuclear ploidy was determined by flow cytometry and plotted on a semilog scale (1 × 106 events/sample). (C) MDS primary cell cultures were cytospun onto glass slides and stained with Wright-Giemsa. The photographs show representative fields of preparations at × 50 magnification. (D) Cell lysates were generated from MDS primary cells under each culture condition. Lyn was immunoprecipitated with anti-Lyn antibody (Santa Cruz Biotech, Santa Cruz, CA), analyzed by Western blot, and probed with antiphospho-Src (Tyr416) (Cell Signaling). Blots were stripped and reprobed with appropriate antibodies to ensure an equal amount of protein in each lane. (E) Purified active Aurora kinase B (lane -: no Aurora kinase B) was preincubated with SU6656 (0-2 μM) prior to the addition of the substrate (dephosphorylated histone H3). Histone H3 phosphorylation detected by Western blotting using a phospho-specific H3 antibody. (F) Aurora B kinase was immunoprecipitated from UT-7/TPO lysates, and kinase activity was assayed using purified histone H3 as a substrate. Phosphorylation was detected by blotting with phospho-specific histone H3 antibody. Blots were reprobed with antibody to histone H3. Control lane contains purified histone H3 alone. IB indicates immunoblot.
Myelodysplastic syndrome (MDS) and SU6656
MDS is a relatively common cause of acquired thrombocytopenia and increases in incidence with advanced age. In many cases, thrombocytopenia results from ineffective thrombopoiesis and abnormal (ie, dysplastic) MKs. Therefore, we tested the hypothesis that SU6656 could improve MK differentiation. Bone marrow cells were obtained from 2 individuals with confirmed MDS and were cultured under serum-free conditions with rhTPO (35 ng/mL) ± SU6656 (2.5 μM). After 72 hours of incubation, polyploid cells (8N and 16N) and cells with increased size and MK morphology were substantially increased in the presence of inhibitor (Figure 2B-C). Of interest, cultured MDS cells demonstrated constitutive activation of Lyn kinase, which was substantially decreased in the presence of SU6656 (Figure 2D). Finally, there were fewer small cells in the presence of SU6656, suggesting that the inhibitor might diminish the growth rate of clonal malignant cells.
In each of the above experiments (Figure 2B-C), the level of Lyn kinase activation was markedly decreased in the presence of SU6656 (data not shown). A similar decrease in intracellular tyrosine phosphorylation was observed in cells grown in the presence of SU6656, compared with our previous results using the Src inhibitor PP2 (data not shown).8 Although SU6656 is clearly a potent inhibitor of the Src family kinases, we have undertaken studies to determine if additional targets can be identified. We found no affect on Janus kinase 2 (Jak2), signal transducer and activator of transcription 3 (STAT3), and STAT5 tyrosine phosphorylation (data not shown). However, the activity of Aurora kinase B was inhibited in vitro by as little as 50 nM SU6656 (Figure 2E). To further extend these observations, we examined the activity of Aurora B in the presence of SU6656 in UT-7/TPO cells. Cells were incubated with SU6656 for 45 minutes before TPO stimulation. Aurora B was immunoprecipitated from the lysates and kinase activity was measured using in vitro phosphorylation assay. As shown in Figure 2F, Aurora B activity was inhibited by SU6656 in the context of intact cells. Previous studies have shown that Aurora B kinase (AIM-1) is down-regulated in human MK and erythromegakaryocytic cell lines during endomitosis and that ectopic expression of AIM-1 blocks phorbol ester-induced polyploidization.17-19 In contrast, a recent observation has shown that Aurora B is expressed and active in polyploidy MKs.20 Until additional studies are carried out, we cannot exclude the possibility that SU6656 inhibits enzymes in addition to Lyn and Aurora kinases, and we are currently studying its effect on other families of kinases. These results demonstrate that SU6656 is an important tool for understanding the molecular basis of MK endomitosis and may also have therapeutic potential.
Acknowledgments
We wish to thank Jennifer Minear for manuscript preparation.
Prepublished online as Blood First Edition Paper, January 27, 2005; DOI 10.1182/blood-2004-10-3934.
Supported by the National Institutes of Health grants R01HL65498-01 and K01DK065129-01.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Vainchenker W, Debili N, Mouthon MA, Wendling F. Megakaryocytopoiesis: cellular aspects and regulation. Crit Rev Oncol Hematol. 1995;20: 165-192. [DOI] [PubMed] [Google Scholar]
- 2.Italiano JE Jr, Shivdasani RA. Megakaryocytes and beyond: the birth of platelets. J Thromb Haemost. 2003;1: 1174-1182. [DOI] [PubMed] [Google Scholar]
- 3.Odell TT Jr, Jackson CW. Polyploidy and maturation of rat megakaryocytes. Blood. 1968;32: 102-110. [PubMed] [Google Scholar]
- 4.Brodsky WY, Uryvaeva IV. Cell polyploidy: its relation to tissue growth and function. Int Rev Cytol. 1977;50: 275-332. [DOI] [PubMed] [Google Scholar]
- 5.Vitrat N, Cohen-Solal K, Pique C, et al. Endomitosis of human megakaryocytes are due to abortive mitosis. Blood. 1998;91: 3711-3723. [PubMed] [Google Scholar]
- 6.Nagata Y, Muro Y, Todokoro K. Thrombopoietin-induced polyploidization of bone marrow megakaryocytes is due to a unique regulatory mechanism in late mitosis. J Cell Biol. 1997;139: 449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lannutti BJ, Shim MH, Blake N, Reems JA, Drachman JG. Identification and activation of Src family kinases in primary megakaryocytes. Exp Hematol. 2003;31: 1268-1274. [DOI] [PubMed] [Google Scholar]
- 8.Lannutti BJ, Drachman JG. Lyn tyrosine kinase regulates thrombopoietin-induced proliferation of hematopoietic cell lines and primary megakaryocytic progenitors. Blood. 2004;103: 3736-3743. [DOI] [PubMed] [Google Scholar]
- 9.Blake RA, Broome MA, Liu X, et al. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20: 9018-9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roginskaya V, Zuo S, Caudell E, Nambudiri G, Kraker AJ, Corey SJ. Therapeutic targeting of Src-kinase Lyn in myeloid leukemic cell growth. Leukemia. 1999;13: 855-861. [DOI] [PubMed] [Google Scholar]
- 11.Luskova P, Draber P. Modulation of the Fcepsilon receptor I signaling by tyrosine kinase inhibitors: search for therapeutic targets of inflammatory and allergy diseases. Curr Pharm Des. 2004;10: 1727-1737. [DOI] [PubMed] [Google Scholar]
- 12.Wolfrum S, Dendorfer A, Rikitake Y, et al. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24: 1842-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Li J, Chakrabarty P, Bu B, Vincent I. Cyclin-dependent kinase inhibitors attenuate protein hyperphosphorylation, cytoskeletal lesion formation, and motor defects in Niemann-Pick Type C mice. Am J Pathol. 2004;165: 843-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talmor-Cohen A, Tomashov-Matar R, Eliyahu E, Shapiro R, Shalgi R. Are Src family kinases involved in cell cycle resumption in rat eggs? Reproduction. 2004;127: 455-463. [DOI] [PubMed] [Google Scholar]
- 15.Tapia JA, Garcia-Marin LJ, Jensen RT. Cholecystokinin-stimulated protein kinase C-delta kinase activation, tyrosine phosphorylation, and translocation are mediated by Src tyrosine kinases in pancreatic acinar cells. J Biol Chem. 2003;278: 35220-35230. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu N, Kunitama M, Yamada M, et al. Establishment and characterization of the thrombopoietin-dependent megakaryocytic cell line, UT-7/TPO. Blood. 1996;87: 4552-4560. [PubMed] [Google Scholar]
- 17.Zhang Y, Sun S, Chen WC, et al. Repression of AIM-1 kinase mRNA as part of a program of genes regulated by Mpl ligand. Biochem Biophys Res Commun. 2001;282: 844-849. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki A, Matsumura I, Miyagawa J, et al. Downregulation of an AIM-1 kinase couples with megakaryocytic polyploidization of human hematopoietic cells. J Cell Biol. 2001;152: 275-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Nagata Y, Yu G, et al. Aberrant quantity and localization of Aurora-B/AIM-1 and survivin during megakaryocyte polyploidization and the consequences of Aurora-B/AIM-1-deregulated expression. Blood. 2004;103: 3717-3726. [DOI] [PubMed] [Google Scholar]
- 20.Geddis AE, Kaushansky K. Megakaryocytes express functional Aurora-B kinase in endomitosis. Blood. 2004;104: 1017-1024. [DOI] [PubMed] [Google Scholar]