Figure 4.
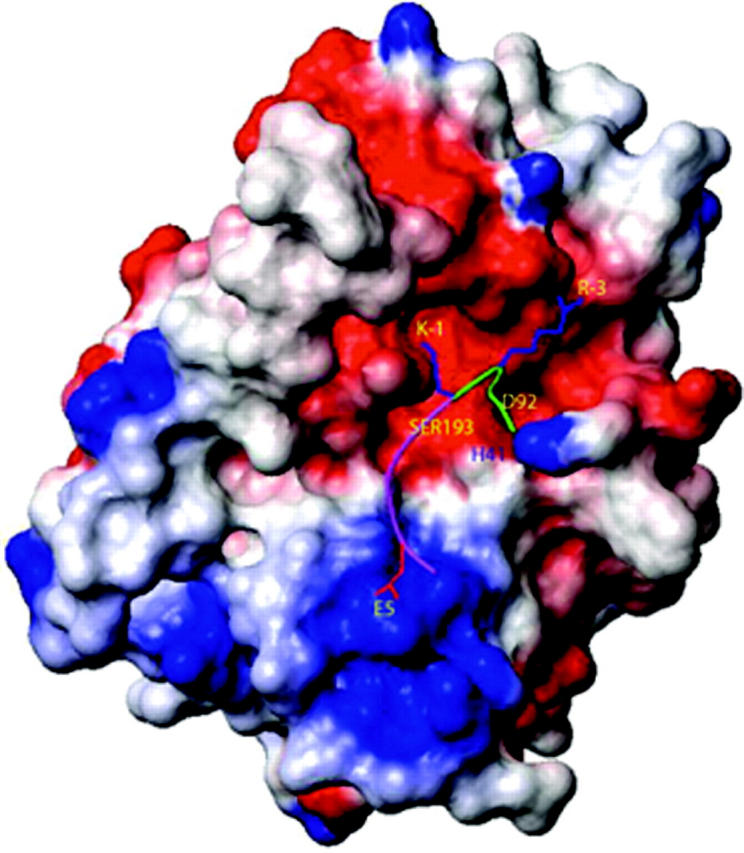
Surface and ball-and-stick representation of the model of tryptase ε binding to pro-uPA. The 13-mer sequence in pro-uPA (residues -7 to +6) recognized by tryptase ε is shown as a green/magenta ribbon with 3 of its side chains (R-3, K-1, and E5) in stick representation. The green portion of the ribbon represents the C-terminal end of pro-uPA's propeptide; the magenta portion of the ribbon represents the N-terminal end of the protease's main-chain, catalytic domain. Tryptase ε is shown in surface representation and is colored according to its electrostatic potential. The red, blue, and white regions are negatively, positively, and neutrally charged, respectively. The active-site triad residues H41, D92, and S193 are labeled in their approximate locations on the surface of tryptase ε. The figure was generated using the MOLMOL program.39
