Abstract
Phosphoinositide 3-kinase gamma (PI3Kγ) in neutrophils plays a critical role in the directed migration of these cells into inflamed tissues. In this study, we demonstrate the importance of the endothelial component of PI3Kγ activity relative to its leukocyte counterpart in supporting neutrophil interactions with the inflamed vessel wall. Despite the reconstitution of class-Ib PI3K function in neutrophils of p110γ–/– mice, we observed a 45% reduction in accumulation of these cells in an acute lung injury model. Mechanistically, this appears to result from a perturbation in selectin-mediated adhesion as manifested by a 70% reduction in wild-type (WT) neutrophil attachment to and 17-fold increase in rolling velocities on p110γ–/– microvessels in vivo in response to tumor necrosis factor alpha (TNFα). This alteration in adhesion was further augmented by a deficiency in p110δ, suggesting that the activity of both catalytic subunits is required for efficient capture of neutrophils by cytokine-stimulated endothelium. Interestingly, E-selectin–mediated adhesion in p110γ–/– mice was impaired by more than 95%, but no defect in nuclear factor kappa B (NF-κB)–induced gene expression was observed. These findings suggest a previously unrecognized partnership between class-I PI3Ks expressed in leukocytes and endothelium, the combination of which is required for the efficient trafficking of immunocompetent cells to sites of inflammation.
Introduction
Class-I phosphoinositide 3-kinases (PI3Ks) play a pivotal role in modulating innate and adaptive immune responses, as they are important transducers of external stimuli to cells such as granulocytes and lymphocytes.1-5 Structurally, they exist as heterodimeric complexes in which a catalytic p110 subunit (designated as α, β, γ, or δ) is in association with a particular regulatory subunit (designated p50, p55, p85, or p101).1,6,7 Functionally, all class-I PI3Ks catalyze the phosphorylation of phosphatidylinositol (4,5)–bisphosphate (PIP2) to form phosphatidylinositol (3,4,5)–trisphosphate (PIP3) in response to activation of either receptor tyrosine kinases (RTKs) or G-protein–coupled receptors (GPCRs), which ultimately regulates a diverse array of biologic functions. In regards to innate immunity, one major role of PI3Ks is to support chemoattractant-directed migration of neutrophils, macrophages, and specific populations of mast cells into sites of inflammation.8-11 Mechanistically, activation of this intracellular signaling pathway is essential for reorganization of cytoskeleton and membrane structure in response to such agonists, events that result in cell polarity and pseudopodia extension.12-14 In particular, it is believed that PI3Kγ,a class-Ib PI3K, predominates in this process, as recruitment of neutrophils into inflamed tissues was reduced by more than 60% in p110γ-null mice as compared with wild-type (WT) controls.9-11 These studies, however, did not take into account the contribution of other cell types required for neutrophil trafficking, including vascular endothelium.
The recruitment of neutrophils to sites of inflammation relies not only on their ability to undergo transendothelial cell migration, but also upon a dynamic interplay between adhesion molecules expressed on these cells and the inflamed vessel wall.15,16 In this regard, the endothelial lining does not act as a permissive barrier between the blood and tissues but rather regulates neutrophil trafficking in response to various stimuli. For instance, endothelial cell activation by the proinflammatory cytokine tumor necrosis factor alpha (TNFα) is a key event for the initiation of a well-orchestrated series of adhesive interactions involved in neutrophil recruitment, a process that relies in part on the de novo expression of E-selectin on the luminal surface of the vessel.17,18 E-selectin is one of 3 structurally related molecules that mediate the reversible capture (ie, tethering) by and subsequent rolling of leukocytes on the inflamed vessel wall in response to hydrodynamic flow.19 It does so by virtue of its ability to interact with constitutively expressed counterreceptors on human neutrophils such as P-selectin glycoprotein ligand-1 (PSGL-1).20 Maximal expression of E-selectin on cytokine-stimulated endothelium occurs within 4 hours and decays by 24 hours; surface expression parallels that of mRNA and is reliant in part on the nuclear factor kappa B (NF-κB) and IκBα regulatory system.21-24 NF-κB is a transcription factor that exists in the cytoplasm of cells in an inactive form due to its association with I kappa B alpha (IκBα). Degradation of IκBα in response to TNFα stimulation enables NF-κB to translocate into the nucleus where it can activate gene expression.
Class-I PI3Ks are known to contribute to specific biologic processes in endothelium such as angiogenesis.25 This signaling pathway, however, was not thought to be a major mediator of proinflammatory processes elicited by TNFα.26 For instance, controversy exists surrounding the role of class-I PI3Ks in regulating NF-κB–mediated gene expression of adhesion molecules such as E-selectin. Previously, it has been reported that the pan–class-I PI3K inhibitor LY294002 did not prevent the transcription of an NF-κB–dependent E-selectin promoter-reporter gene.27 By contrast, transfection of HepG2 cells with a dominant-negative mutant of the p85 regulatory subunit, critical for the function of class-Ia PI3Ks, resulted in both the inhibition of the PI3K signaling pathway and TNF-induced expression of the reporter gene, NF-κB/chloramphenicol acetyltransferase (CAT).28 Recently, we have suggested that class-I PI3Ks may indeed be involved in regulating the proadhesive state of vascular endothelium in response to cytokine stimulation.29 In particular, genetic deletion of the p110δ catalytic subunit in mice significantly reduced neutrophil tethering to and increased rolling velocities of these cells on cytokine-stimulated venular endothelium by a yet-to-be-identified mechanism. The significance of this observation, however, and the potential contribution of its gamma counterpart to this process, remain to be determined.
In the current study, we explore the contribution of PI3Kγ activity in endothelium in supporting neutrophil trafficking into sites of inflammation. This was accomplished using in vitro and in vivo experimental approaches that evaluated the effect that p110γ deficiency in endothelium has on promoting E-selectin expression and interactions with neutrophils. The reported observations broaden our understanding of signal transduction pathways that contribute to the multistep adhesion cascade.
Materials and methods
Reagents
Antibodies used in experiments included CL3 and CL37 (anti–human E-selectin, inhibitory and noninhibitory, respectively; ATCC, Manassas, VA), 9A9 (function-blocking anti–murine E-selectin; Klauss Ley, University of Virginia), platelet-endothelial cell adhesion molecule (PECAM) 1.3 (anti–human PECAM-1; Peter Newman, University of Wisconsin, Milwaukee), and fluorescein isothiocyanate (FITC)–conjugated goat F(ab′)2 anti–mouse immunoglobulin (Ig) (CALTAG Laboratories, Burlingame, CA). The following rat monoclonal antibodies (mAbs) to mouse proteins were purchased from BD Pharmingen (Franklin Lakes, NJ): FITC-conjugated RB6-8C5 (Gr-1) and biotinylated 10E9.6 (E-selectin). Qdot525 streptavidin conjugate was obtained from Quantum Dot (Hayward, CA). Recombinant murine and human TNFα were obtained from PeproTech (Rocky Hill, NJ) and R&D Systems (Minneapolis, MN), respectively. Murine E-selectin, human P-selectin, or human intercellular adhesion molecule 1 (ICAM-1) expressed as Fc chimeric proteins were obtained from R&D Systems, Genetics Institute (Cambridge, MA), or ICOS (Bothell, WA), respectively. Bay 11-7082 and LY294002 were purchased from EMD Biosciences (San Diego, CA). The p110δ inhibitor, IC87114, and recombinant p110 proteins were synthesized and purified as described.30 Rabbit anti-p110δ and p110γ were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA).
Animals
p110δ–/–/GFP–/+ mice and their WT littermate controls have been described29 and were used between 8 and 12 weeks of age. p110γ–/–/GFP–/+ mice were generated in a similar manner.9 Mice in which P-selectin was genetically deleted were obtained from Jackson Laboratories (Bar Harbor, ME) and mated with p110γ–/–/GFP–/+ animals to generate the double knockout. All animals were handled in accordance with policies administered by the National Institutes of Health and the Washington University Institutional Animal Care and Use Committee.
Fetal liver reconstitution
Matings were determined by detection of a copulation plug (designated 0.5 days gestation). All mice were 8 to 10 weeks old with a genetic background of C57BL/6 × 129/Sv. Male mice deficient in p110γ, p110δ, or both catalytic subunits were lethally irradiated (950 rad) and reconstituted with fetal liver cells from WT littermates expressing green fluorescent protein (GFP–/+).31 Briefly, embryos were harvested 14.5 days after coitus, fetal livers dispersed, and the cell suspension centrifuged, washed, and resuspended in Dulbecco modified Eagle medium (DMEM). Cells from each liver were injected into 2 mice that had been irradiated on the same day. Experiments were performed 8 to 10 weeks after injection.
Neutrophil purification
Mouse bone marrow (BM) polymorphonuclear cells (PMNs) were isolated by discontinuous Percoll gradient centrifugation as previously described.29,32,33 The resulting cell populations in all p110 genotypes were equivalent for expression of the granulocyte marker Gr-1 (81% to 86% positive).
LPS-induced lung inflammation
Intratracheal instillation of lipopolysaccharide (LPS; 10 μg/g body weight) was performed as previously described.29 At 6 hours after the challenge, mice were euthanized, bronchoalveolar lavage (BAL) fluid collected, samples centrifuged, and resuspended in 0.6 mL phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 5 mM EDTA (ethylenediaminetetraacetic acid), pH 7.4. Samples were then placed in 24-well tissue-culture plates (Becton Dickinson, Franklin Lakes, NJ) and the number of fluorescent neutrophils determined per unit area (0.7 mm2)by fluorescent microscopy (Nikon X10 and Eclipse TE 300 microscopes; Nikon, Melville, NY). A minimum of 4 fields of view were recorded on Hi-8 videotape and subsequently analyzed using a PC-based interactive image analysis system (Image Pro Plus). For E-selectin–blocking experiments, 100 μg F(ab′)2 9A9 in PBS was administered by intravenous route just prior to instillation of LPS.
TNFα measurement
Whole blood was collected from p110γ–/– mice or WT controls by cardiac puncture. LPS (250 ng/mL final concentration) or PBS was added to equivalent volumes of blood and samples incubated at 37°C for 6 hours. Supernatant was obtained by centrifugation and subsequently analyzed for TNFα by enzyme-linked immunosorbent assay (ELISA; Biosource International, Camarillo, CA). Values were normalized to absolute neutrophil counts contained in each blood sample.
Intravital microscopy
The surgical preparation of animals for all in vivo studies was performed using standard techniques.29 An inflammatory response in the cremaster muscle (CM) venules of mice was induced by an intrascrotal injection of recombinant murine TNFα (20 ng/mouse). The tissue was surgically exposed 3 hours after cytokine stimulation and viewed on an intravital microscope (IV-500; Mikron Instruments, San Diego, CA). The tissue was kept moist by superfusion with thermocontrolled (37°C), bicarbonate-buffered saline. Green fluorescent protein (GFP)–expressing cells were visualized in the microcirculation through a 60×/0.90∞ water immersion objective lens on a LumPlan FL microscope (Olympus, Melville, NY) using an intensified camera (VE1000SIT; Dage mti) and epifluorescence illumination. Rolling fraction was defined as the percentage of cells that interact with a given region of venule as compared with the total number of cells that enter that vessel (interacting and noninteracting) during the same time period. Venular shear rates were determined from optical Doppler velocimeter measurements of centerline erythrocyte velocity. Video images were recorded using a Hi8 VCR (Sony) and analysis of performed using a PC-based image analysis system.
Laminar flow assays
Human umbilical vein endothelial cells (HUVECs; passage 2-3), grown on fibronectin-coated glass coverslips, were pretreated with IC87114 (2 μM), LY294002 (10 μM), Bay 11-7082 (10 μM), or vehicle control (dimethyl sulfoxide [DMSO]) for 1 hour prior to stimulation with TNFα (5 ng/mL, 4 hours). Peripheral blood neutrophils were isolated from healthy volunteers and infused over the endothelial cell monolayer that was incorporated into a parallel plate flow chamber (GlycoTech, Gaithersburg, MD) for 5 minutes at a shear rate of 200 s–1.29 Approval was obtained from the Washington University institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. Neutrophil-endothelial cell interactions were recorded and analyzed as previously described.29 For E-selectin blockade, HUVECs were incubated with mAb CL3 (50 μg/mL, 15 minutes) prior to adhesion assays.
For flow studies involving recombinant protein, polystyrene plates were coated overnight with 100 μg/mL protein A (Sigma) at 4°C, then washed, and finally incubated with E- or P-selectin or ICAM-1-Fc chimeric proteins diluted to a concentration of 20 μg/mL (PBS, 0.1% BSA, pH 7.4) for 2 hours at 37°C. Nonspecific interactions were blocked with rabbit Ig (50 μg/mL) for 30 minutes at 37°C. Murine neutrophils (1 × 106/mL; Hanks balanced salt solution [HBSS], 10 mM Hepes [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 1 mM CaCl2, 0.5% BSA, pH 7.4) were infused over the selectin substrates at a shear rate of 200 s–1. The number of cells that attached over 5 minutes was determined and expressed per unit area.
NF-κB p50 nuclear translocation assay
Cultured HUVECs (passage 3) were incubated for 16 hours in 0.5% fetal calf serum (FCS) containing medium 199. Cells were pretreated with vehicle (DMSO) or 10 μM of IC87 114, LY294002, or BAY 11-7082 for 2 hours before stimulation with TNFα (10 ng/mL) for 30 minutes. Cells were harvested by trypsin digestion and nuclear extracts were prepared by using the TransFactor extraction kit (BD Bioscience/CLONTECH, Mountain View, CA) according to the manufacturer's instructions. After centrifugation at 20 000g for 5 minutes at 4°C, supernatants (nuclear extracts) were assayed for p50 content. An equal amount of nuclear extracts (10 μg) was added to incubation wells precoated with the DNA-binding consensus sequence. The presence of translocated p50 subunit was then assessed by using Mercury TransFactor kit (BD Biosciences/CLONTECH).
Immunoprecipitation and PI3K activity assay
Spleens from WT mice were pulverized in liquid nitrogen–cooled mortar and solubilized in PI3-kinase lysis buffer (50 mM Tris-HCl [pH 7.4]), 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, and a cocktail of inhibitors to serine and cysteine proteases (Complete Mini; Roche Applied Science, Indianapolis, IN). HUVEC lysates were prepared as described.29 Lysates were precleared with protein A–Sepharose and aliquots of the supernatants were incubated with antibodies specific for p110δ and p110γ, or control antibody for 1 hour at 4°C, followed by addition of protein A–Sepharose for 2 hours at 4°C. Precipitates were washed once with lysis buffer, twice with 0.1 M Tris-HCl, pH 7.4; 5 mM LiCl; and 0.1 mM sodium orthovanadate and once with PI3-kinase buffer containing 20 mM Hepes, pH 7.4, 10 μM ATP, 5 mM MgCl2, plus 50 μg/mL horse IgG (Pierce, Rockford, IL). Lipid kinase activity was determined as previously described.30 The radioactive product PIP3 was captured onto a 96-well polyvinylidene difluoride filter plate (Millipore, Billerica, MA) and the bound radioactivity was quantitated with Microbeta Liquid Scintillation Counter (PerkinElmer Life Sciences, Boston, MA).
p110γ Western blot analysis
HUVECs and the murine endothelioma cell line bEND3.1 (ATCC) lysates were prepared as described for the PI3K function assay. Recombinant p110α, β, γ, and δ proteins (20 ng/lane) and cell lysates (100 μg/lane) were electrophoresed in precast 8% polyacrylamide gels (Invitrogen Life Technologies, Carlsbad, CA), transferred electrophoretically to a polyvinylidene difluoride membrane (Invitrogen), and immunoblotted with p110γ antibody as described previously.29
Statistical analysis
A Student t test was used for statistical comparisons. Statistical significance was set at P values less than .05.
Results
LPS-induced recruitment of neutrophils in p110γ–/– chimeric mice
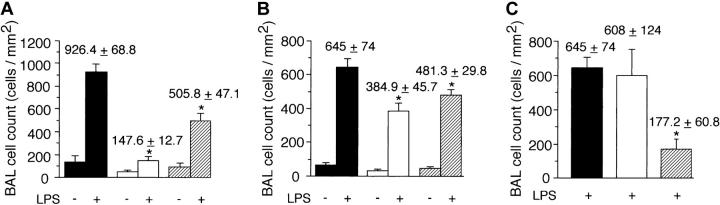
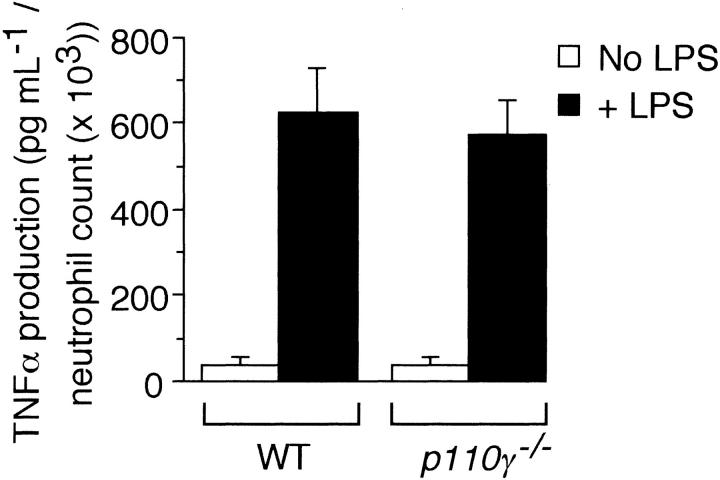
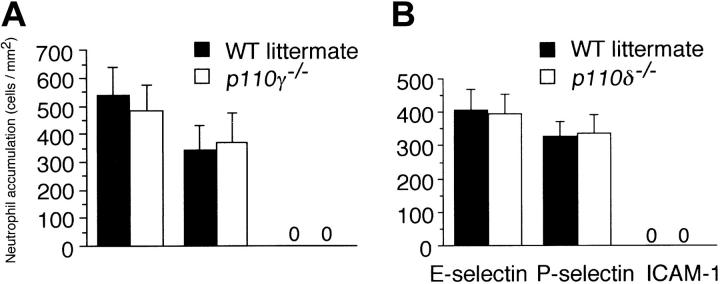
To determine if a “nonleukocyte” component of PI3Kγ activity contributes to neutrophil accumulation at sites of inflammation, we evaluated the recruitment of these cells into LPS-treated lungs of p110γ–/– mice reconstituted with fetal liver cells (FLCs) from GFP-expressing WT littermates. Circulating white blood cell (WBC) counts and absolute neutrophil counts (mean ± standard deviation [SD]) of reconstituted animals were 10.1 K/μL ± 1.4 K/μL and 3.4 K/μL ± 0.8 K/μL, respectively, values equivalent to that of WT-matched controls (10.0 K/μL ± 2.1 K/μL and 2.8 K/μL ± 0.3 K/μL, respectively). Moreover, more than 95% of circulating GR-1 (+) cells in whole blood of all chimeric animals expressed GFP, which is consistent with complete reconstitution of the granulocyte population with p110γ+/+ neutrophils (data not shown). Intratracheal instillation of LPS into WT littermates resulted in an 11.5-fold increase in the number of fluorescent cells in BAL fluid 6 hours after challenge as compared with animals treated with normal saline (Figure 1A). Similar results were obtained in WT mice reconstituted with WT FLCs (data not shown). Complete absence of the p110γ catalytic subunit, however, significantly reduced neutrophil counts (∼84%). Surprisingly, LPS-induced recruitment of these cells was still mitigated (∼45%) despite reconstituting p110γ–/– animals with WT FLCs. This finding was not restricted to PI3Kγ, as the activity of PI3Kδ (class-Ia PI3K) in other cell types also contributes to the inflammatory cell infiltrate, albeit not to the extent observed for the former (Figure 1B). The importance of endothelium in neutrophil recruitment is demonstrated by the ability of a function blocking F(ab′)2 to E-selectin (9A9), an adhesion molecule expressed on inflamed endothelium, to reduce BAL fluid cell counts by 70% (Figure 1C). Although TNFα generated in response to LPS is required for expression of E-selectin, absence of PI3Kγ activity did not alter the ability of leukocytes to secrete this proinflammatory cytokine (Figure 2).34,35
Figure 1.
Contribution of the nonleukocyte component of class-Ib PI3K activity to neutrophil accumulation in response to LPS. GFP-expressing cell counts per unit area obtained 6 hours after intratracheal administration of LPS (10 μg/g) in mice (A) deficient in or chimeric for PI3Kγ activity, (B) deficient in or chimeric for PI3Kδ activity, (C) pretreated with the function-blocking or nonfunction-blocking F(ab')2 9A9 and CL37, respectively. (A) ▪ indicates WT littermate (GFP–/+ p110γ+/+); ▪, GFP–/+ p110γ–/–; and  , p110γ–/– reconstituted with GFP–/+ p110γ+/+ fetal liver cells. (B) ▪ indicates WT littermate (GFP–/+ p110δ+/+); ▪, GFP–/+ p110δ–/–; and
, p110γ–/– reconstituted with GFP–/+ p110γ+/+ fetal liver cells. (B) ▪ indicates WT littermate (GFP–/+ p110δ+/+); ▪, GFP–/+ p110δ–/–; and  , p110δ–/– reconstituted with GFP–/+ p110δ+/+ fetal liver cells. (C) ▪ indicates WT littermate (GFP–/+ p110δ+/+); ▪, WT littermate plus mAB CL37; and
, p110δ–/– reconstituted with GFP–/+ p110δ+/+ fetal liver cells. (C) ▪ indicates WT littermate (GFP–/+ p110δ+/+); ▪, WT littermate plus mAB CL37; and  , WT littermate plus mAb 9A9. Mean values (± SD) are shown for 8 mice in each experimental or control group. *P < .05.
, WT littermate plus mAb 9A9. Mean values (± SD) are shown for 8 mice in each experimental or control group. *P < .05.
Figure 2.
Contribution of p110γ to TNFα production. LPS-induced generation of TNFα in whole blood harvested from either 3 p110γ–/– or WT-matched controls. Mean values (± SE) for experiments performed in triplicate.
p110γ is expressed in vascular endothelium
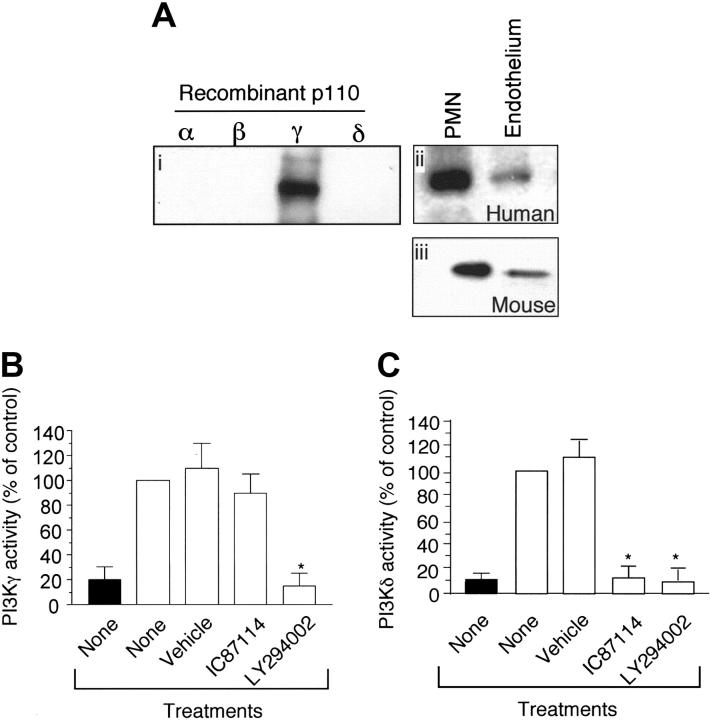
As class-I PI3K activity has been shown to exist in endothelium, it is conceivable that the PI3Kγ may be present in this cell type and thus participate in neutrophil trafficking. To demonstrate for the first time that the p110γ catalytic subunit not only is expressed in endothelium but is functional, we performed immunoprecipitation analysis and measured the activity of PI3Kγ purified from proliferating vascular endothelial cells. Western blot analysis revealed the presence of this class-Ib isoform in both HUVECs and the murine endothelioma cell line bEND3.1 (Figure 3Aii,iii, respectively). Moreover, the immunoprecipitated material was functional as measured by its ability to generate PIP3 (Figure 3B). Importantly, the activity of p110γ could be blocked by pan–class-I PI3K inhibitor LY294002 (10 μM), but not by IC87114 (10 μM) which is selective for p110δ (Figure 3B-C). This is consistent with previous results demonstrating a 58-fold selectivity of IC87114 for p110δ than for p110γ.30 By contrast, inhibitory concentration (IC50) values for LY294004 vary among the 4 class-I PI3Ks by only about 10-fold.
Figure 3.
p110γ is functionally expressed in venular endothelium. (A) Immunoblots of the p110γ catalytic subunit using recombinant proteins (i) and lysates obtained from HUVECs or neutrophils (ii), or the murine endothelioma cell line bEND3.1 (iii). Measurement of (B) PI3Kγ and (C) PI3Kδ kinase activity. Immunoprecipitates of p110γ and p110δ (▪) from HUVEC lysates were assayed for PI3K activity with or without the addition of 10 μM IC87114 or LY294002 as described in “Materials and methods.” ▪ indicates control. The results are expressed as the percent of activity in untreated immunoprecipitates and represent the mean plus or minus SD values of 3 independent determinations in duplicate. *P < .01 as compared with untreated cells.
PI3Kγ in endothelium is required for efficient neutrophil capture and rolling
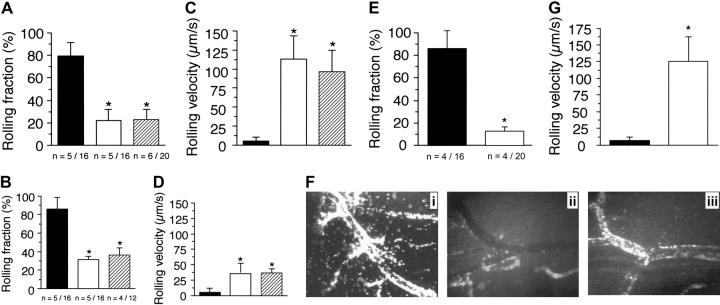
The existence of p110γ as a functional complex in vascular endothelium suggests that it may play an important role in mediating the neutrophil recruitment in response to proinflammatory stimuli. Thus, we sought to elucidate the potential mechanism(s) by which PI3Kγ may regulate such an event by observing the behavior of GFP-expressing granulocytes in microcirculation of TNFα-stimulated venules of mice chimeric for p110γ activity. An absence of this catalytic subunit in endothelium alone resulted in the identical reduction in the number of fluorescent cells that attached to and rolled on the inflamed vessel wall as compared with animals lacking p110γ in both cell types (Figure 4A). This suggests that PI3Kγ in neutrophils does not play a role in this process. Interestingly, there was greater impairment in the attachment of WT neutrophils in p110γ–/– versus p110δ–/– chimeric animals (70% versus 55%), suggesting that the endothelial component of class-I PI3K activity may contribute to the differences observed in the LPS-induced acute lung injury model (Figure 4C,F). In addition to a defect in neutrophil attachment, rolling velocities of p110γ–/– versus p110δ–/– chimeric animals were increased by 17.5- and 7.5-fold, respectively, values also comparable to that observed in their nonreconstituted counterparts (Figure 4B,D). It appears, however, that the activity of both class-I PI3K isoforms is required for optimal attachment and rolling of neutrophils, as there was a greater perturbation in these adhesive parameters in mice lacking both catalytic subunits. Rolling fractions and velocities (mean ± SD) of neutrophils in p110γ–/–/δ–/– mice reconstituted with WT FLCs (GFP–/+) were 12.5% ± 4.3% and 136 μm/s ± 26.6 μm/s, respectively (Figure 4E,G). By contrast, values in reconstituted p110γ-or δ-deficient mice were 24% ± 5.2% versus 44.6% ± 7.7% and 95.1 μm/s ± 29 μm/s versus 44 μm/s ± 12.8μm/s, respectively. Thus, a lack of both p110γ and p110δ resulted in a more than 85% decrease in neutrophil attachment to and an approximately 23-fold increase in rolling velocities as compared with WT controls. We conclude from these observations that PI3Kγ plays a significant role in regulating the proadhesive state of cytokine-stimulated vascular endothelium and that the activity of both class-Ia and Ib PI3Ks are required for optimal interactions between neutrophils and inflamed vessel wall.
Figure 4.
A deficiency in endothelial p110γ impairs neutrophil accumulation on TNFα-stimulated CM venules. Rolling fraction (the number of GFP-expressing cells that attached and rolled on inflamed endothelium divided by the total flux of cells passing through the vessel) determined in mice (A) deficient in or chimeric for p110γ, (B) deficient in or chimeric for p110δ activity. ▪ indicates WT littermate (WT GFP–/+); ▪, GFP–/+ p110γ–/– or GFP–/+ p110δ–/–; and  , p110γ–/– or p110δ–/– reconstituted with WT GFP–/+ fetal liver cells. Results represent the mean plus or minus SD; *P < .05 as compared with WT littermates. n = number of mice/venules analyzed. Rolling velocities for consecutive interacting cells (n = 30 per venule) in mice (C) deficient in or chimeric for p110γ, or (D) deficient in or chimeric for p110δ. Data represent the mean ± SD for more than 150 cells per experimental condition; *P < .05. (E) Rolling fraction and (G) rolling velocities for p110γ–/–/p110δ–/– mice reconstituted with FLC from GFP–/+ WT fetal liver cells (▪). ▪ indicates WT littermates reconstituted with GFP–/+ WT fetal liver cells. *P < .05 as compared with controls. (F) Representative intravital micrographs depicting the extent of neutrophil adhesion to and transmigration across CM venules in WT mice (i) and p110γ–/– (ii) or p110δ–/– (iii) animals reconstituted with WT FLCs (3 hours after stimulation with TNFα).
, p110γ–/– or p110δ–/– reconstituted with WT GFP–/+ fetal liver cells. Results represent the mean plus or minus SD; *P < .05 as compared with WT littermates. n = number of mice/venules analyzed. Rolling velocities for consecutive interacting cells (n = 30 per venule) in mice (C) deficient in or chimeric for p110γ, or (D) deficient in or chimeric for p110δ. Data represent the mean ± SD for more than 150 cells per experimental condition; *P < .05. (E) Rolling fraction and (G) rolling velocities for p110γ–/–/p110δ–/– mice reconstituted with FLC from GFP–/+ WT fetal liver cells (▪). ▪ indicates WT littermates reconstituted with GFP–/+ WT fetal liver cells. *P < .05 as compared with controls. (F) Representative intravital micrographs depicting the extent of neutrophil adhesion to and transmigration across CM venules in WT mice (i) and p110γ–/– (ii) or p110δ–/– (iii) animals reconstituted with WT FLCs (3 hours after stimulation with TNFα).
Role of class-I PI3Ks in E-selectin–dependent adhesion
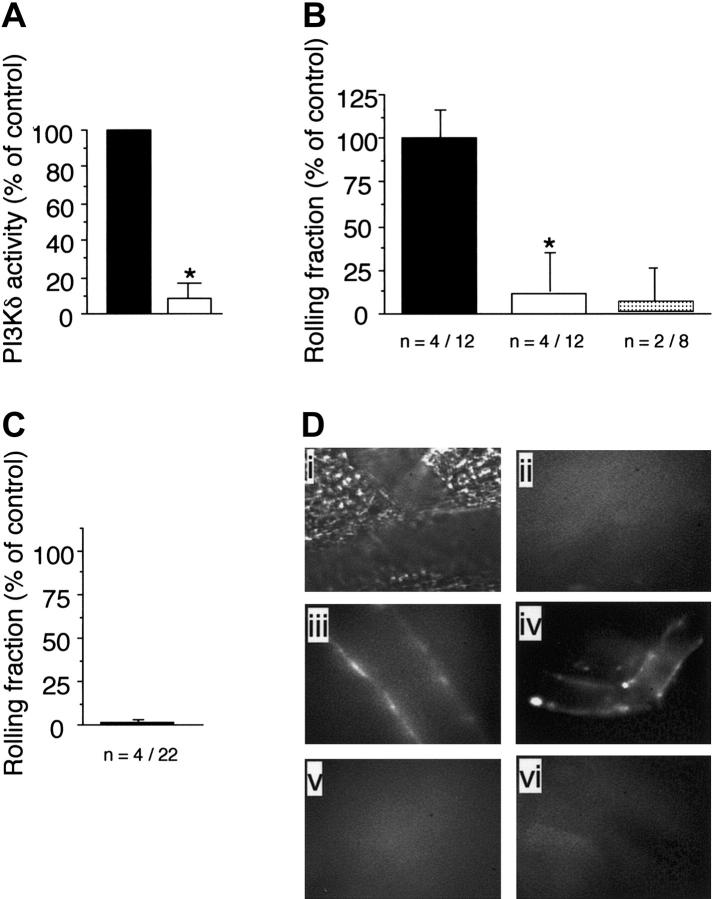
The observed reduction in attachment and increase in rolling velocities of WT neutrophils in animals deficient in class-Ia or Ib PI3Ks suggested that an alteration in selectin-mediated adhesion had occurred. Although P-selectin expressed on endothelium predominates in the initial capture of circulating neutrophils in acute tissue injury, it is E-selectin that accounts for the phenotypically slow rolling movements of these cells in microvessels of TNFα-stimulated CM.36 To determine whether class-I PI3Ks contribute to E-selectin–mediated recruitment of neutrophils, we next evaluated the behavior of GFP-expressing granulocytes in P-selectin–deficient mice that lacked p110γ activity (P-selectin–/–/p110γ–/–) or had received the p110δ-specific inhibitor IC87114. In regards to the latter, we had previously demonstrated the specificity of this compound for this class-Ia PI3K isoform.29 To provide additional evidence that IC87114 directly blocks the function of p110δ, we measured the catalytic activity of this enzyme isolated from spleen extracts of WT animals in the absence or presence of this inhibitor. By comparison to vehicle control, incubation of immunoprecipitated p110δ with 10 μM of IC87114, which inhibits more than 95% of delta activity, reduced PIP3 production by more than 90% (Figure 5A).
Figure 5.
The p110γ is required for efficient capture of neutrophils by E-selectin. (A) Immunoprecipitates of spleens from WT mice using a p110δ-specific antibody that was assayed for PIP3 production in the absence (▪) or presence (▪)of IC87114 (10 μM). The results are expressed as the percent activity in vehicle-treated p110δ immunoprecipitates and represent the mean plus or minus SD values of 3 independent determinations in duplicate. *P < .01 as compared with vehicle-treated WT spleen lysate. Rolling fraction of P-selectin–/–/GFP–/+ animals (B) pretreated with vehicle control (▪), IC87114 (▪), or mAb 9A9 ( ) 1 hour prior to induction of inflammation with TNFα or (C) in those that also lacked p110γ. Data represent the mean plus or minus SD and are normalized as a percentage of control; *P < .05 as compared with control. n = number of mice /venules analyzed. (D) In vivo imaging of E-selectin expression on CM venules in mice with or without prior stimulation with TNFα. Fluorescent Qdots coated with an antibody that recognizes murine E-selectin were injected intravenously into animals and immunolocalization of this adhesion molecule was visualized by intravital microscopy. Transmitted light (i) and epifluorescence (ii) images depict staining of venules in P-selectin–/– mice in the absence of TNFα stimulation. Representative micrographs of inflamed venules in P-selectin–/– animals pretreated with (iii) IC87114 or (iv) lacking p110γ, respectively, or mice deficient in E-selectin in the absence (v) or presence (vi) of IC87114.
) 1 hour prior to induction of inflammation with TNFα or (C) in those that also lacked p110γ. Data represent the mean plus or minus SD and are normalized as a percentage of control; *P < .05 as compared with control. n = number of mice /venules analyzed. (D) In vivo imaging of E-selectin expression on CM venules in mice with or without prior stimulation with TNFα. Fluorescent Qdots coated with an antibody that recognizes murine E-selectin were injected intravenously into animals and immunolocalization of this adhesion molecule was visualized by intravital microscopy. Transmitted light (i) and epifluorescence (ii) images depict staining of venules in P-selectin–/– mice in the absence of TNFα stimulation. Representative micrographs of inflamed venules in P-selectin–/– animals pretreated with (iii) IC87114 or (iv) lacking p110γ, respectively, or mice deficient in E-selectin in the absence (v) or presence (vi) of IC87114.
Oral administration of IC87114 to P-selectin–/– mice 1 hour prior to TNFα stimulation of the CM resulted in a more than 88% reduction in neutrophil attachment to and rolling on inflamed venular endothelium as compared with vehicle treatment alone (Figure 5B). Mean plasma level of the compound 4 hours after oral administration was 4.9 μM ± 2.7 μM, a concentration known to inhibit more than 85% of p110δ but less than 5% of p110γ activity.30 The requirement for E-selectin is demonstrated by the ability of the function-blocking mAb 9A9 to abrogate interactions between circulating granulocytes and the vessel wall in animals that received vehicle control. Thus, p110δ activity is required for E-selectin–dependent adhesion of neutrophils. Moreover, the critical interplay between this adhesion molecule and class-I PI3K activity is further demonstrated in animals deficient in both P-selectin and p110γ. Attachment of GFP-expressing neutrophils to TNFα-stimulated venules in the CM of these animals was impaired by more than 95% (Figure 5C).
To demonstrate that either genetic deletion of p110γ or blockade of p110δ activity in these animals does not prevent surface expression of E-selectin, which could account for the observed reduction in neutrophil adhesion, we next evaluated the accumulation of fluorescent semiconductor nanocrystals encapsulated in phospholipid micelles (Qdots) coupled to an antibody that recognizes this selectin molecule. In the absence of cytokine stimulation, no immunofluorescence was detected on the vessel wall (Figure 5Dii). By contrast, TNFα-induced stimulation resulted in the deposition of the Qdots/antibody conjugate on microvessels in P-selectin–/– animals treated with IC87114 or deficient in p110γ (Figure 5Diii,iv, respectively). The specificity of the interaction was confirmed by the lack of immunofluorescence staining in TNFα-stimulated venules of E-selectin–/– mice treated with vehicle control (Figure 5Dv) or the p110δ selective inhibitor (Figure 5Dvi).
Class-I PI3K activity is not required for NF-κB–mediated expression of E-selectin
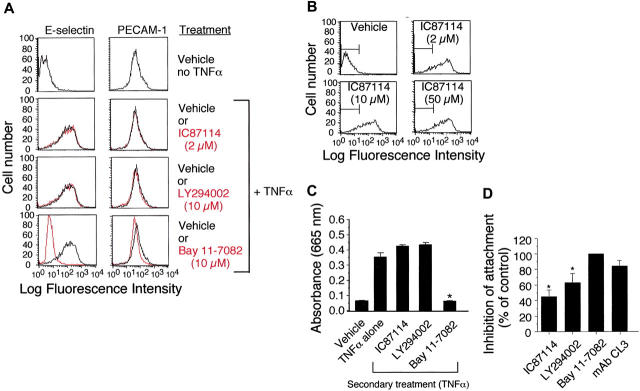
To further extend our in vivo observation that class-I PI3K activity may not be required for cytokine-induced expression of E-selectin, we performed flow cytometric analysis on TNFα-stimulated (4 hours) HUVECs pretreated with vehicle control, IC87114 (2 μM to 50 μM), or LY294002 (10 μM). No difference in E-selectin expression was noted in the presence or absence of the inhibitors (Figure 6A-B). By contrast, TNFα-induced expression of E-selectin was abrogated by Bay 11-7082 (10 μM), a small molecule inhibitor that impairs NF-κB nuclear translocation and thus E-selectin gene transcription.37 Further evidence in support of our finding that class-I PI3K activity does not participate significantly in the expression of this adhesion molecule was provided by evaluating NF-κB nuclear translocation in TNFα-stimulated HUVECs. In contrast to Bay 11-7082, treatment with either the nonspecific or δ isoform selective class-I PI3K inhibitors LY294002 or IC87114, respectively, did not prevent nuclear localization of NF-κB as determined by an ELISA that detects the p50 subunit of this transcription factor (Figure 6C). Both inhibitors nevertheless reduced the ability of TNFα-stimulated HUVEC monolayers to capture untreated neutrophils in vitro by over 45% at a wall shear rate of 200 s–1, whereas the E-selectin–blocking antibody CL3 and Bay 11-7082 (10 μM) impaired attachment by 90% and 100%, respectively (Figure 6D). These results suggest that the role of class-I PI3Ks in E-selectin–mediated neutrophil attachment differs from transcriptional regulation by NF-κB.
Figure 6.
Role of class-I PI3Ks in E-selectin expression in vitro. (A) E-selectin and PECAM-1 surface expression on HUVECs (± 5 μM TNFα) pretreated (1 hour) with the p110δ-specific inhibitor IC87114 (2 μM), the pan–class-I PI3K inhibitor LY294002 (10 μM), or the NF-κB function blocker Bay 11-7082 (10 μM). Adhesion molecule expression on nonstimulated cells is shown for comparison (top panels). Black lines represent vehicle control or cells treated with the indicated inhibitors (gray). (B) E-selectin expression on TNFα-stimulated HUVECs remains unchanged even at doses of IC87114 known to inhibit p110γ (50 μM). (C) TNFα-mediated NF-κB translocation in nuclear extracts from HUVECs pretreated with vehicle control or IC87114 as quantified by ELISA for the p50 subunit. Data represent the mean plus or minus SD for 1 of 3 experiments with similar results. *P < .01 as compared with cells treated with TNFα alone. (D) Percent reduction in attachment of purified human neutrophils to HUVEC monolayer that was pretreated with the indicated inhibitors prior to stimulation with TNFα (4 hours). Results are expressed as the percentage of cells that bound to the vehicle-treated substrate at a wall shear rate of 200 s–1. Data represent the mean plus or minus SD for 3 experiments performed in duplicate. *P < .05 as compared with vehicle control.
To confirm that the lack of PI3Kγ or δ activity in leukocytes does not impair selectin-dependent capture in flow as observed in vivo, we next evaluated the attachment of purified p110γ–/– or p110δ–/– neutrophils to surface-immobilized selectin-Fc chimeras using a parallel plate flow chamber apparatus. Despite the lack of either class-I isoform activity, neutrophils from mutant animals accumulated equally well on these substrate and at levels comparable to that of WT controls (Figure 7A-B).
Figure 7.
Role of class-I PI3Ks in neutrophils in adhesion in vitro. Attachment of (A) p110γ–/– versus (B) p110δ–/– neutrophils to surface-immobilized E- or P-selectin Ig chimeras at a wall shear rate of 200 s–1. Results are compared with neutrophils purified from WT littermates. Adhesion to the β2-integrin ligand ICAM-1-Ig chimera is shown as control. Results represent the mean plus or minus SD for 3 experiments performed in duplicate.
Discussion
PI3Kγ has been shown to be important in a number of biologic processes essential for neutrophil function in an innate immune response. Foremost is its ability to support directed migration of these cells upon GPCR activation by various chemoattractants. Our results provide conclusive evidence that PI3Kγ activity in vascular endothelium is also essential for neutrophil recruitment, as its activity is required for efficient selectin-mediated adhesion, an important event in the multistep process that enables neutrophils to traffick into inflamed tissues. Confirmation that the activity of this class-Ib PI3K in neutrophils did not contribute to the observed impairment in adhesion was demonstrated by (1) similar alterations in rolling fraction and velocities in TNFα-stimulated venules in the CM of p110γ–/– mice as compared with those reconstituted with WT FLCs, and (2) similar levels of attachment of p110γ–/– neutrophils to surface-immobilized E- or P-selectin in flow as compared with WT control cells. The biologic significance of our findings is revealed by the persistent reduction in neutrophil accumulation in an LPS-induced acute lung injury model that used animals chimeric for class-Ib PI3K activity. Moreover, the requirement for E-selectin to promote neutrophil infiltration into inflamed lung and the profound impact that p110γ deficiency has on this interaction supports a defect in endothelial cell–mediated adhesion as the mechanism for the reduced accumulation of neutrophils in this acute lung injury model. That said, the greater perturbation in selectin-dependent adhesion of WT neutrophils on endothelium deficient in p110γ and p110δ suggests that optimal adhesive interactions require the activity of both PI3K isoforms.
How might PI3Kγ participate in regulating the proadhesive state of TNFα-stimulated endothelium? It is conceivable that its activity may contribute to surface expression of adhesion molecules, as it has been suggested that PI3Ks, in general, play a pivotal role in TNFα-mediated activation of NF-κB–dependent genes.23,28 In this scenario, stimulation of the type-1 TNF receptor results in activation of class-I PI3Ks and subsequent generation of PIP3. Consequently, this leads to the phosphorylation of Akt, a downstream target of PI3Ks, which in turn activates NF-κB and thus transcription of genes necessary for key immune and inflammatory responses. Indeed, our previous results concur with the observation that class-I PI3K activity is essential not only for phosphorylation of Akt but also for the activation of PDK1 in endothelium in response to TNFα. Both Akt and PDK1 are cytoplasmic proteins that contain pleckstrin homology domains, enabling them to be recruited to the plasma membrane where they can directly interact with PIP3. Activation of these protein kinases is essential for many of the downstream signaling events associated with PI3K activity. By contrast, our current results do not support a role for class-I PI3K–induced activation of NF-κB, as neither p110γ nor its δ counterpart contributes significantly to this process. This is evident by the inability of the pan–class-I PI3K inhibitor LY249004 or δ-specific inhibitor IC87114 to (1) prevent nuclear translocation of this transcription factor in HUVECs in response to TNFα, and (2) alter the level of expression of E-selectin on this cell type. Moreover, this adhesion molecule was still present on cytokine-stimulated venules in the CM of p110γ–/– and p110δ–/– mice despite the significant reduction in selectin-dependent recruitment of neutrophils. Thus, an NF-κB–independent mechanism must account for the majority of alteration in E-selectin–mediated adhesion in animals lacking class-Ia or Ib PI3Ks.
In addition to gene regulation, it has been shown that cytoskeletal-mediated distribution of adhesion molecules such as E-selectin on the surface of cytokine-stimulated endothelium plays a role in regulating its proadhesive state. Disruption of microtubule assembly in endothelial cells with colchicine before or after TNFα activation altered the location but not expression of E-selectin, an event that reduced neutrophil attachment as assayed under static conditions.38 Moreover, linkage of E-selectin to the cytoskeleton may also be of biologic relevance in terms of strengthening interactions between these cells. In fact, multivalent ligation of E-selectin either by antibodies or surface-bound leukocytes results in its association with components of the actin cytoskeleton via its cytoplasmic domain, an event believed to confer greater resistance to mechanical deformation.39 These data suggest that microtubule dynamics contribute to the functioning of E-selectin on endothelium. In terms of signaling pathways, the small guanosine triphosphate–binding protein Rho has been shown to be required for clustering of E-selectin in the vicinity of leukocytes once they are bound to the endothelial surface.40 Although the involvement of p110γ or δ in this process has not been demonstrated to date, class-I PI3Ks are known to contribute to the clustering of cell surface receptors on chemokine-stimulated lymphocytes.41 Thus, a precedent exists for the involvement of these lipid kinases in regulating the distribution and function of adhesion molecules on cells. Current studies are focused on elucidating the role of PI3Kγ and δ in promoting E-selectin interactions with components of the cytoskeleton and subsequent effects on surface distribution and function.
In conclusion, we have demonstrated that a “nonleukocyte” component of the PI3Kγ activity contributes in part to the innate immune response by regulating the ability of selectin molecules expressed on TNFα-stimulated endothelium to efficiently capture circulating neutrophils. Moreover, we have clarified a controversial issue regarding the involvement of class-I PI3Ks in NF-κB–mediated gene regulation.
Acknowledgments
We thank Ed Kesicki and Jennifer Treiberg for the synthesis of IC87114, Esther Trublood for tissue collection, and Hairu Zhou and Angie Freie for genotyping of animals.
Prepublished online as Blood First Edition Paper, March 15, 2005; DOI 10.1182/blood-2005-01-0023.
Supported by National Institutes of Health grant HL63 244-05 and the American Lung Association (T.G.D.) and the Medical Research Council and Biotechnology and Biological Sciences Research Council (M.T.).
K.D.P., J.D., and J.S.H are employed by ICOS Corporation, the company whose potential product was studied in the present work.
An Inside Blood analysis of this article appears at the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Fruman DA, Cantley LC. Phosphoinositide 3-kinase in immunological systems. Semin Immunol. 2002;14: 7-18. [DOI] [PubMed] [Google Scholar]
- 2.Rickert P, Weiner OD, Wang F, Bourne HR, Servant G. Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends Cell Biol. 2000;10: 466-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wymann MP, Sozzani S, Altruda F, Mantovani A, Hirsch E. Lipids on the move: phosphoinositide 3-kinases in leukocyte function. Immunol Today. 2000;21: 260-264. [DOI] [PubMed] [Google Scholar]
- 4.Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4: 313-319. [DOI] [PubMed] [Google Scholar]
- 5.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3: 317-330. [DOI] [PubMed] [Google Scholar]
- 6.Curnock AP, Logan MK, Ward SG. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology. 2002;105: 125-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanhaesebroeck, B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22: 267-272. [DOI] [PubMed] [Google Scholar]
- 8.Hannigan M, Zhan L, Li Z, Ai Y, Wu D, Huang CK. Neutrophils lacking phosphoinositide 3-kinase gamma show loss of directionality during N-formyl-Met-Leu-Phe-induced chemotaxis. Proc Natl Acad Sci U S A. 2002;99: 3603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki T, Irie-Sasaki J, Jone RG, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287: 1040-1046. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287: 1049-1053. [DOI] [PubMed] [Google Scholar]
- 11.Li, Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287: 1046-1049. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Herzmark P, Weiner OD, Srinivasan S, Servant G, Bourne HR. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol. 2002;4: 513-518. [DOI] [PubMed] [Google Scholar]
- 13.Van Haastert PJ, Devreotes PN. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol. 2004;5: 626-634. [DOI] [PubMed] [Google Scholar]
- 14.Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302: 1704-1709. [DOI] [PubMed] [Google Scholar]
- 15.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272: 60-66. [DOI] [PubMed] [Google Scholar]
- 16.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76: 301-314. [DOI] [PubMed] [Google Scholar]
- 17.Bevilacqua MP, Stengelin S, Gimbrone MA Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243: 1160-1165. [DOI] [PubMed] [Google Scholar]
- 18.Pober JS. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium: physiology and pathology. Am J Pathol. 1988;133: 426-433. [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence MB, Springer TA. Neutrophils roll on E-selectin. J Immunol. 1993;151: 6338-6346. [PubMed] [Google Scholar]
- 20.McEver RP, Cummings RD. Perspectives series: cell adhesion in vascular biology: role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100: 485-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery KF, Osborn L, Hession C, et al. Activation of endothelial-leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Proc Natl Acad Sci U S A. 1991;88: 6523-6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pober JS, Gimbrone MA Jr, Lapierre LA, et al. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986; 137: 1893-1896. [PubMed] [Google Scholar]
- 23.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401: 82-85. [DOI] [PubMed] [Google Scholar]
- 24.Pierce JW, Schoenleber R, Jesmok G, et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272: 21096-21103. [DOI] [PubMed] [Google Scholar]
- 25.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90: 1243-1250. [DOI] [PubMed] [Google Scholar]
- 26.Madge LA, Pober JS. TNF signaling in vascular endothelial cells. Exp Mol Pathol. 2001;70: 317-325. [DOI] [PubMed] [Google Scholar]
- 27.Madge LA, Pober JS. A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apoptosis but does not activate NFkappaB in human endothelial cells. J Biol Chem. 2000;275: 15458-15465. [DOI] [PubMed] [Google Scholar]
- 28.Reddy SA, Huang JH, Liao WS. Phosphatidylinositol 3-kinase as a mediator of TNF-induced NF-kappa B activation. J Immunol. 2000;164: 1355-1363. [DOI] [PubMed] [Google Scholar]
- 29.Puri KD, Doggett TA, Douangpanya J, et al. Mechanisms and implications of phosphoinositide 3-kinase delta in promoting neutrophil trafficking into inflamed tissue. Blood. 2004;103: 3448-3456. [DOI] [PubMed] [Google Scholar]
- 30.Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J Immunol. 2003;170: 2647-2654. [DOI] [PubMed] [Google Scholar]
- 31.Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000:96: 719-726. [PubMed] [Google Scholar]
- 32.Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol. 1996;133: 895-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts AW, Kim C, Zhen L, et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10: 183-196. [DOI] [PubMed] [Google Scholar]
- 34.Faffe DS, Seidl VR, Chagas PS, et al. Respiratory effects of lipopolysaccharide-induced inflammatory lung injury in mice. Eur Respir J. 2000;15: 85-91. [DOI] [PubMed] [Google Scholar]
- 35.Smith S, Skerrett SJ, Chi EY, Jonas M, Mohler K, Wilson CB. The locus of tumor necrosis factor-alpha action in lung inflammation. Am J Respir Cell Mol Biol. 1998;19: 881-891. [DOI] [PubMed] [Google Scholar]
- 36.Kunkel EJ, Ley K. Distinct phenotype of E-selectin-deficient mice: E-selectin is required for slow leukocyte rolling in vivo. Circ Res. 1996;79: 1196-1204. [DOI] [PubMed] [Google Scholar]
- 37.Pierce JW, Schoenleber R, Jesmok G, et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272: 21096-21103. [DOI] [PubMed] [Google Scholar]
- 38.Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. 1995;96: 994-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida M, Westlin WF, Wang N, et al. Leukocyte adhesion to vascular endothelium induces E-selectin linkage to the actin cytoskeleton. J Cell Biol. 1996;133: 445-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol. 1999;145: 1293-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Constantin G, Majeed M, Giagulli C, et al. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13: 759-769. [DOI] [PubMed] [Google Scholar]