Abstract
Hematopoietic stem cells (HSCs) and endothelial progenitors arise from a common embryonic precursor. However, these populations diverge prior to the onset of definitive hematopoiesis, as HSCs become CD45+ and are thought to lose the expression of endothelial markers. After the onset of definitive hematopoiesis, CD144 (vascular endothelial [VE]–cadherin) has been considered a specific marker of endothelial cells. In contrast, we found that virtually all HSC activity from embryonic day 13.5 (E13.5) fetal liver was CD144+. CD144 expression declined on E16.5 fetal liver HSCs and was absent from adult bone marrow HSCs. This identified a new marker that is differentially expressed between fetal and adult HSCs, and enhanced the purification of HSCs from the E13.5 fetal liver. These results emphasize the close developmental relationship between hematopoietic and endothelial cells, while indicating that CD144 is not a specific marker of endothelial cells during fetal development.
Introduction
Primitive hematopoiesis first arises in the yolk sac at embryonic day 7.5 (E7.5) in mice. Definitive hematopoietic stem cells (HSCs) arise in the aorta-gonad-mesonephros (AGM) around the dorsal aorta at E9 to E11, and then migrate to the fetal liver where definitive hematopoiesis arises around E12.1 The yolk sac and AGM are also prominent sites of vasculogenesis and there is a close developmental relationship between endothelial cells and hematopoietic cells.2
Culture studies of mouse embryonic stem (ES) cells suggest that hematopoietic cells and endothelial cells share a common embryonic precursor.3,4 FLK1+CD144+CD45– cells derived from ES cells or E7.5 to E9.5 yolk sac include both endothelial and hematopoietic progenitors.5-7 Prior to the onset of definitive hematopoiesis, CD45– endothelial cells from the dorsal aorta are capable of forming hematopoietic cells in culture8,9 or in vivo.10 The multilineage hematopoietic reconstituting activity from the AGM is initially in the CD45– fraction of cells at E10.5, and in the CD45+CD144+ cell fraction at E11.5.11 However, HSCs appeared to lose CD144 by the time they reached the fetal liver at E12.5.11 These observations suggest that common hematopoietic/endothelial progenitors exist during embryonic development, but that HSCs diverge from these progenitors prior to the onset of definitive hematopoiesis, losing endothelial marker expression as they gain CD45 expression.
Recent studies have challenged this view by showing that definitive HSCs give rise to endothelial cells under some,12,13 but not all,14 conditions. The endothelial marker CD31 (plateletendothelial cell adhesion molecule-1 [PECAM-1]) is also retained on HSCs throughout life.11,15 These observations raise the question of whether definitive HSCs broadly retain the expression of endothelial cell markers. Whereas CD31 is expressed by leukocytes and platelets in addition to endothelial cells, CD144 has been considered a specific marker of endothelial cells after the onset of definitive hematopoiesis.16,17 We have thus approached this question by examining CD144 expression on HSCs.
Study design
Fetal cells were obtained from timed pregnant C57BL/6:Ka-Thy1.1 (CD45.2) females killed 13 (E13.5) or 16 (E16.5) days after a vaginal plug was observed. Adult cells were obtained from 6- to 8-week-old C57BL/6:Ka-Thy1.1 mice. Cells were first stained with unconjugated monoclonal antibody to CD144 (11D4.1; Pharmingen, San Diego, CA) followed by anti–rat immunoglobulin G (IgG) F(ab)2 conjugated to fluorescein isothiocyanate (FITC; Jackson ImmunoResearch, West Grove, PA). Fetal liver cells were stained further with directly conjugated antibodies to Sca-1 (E13-biotin), Mac-1 (M1/70-APC), and phycoerythrin (PE)–conjugated lineage markers including B220 (6B2), CD3 (KT31.1), CD5 (53-7.3), CD8 (53-6.7), CD48 (BCM-1), Gr-1 (8C5), and Ter119 (Ly76). Adult marrow cells were stained with directly conjugated antibodies to Sca-1 (E13-APC), c-kit (2B8-biotin), Mac-1 (M1/70-PE), and the PE-conjugated lineage markers. Streptavidin-PharRed (APC-Cy7) was used to visualize biotinylated antibodies. Cells were resuspended in 2 μg/mL 7-aminoactinomycin D (7-AAD; Molecular Probes, Eugene, OR) to discriminate live from dead cells.
In long-term competitive reconstitution assays, adult C57BL/6:Ka-CD45.1 recipients (> 8 weeks old) were lethally irradiated with an x-ray source delivering 75 rads/min. The mice were administered 2 doses of 570 rad each, delivered at least 3 hours apart. CD45.2+ cells were sorted and then resorted into individual wells of a 96-well plate containing 2 × 105 CD45.1+ whole bone marrow cells. The contents of individual wells were injected into the retro-orbital venous sinus of anesthetized CD45.1+ recipients. Mice were maintained on antibiotic water (1.1 g neomycin sulfate and 106 U/L polymixin B sulfate; Sigma, St Louis, MO). After 1 to 6 months, blood was obtained from the tail veins of recipients, subjected to ammonium-chloride red cell lysis, and analyzed to determine the level of donor reconstitution.
Results and discussion
We compared the gene expression profiles of highly purified E14.5 fetal liver Thy-1lowSca-1+Lineage–Mac-1+ HSCs18 and adult bone marrow Thy-1lowSca-1+Lineage–c-kit+ HSCs.19,20 One of the genes that appeared differentially expressed between fetal and adult HSCs was Cd144 (vascular endothelial [VE]–cadherin), which was 13.6 ± 0.7-fold up-regulated in fetal liver HSCs by microarray analysis and 50.9 ± 23-fold up-regulated in fetal liver HSCs by quantitative polymerase chain reaction (qPCR; data not shown). This was interesting given that CD144 has been considered a specific marker of endothelial cells.16,17
By flow cytometry, CD144+ cells represented 5.0% ± 2.6% of cells in the E13.5 fetal liver, but this declined with each day of development, such that CD144+ cells represented only 1.9% of cells in the E16.5 fetal liver and 0.9% of cells in adult bone marrow (Figure 1A). To test whether CD144+ cells included HSCs, we sorted CD144+ and CD144– fractions of E13.5 fetal liver, E16.5 fetal liver, and adult bone marrow (Figure 1A) and injected these cells into lethally irradiated recipient mice in competitive reconstitution assays. In the E13.5 fetal liver, all detectable HSC activity fell within the CD144+ fraction, whereas in the E16.5 fetal liver there was HSC activity in both the CD144+ and CD144– fractions, and in adult bone marrow all the HSC activity was in the CD144– fraction (Figure 1B). This demonstrates that HSCs were CD144+ in the E13.5 fetal liver, but that some HSCs were CD144– by E16.5, and all HSCs were CD144– by adulthood.
Figure 1.
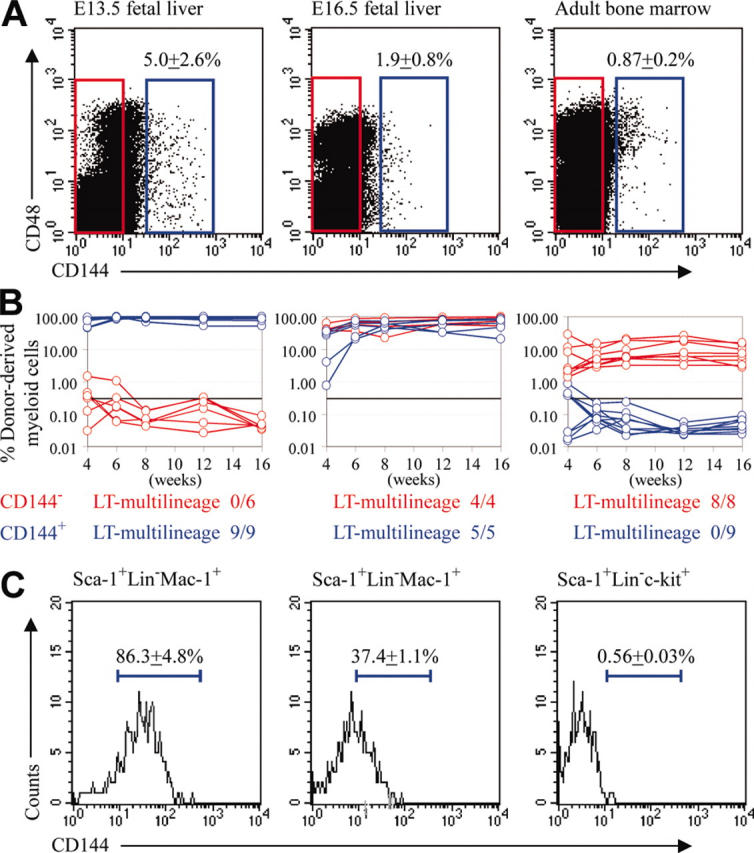
E13.5 fetal liver HSCs express the endothelial marker CD144 (VE-cadherin), but CD144 expression by HSCs declines over time and is no longer detected on adult bone marrow HSCs. (A) The frequency of CD144+ cells in the fetal liver declines over time. CD144– cells (red boxes) and CD144+ cells (blue boxes) were fractionated for functional studies. (B) Irradiated mice were competitively reconstituted with 1350 CD144+ cells or 48 650 CD144– cells from E13.5 liver, 500 CD144+ cells or 49 500 CD144– cells from E16.5 liver, and 2000 CD144+ cells or 198 000 CD144– cells from adult bone marrow. These cell doses were based on the proportion of CD144+ versus CD144– cells in 50 000 fetal liver cells, or 200 000 bone marrow cells as done in previous studies of marker expression by HSCs.18,21 The percentage of donor-derived myeloid (panels in B), B, and T (not shown) cells was analyzed in mice that underwent transplantation with CD144+ (blue lines) or CD144– (red lines) cells. Each line represents the level of donor myeloid cells in a mouse from 4 to 16 weeks after transplantation. The black line at 0.3% in each panel represents the background threshold observed in negative control mice, such that donor cell reconstitution could not be detected below this line. Mice that were long-term reconstituted by donor myeloid cells were always long-term multilineage reconstituted. The proportions of mice that became long-term multilineage reconstituted are indicated under each panel. (C) CD144 expression was analyzed in Sca-++Lineage–Mac-1+ HSCs from E13.5 and E16.5 fetal liver and in Sca-1+Lineage–c-kit+ HSCs from adult bone marrow.18,21
This temporal change in CD144 expression by HSCs was confirmed by CD144 staining of HSCs. HSCs were enriched as Sca-1+Lineage–Mac-1+ cells from the fetal liver18 or Sca-++Lineage–c-kit+ cells from adult bone marrow.19 These populations in the fetal liver and bone marrow give long-term multilineage reconstitution of both primary and secondary recipients.18,19 While most of the HSCs from the E13.5 fetal liver were CD144+, HSCs from E16.5 fetal liver exhibited reduced staining, and adult bone marrow HSCs appeared uniformly CD144– (Figure 1C).
Consistent with this phenotypic analysis, most of the HSC activity within the Sca-1+Lineage–Mac-1+ population fell within the CD144+ fraction at E13.5, but within both CD144+ and CD144– fractions at E16.5. 5 donor type CD144–Mac-++Lineage–Sca-1+ cells or CD144+Mac-1+Lineage–Sca-1+ cells from the E13.5 or E16.5 fetal liver were injected along with 200 000 recipient type bone marrow cells into lethally irradiated mice. At E13.5 most of the long-term multilineage reconstituting activity fell within the CD144+ fraction of Mac-1+Lineage–Sca-1+ cells, but at E16.5, there was strong long-term reconstituting activity in both CD144+ and CD144– fractions (Table 1). Whole bone marrow cells from primary recipients that had been reconstituted for 6 months by CD144–Mac-1+Lineage–Sca-1+ cells (n = 3 mice) or CD144+Mac-1+Lineage–Sca-1+ cells (n = 4 mice) were transplanted into secondary recipients (4 secondary recipients per primary recipient). The secondary recipients were always long-term multilineage reconstituted by donor cells, irrespective of whether the primary recipients had been reconstituted by CD144+ or CD144– cells. These results confirm that CD144 is expressed by most E13.5 HSCs but that CD144 expression is extinguished by a subset of HSCs by E16.5.
Table 1.
Even when highly enriched fetal liver Mac-1+Lineage-Sca-1+ HSCs were tested, most HSC activity was in the CD144+ (VE-cadherin+) fraction at E13.5, but both CD144+ and CD144- fractions had HSC activity by E16.5
| Fetal liver Mac-1+Lin-Sca-1+ cells | Mice that engrafted (%) | Long-term multilineage reconstituted (%) | Transient multilineage reconstituted (%) | Oligopotent reconstituted (%) | Frequency of HSCs |
|---|---|---|---|---|---|
| E13.5 CD144- | 5 of 8 (63) | 1 of 8 (13) | 2 of 8 (25) | 2 of 8 (25) | 1 in 38 |
| E13.5 CD144+ | 9 of 11 (82) | 5 of 11 (45) | 3 of 11 (27) | 1 of 11 (9) | 1 in 8.8 |
| E16.5 CD144- | 13 of 15 (87) | 10 of 15 (67) | 2 of 15 (13) | 1 of 15 (7) | 1 in 5.1 |
| E16.5 CD144+ | 5 of 6 (83) | 4 of 6 (67) | 0 of 6 (0) | 1 of 6 (17) | 1 in 5.1 |
Five CD45.2+CD144-Mac-1+Lineage-Sca-1+ cells or CD144+Mac-1+Lineage-Sca-1+ cells from the E13.5 or E16.5 fetal liver were injected along with 200 000 CD45.1+ cells into lethally irradiated CD45.1+ mice. To be considered reconstituted, donor-type cells of a particular lineage had to represent greater than 0.3% of cells. Mice were considered long-term multilineage reconstituted when donor-derived myeloid, B, and T cells were observed in the blood for at least 6 months after reconstitution. Mice were considered transiently multilineage reconstituted if donor-derived myeloid, B, and T cells were observed in the blood, but donor-type myeloid cells could no longer be detected by 16 weeks. Oligopotent reconstitution was characterized by transient donor reconstitution of 1 or 2 lineages. The frequency of HSCs was calculated by Poisson limit-dilution statistics18,21 based on the frequency of mice that became long-term multilineage reconstituted.
At E13.5, 1 of every 8.8 CD144+Mac-1+Lineage–Sca-1+ cells gave long-term multilineage reconstitution, whereas only 1 in 38 CD144–Mac-1+Lineage–Sca-1+ cells did (Table 1). At E16.5, 1 of every 5.1 CD144+Mac-1+Lineage–Sca-1+ cells gave long-term multilineage reconstitution, though by this point similar purity was observed in the CD144– fraction. These results provide a new marker that enhances HSC purity at early stages of fetal liver development.
Targeted deletion of CD144 disrupts the function and survival of endothelial cells, without affecting hematopoiesis from embryoid bodies in culture22 or the yolk sac in vivo.23 However, these mice die by E9.5, precluding an analysis of definitive HSCs in vivo.
It also remains to be determined whether fetal liver HSCs, and CD144+ HSCs in particular, retain the potential to generate endothelial cells. Some prior studies have observed that adult HSCs could make endothelial cells,12,13 while other studies have not.14 In future studies it will be necessary to use a variety of assays in order to address this question definitively.
The discovery that CD144 marks fetal liver HSCs will be important to consider in studies of the relationship between the hematopoietic and endothelial lineages. Together with the expression of CD3115 and endoglin24 by HSCs, the expression of CD144 by fetal liver HSCs emphasizes the close developmental relationship between the hematopoietic and endothelial cell lineages. However, it indicates that CD144 is not a specific marker of endothelial cells during fetal development and that fate mapping or lineage studies that employ CD144 as an endothelial marker must be interpreted with caution.
Acknowledgments
Thanks to Martin White and David Adams of the University of Michigan Flow-Cytometry Core Facility. Thanks to Elizabeth Smith for antibody production.
Prepublished online as Blood First Edition Paper, April 14, 2005; DOI 10.1182/blood-2004-12-4960.
Supported by the National Institutes of Health (NIH) (R21 HD40 760-02), the Howard Hughes Medical Institute, and the U.S. Army Research Laboratory/Research Office under grant number DAAD19-03-1-0168. Flow cytometry was partially supported by the University of Michigan (UM)–Comprehensive Cancer NIH CA46 592, and the UM-Multipurpose Arthritis Center NIH AR20 557. Antibody production was partially supported by the Rheumatic Core Disease Center (1 P30 AR48 310). I.K. was supported by a postdoctoral fellowship from the Korea Science and Engineering Foundation (KOSEF). O.H.Y. was supported by a predoctoral fellowship from the UM Institute of Gerontology.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Medvinsky AL. Ontogeny of the mouse hematopoietic system. Sem Devel Biol. 1993;4: 333-340. [Google Scholar]
- 2.Kubo H, Alitalo K. The bloody fate of endothelial stem cells. Genes Dev. 2003;17: 322-329. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy M, Firpo M, Choi K, et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386: 488-493. [DOI] [PubMed] [Google Scholar]
- 4.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125: 725-732. [DOI] [PubMed] [Google Scholar]
- 5.Fraser ST, Ogawa M, Yu RT, Nishikawa S, Yoder MC. Definitive hematopoietic commitment within the embryonic vascular endothelial-cadherin(+) population. Exp Hematol. 2002;30: 1070-1078. [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125: 1747-1757. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Li L, Shojaei F, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21: 31-41. [DOI] [PubMed] [Google Scholar]
- 8.Minasi MG, Riminucci M, De Angelis L, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129: 2773-2783. [DOI] [PubMed] [Google Scholar]
- 9.Oberlin E, Tavian M, Blazsek I, Peault B. Blood-forming potential of vascular endothelium in the human embryo. Development. 2002;129: 41474157. [DOI] [PubMed] [Google Scholar]
- 10.Jan YN, Jan LY. Genes required for specifying cell fates in Drosophila embryonic sensory nervous system. Trends Neurosci. 1990;13: 493-498. [DOI] [PubMed] [Google Scholar]
- 11.North TE, de Bruijn MF, Stacy T, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16: 661-672. [DOI] [PubMed] [Google Scholar]
- 12.Bailey AS, Jiang S, Afentoulis M, et al. Transplanted adult hematopoietic stem cells differentiate into functional endothelial cells. Blood. 2004;103: 13-19. [DOI] [PubMed] [Google Scholar]
- 13.Grant MB, May WS, Caballero S, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8: 607-612. [DOI] [PubMed] [Google Scholar]
- 14.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428: 668-673. [DOI] [PubMed] [Google Scholar]
- 15.Baumann CI, Bailey AS, Li W, Ferkowicz MJ, Yoder MC, Fleming WH. PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood. 2004;104: 1010-1016. [DOI] [PubMed] [Google Scholar]
- 16.Breier G, Breviario F, Caveda L, et al. Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood. 1996;87: 630-641. [PubMed] [Google Scholar]
- 17.Corada M, Mariotti M, Thurston G, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96: 9815-9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci U S A. 1995;92: 10302-10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1: 661-673. [DOI] [PubMed] [Google Scholar]
- 20.Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124: 1929-1939. [DOI] [PubMed] [Google Scholar]
- 21.Uchida N, Weissman IL. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin– Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J Exp Med. 1992;175: 175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci U S A. 1997;94: 6273-6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmeliet P, Lampugnani MG, Moons L, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98: 147-157. [DOI] [PubMed] [Google Scholar]
- 24.Chen CZ, Li M, de Graaf D, et al. Identification of endoglin as a functional marker that defines long-term repopulating hematopoietic stem cells. Proc Natl Acad Sci U S A. 2002;99: 15468-15473. [DOI] [PMC free article] [PubMed] [Google Scholar]


