Abstract
Disruption of stromal cell-derived factor-1 (SDF-1/CXCL12 [CXC chemokine ligand 12]) interaction leads to mobilization of stem/progenitor cells from bone marrow to circulation. However, prolonged exposure of CD34+ cells to SDF-1 desensitizes them to SDF-1. So how do cells remain responsive to SDF-1 in vivo when they are continuously exposed to SDF-1? We hypothesized that one or more mechanisms mediated by cytokines exist that could modulate SDF-1 responsiveness of CD34+ cells and the desensitization process. We considered transforming growth factor-β1 (TGF-β1) a possible candidate, since TGF-β1 has effects on CD34+ cells and is produced by stromal cells, which provide niches for maintenance and proliferation of stem/progenitor cells. TGF-β1 significantly restored SDF-1–induced chemotaxis and sustained adhesion responses in cord blood CD34+ cells preexposed to SDF-1. Effects of TGF-β1 were dependent on the dose and duration of TGF-β1 pretreatment. Phosphorylation of extracellular signal-regulated kinase 1 (Erk1)/Erk2 was implicated in TGF-β1 modulation of migratory and adhesion responses to SDF-1. Our results indicate that low levels of TGF-β1 can modulate SDF-1 responsiveness of CD34+ cells and thus may facilitate SDF-1–mediated retention and nurturing of stem/progenitor cells in bone marrow.
Introduction
Hematopoiesis is a complex process whereby proliferation of hematopoietic stem/progenitor cells (HSPCs) ensures continuous renewal of mature blood cells. Stem cells engraft bone marrow following lethal irradiation/chemotherapy and reconstitute hematopoietic and immune compartments.1 Homing of HSPCs to marrow requires concerted action among factors essential for cellular migration, such as adhesion molecules and their ligands as well as chemotactic factors that lead them to a microenvironment for their growth and survival.2-4 In the bone marrow it is important that cells lodge in proper niches. Their retention depends on adhesive interactions between HSPCs and marrow stroma. The CXC chemokine receptor 4 (CXCR4)–stromal cell-derived factor-1 (SDF-1)/CXC chemokine ligand 12 (CXCL12) axis plays a critical role in homing and retention of HSPCs in the bone marrow.5-7
SDF-1/CXCL12 is a potent chemoattractant for primitive and more mature hematopoietic cells. Stromal cells are a potent source of SDF-1. SDF-1 plays a key role during ontogeny of hematopoietic system.8,9 In adults, it promotes engraftment of transplanted HSPCs and hematopoietic reconstitution.3,7,10,11 The chemotactic effect of SDF-1 is mediated through the G protein-coupled receptor CXCR4.12,13 CXCR4, upon ligand binding, activates integrin-mediated firm adhesion and transmigration of HSPCs through bone marrow endothelium and stromal cells.14,15
Rapid desensitization is a hallmark of G-protein–coupled receptors, leading to transient responses of short duration.16 During steady-state hematopoiesis, CXCR4/SDF-1 interactions restrict HSPCs cells in marrow and disruption of the SDF-1/CXCR4 axis leads to their egress into the circulation. This raises an important question. How do HPSCs remain responsive to SDF-1 in vivo when HSPCs are being continuously exposed to SDF-1? We considered the possibility that responses of HSPCs to SDF-1 could be modulated by other cytokines, and one of these may be transforming growth factor-β1 (TGF-β1).
TGF-β1, a pleiotropic factor, plays an important role in regulating the balance between proliferation and differentiation of hematopoietic cells.17,18 TGF-β type I and type II receptors are expressed on primitive progenitors and throughout stages of their maturation and development.19 Like SDF-1, TGF-β1 is produced by stromal cells.20 TGF-β1 induces chemotaxis of human mast cells, monocytes, and monocytic dendritic cells and increases expression of integrin receptors very late antigen-4 (VLA-4) and VLA-5 on many cell types.21-25
We examined the effect of prolonged exposure of CD34+ cells to SDF-1 on their chemotactic and adhesion responses to further SDF-1 stimulation and whether these responses could be modulated by TGF-β1. Our results demonstrate cross talk between TGF-β1 and SDF-1–CXCR4 interactions and suggests one means by which CD34+, CD34+CD38–, and myeloid progenitor cells may retain responsiveness to SDF-1 even in the presence of sustained exposure to SDF-1.
Materials and methods
Antibodies and reagents
The following antibodies were used for cell staining: fluorescein isothiocyanate (FITC)–conjugated CD34 (Miltenyi Biotec, Auburn, CA), phycoerythrin (PE)–conjugated CXCR4, and allophycocyanin (APC)–conjugated CD38 (BD Pharmingen, San Diego, CA). For blocking integrin-mediated adhesion we used anti–VLA-4 (Clone HP1/2; Beckman Coulter, Fullerton, CA) and anti–VLA-5 (clone II A1; BD Pharmingen). Antibodies used for Western blot were anti–phospho Erk1/2 mouse monoclonal antibody, Erk1/Erk2 rabbit polyclonal antibody, anti–phospho Akt (protein kinase B; Ser473) rabbit antibody and anti–Akt rabbit polyclonal antibody (Cell Signaling Tech, Beverly, MA). The secondary antibodies were peroxidase-labeled anti–mouse immunoglobulin G (IgG) and anti–rabbit IgG horseradish peroxidase (HRP)–linked antibodies (Amersham Biosciences, Piscataway, NJ). Mitogen-activated protein/extracellular signal regulated kinase/kinase (MEK) inhibitor, PD98059, was purchased from Cell Signaling. TGF-β1 was purchased from R&D (R&D Systems, Minneapolis, MN) and resuspended and stored as per the manufacturer's instruction. Human recombinant SDF-1 was purchased from BioVision (Mountain View, CA).
Cells
Human cord blood was obtained with institutional review board approval. Low-density mononuclear cells were isolated by density gradient centrifugation over Ficoll-Paque Plus (Amersham Pharmacia Biotech). CD34+ cells were enriched using direct CD34 magnetic separation kit (Miltenyi Biotec) according to the manufacturer's instructions. Purity of the enriched CD34+ cells was 95% to 98% after cells were sequentially passed over 2 columns.
Expansion and pretreatment of CD34+ cord blood (CB) cells
Enriched CD34+ cells were expanded by growing them in Iscoves modified Dulbecco medium (IMDM; Gibco, Grand Island, NY) containing 10% fetal calf serum (FCS), 2 mM glutamine, and including 100 ng/mL recombinant human (rh) thrombopoietin (TPO), 100 ng/mL rhFlt-3 ligand, and 50 ng/mL rh stem cell factor (SCF) for 4 days at 37°C in a humidified atmosphere containing 5% CO2. After 4 days, cells were centrifuged, and the pellet was resuspended in medium containing the above-mentioned cytokines. Cells were pretreated in medium (containing SCF, TPO, Flt-3 ligand) alone or in medium (containing SCF, TPO, and Flt-3 ligand) along with SDF-1 (200 ng/mL) with or without TGF-β1 (0.5 ng/mL) for 24 hours, unless otherwise indicated. All pretreatments contained SCF, TPO, and Flt-3 ligand at the above-mentioned doses. After incubation, cells were harvested, washed, and used in different assays. In some experiments, freshly isolated CD34+ cells were cultured in IMDM containing 2 mM/L L-glutamine and 20% FCS alone or containing SDF-1 (200 ng/mL) with or without TGF-β1 (0.5 ng/mL) for 20 to 24 hours and used in chemotaxis and adhesion assays.
Migration assay
Chemotaxis assays were performed using 96-well chemotaxis chambers (NeuroProbe, Gaithersburg, MD) in accordance with the manufacturer's instructions with minor variations.26 CD34+ cells in 30 μL chemotaxis medium (IMDM + 1% FCS) were added to the membrane. Chemotaxis medium alone (300 μL) or containing 200 ng/mL SDF-1 was added to the bottom well. After 4 hours of incubation at 37°C in 5% CO2, cells migrated to the lower chamber, and nonmigrated cells in the upper chamber were counted for 30 seconds using fluorescence-activated cell scan (FACScan) under identical flow conditions. In experiments designed to determine migration of progenitor cells to SDF-1, both input and migrated cells were counted using a Coulter counter prior to plating cells in semisolid culture.
Progenitor cell colony assay
To assess clonogenic ability, methylcellulose progenitor assay was performed as described previously.27 Briefly, 1 to 5 × 102 CD34+ cells/mL were plated in 1% methyl cellulose, 10–4 M 2-mercaptoethanol, 2 nmol/L L-glutamine, 30% fetal calf serum, 1 U/mL recombinant human (rh) erythropoietin, 10 ng/mL rh granulocyte-macrophage colony-stimulating factor (GM-CSF), 10 ng/mL rh interleukin-3, and 50 ng/mL rhSCF. Cultures, set up in duplicate or triplicate, were incubated in a humidified atmosphere, 5% CO2 and 5% O2 at 37°C for 14 days. Colonies from erythroid burst colony-forming units (BFU-Es), and mixed erythroid-myeloid colony-forming units (CFU-GEMMs) were scored.28
Flow cytometry analysis of cell surface marker
Freshly enriched CD34+ cells or cytokine-expanded CD34+ cells were washed, resuspended in FACScan buffer (PBS (phosphate-buffered saline) + 2 mM EDTA (ethylenediaminetetraacetic acid) + 2% FCS), incubated for 5 minutes at room temperature with Fc-block, and then incubated with a recommended volume of antihuman antibodies: CD34 conjugated to FITC, CXCR4 conjugated to PE and CD38 conjugated to APC. Cells were incubated for 30 minutes at 4°C, washed, and fixed in 2% paraformaldehyde prior to analysis by FACScan using CellQuest software (BD Biosciences Immunocytometry Systems, San Jose, CA).
Adhesion assay
Adhesion assays were performed in 96-well plates (high-binding; Costar, Cambridge, MA) coated with purified plasma fibronectin (Sigma, St Louis, MO; 10 μg/mL) overnight at 4°C in 100 mM bicarbonate buffer (pH 8.8), followed by 2 hours of incubation at 37°C. To block nonspecific binding sites, plates were subsequently incubated for another 2 hours at 37°C with 2% bovine serum albumin (BSA) in the same solution. CD34+ cells were added to wells and incubated at 37°C in a humidified atmosphere containing 5% CO2 for 30 minutes. To distinguish between transient and sustained adhesion mediated by SDF-1, SDF-1 was added to the wells either in the last 2 minutes of incubation (transient response) or at the start of the incubation and kept there until the end of the incubation period (sustained adhesion). In certain experiments, cells were preincubated with either 10 μg/mL human integrin-specific antibody or isotype control antibody at 4°C for 30 minutes or with PD98059 (40 μM) at 37°C for 30 minutes. Cells were washed and plated for adhesion assay. Antibodies did not contain azide. After incubation, nonadherent cells were removed, and the wells were washed 2 to 3 times with medium. Adherent cells were recovered by detaching them using Cell Dissociation Buffer (Gibco).
Actin polymerization
Actin polymerization assays were performed as described by others29 with a few modifications. Cells pretreated with different stimulants were washed and resuspended in IMDM supplemented with 0.1% BSA at 106 cells/mL. SDF-1 (200 ng/mL) was added to the cell suspension. At various time intervals, cells were permeabilized and fixed using 0.2 mL permeabilizing/fixing solution (BD Cytofix/Cytoperm; BD Biosciences Pharmingen) and stained with 0.1 mL Phalloidin-FITC solution (4 × 10–7 M; Sigma). Cells were incubated for 10 minutes at 37°C, and washed twice with BD wash buffer (BD Biosciences Pharmingen, Palo Alto, CA). Pellets were resuspended in 500 μL 2% paraformaldehyde solution. Mean fluorescence was measured by FACScan.
Calcium influx
Calcium flux induced by SDF-1 in CD34+ cells was studied by flow cytometry.30 An equal volume of Fluo-3AM (stock concentration 2 mM; Molecular Probes, Eugene, OR) and Pluronic acid (stock concentration 20% wt/vol; Molecular Probes) were mixed just before use. Cells were washed and resuspended in IMDM + 2% BSA, and Fluo-3AM/Pluronic acid mix was added for a final Fluo-3/AM concentration of 4 μM. After incubation for 45 minutes at room temperature, cells were washed in Ca2+ flux assay buffer (Hanks Balanced Salt solution containing 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and 0.2% BSA, pH 7.4) to remove extracellular dye, incubated for 10 minutes at room temperature, and analyzed by FACScan. Background fluorescence of each sample was measured, and SDF-1 was then added to samples. Samples were quickly mixed by vortex, and Ca2+ influx was recorded.
Western blot
Cells pretreated with different stimuli were washed, resuspended in IMDM + 0.5% BSA, and either left unstimulated or stimulated with SDF-1 for the indicated time period. Alternatively, cells were first incubated with PD98059 for 45 minutes at 37°C before SDF-1 stimulation. Cells were immediately centrifuged at 4°C and washed with ice-cold PBS prior to lysis, followed by centrifugation at 14 000g for 30 minutes at 4°C. Protein content of lysates was quantified using bicinchoninic acid protein assay reagent (Pierce, Rockford, IL), and samples were adjusted to equal protein concentration and volume. Total cell lysates were resolved in 10% sodium dodecyl sulfate (SDS)–polyacrylamide gels (Novex, San Diego, CA) and transferred to Hybond membrane (Millipore, Bedford, MA). Filters were blocked using 3% BSA in Tris (tris(hydroxymethyl)aminomethane)–buffered saline/Tween-20 (TBST) for 1 hour and incubated overnight with antibodies. Membranes were washed with TBST and incubated with secondary antibodies conjugated to horseradish peroxidase, and antibody binding was detected by enhanced chemiluminescence (ECL) reaction (Amersham Life Science, Arlington Heights, IL).
Statistical analysis
Student 2-tailed t test was used for statistical analysis. The level of significance is indicated by P value.
Results
Effect of in vitro expansion of CD34+CB cells on CXCR4 and CD34 expression
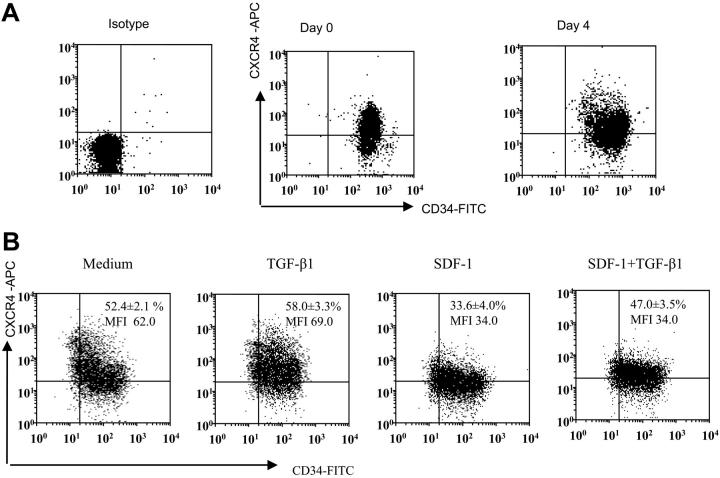
Earlier studies demonstrated that the cocktail of TPO, Flt-3 ligand, and SCF induces increased numbers of CD34+ and hematopoietic progenitor cells from human CD34+ cells.31,32 Expanded progenitor cells using the above-mentioned cytokines retained the ability to engraft in vivo. In this study we have used cytokine-expanded CD34+ cells, and selected experiments were performed using freshly isolated CD34+ cells. We expanded freshly isolated CD34+ CB cells for 4 days using the combination of TPO, Flt-3 ligand, and SCF. After 4 days of expansion, there was an approximately 3.5-fold (3.43 ± 1.2, mean ± SD of 8 experiments) increase in total number of cells. Moreover, the cells still remained essentially CD34+ positive, albeit levels of CD34 and CXCR4 on the expanded population were more heterogeneous than freshly enriched CD34+ cells (Figure 1A). Expanded CD34+ cells were then treated with the same cytokine cocktail (TPO, Flt-3, SCF) either alone or containing TGF-β1, SDF-1, or SDF-1 and TGF-β1 for 24 hours. SDF-1 pretreatment decreased the proportion of cells expressing CXCR4 and also decreased CXCR4 expression (Figure 1B). However, SDF-1 along with TGF-β1 pretreatment increased the proportion of cells expressing CXCR4 but did not change CXCR4 expression level (Figure 1B).
Figure 1.
CD34 and CXCR4 expression on freshly isolated versus cytokine-expanded CD34+-enriched cord blood cells. (A) Representative dot blot of CD34 and CXCR4 expression on freshly isolated CD34+ cells and CD34+ cells expanded in a cocktail of cytokines (see “Materials and methods”) for 4 days. Freshly isolated and expanded CD34+ cells were stained using CD34-FITC, CXCR4-APC monoclonal antibodies (mAbs) and analyzed by multivariant flow cytometry. Gates were set on the basis of cell staining with matched-isotype control mAbs. Similar results were obtained in 8 other experiments performed independently. (B) Representative dot blot showing expression of CD34 and CXCR4 on ex vivo–expanded CD34 cells after 24 hours of culture in cytokine cocktail alone (medium) or along with TGF-β1 (0.5 ng/mL), SDF-1 (200 ng/mL), or the combination of SDF-1 (200 ng/mL) + TGF-β1 (0.5 ng/mL). CXCR4 expression on CD34+ cells is presented as mean ± SD of 3 independent experiments. MFI indicates mean fluorescence intensity.
Desensitization of CD34+ cells to SDF-1
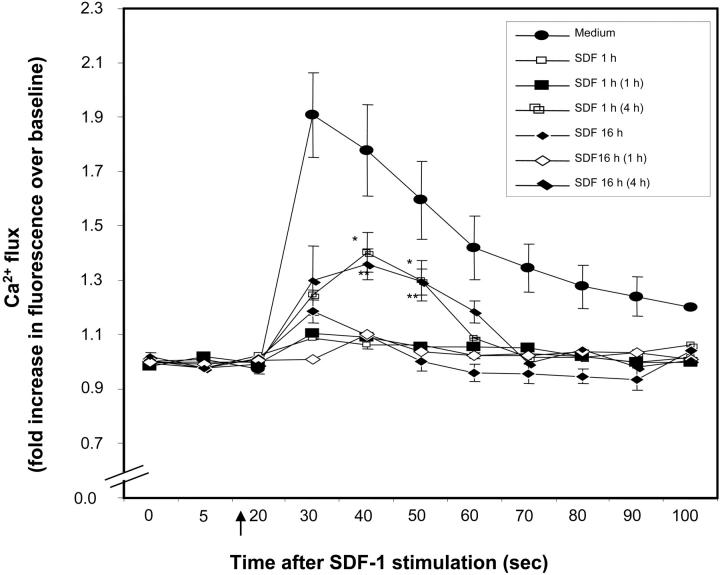
Prolonged exposure of cells to SDF-1 induces desensitization and internalization of CXCR4,33 leading to reduced SDF-1–induced chemotaxis. Similarly, we found that calcium mobilization in response to SDF-1 was significantly desensitized in freshly isolated CD34+ cells by prior treatment with SDF-1, either for 1 hour or 16 hours (Figure 2). Further to determine how long this desensitization lasts, SDF-1–pretreated CD34+ cells were kept in medium alone for varying lengths of time, and then calcium mobilization in response to SDF-1 was analyzed. As seen in Figure 2, SDF-1 withdrawal for 4 hours, but not 1 hour, partially restored SDF-1–induced calcium mobilization in SDF-1–pretreated CD34+ cells.
Figure 2.
Desensitization of CXCR4. Nonexpanded CD34+ cells were pretreated with SDF-1 (200 ng/mL) for 1 hour, 20 hours, or kept in medium alone. Cells were washed and either immediately loaded with Fluo-3AM or kept in medium for 1 hour or 4 hours (in the absence of SDF-1) and then loaded with Fluo-3AM. The time noted in parentheses in the insert key box denotes the time for which SDF-1–pretreated cells were kept in medium prior to SDF-1 stimulation. Baseline fluorescence was first recorded and calcium flux following SDF-1 (200 ng/mL) stimulation (indicated by ↑) was analyzed by FACScan. Increase in fluorescence intensity following SDF-1 addition (compared with baseline), represents the calcium flux in cells and is shown on the y-axis of the plot. Data represent mean ± SEM of 3 independent experiments. *P < .05 and **P < .05 compared with cells pretreated in with SDF-1 for 1 hour and 20 hours, respectively.
TGF-β1 modulates chemotactic response of CD34+ cells to SDF-1
Considering the importance of SDF-1/CXCR4 interactions in vivo,5,34 we investigated the chemotactic response of ex vivo–expanded CD34+ cells, prestimulated with SDF-1 for 24 hours, to SDF-1 in an in vitro chemotaxis assay. SDF-1 pretreatment reduced the chemotactic response of CD34+ CB cells to SDF-1 dose dependently (Figure 3A). Down-modulation in chemotactic response of the SDF-1–prestimulated CD34+ cells to SDF-1 was significantly overcome when the cells were prestimulated with SDF-1 in the presence of TGF-β1 (Figure 3B). The effect of TGF-β1 was dose dependent; 0.5 ng/mL TGF-β1 was optimal (Figure 3C). Reduction in SDF-1–induced migratory response in SDF-1–pretreated cells could be seen as early as 2 hours and lasted up to 24 hours of SDF-1 pretreatment (Figure 3D). To determine whether the effect of TGF-β1 was time dependent, CD34+ cells were coincubated with TGF-β1 and SDF-1 for varying lengths of time up to 24 hours before being subjected to SDF-1–induced chemotaxis. Maximal restoration of SDF-1–induced chemotactic response was observed when cells were cultured with TGF-β1 and SDF-1 for 24 hours (Figure 3D). Shorter coincubation of cells with TGF-β1 and SDF-1 restored partially, but not significantly, SDF-1–induced chemotactic response.
Figure 3.
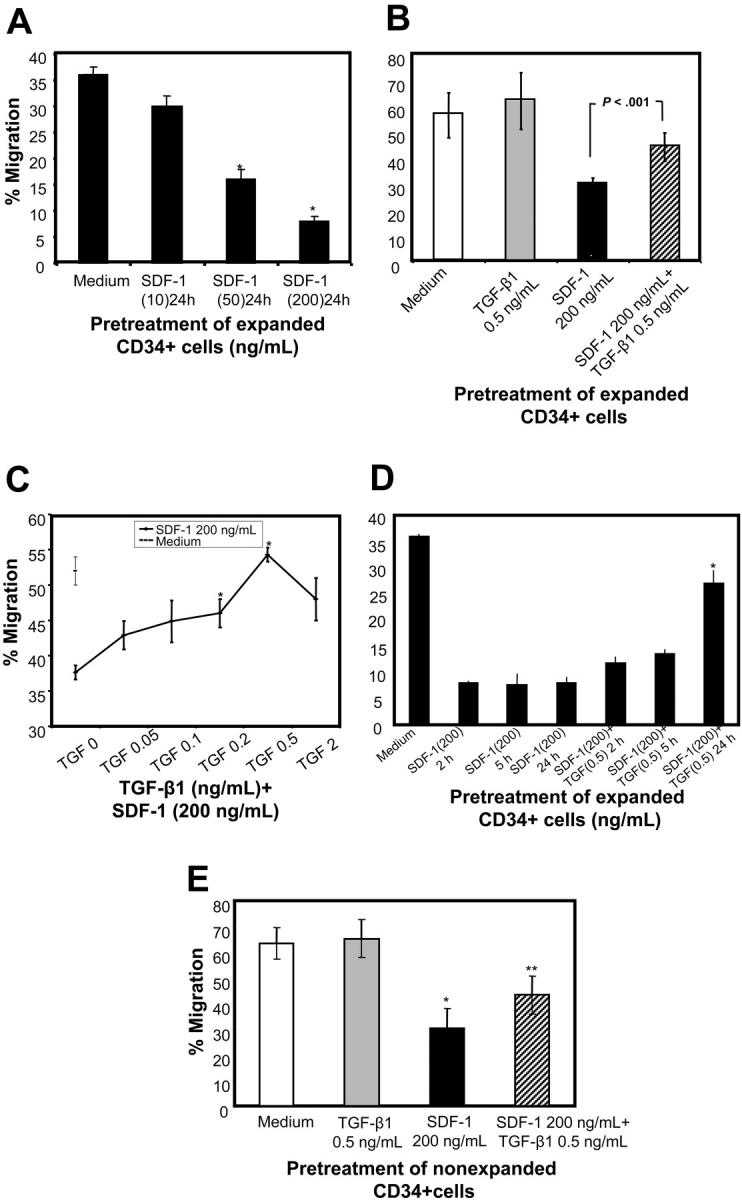
Effect of TGF-β1 on chemotactic response to SDF-1 of CD34+ cells preexposed to SDF-1. (A) CB CD34+ cells were expanded for 4 days as described in “Materials and methods.” Expanded CD34+ cells were first pretreated with indicated doses of SDF-1 for 24 hours, and then after washing the cells their chemotactic activity toward SDF-1 (200 ng/mL) was determined. *P < .05 compared with cells cultured in cytokine cocktail. (B) CD34+ cells were cultured in a cytokine cocktail (SCF, TPO, and Flt-3 ligand) alone, or along with TGF-β1, SDF-1, or SDF-1 and TGF-β1 for 24 hours. Cells were washed, and their chemotactic activity to SDF-1 (200 ng/mL) was determined in migration assay. (C) CD34+ cells were pretreated with SDF-1 (200 ng/mL) in the presence of various doses of TGF-β1 for 24 hours, and their chemotactic response to SDF-1 (200 ng/mL) activity was determined. *P < .05 compared with cells pretreated with SDF-1. (D) Cytokine-expanded CD34+ cells were coincubated with SDF-1 and TGF-β1 or SDF-1 alone for the indicated time periods, and SDF-1 (200 ng/mL) directed chemotactic activity of the cells was assessed. *P < .05 compared with cells pretreated with SDF-1 alone. (E) Freshly isolated CD34+ cells were cultured in medium alone or along with TGF-β1, SDF-1, or SDF-1 and TGF-β1 for 20 hours. Cells were washed, and their chemotactic activity to SDF-1 (200 ng/mL) was determined in migration assay. (A-D) Data are presented as mean ± SD of 4 separate experiments. (E) Data are presented as mean ± SEM for 3 separate experiments. Of 3 experiments shown, 2 were conducted by pooling cells from different CB collections (after pretreatments), and in 1 experiment cells from a single CB collection were used. *P < .05 compared with cells cultured in medium alone and **P < .05 compared with SDF-1–pretreated cells.
To explore whether this phenomenon was observed in fresh CD34+ cells, we pretreated nonexpanded CD34+ cells with SDF-1 alone or SDF-1 and TGF-β1 for 20 hours and compared their chemotactic activity toward SDF-1. As shown in Figure 3E, similar to cytokine-expanded CD34+ cells, SDF-1–pretreated cells had reduced chemotactic activity compared with CD34+ cells cultured in medium alone, and this was partially restored by TGF-β1.
Analysis of migrated cells–cell surface maker and clonogenic potential
We next examined whether this effect was reproduced in a more primitive hematopoietic cell compartment. CD34++/CD38lo cells identify primitive hematopoietic populations.35 Similar to total CD34+ population, SDF-1–directed migratory response of CD34++/CD38lo cells was reduced by SDF-1 prestimulation (Figure 4). This effect was observed for both cytokine-expanded (Figure 4A) and freshly isolated cells (Figure 4B). Reduction in migration potential of CD34++/CD38lo cells was significantly reversed when these cells were exposed simultaneously to SDF-1 and TGF-β1. SDF-1–induced chemotactic response of erythroid and multipotential progenitors was significantly reduced by SDF-1 pretreatment but was restored when CD34+ cells were preincubated with SDF-1 in the presence of TGF-β1 (Figure 4C-D).
Figure 4.
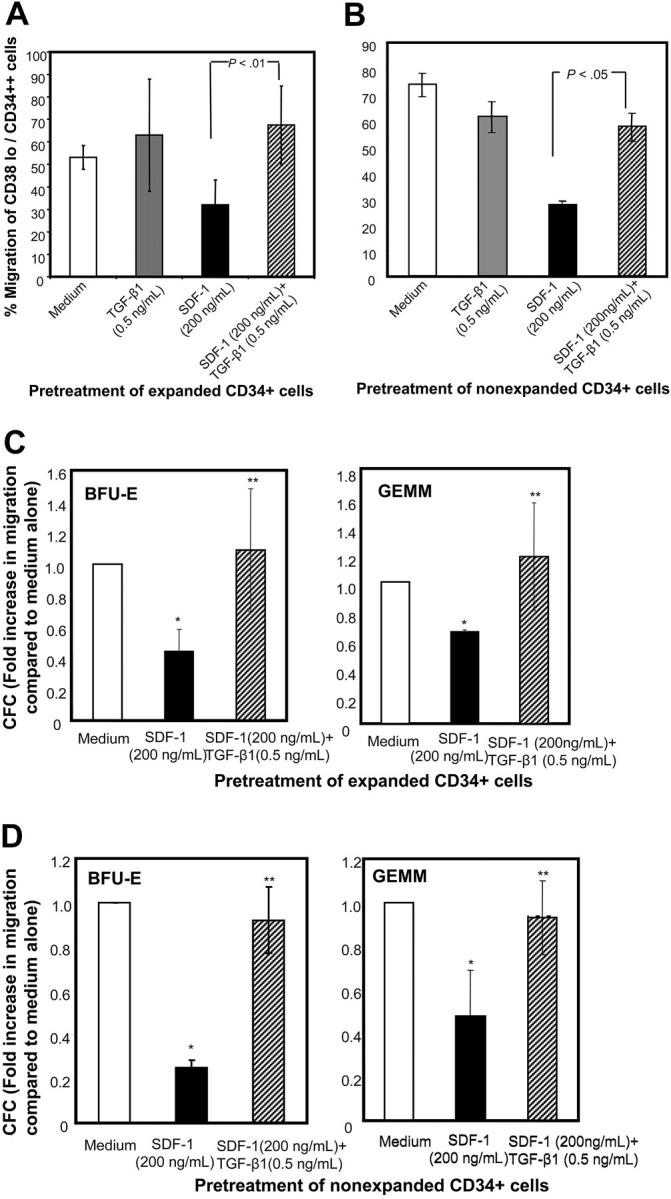
Migration potential of CD38lo/CD34++ cells and myeloid progenitors pretreated with TGF-β1 and SDF-1. (A) Ex vivo–expanded CD34+ cells cultured in cytokine cocktail alone (medium) or containing TGF-β1 (0.5 ng/mL), SDF-1 (200 ng/mL), or SDF-1 (200 ng/mL) along with TGF-β1 (0.5 ng/mL) for 24 hours were assayed for their chemotactic activity toward SDF-1 (200 ng/mL). The proportion of CD38lo/CD34++ cells that migrated was determined by staining aliquots of input and migrated cells with CD34-FITC and CD38-APC and isotype-matched control antibodies. Data are presented as mean ± SD of 3 independent experiments. (B) Freshly isolated CD34+ cells cultured in medium alone or containing TGF-β1 (0.5 ng/mL), SDF-1 (200 ng/mL), or SDF-1 (200 ng/mL) along with TGF-β1 (0.5 ng/mL) for 20 hours were assayed for their chemotactic activity toward SDF-1 (200 ng/mL). The proportion of CD38lo/CD34++ cells that migrated was determined by staining aliquots of input and migrated cells with CD34-FITC and CD38-APC and isotype-matched control antibodies. Data are presented as mean ± SEM of 3 independent experiments. (C) Cytokine-expanded and (D) nonexpanded, myeloid in input (upper chamber), and migrated cells (lower chamber) were assayed by methylcellulose colony assay. The fold increase in progenitor cell migration for SDF-1 and SDF-1 + TGF-β1 pretreatment was normalized with respect to the efficiency of progenitor migration in untreated CD34+ cells. (C) Data are represented as mean ± SD of 3 independent experiments, and (D) data are represented as mean ± SEM of 3 independent experiments. *P < .05 compared with cells cultured in medium and **P < .05 compared with cells pretreated with SDF-1.
Actin polymerization and calcium flux
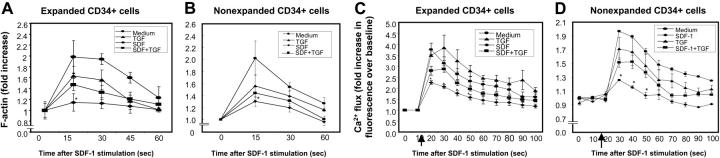
Transient actin polymerization in response to chemokines is an important cellular event for cell polarization, formation of cell membrane structures such as uropods, and chemotaxis.36,37 Therefore, we examined whether SDF-1–induced actin polymerization (F-actin) was affected by preexposure of CD34+ cells to SDF-1. SDF-1 pretreatment led to a significant reduction in SDF-1–induced actin polymerization (F-actin) in both ex vivo cytokine-expanded (Figure 5A) and freshly isolated (nonexpanded) CD34+ cells (Figure 5B) prestimulated with SDF-1, compared with cells cultured in SDF-1 and TGF-β1. Although the amount of SDF-1–induced actin polymerization in CD34+ cells pretreated with SDF-1 and TGF-β1 was reproducibly and significantly higher than that induced in CD34+ cells pretreated with SDF-1 alone, it was lower than SDF-1–induced actin polymerization in cells cultured in medium alone (Figure 5A-B).
Figure 5.
SDF-1–induced actin polymerization and calcium (Ca2+) flux in CD34+ cells. (A) Ex vivo–expanded CD34+ cells and (B) nonexpanded CD34+ cells were pretreated with TGF-β1 (0.5 ng/mL), SDF-1 (200 ng/mL), SDF-1 (200 ng/mL) + TGF-β1 (0.5 ng/mL), or cytokine cocktail alone (medium) for 20 to 24 hours. Cells were then washed and stimulated with 200 ng/mL SDF-1 for the indicated times to induce actin polymerization. Cells were stained with FITC-phalloidin and then subjected to flow cytometry. Data represent the mean ± SD of 3 independent experiments. *P < .05 compared with CD34+ cells pretreated with medium, TGF-1, or SDF-1 + TGF-β1. SDF-1–mediated calcium flux in (C) ex vivo–expanded CD34+ cells and (D) nonexpanded CD34+ cells pretreated with medium alone, TGF-β1 (0.5 ng/mL), SDF-1 (200 ng/mL), or TGF-β1 (0.5 ng/mL) along with SDF-1 (200 ng/mL). Cells were loaded with Fluo-3AM. Baseline fluorescence was first recorded and then calcium flux following SDF-1 (200 ng/mL) stimulation (indicated by ↑) was analyzed by FACScan. Increase in fluorescence intensity following SDF-1 addition (compared with baseline) represented the calcium flux in the cells and is shown on the y-axis of the plot. Data represent the mean ± SEM of 3 independent experiments. *P < .05 compared with cells pretreated in cytokine cocktail alone or along with TGF-β1.
Consistent with a previous report,38 we found that the CXCR4-mediated rise in cytosolic free calcium is desensitized by prior exposure of cells to SDF-1. This activity was only partially restored when cells were exposed to SDF-1 in the presence of TGF-β1 (Figure 5C [expanded] and D [nonexpanded]).
SDF-1–induced Erk1/Erk2 but not protein kinase B (PKB)/AKt phosphorylation is down-modulated by SDF-1 prestimulation
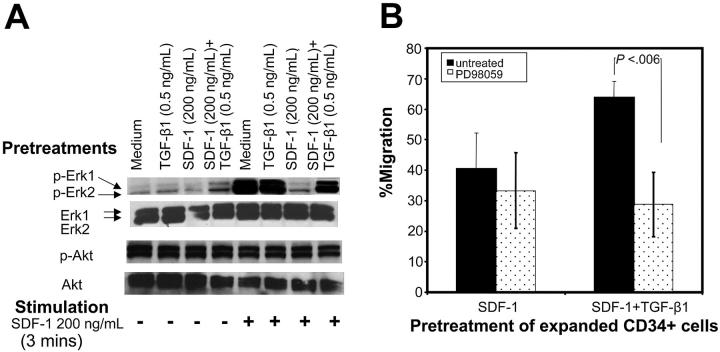
Erk-mitogen kinase pathway has been implicated in cell migration39-41 and can be activated by both SDF-142 and TGF-β1.43 Therefore, we examined the levels of SDF-1–induced Erk1/Erk2 phosphorylation in cells pretreated under various conditions. The basal level of Erk1/Erk2 phosphorylation was comparable among the cells pretreated under different conditions (Figure 6A). CD34+ cells pretreated with cytokine cocktail alone or along with TGF-β1 displayed high levels of SDF-1–induced Erk1/Erk2 phosphorylation, whereas cells pretreated with SDF-1 failed to do so. When CD34+ cells were pretreated with SDF-1 in the presence of TGF-β1, they retained the ability to undergo robust Erk1/Erk2 phosphorylation in response to SDF-1 stimulation.
Figure 6.
Improved chemotactic activity of cells pretreated with SDF-1 and TGF-β1 is associated with enhanced Erk1/Erk2 phosphorylation in response to SDF-1. (A) Ex vivo–expanded CD34+ cells were cultured in cytokine cocktail alone (medium) or along with TGF-β1, SDF-1, or SDF-1 and TGF-β1 for 24 hours. Cells were harvested, washed, and either left unstimulated or stimulated with 200 ng/mL SDF-1 for 3 minutes. Cells were solubilized, and extracts were subjected to immunoblotting by using anti–phospho Erk1/Erk2 or anti–phospho-Akt (Ser473) antibodies. After stripping and saturating nonspecific protein binding, we reprobed the same blots with anti–Erk1/Erk2 or anti-Akt antibody. Blots were developed by a chemiluminescence reaction and exposed to radiographic film. A representative experiment of 1 of 3 experiments performed is shown. (B) Effect of MEK inhibitor, PD98059, on migration activity of CD34+ cells pretreated with cytokine cocktail along with SDF-1 (200 ng/mL) or SDF-1 (200 ng/mL) + TGF-β1 (0.5 ng/mL) SDF-1 for 24 hours. Cells were tested for their migration response to SDF-1 (200 ng/mL). Data represent the mean ± SD of 4 independent experiments.
Unlike SDF-1–induced Erk1/Erk2 phosphorylation, SDF-1 did not induce any Akt phosphorylation above the background level in cells pretreated under different conditions (Figure 6A). Moreover background Akt phosphorylation levels were comparable among cells pretreated with cytokine cocktail alone or cytokine cocktail and SDF-1 alone, TGF-β1 alone, or SDF-1 along with TGF-β1.
To examine the requirement for Erk activation in SDF-1–induced migration, the migration assays were performed in the presence of PD98059, a MEK inhibitor. PD98059-inhibited migration potential of CD34+ cells cultured in cytokine cocktail alone or pretreated with TGF-β1 or with SDF-1 and TGF-β1 was significantly reduced and was comparable to cells pretreated with SDF-1 alone (data not shown). We consistently observed that the migration potential of cells pretreated with SDF-1 and TGF-β1 was significantly inhibited in the presence of PD98059, whereas PD98059 did not have much effect on the residual migration potential of SDF-1–pretreated cells (Figure 6B). This suggests that phosphorylation of Erk1/Erk2 is required for maximal SDF-1–induced chemotaxis. Residual chemotactic activity seen in CD34+ cells pretreated with SDF-1 was unaffected by Erk1/Erk2 phosphorylation and possibly involves other signaling pathways.
Short-term and long-term adhesion mediated by SDF-1
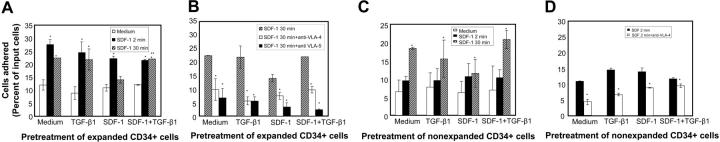
HSPCs in the bone marrow (BM) are thought to be positioned within specific stromal niches that provide the microenvironment for their proliferation and differentiation.44 As HSPCs differentiate, they move from one type of niche to another.45,46 For HSPCs to remain in bone marrow for prolonged periods, a sustained adhesive response needs to be triggered. We examined the effect of SDF-1 preexposure on both SDF-1–induced transient (short-term, 2-minute SDF-1 stimulation) and sustained adhesion (long-term, 30-minute SDF-1 stimulation) responses. In expanded CD34+ cells, SDF-1 induced a significant increase in transient adhesion response, irrespective of pretreatment conditions. This response was not affected by SDF-1 preexposure (Figure 7A). Pretreatment of cells with TGF-β1 along with SDF-1 did not further enhance this response. Next, to simulate the bone marrow situation whereby the stem/progenitor cells are continuously exposed to SDF-1, we evaluated the effect of long-term SDF-1 stimulation of cytokine-expanded CD34+ cells on their adhesive response. We found that the sustained adhesion induced by long-term SDF-1 stimulation was reduced by prior exposure of the cells to SDF-1, and it could be significantly recovered if the cells were pretreated with SDF-1 in the presence of TGF-β1. To examine whether the SDF-1–induced sustained adhesion was dependent on VLA-4/VLA-5 activation, cells were incubated with either anti–VLA-4 or anti–VLA-5 antibodies before the adhesion assay. Irrespective of the pretreatment conditions, we found that SDF-1–induced sustained adhesion could be significantly inhibited by both anti–VLA-4 and anti–VLA-5 antibodies (Figure 7B). We also evaluated the role of Erk, an important molecule in SDF-1 and integrin-triggered signaling pathways.42,47 We used PD98059, a MEK inhibitor, at a concentration of 40 μM. PD98059 significantly inhibited SDF-1–induced sustained adhesion by 59.37%, 43.57%, 60.4%, and 57.3% (P < .05) for cells pretreated with cytokine cocktail alone or along with TGF-β1, SDF-1, or SDF-1 and TGF-β1, respectively.
Figure 7.
SDF-1 induces adhesion of CD34+ cells to fibronectin. (A) Ex vivo–expanded CD34+ cells were pretreated with cytokine cocktail alone (medium) or containing TGF-β1 (0.5 ng/mL), SDF-1 (200 ng/mL), or SDF-1 (200 ng/mL) + TGF-β1 (0.5 ng/mL) for 24 hours. The cells were washed and then plated on fibronectin (10 μg/mL) coated wells and incubated for 30 minutes. Cells were either left unstimulated (□) or were stimulated with SDF-1 (200 ng/mL) for the full 30 minutes (▪) or only for the last 2 minutes ( ) of the adhesion assay. Data represent the mean ± SD of 3 independent experiments. *P < .05 compared with adhesion response in absence of SDF-1 stimulation (basal adhesion) for respective pretreatment and **P < .05 compared with cells pretreated with SDF-1 alone. (B) CD34+ cells pretreated under various conditions were preincubated with anti–VLA-4 and anti–VLA-5 antibodies and assayed in adhesion assay. The cells were plated in fibronectin-coated plates and stimulated with SDF-1 for 30 minutes. Data represent the mean ± SD of 3 independent experiments. *P < .05 compared with adhesion response in the absence of antibodies. (C) Nonexpanded CD34+-enriched cord blood cells were cultured in medium (containing 20% FCS) alone or along with TGF-β1 (0.5 ng/mL), SDF-1 (200 ng/mL), or SDF-1 (200 ng/mL) and TGF-β1 (0.5 ng/mL) for 24 hours. These cells were then assayed for their ability to bind to fibronectin in the absence or presence of 200 ng/mL SDF-1 for 2 minutes (transient response) and 30 minutes (sustained response). Nonadherent cells were removed, and adherent cells were quantified as described in “Materials and methods.” Data represent the mean ± SEM of 3 independent experiments. *P < .05 compared with adhesion response in absence of SDF-1 stimulation (basal adhesion) for respective pretreatment. (D) Fresh CD34+ cells pretreated under various conditions for 24 hours were preincubated with anti–VLA-4 antibody and assayed in adhesion assay as described in “Materials and methods.” Data represent mean ± SEM of 1 experiment. *P < .05 compared with adhesion response in absence of antibodies.
) of the adhesion assay. Data represent the mean ± SD of 3 independent experiments. *P < .05 compared with adhesion response in absence of SDF-1 stimulation (basal adhesion) for respective pretreatment and **P < .05 compared with cells pretreated with SDF-1 alone. (B) CD34+ cells pretreated under various conditions were preincubated with anti–VLA-4 and anti–VLA-5 antibodies and assayed in adhesion assay. The cells were plated in fibronectin-coated plates and stimulated with SDF-1 for 30 minutes. Data represent the mean ± SD of 3 independent experiments. *P < .05 compared with adhesion response in the absence of antibodies. (C) Nonexpanded CD34+-enriched cord blood cells were cultured in medium (containing 20% FCS) alone or along with TGF-β1 (0.5 ng/mL), SDF-1 (200 ng/mL), or SDF-1 (200 ng/mL) and TGF-β1 (0.5 ng/mL) for 24 hours. These cells were then assayed for their ability to bind to fibronectin in the absence or presence of 200 ng/mL SDF-1 for 2 minutes (transient response) and 30 minutes (sustained response). Nonadherent cells were removed, and adherent cells were quantified as described in “Materials and methods.” Data represent the mean ± SEM of 3 independent experiments. *P < .05 compared with adhesion response in absence of SDF-1 stimulation (basal adhesion) for respective pretreatment. (D) Fresh CD34+ cells pretreated under various conditions for 24 hours were preincubated with anti–VLA-4 antibody and assayed in adhesion assay as described in “Materials and methods.” Data represent mean ± SEM of 1 experiment. *P < .05 compared with adhesion response in absence of antibodies.
In nonexpanded CD34+ cells, SDF-1 induced a significant sustained adhesion response irrespective of pretreatment conditions; however, the greatest effect was observed when cells were pretreated with SDF-1 and TGF-β1 (Figure 7C). Short-time exposure (2 minutes) of nonexpanded CD34+ cells to SDF-1 induced only marginal increases in adhesion response above the basal response. The SDF-1–induced transient adhesion response of CD34+ cells could be significantly blocked by anti–VLA-4 antibody (Figure 7D).
Discussion
Stem cells are localized in a microenvironment known as the stem cell niche, where they are maintained in an undifferentiated and quiescent state. During development of blood cells, stem and progenitor cells translocate from a quiescent into a permissive proliferative vascular niche.48,49 Several studies in mice have demonstrated that both the SDF-1/CXCR4 and VLA4/VCAM-1 (vascular cell adhesion molecule-1) axes are critical for hematopoiesis and retention of hematopoietic stem/progenitor cells in bone marrow.50,51 SDF-1 transiently activates cell surface integrins in CD34+ bone marrow progenitor cells.52,53 Although transient activation of SDF-1–mediated adhesion may explain the homing of progenitor cells to bone marrow and also trafficking of blood cells to inflammation sites,52,54,55 it does not explain the scenario in bone marrow microenvironment where stem/progenitor cells are retained for prolonged periods. In bone marrow, stem and developing progenitor cells need to be retained in appropriate niches for survival, proliferation, and/or differentiation.49 Unlike the peripheral vasculature, there is constitutive high expression of VCAM-1 and SDF-1 in bone marrow.53,56 These findings suggest that in bone marrow, although cells are exposed to high concentrations of SDF-1, they remain responsive to SDF-1 and are not only retained in the appropriate niches, but, when required, they can move from one niche to the other and finally exit into the peripheral blood.57 Paradoxically, it has been shown that exposure of cells to SDF-1 makes them unresponsive to SDF-1 because of CXCR4 receptor desensitization.33 Given these opposing findings, it appeared that in bone marrow, responsiveness of the stem/progenitor cells to SDF-1 must be modulated by other factors. In this study we describe TGF-β1 as a modulator of long-term responsiveness of CD34+ stem/progenitor cells to SDF-1. In the presence of TGF-β1, CD34+ cells preexposed to SDF-1 remain chemotactically responsive to SDF-1 for a prolonged period of time and retain sustained adhesion response mediated by SDF-1.
Consistent with earlier reports33,38,58 we found that calcium mobilization in response to SDF-1 was significantly dampened by prior exposure of CD34+ cells to SDF-1. Furthermore when CD34+ cells are exposed to SDF-1 for 20 to 24 hours, their migration response to SDF-1 is significantly reduced. This phenomenon was observed for both freshly isolated (nonexpanded) and cytokine-expanded CD34+ cells. However, TGF-β1 significantly restored chemotactic response in both freshly isolated and cytokine-expanded SDF-1 preexposed CD34+ cells. The effect of TGF-β1 was most significant when cells were preexposed to 0.5 ng/mL TGF-β1 for 18 hours. Using cytokine-expanded CD34+ cells we found that similar to total CD34+ cells, SDF-1–directed chemotaxis of the CD38lo/CD34++ subpopulation, representing the primitive hematopoietic cell population, was reduced by prior exposure to SDF-1. Since it is possible that the CD38 antigen is shed from the cell surface during the expansion period of CD34+ cells in culture, we evaluated the effect of SDF-1 pretreatment of nonexpanded CD38lo/CD34++ cells on their SDF-1–directed chemotaxis. We found that similar to expanded CD38lo/CD34++ cells, prior exposure of nonexpanded CD38lo/CD34++ to SDF-1 reduced their chemotaxis toward SDF-1. In addition to CD38lo/CD34++ cells, the chemotactic response of multipotential progenitor cells (both expanded and nonexpanded) to SDF-1 was reduced by prior exposure to SDF-1, and this effect was ameliorated at least in part by TGF-β1.
Compared with CD34+ cells pretreated with SDF-1 alone, pretreatment with SDF-1 in the presence of TGF-β1 resulted in a greater proportion of CD34+ cells expressing CXCR4 and a greater phosphorylation of SDF-1–mediated Erk1/Erk2 phosphorylation. It is possible that these effects mediated by TGF-β1 may provide the possible mechanism(s) as to how TGF-β1 counteracts the decreased responses of CD34+ cells to SDF-1 because of preexposure of the cells to SDF-1.
Earlier studies involving monocytic dendritic cells, T cells, and eosinophils59-61 had reported that TGF-β1 modulates chemotactic response to SDF-1 by up-regulating CXCR4 expression. We found that pretreatment of CD34+ cells with SDF-1 in the presence of TGF-β1 increased the proportion of cells expressing CXCR4 without affecting CXCR4 expression level. The difference between our study and previous reports is that we have pretreated the CD34+ cells with SDF-1 in the presence of TGF-β1 since we were interested in investigating whether TGF-β1 could overcome partially/fully the desensitization of CD34+ cells to SDF-1 because of prior prolonged exposure to SDF-1. Whereas earlier studies had investigated the effect of TGF-β1 pretreatment on SDF-1–induced chemotactic response, and in these studies cells were pretreated with TGF-β1 alone.
Using cytokine-expanded CD34+ cells we further investigated the mechanisms whereby the CD34+ cells pretreated with SDF-1 in the presence of TGF-β1 remained responsive to SDF-1. We found that these cells retained the ability to undergo Erk phosphorylation when stimulated with SDF-1, unlike CD34+ cells exposed to SDF-1 alone. The Erk pathway has been linked with cell migration in different cellular models.62 Like SDF-1, TGF-β1 has been shown to activate this pathway.63 Since MEK inhibitor, PD98059, could significantly inhibit chemotaxis to SDF-1 of CD34+ cells pretreated with TGF-β1 and SDF-1, this demonstrates that cross talk between TGF-β1 and SDF-1 involved the mitogen-activated protein (MAP) kinase pathway. Further, it suggests that Erk1/Erk2 phosphorylation plays an important role in the ability of CD34+ cells to migrate in response to SDF-1. Unlike our earlier report,42 we did not find an increase in Akt/PKB phosphorylation above background level when the cytokine-expanded CD34+ cells were stimulated with SDF-1. However, in our present study the cells were cultured in optimal concentrations of TPO, SCF, and Flt-3 ligand, prior to SDF-1 stimulation. These cytokines promote CD34+ cell survival through the Akt/PKB pathway.64-66 Indeed the background Akt/PKB phosphorylation was significant and could possibly be optimal, and it may explain why SDF-1 did not induce any further phosphorylation of Akt/PKB.
CXCR4 signaling is involved in the regulation of integrin function.15,53 We found that SDF-1 induced both sustained and transient adhesion responses to fibronectin in cytokine-expanded CD34+ cells. The SDF-1–induced sustained adhesion response was impaired by SDF-1 preexposure but near normal if cells were pretreated with SDF-1 in the presence of TGF-β1. This effect was seen for both expanded and nonexpanded CD34 cells. Unlike sustained adhesion, SDF-1–mediated transient adhesion response in CD34+ cells remained unaffected by SDF-1 preexposure. These findings suggest that not all responses in CD34+ cells mediated by SDF-1 are attenuated by preexposure to SDF-1. Since SDF-1 activates different signaling pathways, it is possible that some pathways are more sensitive to receptor desensitization than the others and/or are likely to be regulated at different levels. An earlier study showed that in B cells, although both SDF-1–mediated transient and sustained adhesion involved focal adhesion kinase (FAK) phosphorylation, upstream signaling pathways were qualitatively different.67
It has been recently reported that TGF-β1 down-regulates expression of SDF-1 by stromal cells, resulting in reduced cell migration and adhesion of BM cells, and it was suggested that this could affect the motility of cells in the bone marrow68; a concentration of 2 ng/mL TGF-β1 was used in that study. We found that 0.5 ng/mL TGF-β1 was optimal for improving SDF-1–mediated responses in CD34+ cells (preexposed to SDF-1). Increasing the dose of TGF-β1 not only resulted in reduced migration but also led to apoptosis (data not shown) in these cells. In physiologic settings the level of active TGF-β1 is low and highly regulated.69 However, in several pathologic conditions it is overproduced and leads to failure of early hematopoietic progenitors.70,71 It is, therefore, likely that TGF-β1 modulates SDF-1–mediated responses, but the outcome depends on the dose of TGF-β1. The dose-dependent effect of TGF-β1 has also been seen in its ability to support myeloid cell proliferation.72
In summary, we show that both SDF-1–stimulated chemotactic activity and sustained adhesion of CD34+ cells is down-regulated by preexposure of CD34+ cells to SDF-1, and these responses are modulated by TGF-β1. Since SDF-1 is constitutively present at high concentrations in the bone marrow compartment, modulation of SDF-1 responses by TGF-β1 may explain, at least in part, how hematopoietic cells remain responsive to SDF-1 in bone marrow. Therefore, modulation of SDF-1 activity by TGF-β1 may be an essential physiologic process that plays a role in retention of stem/progenitor cells in their niches and their movement among the niches.
Prepublished online as Blood First Edition Paper, March 29, 2005; DOI 10.1182/blood-2004-10-4145.
Supported by Public Health Science Grants from the National Institutes of Health (RO1 DK 53674, RO1 HL 67384, and RO1 HL 56416) (H.E.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273: 242-245. [DOI] [PubMed] [Google Scholar]
- 2.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci U S A. 1995;92: 9647-9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16: 1992-2003. [DOI] [PubMed] [Google Scholar]
- 4.Christopherson KW, Broxmeyer HE. Hematopoietic stem and progenitor cell homing, engraftment, and mobilization in the context of CXCL12/SDF-1-CXCR4 axis. In: Broxmeyer HE, ed. Cord Blood Biology, Immunology, Banking, and Clinical Transplantation. Bethesda, MD: AABB Press; 2004: 65-86.
- 5.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111: 187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3: 687-694. [DOI] [PubMed] [Google Scholar]
- 7.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283: 845-848. [DOI] [PubMed] [Google Scholar]
- 8.Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in C. Proc Natl Acad Sci U S A. 1998;95: 9448-9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382: 635-638. [DOI] [PubMed] [Google Scholar]
- 10.Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305: 1000-1003. [DOI] [PubMed] [Google Scholar]
- 11.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16: 1992-2003. [DOI] [PubMed] [Google Scholar]
- 12.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185: 111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim CH, Broxmeyer HE. In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow environment. Blood. 1998;91: 100-110. [PubMed] [Google Scholar]
- 14.Peled A, Grabovsky V, Habler L, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104: 1199-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peled A, Kollet O, Ponomaryov T, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95: 3289-3296. [PubMed] [Google Scholar]
- 16.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53: 1-24. [PubMed] [Google Scholar]
- 17.Fortunel N, Hatzfeld J, Aoustin L, et al. Specific dose-response effects of TGF-beta1 on developmentally distinct hematopoietic stem/progenitor cells from human umbilical cord blood. Hematol J. 2000;1: 126-135. [DOI] [PubMed] [Google Scholar]
- 18.Sporn MB, Roberts AB. Peptide growth factors are multifunctional. Nature. 1988;332: 217-219. [DOI] [PubMed] [Google Scholar]
- 19.Fortunel N, Batard P, Hatzfeld A, et al. High proliferative potential-quiescent cells: a working model to study primitive quiescent hematopoietic cells. J Cell Sci. 1998;111(Pt 13): 1867-1875. [DOI] [PubMed] [Google Scholar]
- 20.Gimble JM, Pietrangeli C, Henley A, et al. Characterization of murine bone marrow and spleen-derived stromal cells: analysis of leukocyte marker and growth factor mRNA transcript levels. Blood. 1989; 74: 303-311. [PubMed] [Google Scholar]
- 21.Olsson N, Piek E, ten Dijke P, Nilsson G. Human mast cell migration in response to members of the transforming growth factor-beta family. J Leukoc Biol. 2000;67: 350-356. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Kawasaki H, Nagayama H, et al. TGF-beta 1 reciprocally controls chemotaxis of human peripheral blood monocyte-derived dendritic cells via chemokine receptors. J Immunol. 2000;164: 2285-2295. [DOI] [PubMed] [Google Scholar]
- 23.Wahl SM, Hunt DA, Wakefield LM, et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987;84: 5788-5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartolome RA, Sanz-Rodriguez F, Robledo MM, Hidalgo A, Teixido J. Rapid up-regulation of alpha4 integrin-mediated leukocyte adhesion by transforming growth factor-beta1. Mol Biol Cell. 2003;14: 54-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nesti LJ, Caterson EJ, Wang M, et al. TGF-beta1 calcium signaling increases alpha5 integrin expression in osteoblasts. J Orthop Res. 2002;20: 1042-1049. [DOI] [PubMed] [Google Scholar]
- 26.Christopherson KW, Campbell JJ, Hromas RA. Transgenic overexpression of the CC chemokine CCL21 disrupts T-cell migration. Blood. 2001;98: 3562-3568. [DOI] [PubMed] [Google Scholar]
- 27.Broxmeyer HE, Hangoc G, Cooper S, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci U S A. 1992;89: 4109-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86: 3828-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howard TH, Meyer WH. Chemotactic peptide modulation of actin assembly and locomotion in neutrophils. J Cell Biol. 1984;98: 1265-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Princen K, Hatse S, Vermeire K, De CE, Schols D. Evaluation of SDF-1/CXCR4-induced Ca2+ signaling by fluorometric imaging plate reader (FLIPR) and flow cytometry. Cytometry. 2003; 51A: 35-45. [DOI] [PubMed] [Google Scholar]
- 31.Luens KM, Travis MA, Chen BP, et al. Thrombopoietin, kit ligand, and flk2/flt3 ligand together induce increased numbers of primitive hematopoietic progenitors from human CD34+Thy-1+Lin– cells with preserved ability to engraft SCID-hu bone. Blood. 1998;91: 1206-1215. [PubMed] [Google Scholar]
- 32.Bhatia M, Bonnet D, Kapp U, et al. Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture. J Exp Med. 1997;186: 619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Signoret N, Oldridge J, Pelchen-Matthews A, et al. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139: 651-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999; 10: 463-471. [DOI] [PubMed] [Google Scholar]
- 35.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997;94: 5320-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279: 509-514. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18: 501-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382: 833-835. [DOI] [PubMed] [Google Scholar]
- 39.Sotsios Y, Whittaker GC, Westwick J, Ward SG. The CXC chemokine stromal cell-derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J Immunol. 1999;163: 5954-5963. [PubMed] [Google Scholar]
- 40.Vlahakis SR, Villasis-Keever A, Gomez T, et al. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol. 2002;169: 5546-5554. [DOI] [PubMed] [Google Scholar]
- 41.Uchida T. Introduction of macromolecules into mammalian cells by cell fusion. Exp Cell Res. 1988;178: 1-17. [DOI] [PubMed] [Google Scholar]
- 42.Lee Y, Gotoh A, Kwon HJ, et al. Enhancement of intracellular signaling associated with hematopoietic progenitor cell survival in response to SDF-1/CXCL12 in synergy with other cytokines. Blood. 2002;99: 4307-4317. [DOI] [PubMed] [Google Scholar]
- 43.Yang YC, Piek E, Zavadil J, et al. Hierarchical model of gene regulation by transforming growth factor beta. Proc Natl Acad Sci U S A. 2003;100: 10269-10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97: 2293-2299. [DOI] [PubMed] [Google Scholar]
- 45.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109: 625-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20: 707-718. [DOI] [PubMed] [Google Scholar]
- 47.McGilvray ID, Lu Z, Bitar R, et al. VLA-4 integrin cross-linking on human monocytic THP-1 cells induces tissue factor expression by a mechanism involving mitogen-activated protein kinase. J Biol Chem. 1997;272: 10287-10294. [DOI] [PubMed] [Google Scholar]
- 48.Gong JK. Endosteal marrow: a rich source of hematopoietic stem cells. Science. 1978;199: 1443-1445. [DOI] [PubMed] [Google Scholar]
- 49.Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10: 64-71. [DOI] [PubMed] [Google Scholar]
- 50.Miyake K, Weissman IL, Greenberger JS, Kincade PW. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991; 173: 599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papayannopoulou T, Priestley GV, Nakamoto B. Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. Blood. 1998;91: 2231-2239. [PubMed] [Google Scholar]
- 52.Hidalgo A, Sanz-Rodriguez F, Rodriguez-Fernandez JL, et al. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-dependent adhesion to fibronectin and VCAM-1 on bone marrow hematopoietic progenitor cells. Exp Hematol. 2001;29: 345-355. [DOI] [PubMed] [Google Scholar]
- 53.Peled A, Grabovsky V, Habler L, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104: 1199-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan JR, Hyduk SJ, Cybulsky MI. Chemoattractants induce a rapid and transient upregulation of monocyte alpha4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest: an early step in the process of emigration. J Exp Med. 2001;193: 1149-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell JJ, Hedrick J, Zlotnik A, et al. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279: 381-384. [DOI] [PubMed] [Google Scholar]
- 56.Schweitzer KM, Drager AM, van der Valk P, et al. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol. 1996;148: 165-175. [PMC free article] [PubMed] [Google Scholar]
- 57.Hattori K, Heissig B, Rafii S. The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leuk Lymphoma. 2003;44: 575-582. [DOI] [PubMed] [Google Scholar]
- 58.Kahn J, Byk T, Jansson-Sjostrand L, et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103: 2942-2949. [DOI] [PubMed] [Google Scholar]
- 59.Sato K, Kawasaki H, Nagayama H, et al. TGF-beta 1 reciprocally controls chemotaxis of human peripheral blood monocyte-derived dendritic cells via chemokine receptors. J Immunol. 2000;164: 2285-2295. [DOI] [PubMed] [Google Scholar]
- 60.Nagase H, Miyamasu M, Yamaguchi M, et al. Expression of CXCR4 in eosinophils: functional analyses and cytokine-mediated regulation. J Immunol. 2000;164: 5935-5943. [DOI] [PubMed] [Google Scholar]
- 61.Franitza S, Kollet O, Brill A, et al. TGF-beta1 enhances SDF-1alpha-induced chemotaxis and homing of naive T cells by up-regulating CXCR4 expression and downstream cytoskeletal effector molecules. Eur J Immunol. 2002;32: 193-202. [DOI] [PubMed] [Google Scholar]
- 62.Veit C, Genze F, Menke A, et al. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 2004;64: 5291-5300. [DOI] [PubMed] [Google Scholar]
- 63.Qian D, Lin HY, Wang HM, et al. Involvement of ERK1/2 pathway in TGF-beta1-induced VEGF secretion in normal human cytotrophoblast cells. Mol Reprod Dev. 2004;68: 198-204. [DOI] [PubMed] [Google Scholar]
- 64.Karlsson R, Engstrom M, Jonsson M, et al. Phosphatidylinositol 3-kinase is essential for kit ligand-mediated survival, whereas interleukin-3 and flt3 ligand induce expression of antiapoptotic Bcl-2 family genes. J Leukoc Biol. 2003;74: 923-931. [DOI] [PubMed] [Google Scholar]
- 65.Jonsson M, Engstrom M, Jonsson JI. FLT3 ligand regulates apoptosis through AKT-dependent inactivation of transcription factor FoxO3. Biochem Biophys Res Commun. 2004;318: 899-903. [DOI] [PubMed] [Google Scholar]
- 66.Majka M, Ratajczak J, Villaire G, et al. Thrombopoietin, but not cytokines binding to gp130 protein-coupled receptors, activates MAPKp42/44, AKT, and STAT proteins in normal human CD34+ cells, megakaryocytes, and platelets. Exp Hematol. 2002;30: 751-760. [DOI] [PubMed] [Google Scholar]
- 67.Glodek AM, Honczarenko M, Le Y, Campbell JJ, Silberstein LE. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. J Exp Med. 2003;197: 461-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright N, de Lera TL, Garcia-Moruja C, et al. Transforming growth factor-beta1 down-regulates expression of chemokine stromal cell-derived factor-1: functional consequences in cell migration and adhesion. Blood. 2003;102: 1978-1984. [DOI] [PubMed] [Google Scholar]
- 69.Junker U, Knoefel B, Nuske K, et al. Transforming growth factor beta 1 is significantly elevated in plasma of patients suffering from renal cell carcinoma. Cytokine. 1996;8: 794-798. [DOI] [PubMed] [Google Scholar]
- 70.Lagneaux L, Delforge A, Dorval C, Bron D, Stryckmans P. Excessive production of transforming growth factor-beta by bone marrow stromal cells in B-cell chronic lymphocytic leukemia inhibits growth of hematopoietic precursors and interleukin-6 production. Blood. 1993;82: 2379-2385. [PubMed] [Google Scholar]
- 71.Urashima M, Ogata A, Chauhan D, et al. Transforming growth factor-beta1: differential effects on multiple myeloma versus normal B cells. Blood. 1996;87: 1928-1938. [PubMed] [Google Scholar]
- 72.Ruscetti FW, Bartelmez SH. Transforming growth factor beta, pleiotropic regulator of hematopoietic stem cells: potential physiological and clinical relevance. Int J Hematol. 2001;74: 18-25. [DOI] [PubMed] [Google Scholar]