Abstract
We detected circulating plasma cells (PCs) by flow cytometry in 302 patients with newly diagnosed multiple myeloma (MM) by gating on CD38+CD45- cells. The number of circulating PCs per 50 000 mononuclear cells was reported. In 80 (27%) patients, no circulating PC were seen; 106 (35%) patients had 1 to 10 and 115 (38%) patients had more than 10 circulating PCs. Median overall survival for the 302 patients was 47 months. Patients with 10 or fewer circulating PCs had a median survival of 58.7 months, whereas patients with more than 10 circulating PCs had a median survival of 37.3 months (P = .001). On multivariate analysis, the prognostic value of circulating PCs was independent of β2-microglobulin, albumin, and C-reactive protein. There was only a weak correlation between tumor mass and circulating PCs, suggesting that the appearance of circulating PCs may be a reflection of tumor biology. We conclude that the number of circulating PCs measured by flow cytometry in patients with newly diagnosed MM is an independent predictor of survival.
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy, with 15 000 cases diagnosed in the United States each year. Despite the development of new, targeted treatment strategies, MM remains incurable, with a median survival time of 3 to 4 years.1 However, the disease is biologically diverse, and there is a significant variation in survival time of patients with MM. Some patients survive 7 to 10 years or longer.2 This variation in survival time was recognized early, and over the years a number of prognostic factors have been identified.3 The Durie-Salmon staging system incorporates a number of traditionally recognized prognostic factors.4 More recently, the International Staging System (ISS) was introduced and uses β2-microglobulin (B2M) and albumin levels, each of which is easy to measure and is widely available.5 The rapid development of therapeutics and combination therapy for the treatment of MM requires the use of well-validated and accessible prognostic factors for risk stratification in clinical trials to ensure that treatment arms are truly comparable. It is essential that prognostic factor assays used for risk stratification be easily available in multiple institutions.
Circulating plasma cells (PCs) can be detected in the peripheral blood of a significant proportion of patients with MM.6,7 Although the appearance of circulating PCs in the blood may be simply a reflection of a tumor mass, it could also represent differences in the disease biology. Cell-to-cell adhesion and cell-to-stroma adhesion are required for myeloma progression,8 and they mediate resistance to therapy.9 We hypothesized that the appearance of circulating PCs in the blood identifies relative independence from adhesion to the microenvironment and, therefore, signifies more aggressive disease. The presence of circulating PCs in peripheral blood detected by slide immunofluorescence has been shown to be a risk factor in patients with newly diagnosed MM.10 The presence of circulating PCs detected by slide immunofluorescence has also been associated with shortened progression-free survival in patients with smoldering myeloma.11
Unfortunately, the slide-based immunofluorescence assay to detect circulating PCs is complex and time and labor intensive, and it requires fluorescence microscopy, which limits the clinical availability of this test. Flow cytometric analysis is rapid and readily available, and it can be used to detect circulating PCs with good correlation with the immunofluorescence-based method.6,7 We investigated the prognostic value of circulating PCs detected by flow cytometry in patients with newly diagnosed MM.
Patients, materials, and methods
Patients
Approval for the study was obtained from the Mayo Clinic Institutional Review Board in accordance with federal regulations. Informed consent was provided according to the Declaration of Helsinki. Blood samples from patients with newly diagnosed MM seen at Mayo Clinic Rochester within the first week of diagnosis and before the initiation of treatment were collected and analyzed. Clinical data including age, sex, and disease stage and prognostic factors such as B2M, albumin, C-reactive protein (CRP), lactate dehydrogenase (LDH) levels, bone marrow PC percentage, and bone marrow PC labeling index (PCLI) were collected. Data with regard to the type of initial and salvage therapies and the use of stem-cell transplantation were also collected. Patients were followed up for the response to treatment and for progression-free survival (PFS) and overall survival (OS).
Quantification of circulating PCs
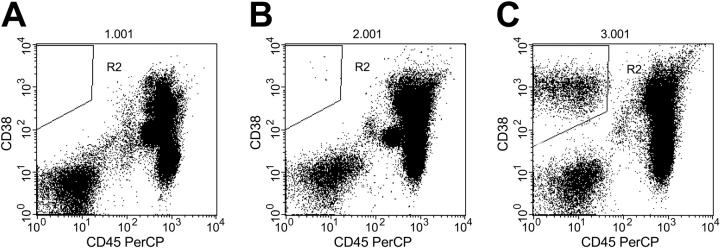
Flow cytometry analysis was conducted as previously described.6 Briefly, mononuclear cells were isolated by density gradient centrifugation on Ficoll (Amersham Biosciences, Piscataway, NJ) for 15 minutes at 1500 rpm (300g) and stained with fluorescence-labeled CD38 and CD45 antibodies (both from BD Biosciences, San Jose, CA). The number of circulating PCs was measured by gating on CD38+CD45- mononuclear cells. Fifty thousand events were analyzed for each patient, and the number of PCs detected among these 50 000 events was recorded. Although the circulating PC population is usually relatively small, it can be clearly identified and quantitated.6 Typical flow cytometry patterns are shown in Figure 1. Circulating PCs were also estimated using a slide based-immunofluorescence method, as previously described.12
Figure 1.
Flow cytometric analysis of peripheral blood in 3 patients with MM. (A) Patient with no circulating PCs (R2). (B) Patient with 7 circulating PCs per 50 000 events (R2). (C) Patient with high number of circulating PCs (R2; 2946 per 50 000 events).
Statistical analysis
Overall survival was calculated from the date of diagnosis to the date of death or date last known alive. Survival analysis was performed using the method described by Kaplan and Meier.13 Differences between survival curves were tested for statistical significance using the 2-tailed Wilcoxon rank sum test. Multivariate analysis was conducted using Cox proportional hazards model.14 The Spearman rank test was used for correlation analyses.
Results
Patient characteristics
Between May 1998 and January 2003, flow cytometry was performed on 302 patients with newly diagnosed MM. Median patient age was 65 years, and there was a slight male predominance (60%). Clinical and laboratory data and stage by ISS are summarized in Table 1. Most (42%) of the patients had ISS stage II disease, and there was an equal distribution of patients with ISS stage I and III disease (26% each). Induction therapies were vincristine, doxorubicin, dexamethasone (VAD) (25%), single-agent dexamethasone (23%), melphalan/prednisone (MP) (23%), thalidomide and dexamethasone (16%), and others (13%). One hundred twenty-two patients (40%) underwent stem-cell transplantation after induction therapy.
Table 1.
Patient characteristics
| No. patients (%) | Median | Range | |
|---|---|---|---|
| Age, y | 302 | 65 | 29-94 |
| Sex | |||
| Male | 180 (60) | NA | NA |
| Female | 123 (40) | NA | NA |
| B2M, mg/L | 275 | 3.68 | 0.98-63.8 |
| LDH, U/L | 275 | 141 | 60-963 |
| CRP, mg/dL | 249 | 0.4 | 0.01-20.00 |
| Albumin level, g/dL | 302 | 3.6 | 1.8-5.4 |
| PC labeling index, % | |||
| Less than 1 (low) | 172 | NA | NA |
| 1 or more (high) | 84 | NA | NA |
| BM PC, % | 255 | 45 | 11-90 |
| ISS stage | |||
| I | 69 (26) | NA | NA |
| II | 128 (42) | NA | NA |
| III | 78 (26) | NA | NA |
| Unknown | 28 (9) | NA | NA |
NA indicates not applicable.
Circulating PCs
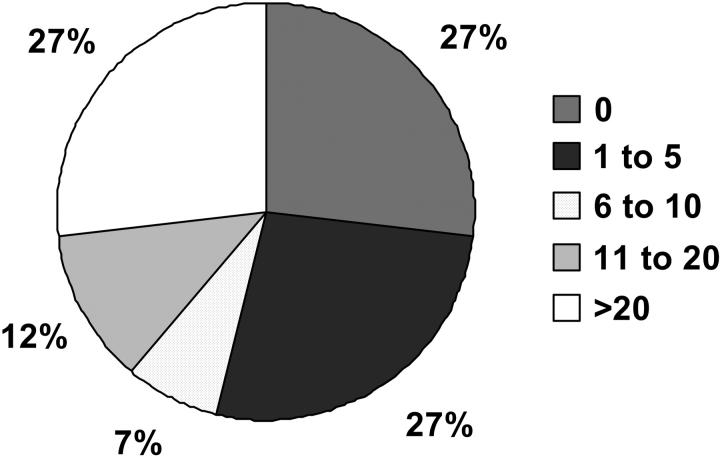
Circulating PCs were detected in 222 patients (73%) with the median PC count of 4 per 50 000 events (range, 1-28 692 events). In 80 patients (27%), no circulating PCs were seen; 106 patients (35%) had 1 to 10 circulating PCs. The remaining 115 patients (38%) had more then 10 circulating PCs (Figure 2). There was a good correlation between the number of circulating PCs detected by flow cytometry and immunofluorescence microscopy (ρ = 0.77; P < .001). There was a weak correlation (17%) between the degree of marrow involvement by PCs and the number of circulating PCs (ρ = 0.32; P < .001). There was also only a weak correlation between circulating PCs and B2M (ρ = 0.29; P < .001)
Figure 2.
Distribution of circulating PCs in 302 study patients. Patients were divided into 5 groups based on the number of PCs.
Follow-up and survival
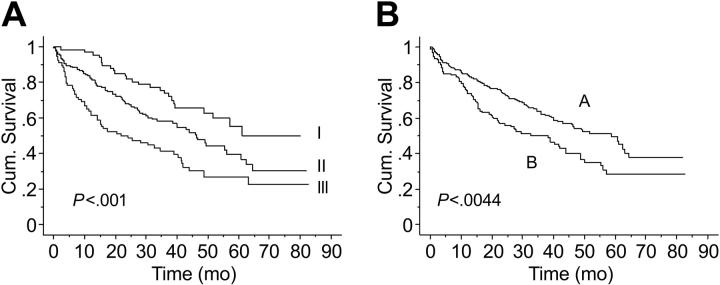
Median follow-up was 33.5 months (range, 1-83 months), and 158 (52%) patients have died. Median overall survival time for the whole cohort was 46 months. Survival time by ISS stage differed significantly, with median survival times of 61, 46, and 22 months for patients with stage I, II, and III stage disease, respectively (P < .001; Figure 3A).
Figure 3.
Kaplan-Meier estimates of OS. (A) Survival by ISS (ISS 1-I, ISS 2-II, ISS 3-III). (B) Survival by number of circulating PCs (A, 10 or less; B, more than 10).
Effect of circulating PCs on outcome
There was a continuous correlation between the number of circulating PCs and shortened OS (correlation coefficient 1.059, P < .001). We tested different cut-off values for circulating PCs and used a cutoff of 10 cells per 50 000 events for subsequent analyses because of the similar proportions of patients (10 circulating PCs or fewer vs more than 10 circulating PCs; 62% vs 38%).
Median OS was significantly longer in patients with 10 or fewer circulating PCs than in those with more than 10 circulating PCs (59 vs 37 months, respectively; P = .001) (Figure 3B). The prognostic value of circulating PCs and other commonly used factors, including B2M, albumin, and PCLI, on univariate analysis is summarized in Table 2.
Table 2.
Univariate analysis of common prognostic markers and circulating PCs as predictors of OS
| Prognostic variable | Proportional hazard ratio | 95% confidence interval | P |
|---|---|---|---|
| PCLI, 1% or greater | 1.88 | 1.31-2.68 | .004 |
| Albumin level, less than 3.5 g/dL | 1.71 | 1.24-2.36 | < .001 |
| CRP, more than 0.80 mg/dL | 1.58 | 1.10-2.27 | .007 |
| B2M, more than 3.5 mg/L | 1.57 | 1.05-2.35 | .026 |
| Age, older than 60 y | 1.52 | 1.07-2.15 | .018 |
| Circulating PCs, more than 10 | 1.57 | 1.14-1.57 | .004 |
On multivariate analysis, the prognostic value of circulating PCs was independent of B2M, albumin level, and age (Table 3). The addition of CRP was not significant in multivariate analysis. In contrast, the inclusion of bone marrow PCLI caused a loss of significance of other variables, including B2M and circulating PCs.
Table 3.
Multivariate analysis of prognostic variables and circulating PCs
| Prognostic variable | Proportional hazard ratio | 95% confidence interval | P |
|---|---|---|---|
| Albumin level, less than 3.5 g/dL | 2.03 | 1.45-2.83 | < .001 |
| B2M, more than 3.5 mg/L | 1.51 | 1.01-2.25 | .04 |
| Age, older than 60 y | 1.47 | 1.02-2.12 | .038 |
| Circulating PCs, more than 10 | 1.42 | 1.01-1.99 | .039 |
Values were determined by the Cox regression model.
Circulating PCs and the ISS
Survival difference was the greatest among patients with ISS stage II or III disease. Patients with ISS stage II and III disease who had more than 10 circulating PCs experienced significantly poorer median OS than did those with 10 or fewer circulating PCs (24 vs 46 months, respectively; P = .024). A similar trend was seen among patients with ISS stage I disease (median survival, 56 vs 79+ months). However, the median survival in this group has not been reached, and the OS difference did not reach statistical significance.
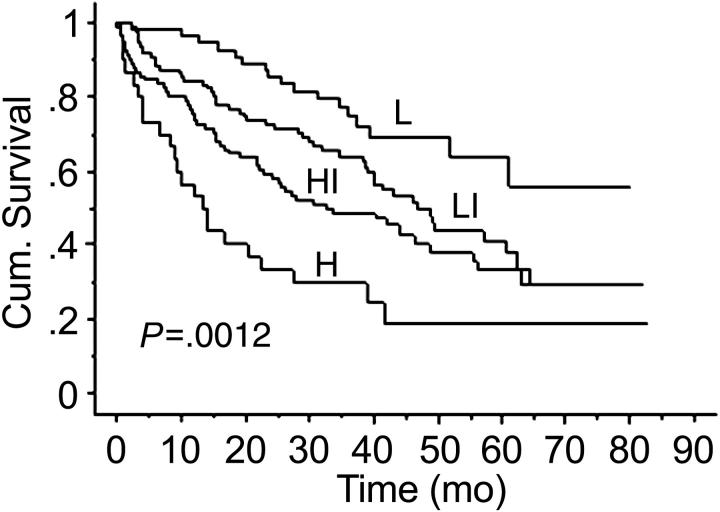
We developed a risk stratification model that incorporated factors used in ISS (B2M and albumin levels) and circulating PCs. This model has a prognostic value exceeding that of the ISS alone. Adverse prognostic factors were presence of more than 10 circulating PCs, B2M greater than 3.5, and albumin level less than 3.5 (Table 4). Four groups of patients were defined: those at low risk (none of the adverse factors present), low-intermediate risk (1of 3 adverse factor present), intermediate-high risk (2 of 3 factors present), and high risk (all 3 factors present). The low-risk group had a median survival of more than 79 months (not reached), whereas patients in the high-risk group had a median survival of only 13.3 months (Figure 4).
Table 4.
Risk stratification groups based on the circulating PCs and risk factors used in ISS
| No. patients at risk (%) | Median survival, mo | |
|---|---|---|
| Risk factor | ||
| B2M, more than 3.5 mg/L | 190 | 41 |
| Albumin level, less than 3.5 g/dL | 127 | 28 |
| Circulating PCs, more than 10 | 115 | 37 |
| Risk stratification group | ||
| Low-risk (none of the risk factors present) | 56 (19) | 79+ |
| Low-intermediate risk (1 of the risk factors present) | 98 (32) | 48 |
| High-intermediate risk (2 of the risk factors present) | 91 (30) | 32 |
| High risk (3 risk factors present) | 57 (19) | 13 |
Figure 4.
Kaplan-Meier estimates of OS in the risk stratification model using B2M, albumin levels, and circulating PCs. L indicates low-risk group; LI, low-intermediate risk group; HI, high-intermediate risk group; H, high-risk group.
Discussion
In defining new prognostic factors, availability and reproducibility are key factors. The Durie-Salmon staging system is based on easily available tests and, despite its limitations and complexity, had been widely used.4 Similarly, the ISS uses widely accessible laboratory values.5 In contrast, PC labeling index, though one of the strongest independent predictors of survival,12,15 has not been incorporated widely into clinical practice and trials because of its limited availability. Similarly, the detection of circulating PCs by a slide-based immunofluorescence assay has been repeatedly shown to be a risk factor in myeloma10,11,16 and other PC disorders.17 However, the limited availability of the immunofluorescence microscopy-based method of PC quantification limits the use of this test in clinical studies.
In the current study, we demonstrate that the number of circulating PCs detected by flow cytometry is an independent prognostic factor in patients with newly diagnosed MM. There was an inverse correlation between the number of circulating PCs and OS. Overall survival in our cohort was higher than reported in other series. However, survival by ISS stage was similar to that observed in the original ISS description.5 The prognostic value of circulating PCs was independent of other well-established prognostic markers, such as age, albumin, and B2M. The latter 2 variables are used in ISS.5 Although the prognostic value of circulating PCs was not independent of bone marrow PCLI, this is not a major limitation because the PCLI is not routinely available in most centers and is infrequently used in clinical studies. Further, risk factors that are biologically related may not show independent significance because of the interaction between the variables.
In this study, we have also developed a risk stratification model using factors incorporated in ISS (B2M and albumin) and circulating PCs. This model appears to enhance the ISS because it enables identification, using a readily available laboratory test, of a subset of patients with a particularly poor prognosis. Median survival of patients in the high-risk group was only 13 months compared with more than 79 months for patients in the low-risk score group.
Besides its clinical usefulness, this study also has important biologic implications. The level of circulating PCs appears to be largely independent of tumor burden reflected by B2M or bone marrow PC involvement. It is well established that cell-to-cell and cell-to-stroma adhesion are required for myeloma progression8 and that they mediate resistance to therapy.9 Given the findings of this study, the appearance of circulating PCs in patients with myeloma likely reflects independence from adhesion to stroma rather than merely tumor burden. Future studies of the expression of adhesion receptors in patients with circulating PCs could provide us with additional insight into the biology of myeloma. Because patients in our study were not treated or evaluated for a response in the uniform fashion, we did not analyze the response to treatment rate. However, it will also be important to determine whether there are differences in treatment response rates in patients with and without circulating PCs, particularly with therapies targeting interactions between myeloma and microenvironment.
Given that flow cytometry is available in most medical centers, incorporating this assay as a pretreatment evaluation in clinical trials is feasible. The assay can then be validated in patients treated in a uniform fashion. Our results suggest that the assay can be clinically useful for risk stratification; future studies could help determine whether there is a differential response to various therapeutic agents based on the presence of absence of circulating PCs. For example, it has already been shown that the presence of circulating PCs detectable by immunofluorescence at the time of stem cell harvest is associated with significantly shortened relapse-free survival after transplantation.18
Although flow cytometry has the advantage of lower cost and greater availability, the assay needs development and validation in other centers. In spite of these limitations, we believe that the findings described and the risk stratification model proposed have unique clinical and biologic significance.
We conclude that the number of circulating PCs measured by flow cytometry in patients with newly diagnosed MM is an independent prognostic factor for overall survival. The appearance of circulating PCs is independent of tumor mass and likely reflects differences in tumor biology.
Prepublished online as Blood First Edition Paper, June 16, 2005; DOI 10.1182/blood-2005-05-1858.
Supported in part by research grants CA 107476 and CA 62242 and by Specialized Programs of Research Excellence Grant CA100707 from the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78: 21-33. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA. Long-term survival in multiple myeloma. N Engl J Med. 1983;308: 314-316. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar SV, Greipp PR. Prognostic factors in multiple myeloma. Hematol Oncol Clin North Am. 1999;13: 1295-1314, xi. [DOI] [PubMed] [Google Scholar]
- 4.Durie BG, Salmon SE. A clinical staging system for multiple myeloma: correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36: 842-854. [DOI] [PubMed] [Google Scholar]
- 5.Greipp PR, San Miguel J, Durie BG, et al. International Staging System for Multiple Myeloma. J Clin Oncol. 2005;23: 3412-3420. [DOI] [PubMed] [Google Scholar]
- 6.Witzig TE, Kimlinger TK, Ahmann GJ, Katzmann JA, Greipp PR. Detection of myeloma cells in the peripheral blood by flow cytometry. Cytometry. 1996;26: 113-120. [DOI] [PubMed] [Google Scholar]
- 7.Rawstron AC, Owen RG, Davies FE, et al. Circulating plasma cells in multiple myeloma: characterization and correlation with disease stage. Br J Haematol. 1997;97: 46-55. [DOI] [PubMed] [Google Scholar]
- 8.Teoh G, Anderson KC. Interaction of tumor and host cells with adhesion and extracellular matrix molecules in the development of multiple myeloma. Hematol Oncol Clin North Am. 1997;11: 27-42. [DOI] [PubMed] [Google Scholar]
- 9.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93: 1658-1667. [PMC free article] [PubMed] [Google Scholar]
- 10.Witzig TE, Gertz MA, Lust JA, Kyle RA, O'Fallon WM, Greipp PR. Peripheral blood monoclonal plasma cells as a predictor of survival in patients with multiple myeloma. Blood. 1996;88: 1780-1787. [PubMed] [Google Scholar]
- 11.Witzig TE, Kyle RA, O'Fallon WM, Greipp PR. Detection of peripheral blood plasma cells as a predictor of disease course in patients with smouldering multiple myeloma. Br J Haematol. 1994;87: 266-272. [DOI] [PubMed] [Google Scholar]
- 12.Witzig TE, Gonchoroff NJ, Katzmann JA, Therneau TM, Kyle RA, Greipp PR. Peripheral blood B cell labeling indices are a measure of disease activity in patients with monoclonal gammopathies. J Clin Oncol. 1988;6: 1041-1046. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan E, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;58: 457-481. [Google Scholar]
- 14.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34 (series B): 187-220. [Google Scholar]
- 15.Greipp PR, Lust JA, O'Fallon WM, Katzmann JA, Witzig TE, Kyle RA. Plasma cell labeling index and beta 2-microglobulin predict survival independent of thymidine kinase and C-reactive protein in multiple myeloma. Blood. 1993;81: 3382-3387. [PubMed] [Google Scholar]
- 16.Omede P, Boccadoro M, Gallone G, et al. Multiple myeloma: increased circulating lymphocytes carrying plasma cell-associated antigens as an indicator of poor survival. Blood. 1990;76: 1375-1379. [PubMed] [Google Scholar]
- 17.Pardanani A, Witzig TE, Schroeder G, et al. Circulating peripheral blood plasma cells as a prognostic indicator in patients with primary systemic amyloidosis. Blood. 2003;101: 827-830. [DOI] [PubMed] [Google Scholar]
- 18.Gertz MA, Witzig TE, Pineda AA, Greipp PR, Kyle RA, Litzow MR. Monoclonal plasma cells in the blood stem cell harvest from patients with multiple myeloma are associated with shortened relapse-free survival after transplantation. Bone Marrow Transplant. 1997;19: 337-342. [DOI] [PubMed] [Google Scholar]