Abstract
Lipopolysaccharide (LPS), a well-known bacterial pyrogen, is recognized by several receptors, including the Toll-like receptor 4 (TLR4), on various cells. Which of these receptors and cells are linked to fever production is unknown. By constructing 4 mouse chimeras and studying their thermoregulatory responses, we found that all 3 phases of the typical LPS fever depend on TLR4 signaling. The first phase is triggered via the TLR4 on hematopoietic cells. The second and third phases involve TLR4 signaling in both hematopoietic and nonhematopoietic cells.
Introduction
Fever is a hallmark of infection and an important host defense response. It is triggered by microbial products, among which the lipopolysaccharide (LPS) of the outer envelope of Gramnegative bacteria is the most studied. In experimental animals, LPS causes a typical polyphasic fever, at least 3 phases of which have been characterized in rats1 and mice.2,3 LPS has been shown, mostly in vitro, to signal via several receptors, including Toll-like receptor 4 (TLR4), CD11/CD18 β2 integrin, and scavenger receptors,4-7 but which of these receptors is linked to any of the febrile phases is unknown. The present study addressed 2 questions: is TLR4 signaling essential for any of the febrile phases, and if it is, does the signaling originate in hematopoietic or nonhematopoietic cells?
Study design
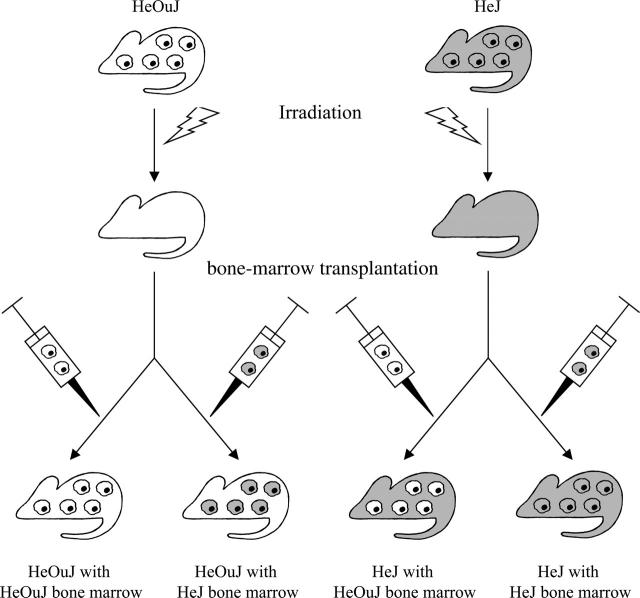
The experimental protocols were approved by the Saint Joseph's Hospital and National Institutes of Health Animal Care and Use Committees. Chimeric mice were generated from 2 strains: C3H/HeOuJ (expresses a fully functional TLR4) and C3H/HeJ (expresses a nonfunctional TLR4 due to a homozygous missense mutation in the Tlr4 gene8). The C3H/HeOuJ and C3H/HeJ mice (both from Jackson Laboratories, Bar Harbor, ME) had their bone marrow destroyed by 137Cs γ-irradiation (9 Gy). They then received an intravenous transplant of 1 to 4 million bone marrow cells obtained from the femur and tibia of nonirradiated C3H/HeOuJ or C3H/HeJ donor mice (Figure 1). This procedure9 resulted in 4 chimeric constructs: HeOuJ mice with HeOuJ bone marrow (functional TLR4 in both nonhematopoietic and hematopoietic cells), HeOuJ mice with HeJ bone marrow (functional TLR4 in radioresistant nonhematopoietic cells but nonfunctional TLR4 in transplanted hematopoietic cells), HeJ mice with HeOuJ bone marrow (nonfunctional TLR4 in nonhematopoietic cells but functional TLR4 in hematopoietic cells), and HeJ mice with HeJ bone marrow (nonfunctional TLR4 in both nonhematopoietic and hematopoietic cells). Transplanted hematopoietic progenitors typically do not transdifferentiate into nonhematopoietic cells.10 Twelve weeks after bone marrow transfusion, that is, when more than 95% of the hematopoietic cells in the recipients are donor-derived,9 the febrile response of the chimeras to LPS (50 μg/kg, intravenously) derived from a “smooth” Escherichia coli colony (055:B5; Sigma-Aldrich, St Louis, MO) was tested according to Rudaya et al.3 The test involved nonstressful administration of LPS via a preimplanted jugular catheter to freely moving mice kept inside an environmental chamber at a tightly controlled, neutral ambient temperature (31°C). Deep body temperature of the mice was monitored by telemetry (series 4000 E-Mitter; Mini Mitter, Bend, OR). The deep body temperature responses were compared across phenotypes and time points by a 2-way ANOVA for repeated measures followed by the Tukey test (Statistica AX'99; StatSoft, Tulsa, OK).
Figure 1.
Generation of 4 types of chimeric mice. The images of individual cells refer to hematopoietic (bone marrow–derived) cells; the rest of the mouse body represents nonhematopoietic tissues. The presence of a nonfunctional TLR4 is coded by gray; the presence of a fully functional TLR4 is coded by white.
Results and discussion
As expected,3 the “controls” (HeOuJ mice with HeOuJ bone marrow) with a fully functional TLR4 responded to the dose of LPS used with a triphasic fever: deep body temperature peaked at about 40, 150, and 300 minutes after injection (Figure 2). In the mice bearing a nonfunctional TLR4 throughout the body (HeJ mice with HeJ bone marrow), all 3 phases of LPS fever were absent (Figure 2; P < .001 compared with the controls). This finding indicates that the TLR4 plays an indispensable role in triggering LPS fever. Importantly, the TLR4-deficient mice failed to respond to LPS with fever even though C3H/HeJ mice are capable of rapidly increasing their deep body temperature in response to other stimuli, such as psychologic stressors.11
Figure 2.
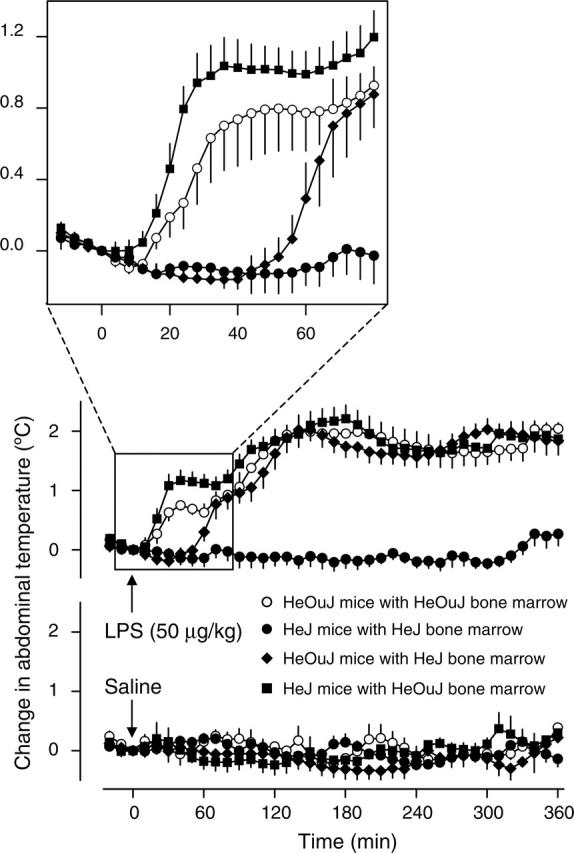
Fever responses of the chimeric mice. Abdominal temperature (mean ± SE) responses of the 4 indicated types of chimeric mice to LPS (50 μg/kg, intravenously) or saline (1 mL/kg, intravenously) are shown. At the time of the injection, the values of abdominal temperature of LPS- and saline-treated mice were, respectively, 35.7 ± 0.2°C (n = 7) and 35.9 ± 0.2°C (n = 6) for the HeOuJ mice with HeOuJ bone marrow, 36.0 ± 0.2°C (n = 7) and 36.2 ± 0.4°C (n = 7) for the HeJ mice with HeJ bone marrow, 35.9 ± 0.2°C (n = 9) and 35.9 ± 0.2°C (n = 7) for the HeOuJ mice with HeJ bone marrow, and 36.2 ± 0.3°C (n = 7) and 35.9 ± 0.2°C (n = 5) for the HeJ mice with HeOuJ bone marrow. These values did not differ statistically from each other.
In the past, numerous studies were aimed at identifying the cellular basis of immune-to-brain febrigenic signaling, either by eliminating a selected cellular population and studying fever under such conditions,12,13 or by identifying cells that produce and release febrigenic mediators, such as prostaglandin E2 (PGE2), in response to LPS.14-18 However, no study was able to determine whether the role of certain cells in fever is related to LPS recognition by these cells or to their activation by endogenous mediators induced by LPS. We found (Figure 2) that the first phase of LPS fever was absent (P < .001) in the mice bearing a nonfunctional TLR4 selectively in hematopoietic cells (HeOuJ mice with HeJ bone marrow), but was present, and even enhanced (P = .043), in the mice bearing a nonfunctional TLR4 in nonhematopoietic cells (HeJ mice with HeOuJ bone marrow). This finding shows that LPS recognition by the TLR4 in hematopoietic cells is pivotal for the development of the first febrile phase. Although future studies are necessary to determine the subpopulation of hematopoietic cells involved, it is tempting to speculate that it consists of macrophages of the LPS-processing organs (such as the liver and lung). Two experimental observations are in line with this scenario. First, the onset of the first phase of LPS fever in rats is associated with up-regulation of PGE2-synthesizing enzymes in the liver and lung but not in the brain.19 Second, pretreatment with drugs causing, among other effects, depletion of macrophages in peripheral tissues results in an attenuation of the first phase of LPS fever in rats12 and guinea pigs.13
Surprisingly, the late (second and third) phases of LPS fever were affected neither in the chimeras bearing a nonfunctional TLR4 selectively in hematopoietic cells nor in the chimeras bearing a nonfunctional TLR4 in nonhematopoietic cells (Figure 2). Only when both hematopoietic and nonhematopoietic cells carried a nonfunctional TLR4 (HeJ mice with HeJ bone marrow) were the second and third febrile phases suppressed. Because the absence of the first phase in the HeOuJ mice with HeJ bone marrow did not result in any attenuation of the second or third phase, the triggering mechanisms of the late febrile phases appear to be independent of those of the first phase. Our results also indicate that hematopoietic and nonhematopoietic cells in the chimeras can compensate for each other's inability to sense LPS when it comes to bringing about the late febrile phases. The relative contribution of each cell population remains unknown. In our previous study,9 the peripheral production of pyrogenic cytokines at the time corresponding to the second and third febrile phases (2-6 hours after LPS) was attributed mostly to hematopoietic cells (eg, macrophages), whereas both nonhematopoietic cells (eg, endotheliocytes, microglia, and astroglia) and hematopoietic cells (eg, perivascular and meningeal macrophages) were found to be responsible for cytokine production within the brain. In the same time window, PGE2 synthesis is activated both in the periphery and in the brain.19 Perhaps the late (second and third) phases of fever depend on redundant mechanisms that involve TLR4-dependent activation of hematopoietic cells (in the periphery and brain) and nonhematopoietic cells (mainly in the brain).
We conclude that all 3 phases of the typical polyphasic febrile response to LPS depend entirely on the TLR4. The first phase of LPS fever is triggered by TLR4 signaling in hematopoietic cells. The mechanisms of the second and third phases involve both hematopoietic and nonhematopoietic cells.
Prepublished online as Blood First Edition Paper, January 10, 2006; DOI 10.1182/blood-2005-11-4743.
Supported by grant NS-41233 (A.A.R.) from the National Institute of Neurological Disorders and Stroke and by a St Joseph's Foundation grant (A.A.R.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Romanovsky AA, Kulchitsky VA, Simons CT, Sugimoto N. Methodology of fever research: why are polyphasic fevers often thought to be biphasic? Am J Physiol. 1998;275: R332-R338. [DOI] [PubMed] [Google Scholar]
- 2.Oka T, Oka K, Kobayashi T, et al. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J Physiol. 2003;551: 945-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2005;289: R1244-R1252. [DOI] [PubMed] [Google Scholar]
- 4.Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14: 123-128. [DOI] [PubMed] [Google Scholar]
- 5.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4: 903-914. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5: 987-995. [DOI] [PubMed] [Google Scholar]
- 7.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430: 257-263. [DOI] [PubMed] [Google Scholar]
- 8.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282: 2085-2088. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25: 1788-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallieres L, Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J Neurosci. 2003;23: 5197-5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002;136: 489-501. [DOI] [PubMed] [Google Scholar]
- 12.Derijk RH, Strijbos PJ, van Rooijen N, Rothwell NJ, Berkenbosch F. Fever and thermogenesis in response to bacterial endotoxin involve macrophage-dependent mechanisms in rats. Am J Physiol. 1993;265: R1179-R1183. [DOI] [PubMed] [Google Scholar]
- 13.Sehic E, Li S, Ungar AL, Blatteis CM. Complement reduction impairs the febrile response of guinea pigs to endotoxin. Am J Physiol. 1998; 274: R1594-R1603. [DOI] [PubMed] [Google Scholar]
- 14.Elmquist JK, Breder CD, Sherin JE, et al. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J Comp Neurol. 1997;381: 119-129. [DOI] [PubMed] [Google Scholar]
- 15.Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson PJ, Ericsson-Dahlstrand A. Inflammatory response: pathway across the blood-brain barrier. Nature. 2001;410: 430-431. [DOI] [PubMed] [Google Scholar]
- 16.Yamagata K, Matsumura K, Inoue W, et al. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J Neurosci. 2001;21: 2669-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci. 2002;22: 5606-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472: 113-129. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov AI, Pero RS, Scheck AC, Romanovsky AA. Prostaglandin E2-synthesizing enzymes in fever: differential transcriptional regulation. Am J Physiol Regul Integr Comp Physiol. 2002;283: R1104-R1117. [DOI] [PubMed] [Google Scholar]