Abstract
Rare cases of somatic mosaicism resulting from reversion of inherited mutations can lead to the attenuation of blood-cell disorders, including Wiskott-Aldrich syndrome (WAS). The impact of the revertant hematopoietic stem or progenitor cells, particularly their representation in blood-cell populations, is of interest because it predicts the outcome of gene therapy. Here we report an 8-year-old patient with WAS caused by a single nucleotide insertion in the WASP gene that abrogates protein expression. The patient nonetheless had mild disease. We found reversion of the mutation in a fraction of patient lymphocytes. Forty percent of natural killer (NK) cells expressed Wiskott-Aldrich syndrome protein (WASP), and NK cells contained both mutated and revertant (normal) sequences. WASP was not expressed in patient T or B cells; T cells contained only the mutated sequence. The selective advantage of WASP+ NK cells was also demonstrated for carrier females. The enrichment of WASP+-revertant NK cells indicates that WASP provides a selective advantage in this lineage and predicts the success of gene therapy for reconstituting the NK-cell compartment. The importance of reconstituting the NK-cell lineage is discussed. (Blood. 2005;106:2815-2817)
Introduction
Wiskott-Aldrich syndrome (WAS) is an X-linked disease characterized by thrombocytopenia, small platelets, eczema, and immunodeficiency. The responsible gene encodes Wiskott-Aldrich syndrome protein (WASP), which functions in actin remodeling during the activation of blood cells.1 WASP is decreased or absent in blood cells of patients with WAS. The absence of WASP is associated with more severe disease, including higher frequency of infections, autoimmune disease, and malignancies.2
Several recent reports describe patients with WAS who have spontaneous reversion of mutations or second-site mutations that restore function.3-5 These rare reversions/corrections, which can be studied at the DNA and protein levels, become evident if the corrected cells have a selective advantage leading to increased frequency in peripheral-blood cells. Although reversions/corrections have been found both (and only) in T- and B-cell lineages, only T cells demonstrate selective advantage. The frequency of corrected cells was highest for memory T cells, suggesting that selection results from an advantage in proliferation, activation, or both. These studies left unanswered the identity of the reverted progenitor cell and whether reversion, if it were to occur in a multipotent hematopoietic progenitor, would confer proliferative advantage outside the T-cell lineage.
Here we report a novel case of somatic mosaicism resulting from spontaneous reversion of an inherited WASP mutation. The revertant sequence was confined to the patient's NK cells, which were a mixture of WASP-negative and WASP-expressing cells and contained the inherited and the revertant gene sequences. Enrichment of WASP-positive cells arising from one or few revertant NK progenitor cells indicates that WASP confers proliferative advantage to the NK-cell compartment and predicts the success of gene therapy in restoring this compartment.
Patients, materials, and methods
Patients
The patient P1 was evaluated as an infant in 1994 because of thrombocytopenia (platelet count, 20 × 109 to 50 × 109/L) and mild eczema. He did not have recurrent infections. A diagnosis of X-linked thrombocytopenia was considered. Bone marrow aspirate revealed normal trilineage hematopoiesis. Peripheral-blood immunophenotype at age 8 showed a decrease in T cells (22% CD4; 11% CD8) and an increase in NK cells (45% CD16+CD56+). After the birth in 2001 of a brother (P2) with thrombocytopenia and eczema, both boys were reevaluated and diagnosed with WAS based on thrombocytopenia (70 × 109/L or less) and small platelet size (P1, 1.89 μm; P2, 1.81 μm; normal platelet size, 2.2 ± 0.12 μm).6 Diagnosis was confirmed by identifying the mutation, as described.7
Cell, DNA, and protein analysis
Blood was collected in acid-citrate-dextrose under protocols approved by the Institutional Review Board of the CBR Institute for Biomedical Research (Boston, MA), including informed consent, and was fractionated immediately or after overnight shipment at ambient temperature. Peripheral-blood mononuclear cells (PBMCs) were isolated by Histopaque-1077 centrifugation. NK cells were isolated by positive selection with CD56 monoclonal antibody (mAb) (Clone N901; Beckman Coulter, Hialeah, FL) and secondary-antibody-linked magnetic beads (Dynal, Lake Success, NY). T cells were isolated using the T-cell Negative Selection kit (Dynal). DNA was isolated using QiaAmp kits (Qiagen, Valencia, CA) and cloned into pSTBlue-1 vector (Novagen, Madison, WI).
Western blots were stained with rabbit anti-WASP (W485).8 For flow cytometry, blood samples (100 μL) were incubated with fluorescein isothiocyanate (FITC) CD3 (clone UCHT1), phycoerythrin (PE)-Cy5 CD56 (clone N901), and PE-Cy5 CD19 (clone J4.119) mAbs (Beckman Coulter) for 15 minutes at approximately 22°C. Erythrocytes were lysed with FACS Lysing Solution (BD Biosciences, Palo Alto, CA). Intracellular WASP staining was performed with 5 μg/mL PE-labeled B9 or isotype control mAb (Santa Cruz Biotechnology, Santa Cruz, CA) using the Cytofix/Cytoperm kit (PharMingen, Palo Alto, CA). Cells were analyzed immediately on the FACScalibur (Becton Dickinson, Mountain View, CA) after collecting 10 000 cells/sample.
Results
Inherited mutation
Sequencing of whole-blood genomic DNA of both brothers identified the insertion of an adenine in a run of 6 adenines in exon 4 (476-77insA; discussed later). The mutation encodes a frameshift and results in WASP-negative cells.
Protein expression
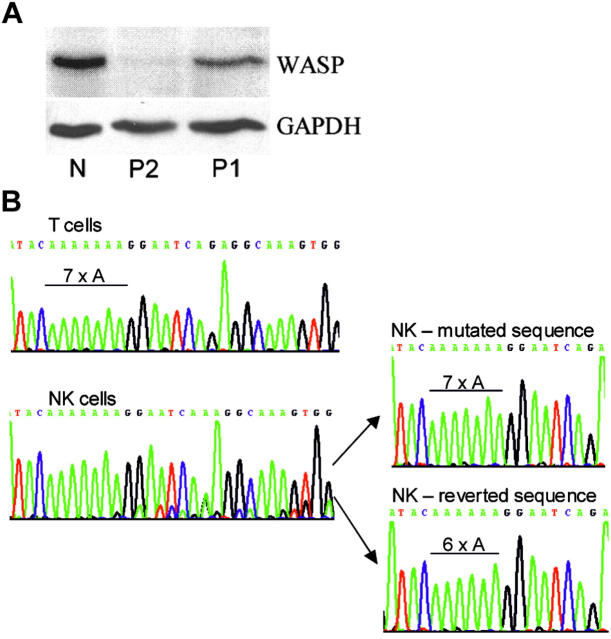
Western blots revealed normal-sized WASP in PBMCs of the patient (Figure 1A; P1). Detection by a C-terminal antibody indicates that the protein has an in-frame C-terminus. Protein expression level was 34% of normal. No WASP was detected in the PBMCs of the patient's infant brother (Figure 1A; P2). These findings suggested a correcting mutation in the cells of P1.
Figure 1.
Western blot and WASP gene analyses. (A). Western blot. Shown are the PBMCs of a genetically normal person (N) and of P2 and P1 stained with rabbit antibodies to WASP C-terminal 15 amino acids8 or GAPDH. (B) Genomic DNA analysis. Shown are amplified WASP exon 4 regions of T cells and NK cells of P1 as well as 2 representative clones of his NK-cell DNA. Only the inherited 7 adenines were found for NK and T cells of the patient's brother, P2 (not shown). Only the 6-adenine sequence was found for normal NK and T cells (not shown).
Gene sequences
Genomic DNA was sequenced from isolated cell populations. Contrary to expectations, patient T-cell DNA had only the inherited sequence with the abnormal run of 7 adenines (Figure 1B). T-cell clones (n = 8) also had only the inherited mutation (not shown). In contrast, the DNA of patient NK cells showed a mixture of 2 sequences, the predominant inherited sequence and a de novo sequence (Figure 1B). Amplification and cloning of NK-cell DNA identified the de novo sequence as the revertant sequence with a normal run of 6 adenines. The revertant sequence was found in 3 of 10 clones, and the remainder had the inherited mutation.
WASP in blood cells
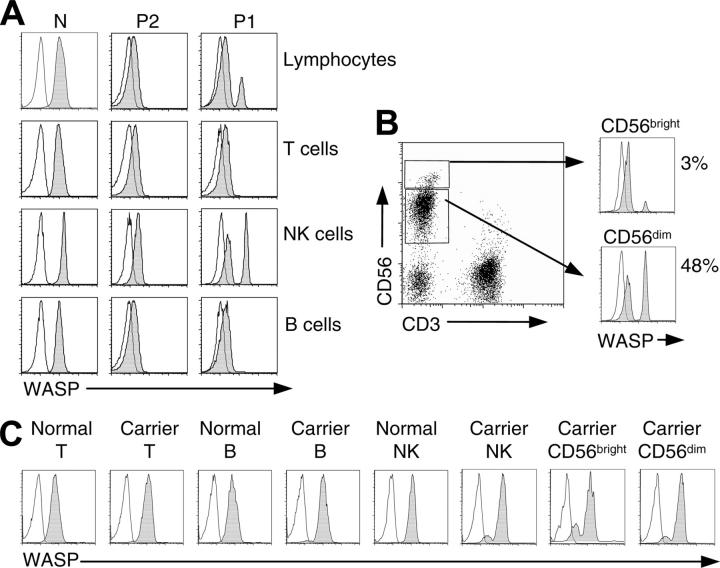
Intracellular WASP staining was performed on the final blood specimens from P1. Total lymphocytes and T, B, and NK cells of genetically normal persons expressed WASP, and the same cell types from P2 showed no detectable WASP (Figure 2A). In contrast, total lymphocytes of P1 showed both WASPdim and WASPbright cells, suggesting somatic mosaicism. On subset analysis, WASP-expressing cells were present only among patient NK cells (CD3-CD56+), where they amounted to 40% to 48%. Patient T (CD3+) and B (CD19+) lymphocytes (Figure 2A) were WASP-negative. Among patient NK cells, WASP-expressing cells were frequent among CD56dim cells (48%), whereas only 3% of CD56bright cells were WASP+ (Figure 2B).
Figure 2.
Flow-cytometric analysis. Cells in whole blood were stained for surface antigens, fixed, and permeabilized and then stained with PE-labeled WASP (shaded curves) or isotype control (open curves) mAbs. (A) Shown are total lymphocytes, T lymphocytes (CD3+), B lymphocytes (CD19+), and NK cells (CD3-CD56+) of a genetically normal person (N), the revertant patient (P1), and his brother (P2). (B) Dot plot of patient (P1) NK cells stained with CD3 and CD56 and histograms showing WASP staining of CD56bright and CD56dim subpopulations. (C) Analysis of T lymphocytes, B lymphocytes, NK cells, and NK subsets of an (age-matched) genetically normal female and a female WAS carrier (large deletion of WASP gene). Note the presence among cells of the female carrier of approximately 88% WASP+ CD56dim cells but only 78% WASP+ CD56bright cells. Similar findings were obtained for a second carrier female (intron 6 + 5 g→a), 90% WASP+CD56dim NK cells, 78% WASP+ CD56bright cells.
We also examined NK cells of female carriers of WAS as an independent measure of the selective advantage conferred in this lineage by WASP expression. A nonrandom pattern of X-chromosome inactivation has been demonstrated for mononuclear cells of carriers of WAS (reviewed in Wengler9). On analysis of WASP expression in the female carrier, selective advantage for WASP+ NK cells was demonstrated by the approximately 90% WASP+NK cells (Figure 2C). Consistent with the findings for the revertant patient, the percentage of WASP+ cells was greater for the CD56dim population than for the CD56bright population. In contrast, T and B cells were 100% WASP+. Similar findings were obtained for a second carrier female.
Discussion
Because WASP functions in dynamic cytoskeletal rearrangements, the enrichment of P1's revertant NK cells might have been caused by the selective advantage of WASP+ compared with WASP- progenitor or precursor cells in migratory capacity, chemokine responsiveness, proliferative responsiveness, or survival capacity. The greater frequency of revertant WASP+ cells among the CD56dim subset of terminally differentiated NK cells relative to CD56bright subset suggests that part of the selective advantage is at the level of peripheral cells, as noted also for memory and naive T cells of previous revertant patients. CD56dim cells express a range of activating and inhibitory receptors and homing receptors for inflammatory tissue sites; they are perforin positive and display high cytotoxicity.10 Most CD56bright NK cells reside in secondary lymphoid organs; they are the most potent cytokine producers and acquire a phenotype similar to that of CD56dim NK cells and an ability to lyse target cells on stimulation with interleukin-2 in vitro.11-13 It is possible that the infrequency of revertants in this subset can be attributed in part to revertant WASP-expressing CD56bright NK cells successfully leaving the circulation and homing to secondary lymphoid organs.
Vital functions of NK cells include antiviral and antitumor cytolytic activity and an immunoregulatory role in which NK cells and dendritic cells interact to provide innate protection against pathogens and to generate protective, adaptive immune responses.14-17 WASP plays an important role in the interactions of dendritic cells18 and NK cells.19 In NK cells, WASP colocalizes with F-actin at the activating immunologic synapse with target cells and undergoes phosphorylation.19,20 In patients with WAS, F-actin accumulation at the activating immunologic synapses is less frequent, and both natural and antibody-mediated cytotoxicity are defective.19,20
To assess the impact of the reversion, it is tempting to compare the disease severity of the 2 brothers; however, this approach is not appropriate because of the age-dependent variability of WAS. Nonetheless, the disease of P1, consisting of thrombocytopenia and mild eczema without recurrent infection, is milder than expected for a (now) 11-year-old patient with a truncating mutation.
Because previous reversions/corrections of WASP (n = 8) were enriched in T cells, and barring an impediment to enrichment of revertant T cells in this patient, the restriction of his revertant gene and WASP-expressing cells to NK cells suggests that his reversion occurred in a cell or in cells committed to the NK-cell lineage. The rarity of reverse mutation events suggests that the WASP+ NK cells are derived from one or a few original revertant cells. These findings have implications for gene therapy because they suggest that even the correction of a small number of stem cells with the capacity to differentiate into NK progenitors may suffice to substantially repopulate this lineage. Moreover, because NK-cell-protective capacities are germ line-encoded with specialization determined late in differentiation,21 the selected gene-corrected cells are anticipated to restore all functions of the compartment. Although correction of the NK-cell defect would not be curative, a functioning NK compartment is expected to contribute to protection against viral infections and possibly malignancies, which are serious complications of the immunodeficiency in this disease. Thus, enrichment of gene-corrected cells in the NK compartment should be an integral goal of gene therapy for patients with WAS.
Acknowledgments
We are grateful to the patients and their family as well as the WAS carrier females and other blood donors for their cooperation. We thank Dolores Fici for assistance with blood cell analysis.
Prepublished online as Blood First Edition Paper, June 28, 2005; DOI 10.1182/blood-2004-12-4724.
Supported by grants from the National Institutes of Health (HL59561 and AI39574) and the Jeffrey Modell Foundation.
Fred S. Rosen died on May 21, 2005, after the completion of this manuscript. M.I.L. performed the experiments; M.I.L. and E.R.-O'D. designed the study and wrote the manuscript; D.S.B. and F.S.R. established the diagnosis and interpreted the patient's disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Burns S, Cory GO, Vainchenker W, Thrasher AJ. Mechanisms of WASp-mediated haematological and immunological disease. Blood. 2004;104: 3454-3462. [DOI] [PubMed] [Google Scholar]
- 2.Jin Y, Mazza C, Christie JR, et al. Mutations of the Wiskott-Aldrich syndrome protein (WASP): hot spots, effect on transcription and translation and phenotype/genotype correlation. Blood. 2004;104: 4010-4019. [DOI] [PubMed] [Google Scholar]
- 3.Ariga T, Yamada M, Sakiyama Y, Tatsuzawa O. A case of Wiskott-Aldrich syndrome with dual mutations in exon 10 of the WASP gene: an additional de novo one-base insertion, which restores frame shift due to an inherent one-base deletion, detected in the major population of the patient's peripheral blood lymphocytes. Blood. 1998;92: 699-701. [PubMed] [Google Scholar]
- 4.Ariga T, Kondoh T, Yamaguchi K, et al. Spontaneous in vivo reversion of an inherited mutation in the Wiskott-Aldrich syndrome. J Immunol. 2001;166: 5245-5249. [DOI] [PubMed] [Google Scholar]
- 5.Wada T, Schurman SH, Otsu M, et al. Somatic mosaicism in Wiskott-Aldrich syndrome suggests in vivo reversion by a DNA slippage mechanism. Proc Natl Acad Sci U S A. 2001;98: 8697-8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenney DM. Wiskott-Aldrich syndrome and related X-linked thrombocytopenia. Curr Opin Pediatr. 1990;2: 932-935. [Google Scholar]
- 7.Jones LN, Lutskiy MI, Cooley J, Kenney DM, Rosen FS, Remold-O'Donnell E. A novel protocol to identify mutations in patients with Wiskott-Aldrich syndrome. Blood Cells Mol Dis. 2002;28: 392-398. [DOI] [PubMed] [Google Scholar]
- 8.Remold-O'Donnell E, Cooley J, Shcherbina A, et al. Variable expression of WASP in B cell lines of Wiskott-Aldrich syndrome patients. J Immunol. 1997;158: 4021-4025. [PubMed] [Google Scholar]
- 9.Wengler G, Gorlin JB, Williamson JM, Rosen FS, Bing DH. Nonrandom inactivation of the X chromosome in early lineage hematopoietic cells in carriers of Wiskott-Aldrich syndrome. Blood. 1995;85: 2471-2477. [PubMed] [Google Scholar]
- 10.Ferlazzo G, Munz C. NK cell compartments and their activation by dendritic cells. J Immunol. 2004;172: 1333-1339. [DOI] [PubMed] [Google Scholar]
- 11.Cooper MA, Fehniger TA, Turner SC, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97: 3146-3151. [DOI] [PubMed] [Google Scholar]
- 12.Fehniger TA, Cooper MA, Nuovo GJ, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101: 3052-3057. [DOI] [PubMed] [Google Scholar]
- 13.Ferlazzo G, Thomas D, Lin SL, et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172: 1455-1462. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5: 405-411. [DOI] [PubMed] [Google Scholar]
- 15.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195: 327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195: 335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4: 175-181. [DOI] [PubMed] [Google Scholar]
- 18.Borg C, Jalil A, Laderach D, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104: 3267-3275. [DOI] [PubMed] [Google Scholar]
- 19.Orange JS, Ramesh N, Remold-O'Donnell E, et al. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci U S A. 2002;99: 11351-11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gismondi A, Cifaldi L, Mazza C, et al. Impaired natural and CD16-mediated NK cell cytotoxicity in patients with WAS and XLT: ability of IL-2 to correct NK cell functional defect. Blood. 2004;104: 436-443. [DOI] [PubMed] [Google Scholar]
- 21.Miller JS, McCullar V. Human natural killer cells with polyclonal lectin and immunoglobulinlike receptors develop from single hematopoietic stem cells with preferential expression of NKG2A and KIR2DL2/L3/S2. Blood. 2001;98: 705-713. [DOI] [PubMed] [Google Scholar]