Abstract
Freshly isolated, resting natural killer (NK) cells are generally less lytic against target cells than in vitro interleukin 2 (IL-2)-activated NK cells. To investigate the basis for this difference, the contribution of several receptors to activation of human NK cells was examined. Target-cell lysis by IL-2-activated NK cells in a redirected, antibody-dependent cytotoxicity assay was triggered by a number of receptors. In contrast, cytotoxicity by resting NK cells was induced only by CD16, and not by NKp46, NKG2D, 2B4 (CD244), DNAM-1 (CD226), or CD2. Calcium flux in resting NK cells was induced with antibodies to CD16 and, to a weaker extent, antibodies to NKp46 and 2B4. Although NKp46 did not enhance CD16-mediated calcium flux, it synergized with all other receptors. 2B4 synergized with 3 other receptors, NKG2D and DNAM-1 each synergized with 2 other receptors, and CD2 synergized with NKp46 only. Resting NK cells were induced to secrete tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ), and to kill target cells by engagement of specific, pair-wise combinations of receptors. Therefore, natural cytotoxicity by resting NK cells is induced only by mutual costimulation of nonactivating receptors. These results reveal distinct and specific patterns of synergy among receptors on resting NK cells.
Introduction
Natural killer (NK) cells are characterized by cytolytic activity against susceptible target cells and by the secretion of cytokines, such as tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ). NK cells discriminate between normal and abnormal cells (infected or transformed) through engagement and dynamic integration of multiple signaling pathways, which are initiated by germline-encoded receptors.1-3 Healthy cells are protected from NK-cell-mediated lysis by expression of major histocompatibility complex (MHC) class I ligands for NK-cell-inhibitory receptors.1,4 However, de novo expression of ligands for NK-cell activation receptor NKG2D can trigger natural cytotoxicity against MHC class I+ target cells.5,6 A number of structurally distinct receptors have been implicated in activation of NK-cell effector functions. It is not yet clear if any one receptor is necessary or sufficient to activate NK cells and to what extent activation receptors may be redundant. Activation receptors can be grouped in 3 categories: receptors that signal through immunoreceptor tyrosine-based activation motif (ITAM)-containing subunits (eg CD16, NKp46, NKp44), the DAP10-associated receptor NKG2D, and several other receptors (eg CD2, 2B4, DNAM-1) that signal by different pathways.
CD16 (FcγRIII), a low-affinity receptor for IgG, is associated with the ITAM-containing FcεRI γ chain and T-cell receptor (TCR) ζ chain. NKp46 and NKp30 are associated with the TCR ζ chain.7 NKp44, KIR2DS, and CD94/NKG2C are associated with the ITAM-containing DAP12. Natural cytotoxicity receptors (NCRs), which include NKp46, NKp44, and NKp30, play a major role in NK-cell cytotoxicity against transformed cells.8 Although ligands of NCRs have not been identified, antibodies against NCRs have been used to block lysis of tumor cells by interleukin 2 (IL-2)-activated and resting NK cells.9-11 However, in the mouse, Syk/ZAP70-independent natural cytotoxicity by NK cells was observed, implying that natural cytotoxicity can occur independently of ITAM-based activation signals.12,13
NKG2D can signal through both DAP10 and DAP12 in mice,14,15 whereas human NKG2D associates only with DAP10.16-18 DAP10 is a signaling subunit that carries a phosphatidylinositol-3 kinase-binding motif.16 Ligands for NKG2D, such as MICA and ULBP, are expressed on some tumor cells, and on infected or stressed cells.19 Experiments have suggested that NKG2D signals are sufficient to activate NK-cell functions.20-22 Lysis of certain tumor cells by resting NK cells and by IL-2-activated NK cells can be blocked by antibodies to NKG2D.11,23 The importance of ligands for NKG2D in immune defense is underscored by strategies developed by viruses to interfere with their expression.19,24,25
Several other receptors activate NK cells by signaling through their own cytoplasmic tail. 2B4 (CD244) recruits SAP and Fyn through cytoplasmic tyrosine-based motifs.26,27 The ligand of 2B4 is CD48, which is expressed on hematopoietic cells.28 CD2 and NKp80 signal through largely unknown pathways. CD2 binds to LFA-3 (CD58).29 The ligand of NKp80 is unknown. DNAM-1 is associated with LFA-1 in NK cells,30 is phosphorylated by a PKC,31 and binds to CD155 and CD112.32 Antibodies to DNAM-1 inhibit NK-cell cytotoxicity toward tumor cells.32-34
Anti-CD16 mouse hybridomas are lysed by human NK cells in so-called redirected lysis assays, in which CD16 is cross-linked by surface IgG.35,36 Likewise, lysis of mouse FcR+ P815 cells, in a redirected, antibody-dependent lysis assays, by IL-2-activated human NK cells can be induced by independent engagement of CD16,36,37 NKp46,38 NKp44,39 NKp30,10 NKp80,40 NKG2D,11,41 2B4,42 CD2,43 DNAM-1,31 KIR2DS,44 CD94/NKG2C,45 and KIR2DL4,46,47 suggesting extensive redundancy in activation pathways. Most published studies have used polyclonal or clonal NK cells that have been expanded in IL-2. Because resting NK cells cannot be maintained very long in the absence of cytokines, much less is known about their requirements for activation of cytotoxicity and cytokine secretion. Here, we tested activation of resting NK cells by several receptors and by pair-wise combinations of receptors. For most NK-cell receptors, it is still unknown if they are capable of triggering NK-cell effector function independently or if they can only serve as costimulating receptors. Apart from the FcR CD16, which was sufficient for activation of cytotoxicity and cytokine release by resting NK cells, all NK-cell receptors tested required coengagement of another receptor for activation. Clear synergies between specific pairs of coactivating receptors were observed in the activation of cytotoxicity and cytokine secretion by resting NK cells.
Material and methods
Cells
Human NK-cell populations were isolated from peripheral blood by negative selection using an NK isolation kit (Miltenyi Biotec, Auburn, CA). Polyclonal IL-2-activated NK cells were expanded in Iscove modified Dulbecco medium (IMDM; Invitrogen, Carlsbad, CA) supplemented with 10% human serum (Valley Biomedical, Winchester, VA), 100 U/mL recombinant IL-2 (rIL-2; Hoffmann-La Roche, Basel, Switzerland), and 10% purified human IL-2 (Hemagen, Columbia, MD). Resting NK cells were resuspended in the same medium without IL-2 and were used within 1 to 4 days after isolation. These cells were 95% to 99% CD3-CD56+ as determined by flow cytometry. Prior to experiments, trypan blue exclusion was used to assess cell viability. After 4 days in culture, viability of resting NK cells was typically more than 90%. Cell-surface expression of activating receptors and the relative distribution of CD56dim and CD56bright NK cells did not change significantly during the culture. The mouse mastocytoma cell line P815 and the human erythroleukemia cell line K562 (both from American Type Culture Collection, Manassas, VA) were maintained in RPMI 1640 supplemented with 2 mM l-glutamine and 10% fetal bovine serum (FBS; all from Invitrogen).
Antibodies
For flow cytometric analysis, antibody-dependent redirected lysis, bead stimulation, and calcium flux measurements, the following monoclonal antibodies (mAbs; all mouse IgG1 isotype) were used: anti-CD2 (clone RPA-2.10), anti-CD3 (clone UCHT1), anti-CD16 (clone 3G8), anti-CD56 (clone B159), anti-CD107a (clone H4A3), anti-CD226/DNAM-1 (clone DX11), anti-NKG2D (clone 1D11), and isotype control (clone MOPC-21) from BD Biosciences (Franklin Lakes, NJ). Anti-CD244/2B4 (clone C1.7) and anti-NKp46 (clone BAB281) were from Beckman Coulter (Fullerton, CA). Anti-NKG2D (clone 149810) was from R&D Systems (Minneapolis, MN). Isotype control mouse IgG1 (clone MOPC-21) was from Sigma (St Louis, MO). Secondary goat F(ab′)2 anti-mouse IgG was from Jackson ImmunoResearch (West Grove, PA).
51Cr-release assay
A standard 3-hour 51Cr-release assay was performed as described.48 Briefly, P815 or K562 target cells were labeled with 0.5 mCi/mL (19 MBq/mL) 51Cr (Amersham Pharmacia, Piscataway, NJ) in saline solution for 1 hour at 37°C. Cells were washed 3 times in phosphate-buffered saline (PBS) and resuspended at 1 × 106 cells/mL in IMDM supplemented with 10% FBS. P815 cells were incubated for 30 minutes at room temperature with 10 μg/mL mAb. Cells were spun down, resuspended in medium, and plated at 1 × 104 cells/well in triplicate. NK cells were washed, resuspended in IMDM supplemented with 10% FBS, and added to wells at the indicated effector-to-target (E/T) cell ratios. The supernatants were measured for 51Cr release on a γ-counter (Cobra II Auto-Gamma, Global Medical Instruments, Ramsey, MN).
Flow cytometric and single-cell Ca2+ flux analysis
NK cells were stained with mAbs at 10 μg/mL in 50 μL Hanks buffered salt solution (HBSS) with Ca2+ and Mg2+ (Biosource, Camarillo, CA) supplemented with 1% FBS for 30 minutes on ice. Then 300 μL dye-loading buffer (HBSS 1% FBS, 2 μM Fluo-4 AM, 5 μM Fura Red AM [both Molecular Probes, Eugene, OR] and 5 mM probenecid σ) were added and cells were further incubated for 30 minutes on ice. Cells were pelleted by centrifugation and washed once in HBSS 1% FBS. Cells were kept on ice and resuspended in 300 μL HBSS 1% FBS just prior to a 5-minute incubation in a waterbath at 37°C. Samples were vortexed and analyzed by flow cytometry (FACS Calibur, BD Bioscience). After 30 seconds, tubes were briefly removed, 4 μg goat anti-mouse F(ab′)2 antibodies were added, samples were vortexed, placed back on the flow cytometer, and events were acquired for 4 minutes. Data were analyzed with FlowJo software (Treestar, Ashland, OR). NK cells were gated on forward scatter/side scatter plots. The ratio between the mean fluorescence intensity of FL-1 and FL-3 was calculated and plotted as a function of time. The baseline for isotype control-stimulated cells was set to 1, and this factor was used to normalize the other plots in every batch.
For microscopic single-cell analysis, resting NK cells were resuspended in culture medium containing 0.4 μM Fluo-4 AM, 4 μM Fura Red AM, and 5 mM probenecid at 2 × 106 cells/mL and incubated at room temperature for 30 minutes. The cells were washed once in HBSS 1% FBS and resuspended at 2 × 106 cells/mL in HBSS 1% FBS. Aliquots of 0.5 mL were added to poly-l-lysine-coated (Sigma) wells of Lab-Tek II chambered coverglasses (Nunc, Roskilde, Denmark). The cells were allowed to settle onto the coverglass for 15 minutes at room temperature. The medium was aspirated from the chambers and replaced with 300 μL cold HBSS 1% FBS containing 3 μg of each of the appropriate mAbs. The chambers were incubated for 30 minutes on ice. Cells were washed once with 1 mL cold HBSS 1% FBS, and 400 μL cold HBSS 1% FBS was placed in each chamber. Prior to acquisition on the confocal microscope, each chamber was warmed in a 37°C, 5% CO2 incubator for 5 minutes. Data were acquired on a Zeiss LSM510 confocal microscope using a × 40 oil-immersion lens (Zeiss, Göttingen, Germany). The microscope was set in λ scan mode so that wavelengths from 499 to 670 nm were acquired in a single pass. The pinhole was adjusted so that optic slices of approximately 2 μm were acquired. Scan speed was set so that one scan was completed every 4 seconds. Thirty seconds after the beginning of acquisition, secondary cross-linking goat anti-mouse F(ab′)2 antibody was added to a final concentration of 13 μg/mL. Scanning was continued for 10 minutes. After acquisition was complete, the data were processed by linear unmixing, using control spectra acquired from cells loaded individually with either Fluo-4 or Fura-Red. After unmixing, individual cells were analyzed to give the fluorescence values of the Fluo-4 and Fura Red for the entire time series, which were used to calculate the ratio of the Fluo-4 fluorescence to the Fura Red fluorescence.
CD107a degranulation assay
P815 cells (2 × 105) were incubated for 15 minutes at room temperature with 5 μg/mL mAbs. Cells were spun down and resuspended with 1 × 105 resting NK cells in IMDM supplemented with 10% FBS. Cells were spun down for 3 minutes at 17g and incubated for 2 hours at 37°C. Thereafter, cells were spun down and cell pellets were resuspended in PBS 2% FBS and stained with PE-conjugated anti-CD56 and FITC-conjugated anti-CD107a mAbs, followed by flow cytometric analysis.
Bead stimulation and ELISA
Beads with a diameter of 4.5 μm were used to stimulate NK cells at a ratio of 8 beads per cell. To coat beads with saturating amounts of mAb, 4 × 107 goat anti-mouse-coated beads (Dynabeads M-450; Dynal, Oslo, Norway) were incubated with 3 μg premixed mAbs in PBS 2% FBS for 1 hour at 4°C. Beads were washed 3 times in PBS 2% FBS to remove excess mAbs. For cell stimulation, 4 × 106 beads were incubated with 5 × 105 resting NK cells in 500 μL IMDM supplemented with 10% human serum for 2 or 6 hours at 37°C. The cultures were rotated end-over-end during the stimulation. Supernatants were stored at -20°C and analyzed with TNF-α enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) or IFN-γ ELISA kit (Pierce, Rockford, IL).
Results
Induction of cytotoxicity by receptors on resting NK cells
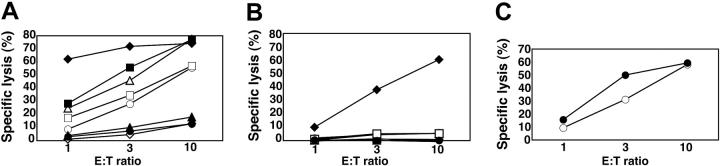
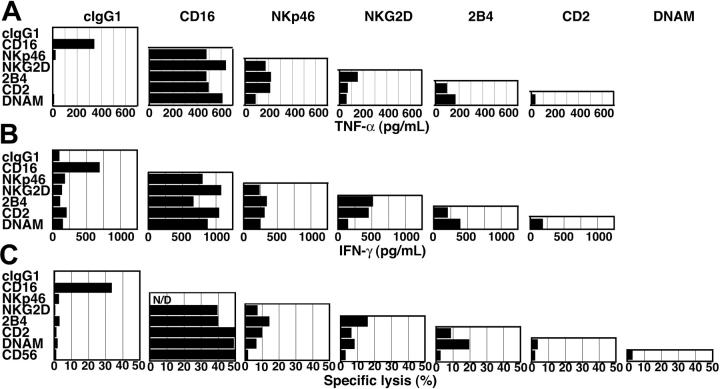
Cytotoxicity by IL-2-activated NK cells and by resting NK cells was compared in redirected, lysis assays. The mouse FcR+ cell line P815 was incubated with mAbs to several NK-cell receptors and mixed with NK cells. 51Cr release by P815 cells at several E/T cell ratios was measured after 3 hours (Figure 1). In several independent experiments, up to 20% lysis was observed when P815 cells were incubated with IL-2-activated NK cells at an E/T cell ratio of 10 (not shown). This lysis was not enhanced by the presence of isotype control IgG1 (11% ± 8.0% [mean ± SD] specific lysis at an E/T ratio of 10:1, 5 independent experiments), and of mAbs to CD56 or CD2 (Figure 1A). The mAbs to CD16, NKp46, NKG2D, 2B4, and DNAM-1 each augmented lysis of P815 target cells in the presence of IL-2-activated NK cells (Figure 1A). Notably, at lower E/T cell ratios, CD16 was the strongest inducer of cytotoxicity by IL-2-activated NK cells (Figure 1A).
Figure 1.
Engagement of CD16, but not NK-cell receptors NKp46, NKG2D, 2B4, DNAM-1, or CD2, induces cytotoxicity by resting NK cells. Redirected lysis assay of P815 target cells with IL-2-activated NK cells (A) or resting NK cells (B) at indicated E/T cell ratios. P815 cells were preincubated with IgG1 mAbs to specific NK-cell receptors, as indicated. ⋄ indicates isotype control antibody; ♦, anti-CD16; □, anti-NKp46; ▪, anti-NKG2D;▵, anti-2B4; ▴, anti-CD2; ○, anti-DNAM-1; and ▪, anti-CD56. (C) Lysis of K562 cells by resting (○) and IL-2-activated (▪) NK cells. Experiments are representative of at least 5 independent experiments.
Freshly isolated, resting NK cells did not induce lysis of P815 cells either in the absence (not shown) or in the presence of isotype control IgG1 (1.0% ± 1.8% specific lysis at an E/T cell ratio of 10:1; n = 7; Figure 1B). A mAb to CD16 induced 35% to 65% lysis of P815 cells at an E/T cell ratio of 10 (51% ± 13% specific lysis; n = 7). The mAbs to NKp46, NKG2D, 2B4, CD2, DNAM-1, or CD56 induced little or no lysis of P815 target cells (Figure 1B). With freshly isolated NK cells from some donors, up to 10% lysis with mAb to 2B4 (6.9% ± 4.7% specific lysis at an E/T cell ratio of 10:1, n = 7) or DNAM-1 (5.2% ± 4.9% specific lysis at an E/T cell ratio of 10:1; n = 6) was observed. Less lysis was observed with a mAb to NKp46 (2.0% ± 1.7% specific lysis at an E/T cell ratio of 10:1, n = 6). No lysis was observed with NK cells from any donor with mAbs to NKG2D, CD2, and CD56 (Figure 1B).
In some instances, natural ligands of NKG2D activated NK cells more efficiently than mAbs to NKG2D.49 We therefore investigated whether incubation of P815 target cells with MICA-Fc or ULBP1-Fc could induce lysis by resting NK cells. MICA-Fc and ULBP1-Fc did not induce lysis by resting NK cells, even though each one augmented the lysis of P815 cells by IL-2-activated NK cells to the same extent as NKG2D mAb (not shown).
The lack of cytotoxicity by receptor engagement on resting NK cells could be explained in different ways. Resting NK cells may be intrinsically less lytic or much stronger signals may be required to induce lysis by resting NK cells. However, resting NK cells readily lysed K562 cells to a similar degree as IL-2-activated NK cells (Figure 1C), indicating that resting NK cells are fully capable of natural cytotoxicity, if provided with sufficient activation signals. Intracellular staining revealed high expression of perforin in both resting and IL-2-activated NK cells (data not shown). A comparison of expression of activating receptors on resting and IL-2-activated NK cells revealed that resting NK cells express high levels of the receptors studied here (Figure 2). Of note, IL-2-activated NK cells expressed more uniformly high levels of CD2 and CD56, as compared to resting NK cells (Figure 2). The small increase in receptor expression on IL-2-activated NK cells (eg, NKp46 and NKG2D) is unlikely to explain the observed differences in cytotoxicity by resting and IL-2-activated NK cells. Additional explanations include that some receptors may not signal in resting NK cells or that resting NK cells may have good cytolytic potential but require additional signals to induce cytotoxicity.
Figure 2.
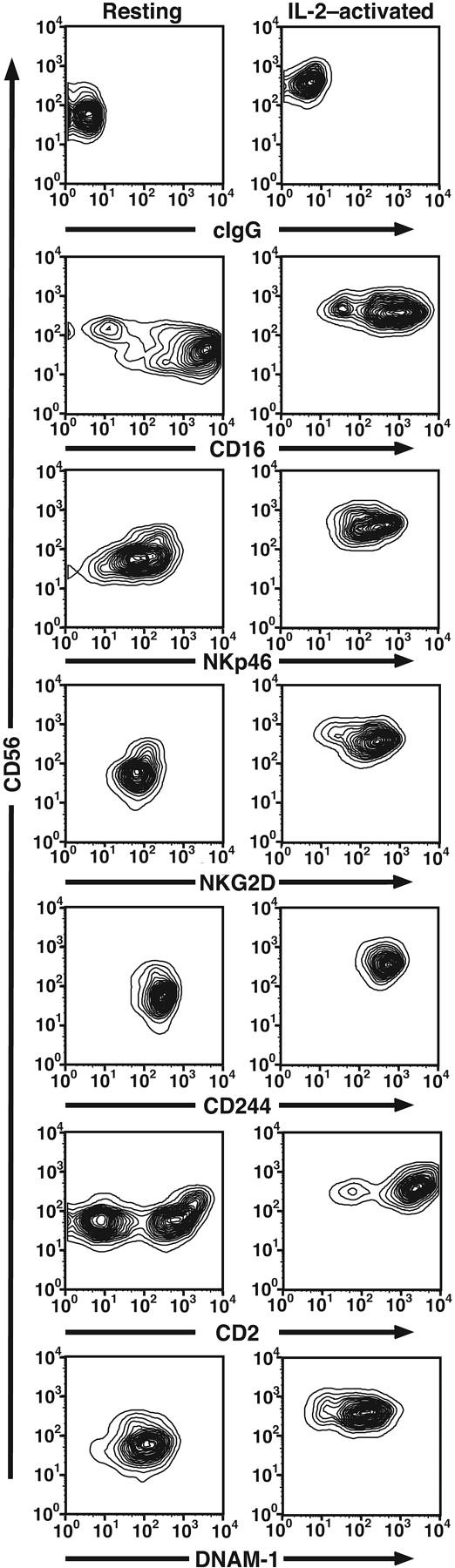
Expression of activating receptors on resting and IL-2-activated NK cells. Freshly isolated, resting NK cells or long-term IL-2-cultured NK cells were stained with directly conjugated mAbs to the indicated receptors (x-axis) and anti-CD56 (y-axis).
Synergistic activation of intracellular Ca2+ mobilization in resting NK cells by pair-wise combinations of receptors
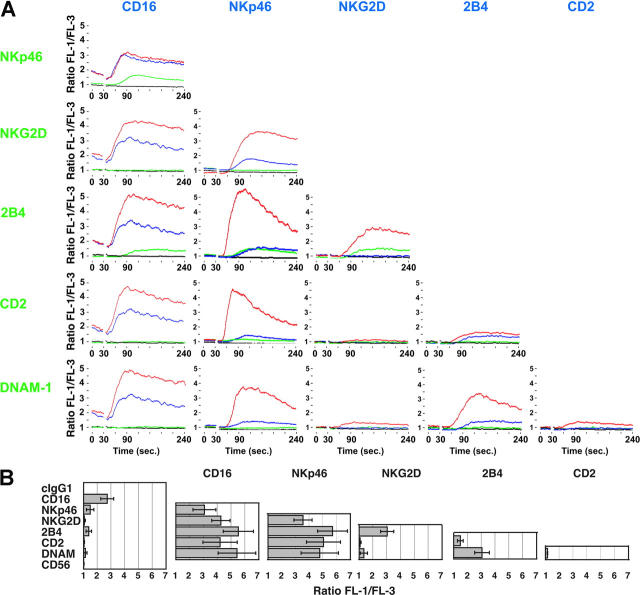
Intracellular Ca2+ concentration was measured in resting NK cells after cross-linking different receptors. NKp46, 2B4, NKG2D, and DNAM-1 are expressed on all NK cells. CD16 is expressed on NK cells of the CD56dim subset, which represented more than 90% of the total NK cell population. Therefore, analysis of activation by these receptors was performed on a forward scatter/side scatter gate for total NK cells. Because CD2 is expressed on a subset of resting NK cells, cells were stained with PE-conjugated anti-CD2 and gated on CD2bright cells. To investigate the relative potency of individual receptors and the effect of co-crosslinking different receptor combinations, time-course analysis was performed with resting NK cells preincubated with mAbs to a single receptor or to pair-wise combinations of receptors. Cells were loaded with the Ca2+ sensitive dyes Fluo-4 and Fura Red and analyzed by flow cytometry (Figure 3). After acquisition of baseline fluorescence, secondary goat F(ab′)2 antimouse fragments were added to cross-link receptors. The FL-1/FL-3 ratio was recorded over 4 minutes (Figure 3A). The mean peak FL-1/FL-3 value from several independent experiments was also calculated (Figure 3B). The mAb to CD16 elevated baseline Ca2+ without cross-linking, and this was further augmented by cross-linking with secondary antibodies (Figure 3A). Cross-linking of NKp46 and of 2B4 also led to rises in intracellular Ca2+, but the peak FL-1/FL-3 ratios were significantly lower and the kinetics were slower than responses elicited by CD16 cross-linking (Figure 3). Cross-linking of mAb to NKG2D or DNAM-1 induced only a very small but reproducible rise in intracellular Ca2+. Cross-linking CD2 produced only minor elevations in intracellular Ca2+ (Figure 3) and cross-linking of CD56 did not produce any rises in Ca2+ (Figure 3B and data not shown).
Figure 3.
Synergistic activation of Ca2+ flux in resting NK cells by co-crosslinking pair-wise combinations of receptors. (A-B) NK cells were preincubated with mAbs to indicated receptors on ice, loaded with Fluo-4 and Fura Red, resuspended in HBSS 1% FBS, and prewarmed at 37°C. Cells were analyzed by flow cytometry. After 30 seconds, secondary F(ab′)2 goat anti-mouse IgG was added to each sample. (A) FL-1/FL-3 ratios are plotted as a function of time. Black lines show activation with isotype control. Blue lines show activation by the single receptors, indicated in blue. Green lines show activation by the single receptors, indicated in green. Red lines show activation by the combination of both receptors. (B) The peak FL-1/FL-3 ratio after cross-linking of indicated receptor combinations was measured in several independent experiments. Bars indicate the SD (≥ 3 independent experiments).
Co-crosslinking CD16 with NKG2D, 2B4, CD2, or DNAM-1 enhanced the peak Ca2+ (Figure 3). Co-cross-linking with the other ITAM-containing receptor NKp46 (Figure 3) or with CD56 (not shown) did not enhance Ca2+ flux. Although NKp46 mAb induced modest elevations in Ca2+ by itself, co-crosslinking with mAbs to NKG2D, 2B4, CD2, or DNAM-1 significantly increased the rise in intracellular Ca2+ (Figure 3). 2B4 induced significant elevations in intracellular Ca2+ in combination with CD16, NKp46, NKG2D, or DNAM-1, but not with CD2 or CD56 (Figure 3 and data not shown). NKG2D cross-linking synergized with CD16, NKp46, or 2B4. A small enhancement was also observed when NKG2D and DNAM-1 were co-crosslinked, but no synergy was observed on cross-linking with CD2 (Figure 3). Cross-linking CD2 and DNAM-1 did not produce any significant rise in intracellular Ca2+ (Figure 3). These results revealed a complex pattern of synergy among receptors in activation of resting NK cells.
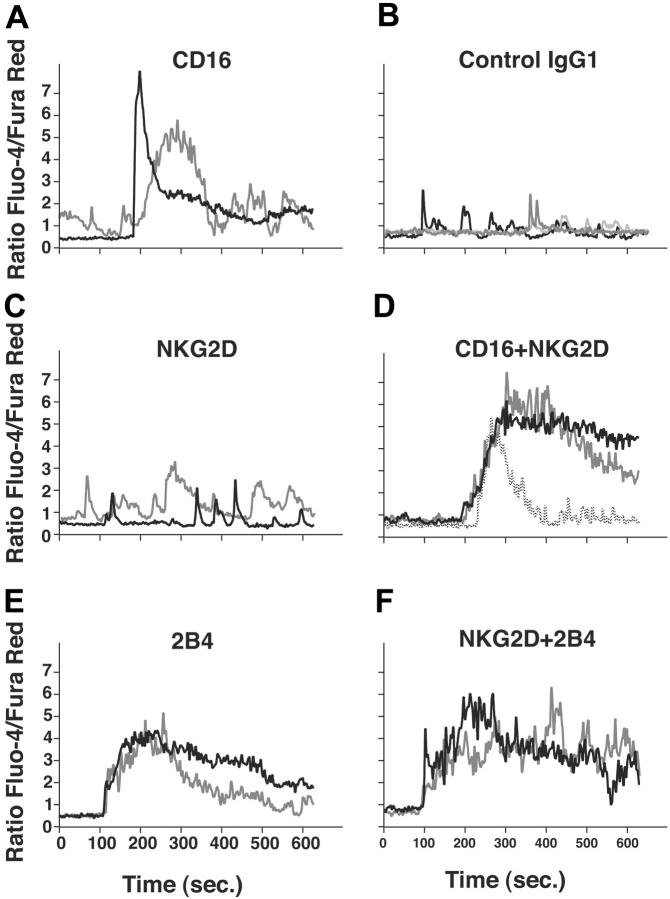
Flow cytometry cannot follow the kinetics of Ca2+ regulation in individual cells. Recording of Ca2+ fluctuations in individual T cells revealed important differences in functional outcome of oscillating versus sustained Ca2+ fluxes.50 We performed microscopy experiments to follow intracellular Ca2+ responses elicited in individual NK cells after receptor cross-linking or co-crosslinking (Figure 4). Resting NK cells were placed on poly-l-lysine-coated coverslips, incubated with mAbs, and loaded with Ca2+-sensitive dyes Fluo-4 and Fura Red. Recordings of individual cells revealed that CD16 cross-linking elicited varied responses (Figure 4A), in comparison to baseline Ca2+ fluctuations observed with isotype control mAb-treated cells (Figure 4B). In some cells, CD16 cross-linking induced a sharp but transient rise in intracellular Ca2+. In other cells, lower peaks but more oscillation was observed. Whereas NKG2D cross-linking produced little or no rise in baseline intracellular Ca2+ (Figure 4C), cross-linking of CD16 and NKG2D resulted in a more sustained elevation in Ca2+ in most cells (Figure 4D). Cross-linking of 2B4 induced sustained rises in intracellular Ca2+ (Figure 4E), and these were further enhanced by co-crosslinking with NKG2D (Figure 4F). Overall, data match those obtained by flow cytometry.
Figure 4.
Single-cell Ca2+ flux analysis. (A-F) Resting NK cells were loaded with Fluo-4 and Fura Red, placed on coverslips, and stained with antibodies as indicated. Cells were washed, resuspended in HBSS 1% FBS, and prewarmed at 37°C. Fluorescence was measured by confocal microscopy. Thirty seconds after the beginning of each scan, secondary F(ab′)2 goat anti-mouse IgG was added to each sample. Traces of the Fluo-4/Fura Red ratio of 2 or 3 representative NK cells are shown.
In conclusion, each receptor is capable of contributing a signal in resting NK cells, which results in Ca2+ mobilization when costimulated by another receptor. Some receptors can elicit Ca2+ flux directly, in the absence of adhesion or coengagement of other receptors.
Synergistic Ca2+ flux correlates with degranulation
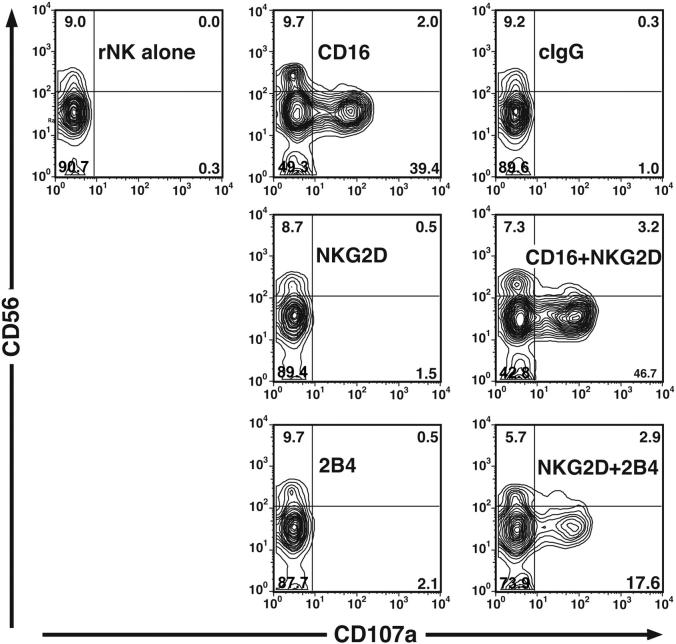
Phospholipase C-γ activation and Ca2+ mobilization are among the early steps leading to perforin-dependent NK-cell cytotoxicity.51 Exocytosis of secretory lysosomes, which are lytic granules, is required to complete this process. An assay was used to measure degranulation based on cell-surface expression of the lysosomal protein CD107a (LAMP-1).52 Resting NK cells did not stain with anti-CD107a mAb, relative to isotype control mAb staining, and incubation with P815 cells and isotype control antibody did not induce CD107a surface expression (Figure 5). When NK cells were incubated with anti-CD16 mAb and FcR+ P815 cells for cross-linking, CD107a expression was detected on 50% of the NK cells (52% ± 9.2% [mean ± SD] of CD107a+CD56+ NK cells, 7 independent experiments; Figure 5). Notably, degranulation was observed on the CD56dimCD16+ but not the CD56brightCD16- NK-cell subset (Figure 5). Alone, anti-NKG2D and anti-2B4 mAbs did not induce surface expression of CD107a (1.3% ± 0.6% and 2.7% ± 1.3%, respectively, 7 independent experiments; Figure 5). Therefore, CD107a surface expression correlated with the observed cytolytic activity by resting NK cells (Figure 1B). When resting NK cells were incubated with a combination of anti-CD16 and anti-NKG2D mAbs, CD107a surface expression increased somewhat relative to that induced by anti-CD16 mAb alone (56% ± 8.3%, 7 independent experiments; Figure 5). Interestingly, the combination of anti-NKG2D and anti-2B4 mAbs induced a synergistic increase in CD107a surface expression (13% ± 6.1%, 7 independent experiments; Figure 5), correlating with the observed synergy for Ca2+ flux.
Figure 5.
Degranulation by resting NK cells induced by NKG2D and 2B4 synergism. NK cells were incubated for 2 hours either alone or with P815 cells and mAbs as specified. Cells were stained with anti-CD56 and isotype control or anti-CD107a mAbs. Plots were gated on a forward scatter/side scatter lymphocyte gate. The experiment is representative of 7 independent experiments.
Stimulation of TNF-α and IFN-γ secretion
To evaluate which receptor or receptor combination could induce cytokine secretion, resting NK cells were incubated for 2 hours at 37°C with beads coated with mAbs to NK-cell receptors. TNF-α secretion was quantified by ELISA. The mAb to CD16 was sufficient to induce TNF-α secretion by resting NK cells (Figure 6A). By comparison, mAbs to other receptors did not induce TNF-α secretion (Figure 6A). Therefore, the difference between CD16 and the other receptors was even more striking than it had been in Ca2+ flux measurements. All pair-wise combinations of mAbs were tested for induction of TNF-α secretion (Figure 6A). In these experiments, mAbs to 2 receptors were added together on the same beads. NKp46 synergized with NKG2D, 2B4, and CD2, and to a lesser extent with DNAM-1 (Figure 6A). In addition, clear synergy was observed between NKG2D and 2B4, 2B4 with DNAM-1, and 2B4 with CD2 (Figure 6A). Weaker stimulations were obtained with combinations of NKG2D, CD2, and DNAM-1 (Figure 6A). Therefore, synergies similar to those observed for the induction of Ca2+ flux occurred for the induction of TNF-α secretion. Secretion of IFN-γ was also measured after a 6-hour stimulation (Figure 6B). In repeated experiments, secretion of IFN-γ responded to the same combinations of receptor signals as TNF-α.
Figure 6.
TNF-α secretion, IFN-γ secretion, and cytotoxicity induced by pair-wise combinations of NK-cell receptors. TNF-α (A) and IFN-γ (B) secretion by resting NK cells after 2 or 6 hours of stimulation, respectively, with beads coated with specific combinations of mAbs, as indicated. (C) Redirected lysis of P815 cells by resting NK cells at an E/T cell ratio of 10, for 3 hours at 37°C. P815 target cells were preincubated with combinations of mAbs to specific NK-cell receptors, as indicated.
Cytotoxicity induced by pair-wise combinations of receptors
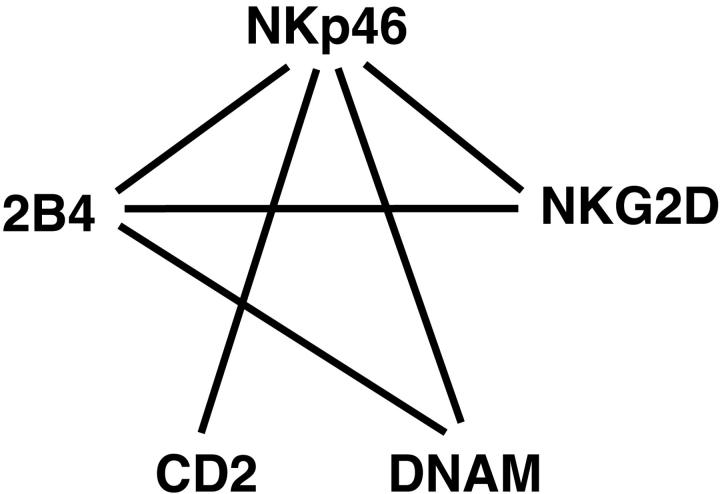
To test whether combinations of receptors that synergize to activate Ca2+ flux and cytokine secretion could also signal for cytotoxicity by resting NK cells, the redirected lysis assay shown in Figure 1 was used. All pair-wise combinations of receptors were tested for lysis at an E/T cell ratio of 10 (Figure 6C). The strongest synergies were observed with 2B4 in combination with NKp46, NKG2D, and DNAM-1. In addition, as seen with induction of Ca2+ flux and cytokine secretion, receptor NKp46 synergized with all other receptors. Overall, the pattern of synergies (Figure 7) was similar in all assays used. We conclude that resting NK cells are quite capable of killing target cells, given the right combination of signals. Furthermore, of all the receptors tested, each one is capable of contributing a signal in resting NK cells.
Figure 7.
Schematic representation of synergies among receptors in resting NK cells. Solid lines represent strong synergies between pairs of receptors.
Discussion
The data presented here show that responses by resting NK cells, in the absence of exogenous cytokines, can be induced by combinations of activation receptors. Responses measured were Ca2+ flux triggered by cross-linked mAbs in solution, TNF-α and IFN-γ secretion induced by mAbs attached to beads, and cytotoxicity against an FcR+ target cell in the presence of mAbs directed to NK-cell activation receptors. Except for the FcR CD16, which mediates antibody-dependent cellular cytotoxicity (ADCC) and not natural cytotoxicity, no single receptor tested—NKp46, NKG2D, 2B4, DNAM-1, or CD2—was sufficient to activate considerable cytotoxicity or cytokine secretion. However, each one of these receptors contributes a signal in resting NK cells, as shown by synergistic activation by specific pair-wise combinations of receptors. Resting NK cells are not inherently less responsive than IL-2-activated NK cells, but the regulation of their activation is more stringent.
A hierarchy among receptors for the activation of resting NK cells was revealed. CD16 was unique in its ability to induce cytokine secretion by resting NK cells without additional signals. With regard to cytotoxicity, it is possible that recognition of mouse ligands on P815 cells by human NK cells contributes to activation. P815 cells express mouse ICAM-1, which is a ligand for human LFA-1.53 Blocking antibodies to LFA-1 reduced the CD16-mediated cytotoxicity toward target cells.35 In Ca2+ flux experiments, activation by CD16 was enhanced by all other receptors, except NKp46, suggesting that ITAM-based signals do not enhance one another. The results imply a difference in signaling via the ITAM chains associated with CD16 and those associated with NKp46. The mAb to NKp46, in the absence of further cross-linking, was not sufficient to mobilize Ca2+ in resting NK cells. Further, cross-linking of NKp46 resulted in lower Ca2+ flux as compared to CD16. CD16 associates with ITAM-containing chain (FcεRI γ and TCR ζ) complexes through a negatively charged aspartate residue in the transmembrane, whereas NKp46 has a positively charged arginine residue in the transmembrane. Cross-linking of NKp46 on resting NK cells was insufficient for full activation, but if provided with additional signals, NKp46 can potentiate activation. NKp46 ranks high in the hierarchy, but below CD16, because it synergizes with all 4 other receptors. The observed synergies among receptors involved in natural cytotoxicity are depicted in Figure 7.
2B4 synergizes with the 3 receptors, NKp46, NKG2D, and DNAM-1. NKG2D and DNAM-1 each synergize with the 2 receptors, NKp46 and 2B4. Finally, CD2 is unique in its exclusive synergy with ITAM-associated receptors. Although the signaling pathways for most of these receptors are still poorly understood, evidence indicates that each receptor signals by different pathways.54,55 The NKG2D/DAP10 complex activates phosphatidylinositol-3 kinase, phospholipase C-γ and Vav.22 2B4 recruits SAP and Fyn, through phosphorylated tyrosine motifs.26,27 DNAM-1 is associated with LFA-1 in NK cells and is phosphorylated by PKC.30,31 CD2 binds the adaptor protein CD2AP, as shown in T cells, and provides a link to Wiskott-Aldrich syndrome protein-mediated actin cytoskeleton remodeling.56,57 The unique pattern of receptor combinations that provide synergy is consistent with the use of different signaling modules by each receptor to induce activation. It will be necessary to identify signaling components for each of the receptors to determine the basis for the synergies.
Our data have revealed a complex hierarchy, synergy, and redundancy among activation receptors on NK cells. A hierarchy of CD16 > NKp46 > 2B4 > NKG2D, DNAM-1 > CD2 can be established on the basis of requirements for NK-cell activation. It should be noted that CD16 is not a natural cytotoxicity receptor. CD16 mediates ADCC, which leads to the elimination of antibody-coated target cells. Because different combinations of different signals can each result in activation of NK-cell function, including natural cytotoxicity, redundancy is apparent. For example, the combination of NKp46 and CD2 is sufficient to activate, but the combination of 2 other signals, such as DNAM-1 and 2B4, can also activate. It is likely that this kind of redundancy forms the basis for the natural cytotoxicity observed with NK cells from mice with a double Syk/ZAP70 deficiency, even on target cells that lacked ligands for NKG2D.12 Several combinations of signals, excluding ITAM- and DAP10-based signals, are still available that can result in activation of cytotoxicity.
Activation of resting NK cells by synergistic signals raises the issue of terminology used to describe NK-cell receptors. NK cells do not have a dominant activation receptor, in the sense used to describe the TCR, except for the ADCC induced by CD16. NK cells use combinations of synergistic receptors to mediate natural cytotoxicity. The term “costimulation” could be confusing because it means something different in the context of T cells. “Coactivation” receptors may be a useful term to describe the NK-cell activation receptors studied here.
The importance of a tight regulation of natural cytotoxicity by inhibitory receptors on NK cells is obvious, considering that ligands for several of the coactivation receptors are widely expressed. LFA-3 (CD58), the ligand for CD2, is broadly expressed, whereas expression of CD48, a ligand for 2B4, is confined to hematopoietic cells and a subset of endothelial cells.58 Ligands for DNAM-1, CD155 and CD112, are transcribed in a wide variety of tissues.59,60 Ligands of NKp46 and of other NCRs have not been identified yet, but may include a carbohydrate component.61-63 Cells that have up-regulated expression of ligands for NKG2D become sensitive to lysis by NK cells. Our data suggest that NKG2D does not signal alone but provides a coactivation signal to pre-existing signals from other receptors, such as 2B4 and NCRs. Thus, the induced expression of ligands for NK-cell receptors may suffice to override the balance between activation and inhibition. The distribution of ligands in vivo must determine degrees of sensitivity to NK cells. Some of the most widely expressed ligands are those for receptors DNAM-1, CD2, and NKG2D. Combinations of these receptors did not produce robust NK-cell activation. We propose that a more restricted expression of ligands for 2B4, NKp46, and other NK-cell receptors with similar coactivation potential could confine NK-cell alloreactivity to hematopoietic cells.
Acknowledgments
We thank A. Das, S. Rajagopalan, P. Srinivasan, and S. Vielkind for help in NK-cell isolation.
Prepublished online as Blood First Edition Paper, September 8, 2005; DOI 10.1182/blood-2005-04-1351.
Supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), NIH-Karolinska Institutet Graduate Partnership Program (Y.T.B.), and Swedish Foundation for Strategic Research, Research Council, and Cancer Society (H.G.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23: 225-274. [DOI] [PubMed] [Google Scholar]
- 2.Chiesa S, Tomasello E, Vivier E, Vely F. Coordination of activating and inhibitory signals in natural killer cells. Mol Immunol. 2005;42: 477-484. [DOI] [PubMed] [Google Scholar]
- 3.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23: 255-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljunggren HG, Karre K. In search of the `missing self': MHC molecules and NK cell recognition. Immunol Today. 1990;11: 237-244. [DOI] [PubMed] [Google Scholar]
- 5.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A. 2001;98: 11521-11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1: 41-49. [DOI] [PubMed] [Google Scholar]
- 7.Bottino C, Moretta L, Pende D, Vitale M, Moretta A. Learning how to discriminate between friends and enemies, a lesson from natural killer cells. Mol Immunol. 2004;41: 569-575. [DOI] [PubMed] [Google Scholar]
- 8.Moretta A, Bottino C, Vitale M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19: 197-223. [DOI] [PubMed] [Google Scholar]
- 9.Pessino A, Sivori S, Bottino C, et al. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med. 1998;188: 953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pende D, Parolini S, Pessino A, et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190: 1505-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pende D, Cantoni C, Rivera P, et al. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol. 2001;31: 1076-1086. [DOI] [PubMed] [Google Scholar]
- 12.Colucci F, Schweighoffer E, Tomasello E, et al. Natural cytotoxicity uncoupled from the Syk and ZAP-70 intracellular kinases. Nat Immunol. 2002; 3: 288-294. [DOI] [PubMed] [Google Scholar]
- 13.Zompi S, Hamerman JA, Ogasawara K, et al. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases. Nat Immunol. 2003;4: 565-572. [DOI] [PubMed] [Google Scholar]
- 14.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3: 1150-1155. [DOI] [PubMed] [Google Scholar]
- 15.Diefenbach A, Tomasello E, Lucas M, et al. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3: 1142-1149. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Song Y, Bakker AB, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285: 730-732. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Cherwinski H, Spies T, Phillips JH, Lanier LL. DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells. J Exp Med. 2000;192: 1059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen DB, Araki M, Hamerman JA, Chen T, Yamamura T, Lanier LL. A structural basis for the association of DAP12 with mouse, but not human, NKG2D. J Immunol. 2004;173: 2470-2478. [DOI] [PubMed] [Google Scholar]
- 19.Lodoen MB, Lanier LL. Viral modulation of NK cell immunity. Nat Rev Microbiol. 2005;3: 59-69. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland CL, Chalupny NJ, Schooley K, VandenBos T, Kubin M, Cosman D. UL16-binding proteins, novel MHC class I-related proteins, bind to NKG2D and activate multiple signaling pathways in primary NK cells. J Immunol. 2002;168: 671-679. [DOI] [PubMed] [Google Scholar]
- 21.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17: 19-29. [DOI] [PubMed] [Google Scholar]
- 22.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003;4: 557-564. [DOI] [PubMed] [Google Scholar]
- 23.Pende D, Rivera P, Marcenaro S, et al. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62: 6178-6186. [PubMed] [Google Scholar]
- 24.Cosman D, Mullberg J, Sutherland CL, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14: 123-133. [DOI] [PubMed] [Google Scholar]
- 25.Dunn C, Chalupny NJ, Sutherland CL, et al. Human cytomegalovirus glycoprotein UL16 causes intracellular sequestration of NKG2D ligands, protecting against natural killer cell cytotoxicity. J Exp Med. 2003;197: 1427-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen R, Relouzat F, Roncagalli R, et al. Molecular dissection of 2B4 signaling: implications for signal transduction by SLAM-related receptors. Mol Cell Biol. 2004;24: 5144-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eissmann P, Beauchamp L, Wooters J, Tilton JC, Long EO, Watzl C. Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244). Blood. 2005;105: 4722-4729. [DOI] [PubMed] [Google Scholar]
- 28.Brown MH, Boles K, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J Exp Med. 1998; 188: 2083-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvaraj P, Plunkett ML, Dustin M, Sanders ME, Shaw S, Springer TA. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. Nature. 1987;326: 400-403. [DOI] [PubMed] [Google Scholar]
- 30.Shibuya K, Lanier LL, Phillips JH, et al. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity. 1999;11: 615-623. [DOI] [PubMed] [Google Scholar]
- 31.Shibuya A, Lanier LL, Phillips JH. Protein kinase C is involved in the regulation of both signaling and adhesion mediated by DNAX accessory molecule-1 receptor. J Immunol. 1998;161: 1671-1676. [PubMed] [Google Scholar]
- 32.Bottino C, Castriconi R, Pende D, et al. Identification of PVR (CD155) and nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198: 557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibuya A, Campbell D, Hannum C, et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4: 573-581. [DOI] [PubMed] [Google Scholar]
- 34.Pende D, Spaggiari GM, Marcenaro S, et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the poliovirus receptor (CD155) and nectin-2 (CD112). Blood. 2005;105: 2066-2073. [DOI] [PubMed] [Google Scholar]
- 35.Lanier LL, Ruitenberg JJ, Phillips JH. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988;141: 3478-3485. [PubMed] [Google Scholar]
- 36.Bonnema JD, Karnitz LM, Schoon RA, Abraham RT, Leibson PJ. Fc receptor stimulation of phosphatidylinositol 3-kinase in natural killer cells is associated with protein kinase C-independent granule release and cell-mediated cytotoxicity. J Exp Med. 1994;180: 1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perussia B, Trinchieri G. Antibody 3G8, specific for the human neutrophil Fc receptor, reacts with natural killer cells. J Immunol. 1984;132: 1410-1415. [PubMed] [Google Scholar]
- 38.Sivori S, Pende D, Bottino C, et al. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol. 1999;29: 1656-1666. [DOI] [PubMed] [Google Scholar]
- 39.Vitale M, Bottino C, Sivori S, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187: 2065-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitale M, Falco M, Castriconi R, et al. Identification of NKp80, a novel triggering molecule expressed by human NK cells. Eur J Immunol. 2001;31: 233-242. [DOI] [PubMed] [Google Scholar]
- 41.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285: 727-729. [DOI] [PubMed] [Google Scholar]
- 42.Valiante NM, Trinchieri G. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J Exp Med. 1993;178: 1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanier LL, Corliss B, Phillips JH. Arousal and inhibition of human NK cells. Immunol Rev. 1997; 155: 145-154. [DOI] [PubMed] [Google Scholar]
- 44.Moretta A, Sivori S, Vitale M, et al. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J Exp Med. 1995;182: 875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantoni C, Biassoni R, Pende D, et al. The activating form of CD94 receptor complex: CD94 covalently associates with the Kp39 protein that represents the product of the NKG2-C gene. Eur J Immunol. 1998;28: 327-338. [DOI] [PubMed] [Google Scholar]
- 46.Rajagopalan S, Fu J, Long EO. Cutting edge: induction of IFN-gamma production but not cytotoxicity by the killer cell Ig-like receptor KIR2DL4 (CD158d) in resting NK cells. J Immunol. 2001; 167: 1877-1881. [DOI] [PubMed] [Google Scholar]
- 47.Kikuchi-Maki A, Catina TL, Campbell KS. Cutting edge: KIR2DL4 transduces signals into human NK cells through association with the Fc receptor {gamma} protein. J Immunol. 2005;174: 3859-3863. [DOI] [PubMed] [Google Scholar]
- 48.Wagtmann N, Rajagopalan S, Winter CC, Peruzzi M, Long EO. Killer cell inhibitory receptors specific for HLA-C and HLA-B identified by direct binding and by functional transfer. Immunity. 1995;3: 801-809. [DOI] [PubMed] [Google Scholar]
- 49.Andre P, Castriconi R, Espeli M, et al. Comparative analysis of human NK cell activation induced by NKG2D and natural cytotoxicity receptors. Eur J Immunol. 2004;34: 961-971. [DOI] [PubMed] [Google Scholar]
- 50.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19: 497-521. [DOI] [PubMed] [Google Scholar]
- 51.Tassi I, Presti R, Kim S, Yokoyama WM, Gilfillan S, Colonna M. Phospholipase C-{gamma}2 is a critical signaling mediator for murine NK cell activating receptors. J Immunol. 2005;175: 749-754. [DOI] [PubMed] [Google Scholar]
- 52.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281: 65-78. [DOI] [PubMed] [Google Scholar]
- 53.Johnston SC, Dustin ML, Hibbs ML, Springer TA. On the species specificity of the interaction of LFA-1 with intercellular adhesion molecules. J Immunol. 1990;145: 1181-1187. [PubMed] [Google Scholar]
- 54.Leibson PJ. Signal transduction during natural killer cell activation: inside the mind of a killer. Immunity. 1997;6: 655-661. [DOI] [PubMed] [Google Scholar]
- 55.Perussia B. Signaling for cytotoxicity. Nat Immunol. 2000;1: 372-374. [DOI] [PubMed] [Google Scholar]
- 56.Badour K, Zhang J, Shi F, et al. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity. 2003;18: 141-154. [DOI] [PubMed] [Google Scholar]
- 57.Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci U S A. 2003; 100: 14151-14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis SJ, van der Merwe PA. The structure and ligand interactions of CD2: implications for T-cell function. Immunol Today. 1996;17: 177-187. [DOI] [PubMed] [Google Scholar]
- 59.Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56: 855-865. [DOI] [PubMed] [Google Scholar]
- 60.Eberle F, Dubreuil P, Mattei MG, Devilard E, Lopez M. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene. 1995; 159: 267-272. [DOI] [PubMed] [Google Scholar]
- 61.Arnon TI, Achdout H, Lieberman N, et al. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004; 103: 664-672. [DOI] [PubMed] [Google Scholar]
- 62.Mandelboim O, Lieberman N, Lev M, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409: 1055-1060. [DOI] [PubMed] [Google Scholar]
- 63.Cantoni C, Ponassi M, Biassoni R, et al. The three-dimensional structure of the human NK cell receptor NKp44, a triggering partner in natural cytotoxicity. Structure (Camb). 2003;11: 725-734. [DOI] [PubMed] [Google Scholar]