Abstract
Signaling lymphocyte activation molecule (SLAM, CD150, or SLAMF1) is a self-ligand receptor on the surface of activated T- and B-lymphocytes, macrophages, and dendritic cells (DCs). Here we examine the effect of SLAM/SLAM interactions on CD40L-induced CD40 signaling pathways in human DCs. CD40L-expressing L929 cells induced DCs to produce interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and IL-12, which was strongly inhibited by coexpression of SLAM on the surface of the L929 cells. Similarly, transfection of DCs with SLAM strongly reduced CD40L-induced IL-12 production. Furthermore, the negative effect of SLAM/SLAM interactions on CD40L-induced DC activation was also detected in the presence of lipopolysaccharide (LPS). LPS-induced IL-12 secretion, however, was not inhibited by SLAM engagement. CD40L-activated DCs affected by exposure to SLAM/SLAM engagement were impaired in their ability to induce differentiation of naive T lymphocytes into interferon-γ (IFN-γ)–producing T-helper 1 (Th1) effector cells. These inhibitory effects were not the result of a general unresponsiveness of DCs to CD40L, as SLAM/SLAM interactions did not prevent CD40L-induced up-regulation of CD83, CD86, or human leukocyte antigen (HLA)–DQ on the surface of DCs. Taken together, the results indicate that SLAM/SLAM interactions inhibit CD40-induced signal transduction in monocyte-derived dendritic cells, an effect that was not detectable in earlier studies using anti-SLAM monoclonal antibodies.
Introduction
Dendritic cells (DCs) regulate both adaptive and innate immune responses.1 Much of their effects are implemented at the interface of DCs and T lymphocytes, following the integration of multiple activating or inhibitory signals from pattern recognition receptors, cytokine receptors, and coreceptor molecules involved in DC–T-cell communication. Depending on the pathogenic structures, tissue factors, and coreceptor-induced activation signals, DCs can orchestrate the effector response to be appropriate for intracellular or extracellular pathogens, or to suppress self-reactive clones.2-4 Activation of immature DCs (IDCs) by proinflammatory cytokines, Toll-like receptors (TLRs), or interaction with CD40L expressed on activated T cells induces the maturation of DCs.5 Activated DCs express costimulatory receptors essential for efficient T-cell priming.5,6 Activation of IDCs by CD40L or lipopolysaccharide (LPS) induces interleukin-12 (IL-12) secretion, which elevates local tissue inflammation and plays a central role in T-helper 1 (Th1) differentiation.7-9 However, various soluble factors and coreceptor molecules may modulate the ability of DCs to polarize T-cell response.10,11
Signaling lymphocyte activation molecule (SLAM, CD150)12,13 is a self-ligand receptor present on the surface of activated T and B lymhocytes, macrophages, and dendritic cells.14-16 The importance of SLAM signaling in innate and adaptive responses is underscored by the severe immune defect observed in X-linked lymphoproliferative disease in humans17-19 or in several murine models. In SLAM-associated adaptor protein (SAP)–deficient mice the lack of the SLAM/SAP pathway results in defective Th2 polarization,20,21 primary B-cell responses,22,23 and the absence of long-lived plasma cells.24 SLAM-deficient mice have a mild defect in Th2 differentiation and show a profound deficiency in LPS-induced macrophage functions, suggesting that SLAM influences TLR4-mediated signaling in murine macrophages.15 Moreover, SLAM was found to be one of the 2 receptors of measles virus on the surfaces of lymphocytes, macrophages, and dendritic cells.25,26 Modulation of SLAM signaling in DCs might in part mediate the strong immunosuppressive effect characteristic of measles.27-30
The stimuli that induce maturation of immature DCs (eg, TLR agonists, various proinflammatory cytokines, or ligation of CD40) induce cell-surface expression of SLAM.31-33 Triggering of SLAM with a specific antibody augmented IL-12 and IL-8 production, but not IL-10 secretion in CD40L-activated human monocyte-derived DCs, suggesting a proinflammatory effect of SLAM engagement.33 Despite this pioneering study, the function of SLAM in DCs is still elusive. Moreover, several observations indicate that ligation of SLAM with specific antibodies may not mimic, but rather interfere with homotypic SLAM/SLAM interactions. SLAM-specific antibodies induce interferon-γ (IFN-γ) production in Th1 clones, and also in polarized Th2-cell populations isolated from patients with rheumatoid arthritis. These observations suggested that anti-SLAM promotes differentiation of Th1 cells.12,34 In contrast, SLAM homoassociation in the B141 T-cell line expressing SLAM and SAP inhibited IFN-γ production.35 In SLAM-deficient murine T cells IFN-γ production was elevated rather than decreased, indicating that the SLAM/SAP signaling pathway inhibits IFN-γ production. In line with this, Slamnull mice show skewed polarization of T lymphocytes toward Th1.15
To avoid the use of monoclonal antibodies (mAbs), the contribution of SLAM signals to DC effector functions was studied by coculturing activated DCs with cells constitutively expressing human SLAM. To examine the effect of SLAM signals on CD40L-induced DC function, DCs were cocultured with L929 cells (L cells) expressing human CD40L together with SLAM or with activated Jurkat T cells expressing SLAM constitutively. CD40L-induced IL-12, tumor necrosis factor α (TNF-α), and IL-6 production of DCs was inhibited by SLAM engagement. Consequently, DCs that had matured in the presence of SLAM/SLAM interactions were less potent in inducing differentiation of naive T lymphocytes into IFN-γ–producing Th1 effector cells. The effect of SLAM engagement on pathogen-induced activation via TLR signals was measured in the presence of LPS in the cultures. LPS-mediated IL-12 expression was not inhibited in the presence of SLAM/SLAM homoassociation. These findings suggest that SLAM engagement in DCs may induce a negative feedback loop on CD40L-induced inflammatory signals, while such a mechanism does not operate to down-regulate inflammatory responses induced by bacteria. Thus, signaling induced via SLAM/SLAM interactions regulates DC functions in a complex manner that is distinct from the previously described effect of anti-SLAM antibodies.32,33
Materials and methods
Dendritic-cell generation and activation
Monocytes were isolated from buffy coats by Ficoll gradient centrifugation (Amersham Biosciences, Uppsala, Sweden) and immunomagnetic cell separation using anti-CD14–conjugated microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). After magnetic separation, 96% to 99% of the cells were CD14+ monocytes as measured by flow cytometry (data not shown). Monocytes were cultured in 6-well tissue-culture plates at a density of 1.5 × 106 cells/mL in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; Invitrogen, Paisley, Scotland), 75 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Leucomax), and 100 ng/mL IL-4 (Peprotech, London, United Kingdom). Cells were cultured for 5 or 6 days, and new IL-4 and GM-CSF was added at day 2. For DC activation, immature DCs were activated with a mixture of cytokines: 10 ng/mL TNF-α, 5 ng/mL IL-1β, 20 ng/mL IL-6 (Peprotech), and 1 μg/mL PGE2 (Sigma, St Louis, MO);36 100 ng/mL LPS (Sigma) and 10 ng/mL IFN-γ (Peprotech); 10 μg/mL poly(I:C) (Sigma); or via CD40, with soluble CD40L (Peprotech), or with the addition of CD40L-expressing L cells (CD40L-L) using a 4:1 DC/CD40L-L cell ratio. In some experiments DCs were activated in the presence of Jurkat (E6-1) or SLAM-expressing Jurkat cells using 2:1 DC/Jurkat ratio. Jurkat cells were activated by anti-CD3 (5 μg/mL) in these cultures. Activation signals were present for 24 hours unless indicated otherwise. CD40L expression on Jurkat and Jurkat/SLAM cells upon activation with anti-CD3 antibody was verified by flow cytometry (data not shown).
Generation of cell lines expressing SLAM
L929 mouse fibroblast cells (3 × 106) expressing the human CD40L or the parental cells (gifts of G. Trinchieri, Schering-Plough, Dardilly, France) were transfected with a pCI-SLAM construct expressing full-length human SLAM using Fugene6 (Roche Diagnostics, Indianapolis, IN). After 2 passages, on day 10, transfectants were incubated with PE-labeled SLAM-specific antibody (A12) and SLAM-expressing cells (3%-5%) were enriched by cell sorting. Individual SLAM-expressing clones were isolated from the polyclonal population by the standard limiting dilution method and analyzed further. CD40L expression on the L cells was not influenced by the presence of SLAM (data not shown).
FACS analysis and cell sorting
For the characterization of the DC phenotype, cells were stained with PE-conjugated anti-SLAM (BD Pharmingen, San Diego, CA) and anti-CD86 (Immunotech, Marseille, France) mAbs as well as FITC-labeled anti-CD83 (Immunotech) and anti–HLA-DQ (BD Pharmingen) mAbs. When analyzing DCs cocultured with L cells, DCs were distinguished using PE-labeled CD40 (BD Pharmingen) or FITC-labeled HLA-DQ–specific mAbs. Fluorescence intensities were measured with FACStar flow cytometer (Becton Dickinson, San Jose, CA) and data were analyzed using WinMDI software (Joseph Trotter, La Jolla, CA). A FACSvantage DIVA cell sorter (Becton Dickinsson) was used to separate DCs from L cells using anti-SLAM–PE or anti-CD11c–PE mAbs (BD Pharmingen).
Transient transfection of monocyte-derived DCs
Immature DCs (5 × 106) were harvested, washed once with media, and electroporated with 4.5 μg human pCI-SLAM construct (or with pCI as a control) using AMAXA's Nucleofection technology (AMAXA, Cologne, Germany). The pCI-SLAM construct contained the coding sequence of human SLAM cloned into the EcoRI and XbaI site of pCI. Following transfection, cells were cultured in AIM-V media (Invitrogen) supplemented with GM-CSF (75 ng/mL). Five hours after transfection cells were stained with PE-labeled anti-SLAM monoclonal antibodies (BD Pharmingen) and DCs expressing SLAM were sorted. SLAM+ and SLAM– cells were collected and used in parallel for functional assays. Transfection efficiencies were between 5% and 35%. The purity of sorted SLAM– and SLAM+ DCs were more than 99.5% in each experiment (not shown).
T-cell activation by autologous or allogeneic DCs
Allogeneic or autologous naive T cells were enriched using the naive CD4+ T-cell isolation kit (Miltenyi Biotech). T-cell preparations contained 96% to 98% CD3+ cells, of which 90% to 95% were CD45RAhigh as measured with flow cytometry (data not shown). Autologous peripheral-blood mononuclear cells (PBMCs) were frozen during DC differentation. DCs pretreated with CD40L-L cells or L cells for 12 hours were purified by cell sorting. Next, the cells were cultured with the naive T cells on 96-well cell-culture plates for 4 days at cell densities of 2 × 104 DCs/well and 1.5 × 105 T cells/well. T cells were then reactivated for 24 hours on plates coated with 5 μg/mL anti-CD3 mAb (BD Pharmingen). The concentration of various cytokines from the supernatants was determined by enzyme-linked immunosorbent assay (ELISA). Human recombinant IL-12p70 was obtained from Peprotech.
Cytokine production
Production of various cytokines in the supernatant of DCs activated with CD40L-L cells or SLAM/CD40L-L cells was analyzed using a protein array (Raybiotech, Norcross, GA) Briefly, 2 × 106 immature monocyte-derived DCs (MoDCs) were plated on monolayers of L cells expressing CD40L or CD40L and SLAM in AIM-V media (Invitrogen) and incubated for 24 hours on 6-well tissue-culture plates. Supernatants were then analyzed with the RayBiotech cytokine array III as described by the manufacturer. Enhanced chemiluminescence (ECL) signals were developed on Biomax films (Sigma). ECL signals were also detected by a Kodak 2000MM Image Station and quantitated using Kodak 1D Image Analysis software (Kodak, New Haven, CT). Intensities of the spots corresponding to each cytokine were normalized to the mean in intensity of the 6 internal control spots. The effect of SLAM was calculated as the ratio of normalized spot intensities obtained with culture supernatants of SLAM/CD40L-L cell versus CD40L-L cell–activated DCs. Concentrations of individual cytokines in the supernatants of activated DCs or T cells were determined by ELISA. ELISA kits specific for IL-12p70, TNF-α, IL-6, IL-13, and IFN-γ were purchased from BD Pharmingen. Assays were performed according to the manufacturer's instructions. Culture supernatants were stored at –70°C.
Real-time RT-PCR
Real-time polymerase chain reaction (PCR) was done as described previously.37 Briefly, total RNA was isolated with TRIZOL reagent (Invitrogen) and RNA was treated with RNase-free DNase (Ambion, Austin, TX). RNA was further purified on RNeasy columns (Qiagen, Germantown, MD). Reverse transcription was performed from 200 ng total RNA using Superscript II reverse transcriptase (RT) and random hexamer primers (both from Invitrogen) using a standard RT reaction. Amplication reactions were performed in an ABI PRISM 7900 sequence detector (Applied Biosystems, Warrington, United Kingdom) using 40 cycles of 95°C for 12 seconds and 60°C for 30 seconds. All PCR reactions were done in triplicates with 1 control reaction containing no RT enzyme. The comparative Ct method was used to quantify IL-12p40 relative to the endogenous control gene 36B4. 36B4 Ct values did not vary between cell types or treatments.
The following PCR primers and probes were used: forward primer for 36B4 (NM_001 002), AGATGCAGCAGATCCGCAT, reverse primer ATATGAGGCAGCAGTTTCTCCAG; and forward primer for IL-12p40 (NM_002 187), CCCTGACCATCCAAGTCAAAG, reverse primer CAGGAGCGAATGGCTTAGAAC. Probes were FAM-AGGCTGTGGTGCTGATGGGCAAGAA-TAMRA and FAM-CCTCCTTTGTGACAGGTGTACTGGCCA-TAMRA for 36B4 and IL-12p40, respectively.
Results
CD40L-induced maturation of DCs is not affected by SLAM-SLAM interactions
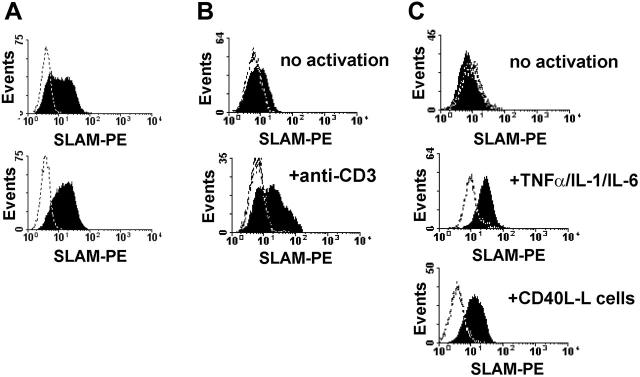
Activation of DCs via CD40/CD40L interaction, delivered by activated T cells, is one of the major pathways that induces DC maturation as well as the production of proinflammatory cytokines.38 SLAM expression is induced upon DC maturation, suggesting a regulatory role for SLAM in mature DCs.32,33 Bleharsky et al reported that SLAM ligation by a specific antibody augments proinflammatory responses.33 As several reports have raised doubts about specific antibodies mimicking the true biologic effect of SLAM family receptors, we decided to set up an in vitro system based on L cells expressing SLAM. To analyze how ligation of SLAM molecules on activated DCs influences DC functions, we transfected CD40L-L with the full-length human SLAM cDNA. Following transfection, SLAM-expressing L cells were enriched by cell sorting (Figure 1A, top panel) and several SLAM-expressing cell lines were established using limiting dilution cloning (Figure 1A, bottom panel, and data not shown). These cell lines, as well as polyclonal L cells, expressed SLAM at comparable surface densities with activated T cells (Figure 1B, bottom panel) or with DCs activated by inflammatory cytokines or via CD40 (Figure 1C). The SLAM-transfected L cells thus provided a useful tool to analyze SLAM/SLAM interaction–induced signal transduction in DCs.
Figure 1.
SLAM-expressing CD40L-L cells induce phenotypic maturation of DCs. (A) Cell-surface expression of SLAM on CD40L-L cells stably transfected with SLAM was measured by flow cytometry. SLAM expression on the surface of a polyclonal population of FACS-sorted stable transfectants (top histogram) and a representative SLAM/CD40L-L cell clone obtained by limiting dilution is shown (bottom histogram). (B) Expression of SLAM on untreated human peripheral-blood T lymphocytes (top histogram) or following activation with 5 μg/mL anti-CD3 mAb (OKT3) for 24 hours (bottom histogram). (C) Expression of SLAM molecules on the surface of monocyte-derived DCs without activation (top histogram), following a 24-hour activation with the indicated inflammatory cytokines (middle histogram), or with CD40L-L cells for 24 hours (bottom histogram). Dashed lines represent staining with isotype control antibodies. Typical results of 4 donors analyzed in independent experiments are shown.
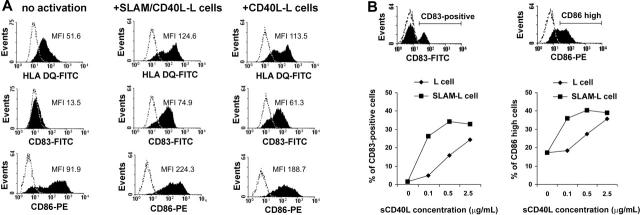
We then examined whether the presence of SLAM on CD40L-L cells would interfere with the activation-induced changes in cell-surface receptor expression. To that end, IDCs were coincubated with either CD40L-expressing L cells (CD40L-L) or with L cells coexpressing CD40L and SLAM (SLAM/CD40L-L cells). Compared with the nonactivated DCs, both CD40L-expressing L cells and SLAM/CD40L double-transfectant L cells readily induced up-regulation of HLA-DQ, CD83, and CD86 (Figure 2A). This result is indicative that phenotypic maturation of DCs upon activation via CD40 is not affected by cell-surface expression of SLAM. The density of CD40L molecules, however, may have an impact on DC maturation. In a report by Mackey et al, supraphysiologic levels of signals via CD40 were shown to induce phenotypic maturation of DCs even when CD40 signaling was partially disrupted.39 Thus, we set up a system in which the level of CD40 signaling could be modulated. Immature DCs were cultured in the presence of graded concentrations of soluble CD40L molecules (sCD40L) and either L cells or L cells expressing SLAM. We found that SLAM/SLAM interactions did not interfere with sCD40L-induced DC maturation. Interestingly, at limiting concentrations of sCD40L, SLAM engagement actually augmented the induction of CD83 and CD86 molecules (Figure 2B). The expression of HLA-DR was minimally influenced by sCD40L concentration (data not shown).
Figure 2.
The effect of SLAM molecules on the induction of cell-surface receptors concomitant with CD40L-induced maturation of DCs. (A) Immature DCs were cultured in the presence of CD40L-L cells or SLAM/CD40L-L cells for 24 hours. Cell-surface expression of HLA-DQ, CD83, and CD86 was determined by flow cytometry. Histograms obtained when using specific antibodies (filled areas) or isotype-identical controls (open areas) are shown. MFI indicates mean fluorescence intensity; FITC, fluorescein isothiocyanate. (B) IDCs were cultured with or without sCD40L for 24 hours in the presence of SLAM-L cells or the SLAM– parental cell line. CD40L was added at concentrations indicated. Cell-surface expression of CD83 and CD86 molecules on DCs was analyzed by FACS. The ratio of CD83+ and CD86high DCs was calculated using the indicated gates (histograms). The ratio of CD83+ and CD86high DCs at different sCD40L concentrations in the presence of SLAM+ or SLAM– L cells is shown. Representative results of 3 independent experiments are shown.
SLAM/SLAM interactions inhibit production of IL-12, IL-6, and TNF-α by CD40L-activated DCs
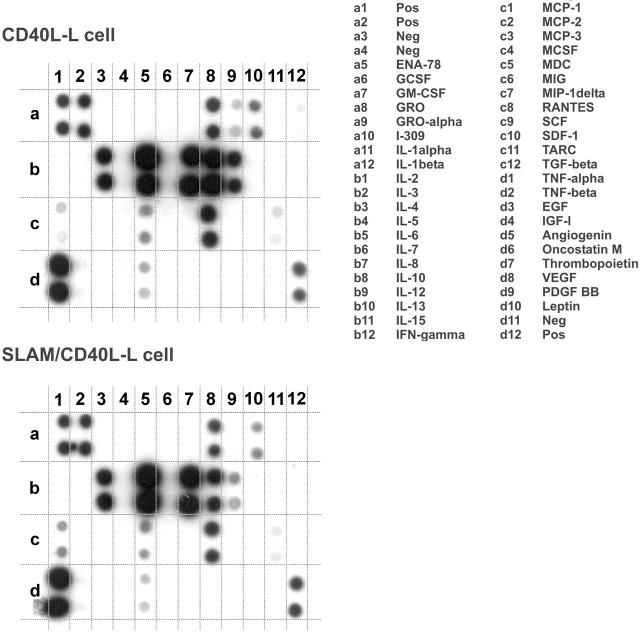
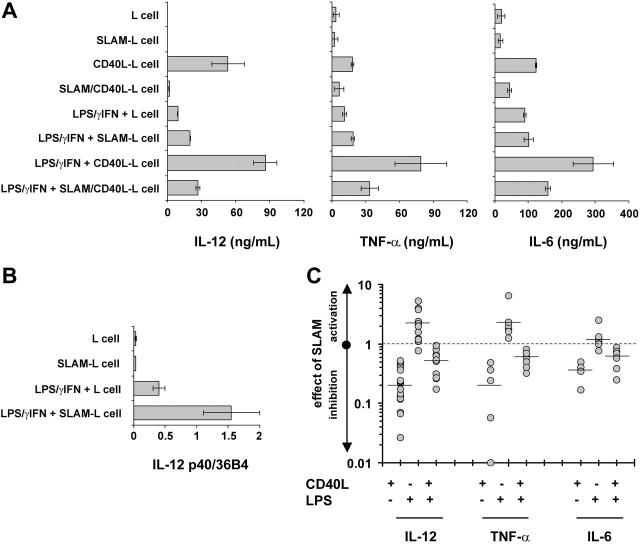
Because DCs orchestrate immune responses in part through soluble mediators acting on other immune cells, the effect of SLAM/SLAM interactions on the secretion of cytokines and chemokines by DCs was determined. First, we analyzed the secretion of 42 cytokines and chemokines by DCs that had been cultured for 24 hours in the presence of SLAM/CD40L-L or CD40L-L cells using a protein array (Figure 3). Interestingly, we found lower levels of IL-12 and TNF-α in the supernatants of DCs activated by SLAM/CD40L-L cells than in the supernatants of DCs that had been cocultured with CD40L-L cells. In 2 of the 8 donors tested, inhibition of secretion of other cytokines (eg, IL-2 and IL-15 [data not shown]) and to a lesser extent IL-10 (Figure 3) was also observed when activation by CD40L was concomitant with SLAM signaling. The relative amounts of cytokines detected by a set of 4 pairs of arrays were determined based on the relative spot densities obtained with DCs activated by SLAM/CD40L-L cells and CD40L-L cells (Table 1). These findings and previously published results33 prompted us to examine the impact of SLAM signaling on the production of inflammatory cytokines by activated DCs. First, several independently cloned cell lines as well as polyclonal cell populations expressing CD40L and SLAM were analyzed for their ability to induce IL-12 production by DCs. In all cases SLAM/ SLAM-induced signaling strongly inhibited IL-12 production of CD40L-activated DCs, suggesting that cloning did not alter the biologic activity of these cells (Figure 4A). Consequently, we used individual SLAM/CD40L-L clones to examine the effect of SLAM on CD40-mediated activation of DCs in all of our experiments. Analysis of IL-12p40 mRNA also identified an approximately 2.5-fold reduction in p40 mRNA expression in DCs that had been cocultured with SLAM/CD40L-L cells compared with DCs cocultured with CD40L-L cells (Figure 4B).
Figure 3.
The effect of SLAM engagement on CD40L-induced cytokine production of DCs. Cytokine production of DCs activated in the presence of CD40L-L cells or SLAM/CD40L-L cells for 24 hours was compared using a protein array (Raybiotech cytokine array III). One representative of 8 experiments is shown with the list of cytokines measured by the array.
Table 1.
SLAM effect
| Proteins | SLAM effect (SD) |
|---|---|
| GRO | 0.9 (0.4) |
| IL-6 | 0.9 (0.3) |
| IL-8 | 0.9 (0.5) |
| IL-10 | 0.7 (0.4) |
| IL-12 | 0.3 (0.2) |
| MDC | 0.6 (0.1) |
| RANTES | 0.9 (0.8) |
| TNF-α | 0.4 (0.1) |
Quantitative data were obtained for 4 experiments. The effect of SLAM on cytokine production was calculated by the following formula: dot intensity in the presence of SLAM/dot intensity in the absence of SLAM. Cytokines detected in at least 3 of the 4 donors are shown.
Figure 4.
Interference of SLAM homoassociation–induced signaling with CD40L-induced IL-12 production of DCs. (A) DCs were activated in the presence of CD40L-L cells or with various SLAM-expressing CD40L-L cells, including polyclonal SLAM-transfected CD40L-L cells (which contained SLAM– cells as well) and 2 independent SLAM/CD40L-L clones (F8 and G4). IL-12 concentrations were measured by ELISA at 24 hours. (B) Relative expression levels of IL-12p40 mRNA in DCs activated with CD40L-L or SLAM/CD40L-L cells determined by quantitative RT-PCR. (C) IL-12p70 production of DCs in the presence of Jurkat T cells, or a Jurkat cell line expressing SLAM in the presence or absence of anti-CD3 mAbs. Error bars represent the standard deviation of triplicate cultures.
Because DCs frequently receive CD40L signals from activated CD4+ T cells, DCs were cocultured with either the human T-cell line Jurkat expressing SLAM or with Jurkat cells. While Jurkat cells activated by anti-CD3 mAb readily induced IL-12 secretion by DCs, the presence of SLAM on the surface of Jurkat cells40 markedly inhibited IL-12 production by DCs (Figure 4C).
Because LPS is known to up-regulate SLAM expression on the surface of human DCs, the effect of SLAM/SLAM interactions on IL-12, IL-6, and TNF-α secretion was measured in the absence or presence of LPS. DCs activated by either CD40L-L cells or SLAM/CD40L-L cells produced more IL-12 in the presence of a combination of LPS and IFN-γ than in its absence (Figure 5A). Interestingly, IL-12 secretion by DCs in cocultures with SLAM-L cells in the presence of LPS/IFN-γ was not inhibited by SLAM-induced signals (Figure 5A). Instead, a twofold increase in IL-12p70 secretion and a similar increase in IL-12p40 transcription were observed (Figure 5A-B; Table 2). Yet, consistent with the other observations, inhibition of IL-12 secretion by DCs that had been cultured in the presence of SLAM/CD40L-L cells compared to those cultured in the presence of CD40L-L cells was detected even in the presence of LPS/IFN-γ (Figure 5A,C; Table 2). Similarly, production of TNF-α or IL-6 was also inhibited by the presence of SLAM regardless whether the culture contained LPS/IFN-γ (Figure 5A,C; Table 2). Since the capacity to produce these cytokines can differ significantly between donors (Table 2), the effect of SLAM-mediated signals on cytokine production by DCs derived from different donors is also shown (Figure 5C). The effects of SLAM signaling on CD40- or on LPS-induced IL-12, TNF-α, or IL-6 production was statistically significant with the exception of enhancing LPS-induced IL-6 production (Table 2). Neither poly(I:C) nor a mixture of inflammatory cytokines (TNF-α, IL-6, and IL-1β) induced IL-12 production by DCs in the presence or absence of SLAM-L cells (data not shown). Taken together, these experiments strongly support the conclusion that SLAM/ SLAM associations inhibit IL-12, TNF-α, and IL-6 secretion by CD40L-activated DCs.
Figure 5.
The effect of SLAM signaling on the IL-12, TNF-α, and IL-6 production of DCs activated by CD40L or LPS. DCs were activated with 100 ng/mL LPS and 10 ng/mL IFN-γ in the presence of L cells or L cells expressing the SLAM receptor, as indicated. As previously described, CD40L-induced activation was delivered by CD40L-L cells or SLAM/CD40L-L cells or by the combination of CD40L- and LPS-induced signaling as indicated. (A) The amount of secreted IL-12, TNF-α, and IL-6 was measured by ELISA 24 hours following activation. The result of a representative experiment is shown. Error bars represent the standard deviation of triplicate cultures. (B) Relative expression levels of IL-12p40 mRNA in DCs activated with LPS and IFN-γ in the presence of SLAM-L cells or L-cell control were determined by quantitative RT-PCR. Error bars represent the standard deviation of triplicate RNA samples. (C) The effect of SLAM/SLAM interactions on IL-12, TNF-α, and IL-6 production of activated DCs derived from individual donors. Donors are represented by filled circles and mean values are indicated with horizontal lines. The effect of SLAM is plotted as fold-activation of cytokine production on a logarithmic scale.
Table 2.
The effect of SLAM signaling on the cytokine production of DCs activated by CD40L, LPS/IFN-γ, or with the mixture of CD40L and LPS/IFN-γ
| CD40L | LPS/IFN-γ | CD40L + LPS/IFN-γ | |
|---|---|---|---|
| IL-12 | |||
| n | 18 | 13 | 10 |
| Production range, ng/mL* (median) | 0.5-56.2 (2.7) | 0.3-26.3 (6.1) | 1.0-146.1 (61.2) |
| SLAM effect, average ± SD† | 0.2 ± 0.1 | 2.3 ± 1.4 | 0.5 ± 0.3 |
| P | < .001 | .005 | .01 |
| TNF-α | |||
| n | 5 | 8 | 6 |
| Production range, ng/mL* (median) | 18-100.1 (52.7) | 0.8-35.0 (11.7) | 78.5-133.7 (99.4) |
| SLAM effect, average ± SD† | 0.2 ± 0.2 | 2.4 ± 1.8 | 0.6 ± 0.1 |
| P | .03 | .04 | .02 |
| IL-6 | |||
| n | 5 | 6 | |
| Production range, ng/mL* (median) | 54.3-197.0 (122.3) | 30.3-140.2 (68.0) | 98.7-209.0 (176.5) |
| SLAM effect, average ± SD† | 0.4 ± 0.1 | 1.3 ± 0.6 | 0.6 ± 0.4 |
| P | .03 | .09 | .02 |
n indicates the number of donors tested.
Production range was calculated in samples activated in the absence of SLAM-SLAM interactions.
SLAM effect was calculated by the following formula: cytokine concentration in the presence of SLAM/SLAM interactions/cytokine concentration in the absence of SLAM-SLAM interaction. P values were calculated with signed-rank test or paired t test according to the distribution of variables.
Expression of SLAM on the surface of immature dendritic cells causes inhibition of CD40L-induced IL-12 production
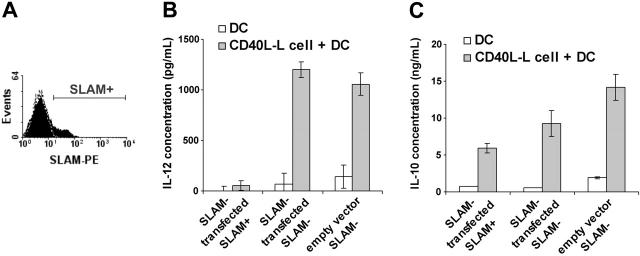
As SLAM/SLAM interactions could take place between activated DCs themselves in these in vitro experiments, nonactivated SLAM– IDCs were transiently transfected with a vector that encoded human SLAM. Five hours after transfection, SLAM+ and SLAM– cell populations were purified by fluorescence-activated cell sorting (FACS). These SLAM+ transfectant cells expressed similar levels of SLAM on their surface (Figure 6A) to DCs that had been activated in vitro (Figure 1C).
Figure 6.
Expression of SLAM molecules on immature DCs inhibits IL-12 production upon CD40 stimulation. Immature (nonactivated), monocyte-derived DCs were transiently transfected with the human SLAM cDNAby electroporation (Nucleofection; AMAXA). SLAM expression was measured 5 hours following transfection by flow cytometry. A representative of 4 independent experiments is shown (A). The filled histogram shows SLAM expression on DCs following transfection; the dashed line represents staining with an isotype control antibody. SLAM-transfected SLAM+ and SLAM– cells were separated 5 hours after transfection on a cell sorter. IL-12 (B) or IL-10 (C) present in the supernatants of CD40L-Lcell–activated, SLAM-sorted SLAM+ and SLAM– populations or vector-transfected controls were measured at 24 hours by ELISA. Error bars represent the standard deviation of triplicate cultures.
The purified SLAM+ and SLAM– transfectant cell populations were then cultured for 24 hours in the presence of L cells expressing CD40L. Thus, SLAM/SLAM interactions could only take place between DCs, and CD40L/CD40 interactions could only take place between the L-cell transfectants and DCs.
As shown in Figure 6B, expression of SLAM on the surface of DCs completely prevented IL-12 production induced by the CD40L-L cells, as judged by comparison with the DCs that were SLAM– after FACS, or with DCs that had been transfected with the empty vector. IL-10 production by SLAM+ DCs was, however, only moderately inhibited compared with the amount of IL-10 secreted by SLAM-DCs (Figure 6C).
DCs activated by SLAM-induced signaling are impaired in their ability to induce differentation of naive CD4+ cells into Th1 cells
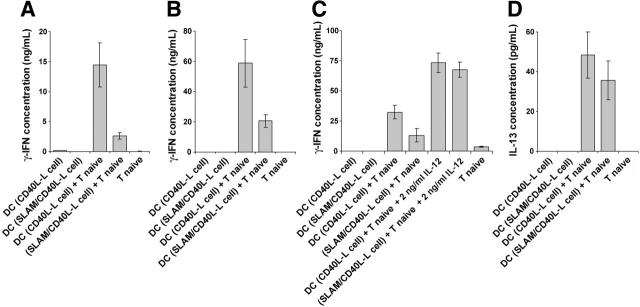
The impact of costimulation by a combination of CD40 and SLAM on IL-12 production by DCs predicts that SLAM-expressing DCs would be impaired in their ability to induce Th1 differentiation of naive CD4+ T cells. To test this hypothesis, DCs were preactivated with the CD40L-L cells or SLAM/CD40L-L cells for 12 hours and were then purified by FACS using anti-CD11c. These preactivated DCs were then cultured with naive CD4+CD45RO– T cells for 5 days. On day 4, T cells were reactivated for 24 hours in the presence of 5 μg/mL plate-bound anti-CD3 mAb. As shown in Figure 7A-B, DCs precultured with the SLAM/CD40L-L cells were poor inducers of IFN-γ production by either allogeneic or autologous T cells compared with DCs activated with CD40L-L cells. Down-regulation of IL-12 production by SLAM-induced signaling in DCs seems to be the principal mechanism of this skewed T-cell polarization, as exogenously added human recombinant IL-12 restored priming in the presence of DCs treated with SLAM-expressing L cells (Figure 7C). Taken together, these results demonstrate that SLAM signaling modulates the function of CD40L-activated DCs by reducing their ability to promote naive T-cell differentiation toward IFN-γ–producing effector cells.
Figure 7.
T cells differentiated in the presence of DCs preactivated by SLAM and CD40 signals produce reduced amounts of IFN-γ. We analyzed whether SLAM-mediated inhibition of DC IL-12 production is reflected in the ability of these cells to promote Th1 differentiation. DCs were activated with CD40L-L cells or with SLAM/CD40L-L cells for 12 hours, then purified by cell sorting for the CD11c+ population. Purified, allogeneic CD4+ naive T cells (A) or autologous CD4+ naive T cells (B) were cultured with these preactivated DCs for 4 days, and then the T cells were activated with anti-CD3 mAb for 24 hours. IFN-γ concentrations were measured in the supernatants by ELISA. To confirm the role of SLAM-mediated inhibition of IL-12 production in Th1 polarization, human recombinant IL-12p70 was exogenously added to cultures of naive CD4+ T cells mixed with autologous CD40L or CD40L/SLAM-activated DCs (C). T-cell priming/activation was analyzed as described for panel B. In the supernatants of cultures obtained from experiments described for panel B the concentration of secreted IL-13 was also determined (D). Representative results of 3 independent experiments are shown. Error bars represent the standard deviation of triplicate cultures.
In the same cultures the amount of IL-4 was below the detection limits of the ELISA, while we found low levels of IL-13, which was modestly reduced by SLAM/SLAM interactions (Figure 7D). The lack of IL-4 production and the low levels of IL-13 in the presence of high concentration of IFN-γ indicate that under these experimental conditions SLAM inhibits the differentiation of CD4+ naive T cells into Th1-type effector cells without promoting their differentiation into Th2 cells. Down-regulation of inflammatory cytokine production and Th1 polarization may, however, indirectly support Th2 differentiation in vivo.
Discussion
In this article, we describe an in vitro model that allows for an assessment of the role of SLAM ligand–induced signal transduction in activated myeloid DCs and activated CD4+ T cells. SLAM/SLAM interactions induced by L cells that coexpress SLAM and CD40L decrease IL-12 production by DCs. Similar results were obtained when DCs were triggered by activated Jurkat cells that coexpressed CD40L and SLAM or by bidirectional SLAM/SLAM interactions between DCs. Because DCs activated via CD40 in the presence of SLAM/SLAM interactions had a reduced ability to promote the differentiation of naive T lymphocytes toward IFN-γ–producing effector CD4+ T cells, we concluded that concomitant CD40 and SLAM signals influenced DC-derived instructive signals. In addition to its inhibitory effect on CD40-induced IL-12 production by DCs, SLAM/SLAM interactions also caused a marked decrease in the secretion of other inflammatory cytokines, such as TNF-α and IL-6, suggesting that SLAM engagement may promote anti-inflammatory functions of DCs. Importantly, these inhibitory effects were not the result of a general unresponsiveness of DCs to CD40L, as SLAM/SLAM interactions did not interfere with the CD40L-induced up-regulation of CD83, CD86, or HLA-DQ on the surface of DCs, and only moderately decreased the production of IL-10. In fact, sCD40L-induced up-regulation of CD83 and CD86 was augmented in the presence of SLAM signaling. Taken together, the results indicate an important role of the SLAM receptor in the modulation of DC functions, such as in the regulation of inflammatory response and in controlling signals that promote the differentiation of naive CD4+ T cells into Th1-type effector cells.
SLAM signaling has been suggested to mediate the strong immunosuppression induced by the measles virus.27-30 In particular, when human SLAM was expressed in murine DCs as a transgene under the control of the Cd11c promoter, measles virus infection of murine splenic DCs caused a marked down-regulation of costimulatory molecules (eg, CD80, CD86, CD40, and major histocompatibility [MHC] class I and class II antigens).30 Our data show that SLAM/SLAM homoassociation, unlike measles virus infection, does not interfere with the normal maturation process induced by CD40L (Figure 2A-B), which suggests that SLAM signaling may only contribute to, but not deliver, the full immunosuppressive potential of the measles virus. The in vivo experiments by Hahm et al,30 or a similar suppression of DC function in vitro reported by Servet-Delprat et al,41 are not directly comparable with our model because in both approaches viral replication takes place, which is likely to modulate multiple signaling pathways independent from SLAM signaling. Yet, modulation of SLAM receptor signaling may be advantageous to the virus by decreasing CD40L-induced DC inflammatory cytokine production. Examination of the signaling events induced by SLAM/SLAM homoassociation versus SLAM/measles virus interactions will clarify the contribution of receptor-induced signaling and viral replication to measles-induced immunosuppression.
The data presented here raise the possibility that SLAM may act as a context-dependent regulator of IL-12 and other inflammatory cytokine production in DCs. However, to prove this, identification of the DC40- and LPS-induced downstream signaling pathways influenced by SLAM is essential. Recently, phosphoinositide 3-kinase (PI3K) has been proposed as an endogenous suppressor of IL-12 production triggered by TLR signaling.42,43 As PI3K was activated upon antibody-mediated SLAM triggering in murine T cells,44 this pathway might be involved in the SLAM-mediated inhibition of IL-12 production by CD40L-activated DCs. However, the PI3K inhibitors LY294002 and wortmannin did not suspend the inhibitory effect of SLAM on CD40L-induced IL-12 production (data not shown). Furthermore, SLAM did not inhibit the LPS-induced IL-12 production, suggesting that PI3K is not involved in the SLAM-mediated regulation of cytokine secretion in DCs. Thus, the mechanism(s) by which SLAM-mediated regulation of CD40 and TLR signaling is implemented remains to be established.
Because SLAM expression on the surface DCs is regulated by a variety of signals (eg, TLR ligands, inflammatory cytokines, or CD40L33) in the course of an immune response, SLAM expressed on activated DCs should not ubiquitously block IL-12 production during subsequent antigen presentation. Indeed, we found that when DCs were coincubated with L cells that expressed CD40L, association of SLAM receptors present on the surface of CD40L-activated DCs did not inhibit IL-12 secretion. However, in cultures in which DCs were coincubated with L cells expressing both CD40L and SLAM, IL-12 secretion was inhibited. Thus, CD40L-induced IL-12 production can be efficiently inhibited in MoDCs by interaction with cells that express both CD40L and SLAM on their surface, and therefore concomitantly activate CD40L and SLAM signaling pathways. High levels of SLAM expression were reported on polarized Th1 cells in mice. Significant but lower levels of SLAM were detected on polarized Th2 cells, but SLAM expression was stably maintained on Th1 but not on Th2 clones.45 Furthermore, ligation of CD3 and CD28 on the surface of naive CD4+ but not CD8+ human peripheral-blood T cells induce the expression of CD40L.46 Therefore, CD4+ Th1 effector-memory cells are particularly suitable to induce contemporary signaling via SLAM and CD40L.
The number of SLAM-expressing, activated, polarized CD4+ T lymphocytes is expected to increase at later stages of the immune response both in lymphoid tissues and at the site of the infection. We propose that SLAM signaling at this point acts as an inhibitory feedback loop to limit production of excessive amounts of inflammatory and Th1-polarizing cytokines by DCs.
As discussed earlier, SLAM expression on DCs induced by maturation signals (eg, by CD40L) does not interfere with IL-12 secretion. However, when SLAM was expressed by transfecting SLAM into immature DCs, subsequent CD40L-induced IL-12 secretion was strongly inhibited, suggesting that SLAM/SLAM association between interacting DCs can transmit an effective inhibitory signal when SLAM is present in immature DCs prior to CD40/CD40L ligation. Regarding the presence of SLAM in immature DCs, 2 groups have shown recently that SLAM expression is induced in MoDCs treated with IL-10, which implicates SLAM as modulator of certain DC functions in immature and/or regulatory-type DCs.47,48
DCs readily produce IL-12 following interaction with various pathogens, such as bacteria or viruses.3,9 Our experiments demonstrated that although CD40L-induced IL-12 production was strongly inhibited even in the presence of LPS, LPS-induced IL-12 production by DCs was not inhibited by SLAM, suggesting that signaling induced via SLAM/SLAM interactions may affect DC functions in a complex, context-dependent manner. SLAM engagement that takes place between activated DCs and CD4+ Th1 cells decreases Th1 polarization and inflammatory responses, although it does not interfere with inflammation or Th1 responses induced by DCs upon recognition of microbial ligands in the peripheral tissues.
Our results clearly indicate that regulation of SLAM expression either on DCs or other cells interacting with DCs may have a strong impact on DC functions. In this regard, SLAM expression was strikingly different on activated macrophages in 2 distinct, Th1 cell–driven inflammatory diseases. SLAM+ macrophages were detected in the gut of patients with Crohn disease but SLAM was not expressed on activated macrophages present in the brain of patients with multiple sclerosis.49 This result indicated that SLAM expression on the antigen-presenting cell might be sensitively modulated possibly by inflammatory mediators or tissue factors.
Importantly, our data based on the described in vitro models are in good correlation with those obtained using Slam- or Sh2d1a-deficient mice.15,21 Furthermore, data presented here suggest that different ligands activating SLAM signaling, such as antibodies33 or SLAM/SLAM association may induce opposing effector functions in DCs, which should be carefully evaluated when modulation of SLAM expression is considered as a therapeutic tool.
Prepublished online as Blood First Edition Paper, November 29, 2005; DOI 10.1182/blood-2005-06-2265.
Supported by grants from the National Insitutes of Health (NIH), Fogarty International Research Collaboration Award RO3TW006472-03 (C.T. and A.L), by the Hungarian Academy of Sciences, OTKA T38353 (A.L.), OTKA T43420 (E.R.), and by the National R&D Program, NKFP 3081 (E.R.). R03TW006472-03 is granted to the Division of Immunology, Beth Israel Deaconess Hospital; other sources of support are granted to the Institute of Immunology, University of Debrecen Medical and Health Science Center. A.L. was also supported by the Bolyai Fellowship From the Hungarian Academy of Sciences.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9: 271-296. [DOI] [PubMed] [Google Scholar]
- 2.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1: 199-205. [DOI] [PubMed] [Google Scholar]
- 3.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol. 2005;26: 289-307. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM. Some interfaces of dendritic cell biology. APMIS. 2003;111: 675-697. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392: 245-252. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18: 767-811. [DOI] [PubMed] [Google Scholar]
- 7.Manetti R, Parronchi P, Giudizi MG, et al. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177: 1199-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260: 547-549. [DOI] [PubMed] [Google Scholar]
- 9.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3: 133-146. [DOI] [PubMed] [Google Scholar]
- 10.Akiba H, Miyahira Y, Atsuta M, et al. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J Exp Med. 2000;191: 375-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong EC, Vieira PL, Kalinski P, et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J Immunol. 2002;168: 1704-1709. [DOI] [PubMed] [Google Scholar]
- 12.Cocks BG, Chang CC, Carballido JM, Yssel H, de Vries JE, Aversa G. A novel receptor involved in T-cell activation. Nature. 1995;376: 260-263. [DOI] [PubMed] [Google Scholar]
- 13.Sidorenko SP, Clark EA. Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J Immunol. 1993;151: 4614-4624. [PubMed] [Google Scholar]
- 14.Veillette A, Latour S. The SLAM family of immune-cell receptors. Curr Opin Immunol. 2003;15: 277-285. [DOI] [PubMed] [Google Scholar]
- 15.Wang N, Satoskar A, Faubion W, et al. The cell surface receptor SLAM controls T cell and macrophage functions. J Exp Med. 2004;199: 1255-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel P, Eck MJ, Terhorst C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat Rev Immunol. 2003;3: 813-821. [DOI] [PubMed] [Google Scholar]
- 17.Sayos J, Wu C, Morra M, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395: 462-469. [DOI] [PubMed] [Google Scholar]
- 18.Coffey AJ, Brooksbank RA, Brandau O, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20: 129-135. [DOI] [PubMed] [Google Scholar]
- 19.Nichols KE, Harkin DP, Levitz S, et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 1998;95: 13765-13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czar MJ, Kersh EN, Mijares LA, et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci U S A. 2001;98: 7449-7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Nguyen KB, Pien GC, et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat Immunol. 2001;2: 410-414. [DOI] [PubMed] [Google Scholar]
- 22.Morra M, Barrington RA, Abadia-Molina AC, et al. Defective B cell responses in the absence of SH2D1A. Proc Natl Acad Sci U S A. 2005;102: 4819-4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hron JD, Caplan L, Gerth AJ, Schwartzberg PL, Peng SL. SH2D1A regulates T-dependent humoral autoimmunity. J Exp Med. 2004;200: 261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421: 282-287. [DOI] [PubMed] [Google Scholar]
- 25.Tatsuo H, Ono N, Yanagi Y. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J Virol. 2001;75: 5842-5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanagi Y. The cellular receptor for measles virus—elusive no more. Rev Med Virol. 2001;11: 149-156. [DOI] [PubMed] [Google Scholar]
- 27.Schneider-Schaulies S, Bieback K, Avota E, Klagge I, ter Meulen V. Regulation of gene expression in lymphocytes and antigen-presenting cells by measles virus: consequences for immunomodulation. J Mol Med. 2002;80: 73-85. [DOI] [PubMed] [Google Scholar]
- 28.Schneider-Schaulies S, Niewiesk S, Schneider-Schaulies J, ter Meulen V. Measles virus induced immunosuppression: targets and effector mechanisms. Curr Mol Med. 2001;1: 163-181. [DOI] [PubMed] [Google Scholar]
- 29.Dubois B, Lamy PJ, Chemin K, Lachaux A, Kaiserlian D. Measles virus exploits dendritic cells to suppress CD4+ T-cell proliferation via expression of surface viral glycoproteins independently of T-cell trans-infection. Cell Immunol. 2001;214: 173-183. [DOI] [PubMed] [Google Scholar]
- 30.Hahm B, Arbour N, Oldstone MB. Measles virus interacts with human SLAM receptor on dendritic cells to cause immunosuppression. Virology. 2004;323: 292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polacino PS, Pinchuk LM, Sidorenko SP, Clark EA. Immunodeficiency virus cDNA synthesis in resting T lymphocytes is regulated by T cell activation signals and dendritic cells. J Med Primatol. 1996;25: 201-209. [DOI] [PubMed] [Google Scholar]
- 32.Kruse M, Meinl E, Henning G, et al. Signaling lymphocytic activation molecule is expressed on mature CD83+ dendritic cells and is up-regulated by IL-1 beta. J Immunol. 2001;167: 1989-1995. [DOI] [PubMed] [Google Scholar]
- 33.Bleharski JR, Niazi KR, Sieling PA, Cheng G, Modlin RL. Signaling lymphocytic activation molecule is expressed on CD40 ligand-activated dendritic cells and directly augments production of inflammatory cytokines. J Immunol. 2001;167: 3174-3181. [DOI] [PubMed] [Google Scholar]
- 34.Carballido JM, Aversa G, Kaltoft K, et al. Reversal of human allergic T helper 2 responses by engagement of signaling lymphocytic activation molecule. J Immunol. 1997;159: 4316-4321. [PubMed] [Google Scholar]
- 35.Latour S, Gish G, Helgason CD, Humphries RK, Pawson T, Veillette A. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat Immunol. 2001;2: 681-690. [DOI] [PubMed] [Google Scholar]
- 36.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27: 3135-3142. [DOI] [PubMed] [Google Scholar]
- 37.Szatmari I, Gogolak P, Im JS, Dezso B, Rajnavolgyi E, Nagy L. Activation of PPARgamma specifies a dendritic cell subtype capable of enhanced induction of iNKT cell expansion. Immunity. 2004;21: 95-106. [DOI] [PubMed] [Google Scholar]
- 38.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22: 307-328. [DOI] [PubMed] [Google Scholar]
- 39.Mackey MF, Wang Z, Eichelberg K, Germain RN. Distinct contributions of different CD40 TRAF binding sites to CD154-induced dendritic cell maturation and IL-12 secretion. Eur J Immunol. 2003;33: 779-789. [DOI] [PubMed] [Google Scholar]
- 40.Howie D, Simarro M, Sayos J, Guirado M, Sancho J, Terhorst C. Molecular dissection of the signaling and costimulatory functions of CD150 (SLAM): CD150/SAP binding and CD150-mediated costimulation. Blood. 2002;99: 957-965. [DOI] [PubMed] [Google Scholar]
- 41.Servet-Delprat C, Vidalain PO, Azocar O, Le Deist F, Fischer A, Rabourdin-Combe C. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J Virol. 2000;74: 4387-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24: 358-363. [DOI] [PubMed] [Google Scholar]
- 43.Fukao T, Tanabe M, Terauchi Y, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3: 875-881. [DOI] [PubMed] [Google Scholar]
- 44.Howie D, Okamoto S, Rietdijk S, et al. The role of SAP in murine CD150 (SLAM)-mediated T-cell proliferation and interferon gamma production. Blood. 2002;100: 2899-2907. [DOI] [PubMed] [Google Scholar]
- 45.Castro AG, Hauser TM, Cocks BG, et al. Molecular and functional characterization of mouse signaling lymphocytic activation molecule (SLAM): differential expression and responsiveness in Th1 and Th2 cells. J Immunol. 1999;163: 5860-5870. [PubMed] [Google Scholar]
- 46.Mailliard RB, Egawa S, Cai Q, et al. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med. 2002;195: 473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velten FW, Duperrier K, Bohlender J, Metharom P, Goerdt S. A gene signature of inhibitory MHC receptors identifies a BDCA3(+) subset of IL-10-induced dendritic cells with reduced allostimulatory capacity in vitro. Eur J Immunol. 2004;34: 2800-2811. [DOI] [PubMed] [Google Scholar]
- 48.McBride JM, Jung T, de Vries JE, Aversa G. IL-10 alters DC function via modulation of cell surface molecules resulting in impaired T-cell responses. Cell Immunol. 2002;215: 162-172. [DOI] [PubMed] [Google Scholar]
- 49.Theil D, Farina C, Meinl E. Differential expression of CD150 (SLAM) on monocytes and macrophages in chronic inflammatory contexts: abundant in Crohn's disease, but not in multiple sclerosis. J Clin Pathol. 2005;58: 110-111. [DOI] [PMC free article] [PubMed] [Google Scholar]