Abstract
Thrombin-mediated endothelial-cell release of von Willebrand factor (VWF) and P-selectin functionally links protease-activated receptors (PARs) to thrombosis and inflammation. VWF release can be stimulated by both Ca2+ and cAMP, and, although both VWF and P-selectin are found in Weibel-Palade bodies (WPBs), we found that their release could be differentially regulated. In these studies, human umbilical vein endothelial cells stimulated with cAMP or PAR2-AP led to a delayed release of VWF and significantly less P-selectin release compared with histamine, thrombin, or PAR1-AP. Dose-response studies revealed that PAR2-AP was significantly less efficacious in promoting the release of P-selectin compared with VWF. PAR2-AP–induced robust stimulation of intracellular Ca2+ coupled with a significantly greater inhibitory effect of calcium chelation on release of VWF compared with cell-surface expression of P-selectin, suggests an additional Ca2+-independent pathway involved in release of P-selectin. PAR2-AP failed to increase global cAMP levels; however, inhibition of protein kinase A led to a significant attenuation of PAR2-AP–mediated release of VWF. Confocal microscopy studies revealed that PAR2 and forskolin caused preferential release of a population of Weibel-Palade bodies (WPBs) consisting of only VWF. Thus, WPBs are pharmacologically and morphologically heterogeneous, and distinct granule populations are susceptible to differential regulation.
Introduction
Thrombin is the major protease in the coagulation cascade whose pleiotropic actions can ultimately lead to thrombosis and tissue injury. Thrombin displays a diverse range of effects in vascular cells that functionally connects tissue damage to both hemostatic and inflammatory responses.1,2 Thrombin stimulation of the release of preformed von Willebrand factor (VWF) and P-selectin from endothelial cells links inflammation and atherosclerosis, because VWF facilitates platelet aggregation at sites of vascular injury,3 and P-selectin is involved in adhesion of leukocytes to endothelial cells.4 Elevated levels of VWF predict adverse outcomes in acute coronary syndromes and may be a biochemical surrogate for endothelial-cell dysfunction.5,6 P-selectin is a transmembrane protein of the selectin family and plays a critical role early in the inflammatory response by mediating the rolling of leukocytes on the endothelium. It has been suggested that P-selectin may be involved in atherosclerosis because P-selectin deficiency delays and reduces atherosclerotic lesion formation.7,8 Much interest has also been given to soluble P-selectin since this was first proposed to be a marker of platelet activation in 1997.9 Recent evidence suggests that soluble P-selectin may play a key role in hemostasis and thrombosis.10-12 The source of soluble P-selectin has been debated because P-selectin is found in both platelets and Weibel-Palade bodies. Most investigators believe the major source of soluble P-selectin is platelets.13
Many of the cellular effects of thrombin are initiated via activation of a family of protease-activated receptors (PARs), which are coupled to heterotrimeric G-proteins. PARs are unique among G-protein–coupled receptors (GPCRs) in that they are activated by thrombin through proteolytic generation of a tethered ligand. Thus far, 4 PARs have been cloned and characterized (PAR1-PAR4). Human umbilical vein endothelial cells (HUVECs) express both PAR1 and PAR2, whereas PAR3 transcripts have been found at considerably lower levels when compared with PAR1.14 Thrombin stimulates PAR1 and PAR3, whereas trypsin/tryptase and coagulation proteases upstream of thrombin (eg, tissue factor/VIIa complex, factor Xa, and the cognate ternary complex) stimulate PAR2.15 PAR2 plays a pivotal, yet undefined, role in inflammation and ischemia with evidence for both proinflammatory and anti-inflammatory roles.15 Many of the studies on thrombin activation of biochemical and functional effects in endothelial cells were done before it was known that multiple PARs existed. Additionally, the agonist peptide used for activation of PAR1, which is referred to as thrombin receptor activating peptide (TRAP, SFLLRNDKYEPF), can also activate PAR2 on endothelial cells.16,17 For example, thrombin and TRAP have been shown to mediate leukocyte rolling in rat mesenteric venules via P-selectin and sialyl Lewis X.18 These effects could be mediated by either PAR1 or PAR2 given that TRAP can activate PAR2. However, substitution of the serine to threonine yields a PAR1-specific peptide (TFLLRNPND).19
Endothelial cells contain elongated endothelial storage vesicles called Weibel-Palade bodies (WPBs) that are involved in the regulated secretion of mature VWF and P-selectin.20,21 VWF and P-selectin are the best-characterized constituents of WPBs, and several lines of evidence exist for their close association. VWF knockout mice also display defective leukocyte attachment, implicating a lack of P-selectin expression.22 More recently P-selectin has been shown to mediate the attachment of ultralarge VWF multimers to the endothelial surface on release.23
VWF is released after fusion with the plasma membrane in response to agents that result in elevated intracellular Ca2+, such as thrombin, histamine, or calcium inonophore.24 In addition, agents that elevate intracellular cAMP such as vasopressin receptor agonists, purine nucleotides, epinephrine, and forskolin can also trigger the release of VWF.25-27 The time course of cAMP-stimulated VWF release is delayed compared with agonists that increase intracellular Ca2+. By inference, stimulation of VWF release should also occur in tandem with translocation of P-selectin to the cell surface, because both factors are located in the same secretory granule. The purpose of this study was to examine release of VWF and P-selectin from endothelial cells stimulated by different G-protein–mediated signaling pathways. Our studies have demonstrated differential effects on secretion of VWF and P-selectin and have shown that distinct populations of WPBs that are susceptible to regulation by different G-protein–mediated signaling pathways.
Materials and methods
α-Thrombin and PTX were obtained from Calbiochem (La Jolla, CA), and PAR-APs were obtained commercially from PeptidoGenic Research (Livermore, CA) and were greater than 95% pure as determined by reverse-phase high-performance liquid chromatography (HPLC). All other chemicals were obtained from Sigma (St Louis, MO) and were the highest grade available.
Cell culture
HUVECs were isolated28 and cultured in medium 199 supplemented with 15% fetal bovine serum (FBS), endothelial mitogen, penicillin, streptomycin, amphotericin B, and heparin. Primary HUVECs were plated out in gelatin-coated 48-well plates and assayed 1 to 2 days after becoming confluent. Cells were washed once with M199/0.1% BSA and were stimulated with indicated agonists, and the conditioned medium was collected at 15 minutes and assayed for VWF activity, and cells were assayed for P-selectin. All experiments were conducted in passage 1 HUVECs except where otherwise noted. The collection of umbilical cords was approved by the Vanderbilt University Medical Center Institutional Review Board, Nashville, TN.
Cell-based P-selectin assay
HUVECs were stimulated with indicated agents and then fixed for 5 minutes with 0.5% paraformaldehyde solution and washed and blocked. Mouse anti–P-selectin antibodies (Research & Diagnostics, Flanders, NJ) were then added and incubated at room temperature for 1 hour, washed, and blocked. Goat anti–mouse horseradish peroxidase (HRP)–conjugated second-degree antibody (KRL) was then added for 1 hour followed by washing and then analyzed at 450 nM after addition of substrate. Soluble P-selectin was measured using the soluble P-selectin kit from R&D Systems (Flanders, NJ).
von Willebrand factor assay
Samples were analyzed using a sandwich enzyme-linked immunosorbent assay (ELISA) for VWF using a polyclonal rabbit anti-VWF antibody (DAKO, Glostrup, Denmark) in combination with HRP-conjugated anti-VWF antibody (DAKO) according to the manufacturer's protocol. A standard curve for VWF was generated from serial dilutions of normal human serum with extrapolation of levels using a standard amount of purified VWF.
Intracellular Ca2+ mobilization
Intracellular Ca2+ mobilization was measured using the fluorescence-based FLEXstation Calcium Plus Assay kit from Molecular Devices (Sunnyvale, CA) according to the manufacturer's protocol. Primary HUVECs were grown to confluence in 96-well plates and washed twice with M199/0.1% BSA before the addition of agonist. All wells first received buffer, resulting in a small rise in Ca2+ mobilization (approximately 1.4- to 1.6-fold increase) which prevented further increases in Ca2+ on a second addition of buffer 60 seconds later.
Immunocytochemistry
Primary HUVECs were plated on gelatin-coated coverslips, washed, and fixed with 3.7% paraformaldehyde for 15 minutes, and then washed and blocked. Samples were then incubated with either rabbit anti-VWF antibody or monoclonal mouse anti–P-selectin antibody (clone AK-6 from Biodesign International, Saco, ME), or both for 1 hour at room temperature. After extensive washing with PBS, samples were then incubated for 1 hour at room temperature with Rhodamine Red–X-conjugated goat anti–rabbit IgG (Jackson ImmunoResearch, Baltimore, PA) for VWF or fluorescein-conjugated goat anti–mouse IgG (Jackson ImmunoResearch) for P-selectin or both. Controls consisted of either incubating cells with one or both first-degree antibodies without fluorescently labeled second-degree antibodies or cells incubated with one or both fluorescently labeled second-degree antibodies in the absence of first-degree antibodies. Samples were then washed extensively and mounted using Vectashield (Vector Laboratories, Burlingame, CA) and sealed. Images were obtained on a Zeiss LSM510 confocal microscope (Zeiss, Jena, Germany) equipped with a 63 ×/1.3 Plan-Neofluar objective lens. No camera was used; images were obtained through the Zeiss laser scanning system. Images were examined using the Zeiss LSM Image examiner (Figure 7) and with Metamorph 6.2 imaging software (Molecular Devices) where indicated.
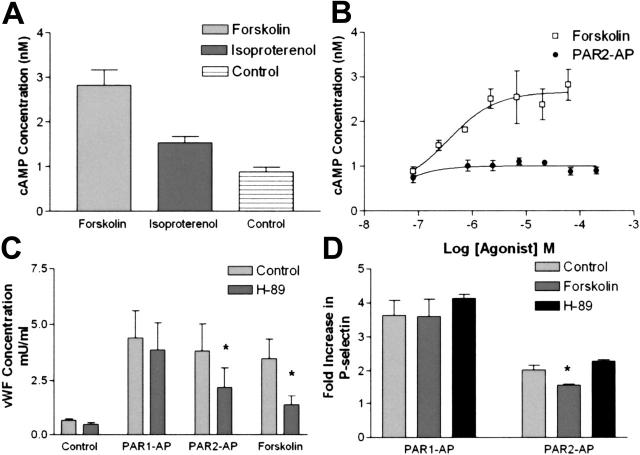
Figure 7.
The role of cAMP in PAR2-mediated release of VWF and P-selectin. (A) HUVECs were stimulated with forskolin (20 μM), isoproterenol (10 μM), or vehicle for 15 minutes and then assayed for cAMP. Figure is representative of an experiment conducted 3 times. (B) Dose response of forskolin and PAR2-AP in HUVECs. Forskolin data are representative of an experiment done 3 times, whereas PAR2-AP data are the average of an experiment done 3 times. Dose-response curves were generated using the area under the curve function in SOFTmax PRO. (A-B) Cells were stimulated for 15 minutes with agonist and IBMX (100 μM) and then were assayed for cAMP. (C) Protein kinase A inhibition with H-89 decreases PAR2-AP and forskolin stimulation of VWF. HUVECs were pretreated with H-89 (10 μM) for 10 minutes and then stimulated with PAR1-AP (100 μM), PAR2-AP (200 μM), or forskolin (20 μM with 100 μM IBMX) for 20 minutes, and medium was collected and assayed for VWF. Figure represents the mean ± SEM of 3 experiments conducted in triplicate. *Statistically significant difference between control and H-89 (P < .05 as determined by 2-tailed t test). (D) Forskolin and H-89 do not significantly alter PAR1-AP–mediated release of P-selectin. HUVECs were pretreated with H-89 (10 μm) for 10 minutes or stimulated with forskolin (20 μM) along with PAR1-AP (100 μM) or PAR2-AP (200 μM) or vehicle for 15 minutes, and P-selectin was measured. Values represent the fold induction and are corrected for the effect of forskolin on basal P-selectin release. Figure represents the mean ± SEM of 3 experiments conducted in triplicate.
cAMP assay
Measurement of intracellular cAMP was performed by use of the Catch-Point Fluorescent Assay kit (Molecular Devices). The assay was performed according to the manufacturer's protocol.
Secretion of sP-selectin from HUVECs
Passage 1 HUVECs were grown in 6-well plates and used at 100% confluence. Cells were washed twice with PBS and were stimulated for 30 minutes with either M199/0.1% BSA medium or medium containing 10 nM thrombin, 100 μM PAR1-AP, or 200 μM PAR2-AP. The conditioned medium from the respective treatments was collected and cleared of cell debris by centrifugation. The medium was concentrated by TCA (trichloroacetic acid) protein precipitation, and sP-selectin was measured using the Parameter Human sP-Selectin Immunoassay Kit (R&D Systems) according to the manufacturer's instruction.
Subcloning of soluble P-selectin
Total RNA was isolated from HUVECs with the total RNA isolation kit (Aurum Total RNA Mini Kit; Bio-Rad, Hercule, CA). Reverse transcriptase–polymerase chain reaction (RT-PCR) was completed using the Thermo-Script RT-PCR system from Invitrogen (Carlsbad, CA), and PCR was performed using 2 specific primers surrounding the transmembrane domain of P-selectin. The primers used were as follows: forward primer, 5′-CAT GTA CTG TAG GCA TCA TC-3′; reverse primer, 5′-CTA GGT GGC TGT GAG GAT TC-3′. The PCR products were analyzed by agarose gel electrophoresis and sequenced.
Construction of P-selectin and soluble P-selectin vectors
Total RNA was isolated from HUVECs with the total RNA isolation kit (Aurum Total RNA Mini Kit; Bio-Rad), and RT-PCR was carried out using the ThermoScript RT-PCR system (Invitrogen). PCR was performed using 2 primers containing BspE1 and Sal1 restriction sites. The primers used were as follows: forward primer, 5′-ATA TCC GGA AGC AGA GTC ACA GAG GAG ATG GC-3′; reverse primer, 5′-AAT GTC GAC GGT CCA GAG CGG GTC CAA CAG-3′. Full-length cDNAs encoding human P-selectin were subcloned into the pECFP-C1 vector at the BspE1 and Sal1 sites. The constructs were digested with the endonuclease Apa 1, and pECFP–P-selectin vectors displayed a band of 763 bp (base pairs), whereas pECFP–soluble P-selectin displayed a band of 643 bp.
Results
Effects of thrombin, PAR-APs, forskolin, and histamine on release of P-selectin and von Willebrand factor
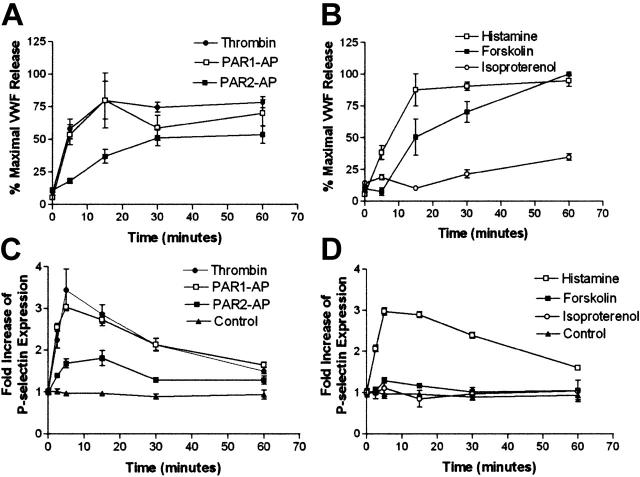
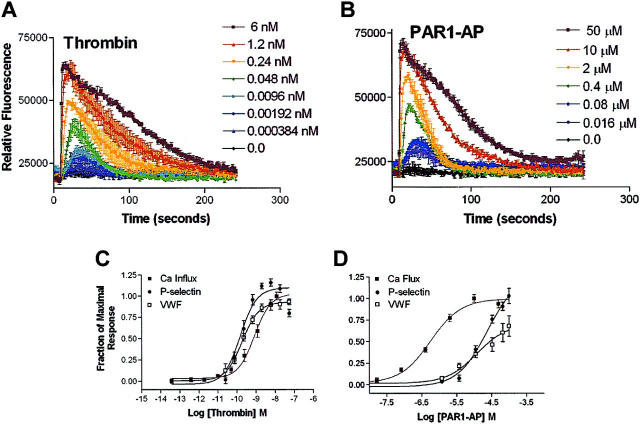
To examine the effects of agonist-mediated release of WPB contents in HUVECs, we conducted time-course experiments in which we measured VWF in the medium while measuring cell-surface expression of P-selectin. Thrombin and PAR1-AP (TFLL-RNPND)–mediated release of VWF is maximal at approximately 15 minutes, whereas PAR2-AP (SLIGKV)–mediated release of P-selectin is delayed compared with PAR1-AP or thrombin (Figure 1A) and more closely resembles forskolin-mediated release of VWF (Figure 1B). Maximal release of P-selectin occurs more rapidly, with maximal levels seen at approximately 5 minutes for all agonists tested (Figure 1C-D). The magnitude of PAR2-AP–mediated P-selectin release was less than PAR1-AP, thrombin, or histamine, whereas forskolin-mediated release of P-selectin was even less compared with PAR2-AP, and isoproterenol had no effect.
Figure 1.
PAR2-AP and cAMP stimulation of release of VWF is delayed and P-selectin is reduced compared with PAR1-AP and thrombin. (A-B). Time course of VWF release in HUVECs: PAR2-AP, forskolin, and isoproterenol-mediated release of VWF is delayed compared with thrombin, PAR1-AP, or histamine. HUVECs were stimulated with thrombin (10 nM), PAR1-AP (100 μM), PAR2-AP (200 μM), histamine (20 μM), forskolin (20 μM with 100 μM IBMX), or isoproterenol (10 μM with 100 μM IBMX), and medium was collected at indicated time points. Data were corrected for the constitutive release of VWF and normalized to percentage of forskolin at 60 minutes, which consistently resulted in the highest levels of VWF. Values represent mean ± SEM. Results shown represent an experiment that was conducted 3 times. (C-D). Time course of cell-surface P-selectin expression in HUVECs: PAR2-AP– and forskolin-mediated P-selectin cell-surface expression is less when compared with PAR1-AP, thrombin, and histamine. HUVECs were stimulated with the above-mentioned concentrations of agonist, and the cell surface was assayed for P-selectin at indicated time points. Values represent mean ± SEM of an experiment that was conducted 3 times.
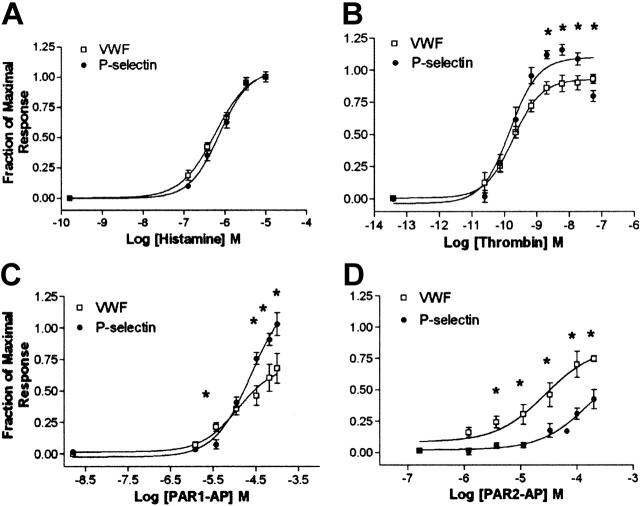
To examine how the differences in the time courses in Figure 1 seen with PAR1-AP and PAR2-AP translated into differences in efficacy, we conducted dose-response studies. Addition of histamine produced nearly identical responses for both VWF release and P-selectin cell-surface expression (Figure 2A). Dose-response studies with thrombin yielded similar EC50s for release of VWF and cell-surface expression of P-selectin; however, the magnitude of response or efficacy was statistically significantly greater for P-selectin cell-surface expression than for VWF release (Figure 2B). Application of PAR1-AP was slightly more efficacious for cell-surface P-selectin expression compared with release of VWF, similar to thrombin (Figure 2C). However, addition of PAR2-AP resulted in an opposite pattern compared with PAR1-AP with a greater efficacy with VWF release compared with P-selectin (Figure 2D). Importantly, HUVECs express only the histamine 1 (H1) receptor subtype as determined pharmacologically,29 which reportedly couples only to Gq.30 This suggests that PARs, which couple to more G-proteins than the histamine receptor, may mediate the release of VWF and P-selectin through multiple G-proteins/pathways.
Figure 2.
Differences in efficacy between P-selectin and VWF release with PAR signaling. Cells were stimulated with indicated concentrations of agonists for 15 minutes, and medium was collected and assayed for VWF while P-selectin was measured on the cell surface. Because of the delayed time course of PAR2-AP–mediated release of VWF, medium was collected and assayed for VWF at 30 minutes. Represented are stimulation with histamine (A), thrombin (B), PAR1-AP (C), and PAR2-AP (D). Data were normalized to maximal does of histamine secondary to variability of fold induction of VWF (3- to 12-fold increase) and P-selectin (2.0- to 3.5-fold increase) observed with different HUVEC preparations. Values represent mean ± SEM of 4 to 6 independent experiments. *Statistically significant difference between release of P-selectin compared with VWF (P < .05 as determined by 2-tailed t test).
Role of Ca2+ mobilization in VWF and P-selectin release in HUVECs
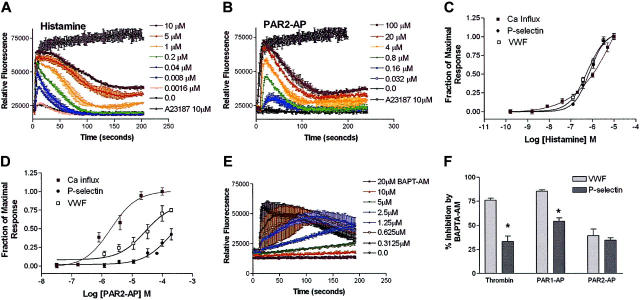
Because the secretion of VWF occurs after intracellular Ca2+ levels rise,24 and peak elevations in cytoplasmic-free Ca2+ correlate with VWF secretion in HUVECs,31 we next examined the ability of the same agonists to mobilize intracellular Ca2+. The peak level of Ca2+ mobilization in response to histamine or PAR2-AP was nearly identical and resulted in nearly a 3-fold increase in intracellular Ca2+ in HUVECs (Figure 3A-B). When dose-response curves were generated for histamine and PAR2-AP–mediated mobilization of Ca2+ and overlaid with the dose-response curves for both VWF and P-selectin release (Figure 2), the PAR2-AP Ca2+ dose-response curve is significantly shifted to the left compared with both VWF and P-selectin. This suggests that PAR2-mediated VWF and P-selectin release requires additional pathways in addition to intracellular Ca2+ mobilization. In contrast, the histamine dose-response curve (Figure 3C) suggests that Ca2+ mobilization is sufficient to mediate both VWF and P-selectin release. Intriguingly, the thrombin dose-response curve for Ca2+ mobilization overlays with both P-selectin and VWF release (Figure 4C), whereas the dose-response curve for PAR1-AP–mediated Ca2+ mobilization is shifted to the left when compared with both VWF and P-selectin release (Figure 4D). This could reflect that peptide activation when compared with activation by thrombin alters receptor/G-protein binding to favor Gαq activation over the other G-proteins involved in signaling as we have shown previously in human microvascular endothelial cells.32 Alternatively, this may suggest that thrombin activates additional non-PAR pathways that may be contributing to this response as previously shown in HUVECs.33
Figure 3.
Agonist-induced Ca2+ mobilization in HUVECs and effect of calcium chelation on thrombin-, PAR1-AP–, and PAR2-AP–mediated release of VWF and P-selectin. Cells were plated into 96-well plates and stimulated with indicated concentrations of agonists, and Ca2+ was measured fluorometrically. Stimulation with indicated doses of histamine and the calcium ionophore A23187 (10 μM) (A) and stimulation with indicated concentrations of PAR2-AP and calcium ionophore A23187 (10 μM) (B) are represented. The y-axis represents the relative fluorescence units (RFUs). Data represent the mean ± SEM of 2 independent experiments for histamine and 3 independent experiments for PAR2-AP. (C-D) Dose-response curves were generated using the area under the curve function in SOFTmax PRO (Molecular Devices) and then normalized to fraction of maximal response and plotted with the data from Figure 2 to graphically represent the different potencies between agonists with respect to Ca2+ mobilization and release of VWF and P-selectin. (E) The effect of BAPTA-AM on agonist-mediated stimulation of Ca2+ mobilization is represented. HUVECs were pretreated with BAPTA-AM at indicated concentrations for 1 hour and stimulated with thrombin (10 nM). Figure represents the mean ± SEM of 2 independent experiments conducted in triplicate. (F) HUVECs that were pretreated either with 10 μM BAPTA-AM for 1 hour and stimulated with thrombin (10 nM), PAR1-AP (100 μM), or PAR2-AP (200 μM), are represented. The y-axis represents percentage of inhibition and was normalized by dividing the agonist stimulation by the basal levels in the presence of BAPTA-AM. Data represent the mean ± SEM of 3 independent experiments conducted in triplicate. *Statistical difference (P < .05 as determined by 2-tailed t test) between VWF and P-selectin in the presence of BAPTA-AM. Figure represents the mean ± SEM of 3 independent experiments conducted in triplicate.
Figure 4.
Thrombin and PAR1-AP induced Ca2+ mobilization in HUVECs and mediated release of VWF and P-selectin. Cells were plated into 96-well plates and stimulated with indicated concentrations of agonists, and Ca2+ was measured fluorometrically. Represented is stimulation with indicated doses of thrombin (A) and with indicated concentrations of PAR1-AP (B). The y-axes represent the relative fluorescence units. Data represent the mean ± SEM of 2 independent experiments for histamine and 3 independent experiments for PAR2-AP. (C-D) Dose-response curves were generated using the area under the curve function in SOFTmax PRO and then normalized to the fraction of maximal response and plotted with the data from Figure 2 to graphically represent the different potencies between agonists with respect to Ca2+ mobilization and release of VWF and P-selectin.
The ability of PAR2-AP to stimulate Ca2+ mobilization to a similar degree as histamine, PAR1-AP, or thrombin, while triggering only minimal release of P-selectin, suggests that alternative Ca2+-independent pathways are involved in the regulation of endothelial release of P-selectin. To further investigate the role of Ca2+ mobilization, HUVECs were treated with the cell-permeable Ca2+ chelator, BAPTA-AM. Ca2+ mobilization studies demonstrate that BAPTA-AM (10 μM) completely inhibits agonist-mediated Ca2+ mobilization (Figure 3E). Preincubation with BAPTA-AM significantly inhibited the effect of PAR1-AP and thrombin on VWF release (85% and 75%, respectively), whereas it had only a modest effect on thrombin and PAR1-AP–mediated cell-surface P-selectin expression (50% and 28%, respectively; Figure 3F). Ca2+ chelation inhibited PAR2-AP stimulation of both VWF and P-selectin release by 39% and 34%, respectively, when compared with control. These data are consistent with the hypothesis that PAR2 activation stimulates additional pathways that regulate the release of both VWF and P-selectin.
Regulated secretion of soluble P-selectin by PARs in HUVECs
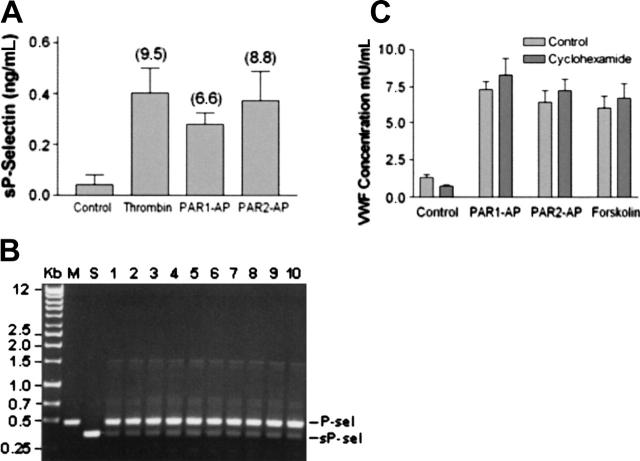
One alternative explanation for the disparate release of VWF and P-selectin caused by PAR1-AP and PAR2-AP is that activation of PAR2 might lead to the preferential release of soluble P-selectin. To address this issue, soluble P-selectin was measured in the medium after stimulation with thrombin, PAR1-AP, or PAR2-AP. Stimulation of HUVECs with thrombin or PAR1-AP or PAR2-AP resulted in a significant increase in soluble P-selectin when compared with control. However, no significant difference was observed when comparing the different agonists (Figure 5A). This suggests that the relatively poor ability of PAR2-AP to stimulate cell-surface expression of P-selectin when compared with PAR1-AP is not secondary to the preferential release of soluble P-selectin by PAR2-AP.
Figure 5.
Regulated secretion of soluble P-selectin from HUVECs. (A) HUVECs were stimulated with thrombin (10 nM), PAR1-AP (100 μM), or PAR2-AP (200 μM) for 20 minutes after which the conditioned medium was collected and assayed for soluble P-selectin. Data represent the mean ± SEM of 3 independent experiments conducted in triplicate. There was no statistical difference between the agonists (P > .05 as determined by 2-tailed t test). (B) Membrane-bound P-selectin predominates over soluble P-selectin in HUVECs. Shown are agarose gel electrophoresis of PCR-amplified P-selectin and soluble P-selectin fragments from HUVECs. Lane M represents PCR amplification of P-selectin constructed into pECFP, whereas lane S represents PCR amplification of soluble P-selectin constructed into pECFP. Lanes 1 to 10 represent PCR amplification using cDNA obtained from HUVECs (10 different cord preparations) as templates. (C) Protein synthesis inhibition does not decrease forskolin or PAR2-AP stimulation of VWF release. HUVECs were preincubated with cycloheximide (10 μM) or vehicle for 30 minutes prior to stimulation with vehicle, PAR1-AP (100 μM), PAR2-AP (200 μM), or forskolin (20 μM with 100μM IBMX). Medium was collected after 30 minutes and assayed for VWF. Values represent the mean ± SEM of an experiment conducted 3 times.
The source of soluble P-selectin is controversial and thought to be either derived from proteolytic removal or shedding of P-selectin from cell-surface P-selectin or derived from alternatively spliced P-selectin that lacks the transmembrane domain.34,35 To address the source of soluble P-selectin in our population of HUVECs, we first cloned the alternatively spliced isoform of P-selectin from HUVEC total RNA. PCR primers were then designed to amplify the region encoding the transmembrane domain so that the 120–base pair difference in size between the full-length and the alternatively spliced different HUVEC preparations of total RNA yielded 2 major bands that corresponded with the full-length and the alternatively spliced isoform of P-selectin. The full-length isoform predominated and, based on our semiquantitative analysis, appears to be present in a 3- to 5-fold excess when compared with the alternatively spliced isoform (Figure 5B).
Because of the ongoing synthesis of VWF evident by the perinuclear staining in HUVECs (Figure 6) and the delayed PAR2-AP–mediated release of VWF (Figure 1A), we needed to exclude the possibility that activation of PAR2 leads to increased synthesis of VWF and subsequent release of VWF via the constitutive pathway. Inhibition of protein synthesis with cycloheximide caused a small decrease in the basal release of VWF but did not decrease forskolin-, PAR1-, or PAR2-AP–mediated release of VWF and caused a small increase in VWF release, which is consistent with previous studies conducted with forskolin (Figure 5C).25
Figure 6.
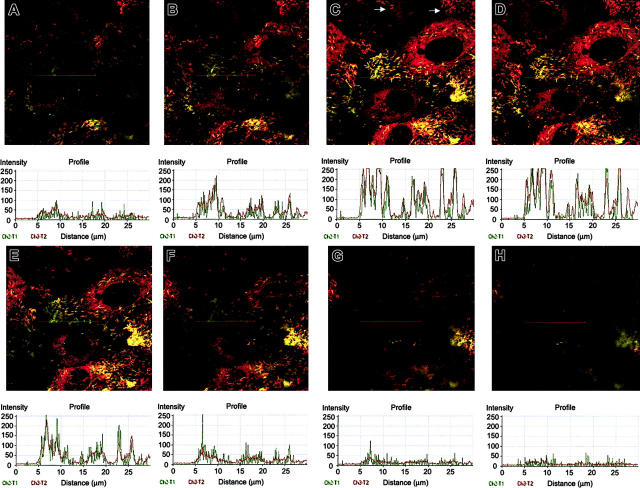
Sequential imaging of HUVECs immunostained for P-selectin and VWF. Shown starting at the apical surface (A-H) and extending to the basal surface imaged every 0.5 μm are z-sections of merged images depicting colocalization of VWF and P-selectin. Histograms were generated using the profile feature in the Zeiss LSM image browser and represent the colocalization of P-selectin and VWF when graphed objectively through the red line. Note that when certain granules appear to be composed of entirely P-selectin (green) and when examined objectively, there is underlying VWF (red). Examination of at least 6 to 8 additional fields yielded similar results. Images were obtained by viewing through a × 63 objective.
Role of cAMP in PAR-mediated release of VWF and P-selectin
PAR2-AP induced a delayed release of VWF and modest cell-surface expression of P-selectin compared with PAR1-AP. This more closely resembled the pattern of forskolin. Because PAR2 has been reported to increase cAMP in certain human cell lines,36 one hypothesis for PAR2-AP's similarity to forskolin might be that PAR2 couples to the Gs/adenylyl cyclase pathway to increase cAMP levels. To address this possibility, we measured agonist-mediated rises in intracellular cAMP in HUVECs. Forskolin caused a 3-fold increase in cAMP levels, whereas isoproterenol caused approximately a 60% to 80% increase (Figure 7A), which correlated with the ability of forskolin to increase VWF levels to a greater extent than isoproterenol (Figure 1B). PAR2-AP failed to increase global intracellular cAMP (Figure 7B). Time-course studies also showed that PAR2-AP did not cause an increase in global cAMP (data not shown). However, inhibition of protein kinase A with H-89 resulted in a 60% decrease in forskolin-mediated VWF release and a 43% decrease in PAR2-AP–mediated VWF release, while not statistically altering PAR1-AP–mediated release of VWF (Figure 7D). Pretreatment with forskolin did not alter PAR1-AP–mediated release of P-selectin but did cause a small but statistically significant decrease (17%) in PAR2-AP–mediated release of P-selectin. However, H-89 did not alter PAR1- or PAR2-AP–mediated release of P-selectin (Figure 7D). In addition, pretreatment with cell-permeable cAMP analogs did not alter PAR1-AP–mediated P-selectin release (data not shown).
Selective release of Weibel-Palade bodies that contain predominately VWF by forskolin and PAR2-AP in HUVECs
The differential responses in the release of VWF and cell-surface expression of P-selectin motivate the hypothesis that VWF and P-selectin are not stored in the same secretory granule (WPB). Differential pathways of VWF and t-PArelease in HUVECs with respect to pertussis toxin sensitivity and levels of intracellular Ca2+ have been described.37 These functional differences may have a structural explanation, because it has been observed that t-PA is stored in small dense vesicles distinct from WPBs.38 However, this observation is controversial because other studies have shown that t-PAis stored in WPBs in HUVECs.39-41 Indeed, some of the observed differences in localization of t-PA have been ascribed to subtle differences in tissue culture methods.39 Accordingly, we examined the localization of VWF and P-selectin in our cell-culture system by performing immunostaining and confocal microscopy. The majority of P-selectin is localized with VWF in the characteristic WPBs in HUVECs; however, some granules appear to be composed of primarily P-selectin (Figure 6, see red line). However, further analysis of the cross-sections (z-sections) reveals intimate colocalization at every level when examined more objectively with the histogram (Figure 6). In contrast, some granules appear to be composed of primarily VWF (Figure 6 and Figure 8A, see white arrows), and, when examined objectively, only stain for VWF (data not shown).
Figure 8.
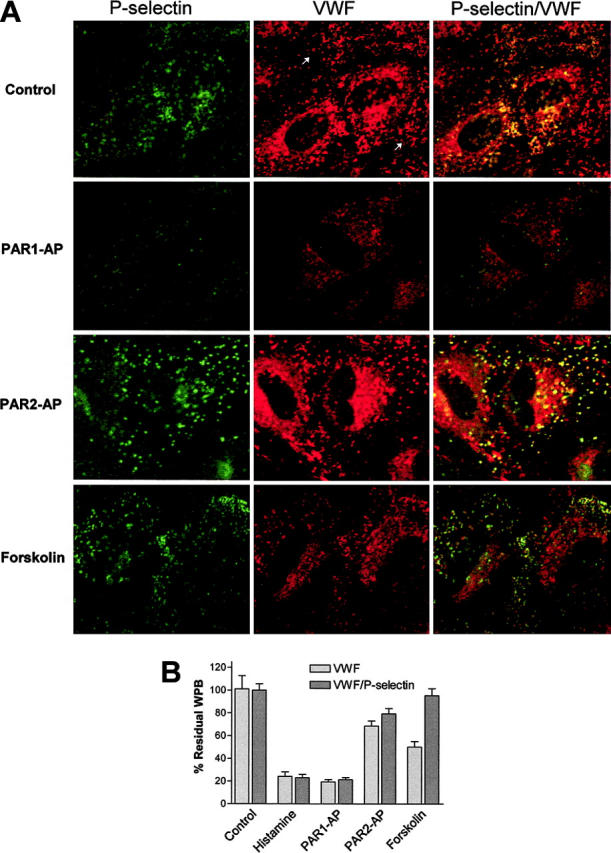
PAR2-AP and forskolin stimulate selective release of Weibel-Palade bodies only containing von Willebrand factor. (A) Projections were taken from HUVECs incubated with monoclonal mouse anti-VWF antibodies (VWF) and rabbit anti–P-selectin antibodies. The first columns or the P-selectin column represent images as projected through the FITC filter (548 nm), whereas the VWF columns are images as projected through the Texas Red channel (488 nm) and represent VWF, and the VWF/P-selectin columns are merged images with yellow representing colocalization of VWF and P-selectin. Negative controls consisting of incubation with both second-degree fluorescently labeled Abs only or incubation with only first-degree Abs failed to demonstrate appreciable fluorescence. Images obtained are representative of at least 6 to 8 fields viewed of an experiment that was performed 3 times. Cells were stimulated with indicated agonists for 20 minutes with PAR1-AP (100 μM), PAR2-AP (200 μM), or forskolin (10 μM) and then fixed and are representative of 3 independent experiments. In addition, HUVECs were stimulated with 20 μM histamine (data not shown). (B) The WPBs were counted after stimulation with the above-listed agonists. WPBs counted per cell for each agonist tested were normalized to control and expressed as the percentage of control. At least 10 to 12 fields at × 63 magnification per agonist tested were used to count the WPBs.
One explanation for the ability of PAR2-AP and forskolin to cause selective release of VWF and only minimal release of P-selectin is that activation of PAR2 and increases in intracellular cAMP cause the selective release of WPBs that contain predominately VWF. To test this hypothesis, HUVECs were stimulated with thrombin, PAR1-AP, PAR2-AP, histamine, or forskolin and then immunostained for VWF and P-selectin (Figure 8A), and the number of WPBs was then quantified (Figure 8B). Through the use of confocal image analysis software (MetaMorph) it was determined that nearly all of the WPBs that stained positive for P-selectin also had underlying VWF, which correlated with the data in Figure 6. In contrast, there exists a population of WPBs that contain only VWF. Therefore, WPBs counted as green were characterized as containing both P-selectin and VWF, whereas those staining red for VWF were characterized as containing VWF. As demonstrated in Figure 8, the response to PAR2-AP is similar to forskolin because there are fewer WPBs containing VWF than control. Application of forskolin resulted in virtually no change in the population of WPBs that contain both P-selectin and VWF. Stimulation with PAR2-AP resulted in a small decrease in WPBs containing both P-selectin and VWF, which more resembled the response of forskolin than PAR-1. In contrast, stimulation with PAR1-AP or histamine depletes both populations of WPBs, which correlates with pharmacologic data (see “Results”).
Discussion
Our studies have demonstrated differential effects on secretion of VWF and P-selectin. This suggests that VWF and P-selectin are not always coreleased. The ability of PAR2 to stimulate selective release of VWF, while causing only minimal release of P-selectin, has shown to be based on its ability to stimulate cAMP and PKA. Although PAR2-AP did not increase global cAMP in HUVECs, PKA inhibition partially attenuated the release of VWF, suggesting that a common downstream pathway is shared between PAR2 and cAMP. Raising intracellular cAMP levels by the addition of forskolin did not alter the ability of PAR1-AP to stimulate P-selectin cell-surface expression, which suggests that cAMP does not inhibit P-selectin release (Figure 5D). Immunostaining studies demonstrated that PAR2 or forskolin causes selective release of WPBs that only contain VWF. These findings suggest that there are distinct populations of WPBs that can be differentially released. Indeed, real-time studies performed with a green-fluorescent protein-VWF fusion protein transfected into HUVECs revealed striking differences in the movement patterns of resting WPBs.42 In the same study, cAMP-raising agonists led to grouping of a subset of WPBs in the perinuclear region that escaped secretion, whereas this functional sequestration was not observed with phorbol ester or thrombin stimulation.42 In addition, it has been shown that cAMP mediates secretion of VWF from peripheral WPBs compared with thrombin/[Ca2+]i-mediated release of both central and peripheral VWF granules, suggesting differences in the mechanism of release of WPBs between the 2 pathways.43 Our findings not only support this mechanistic difference between thrombin/[Ca2+]i- and cAMP-mediated release of WPB but also suggest that WPBs are heterogeneous in nature and can be differentially regulated.
Responses to PAR2-AP differ not only in the time course of VWF secretion compared with PAR1-AP but also in the reduced ability to release P-selectin, as well as sensitivity to protein kinase A inhibition. These findings, taken together, are indicative of differential signaling between the 2 G-protein–coupled PARs. Our findings suggest that the PAR2-AP more closely approximates cAMP-raising agents (ie, forskolin) with respect to its signaling pattern. PAR2-AP has been shown to increase global cAMP levels in melanocytes36; however, in HUVECs PAR2-AP had no such affect (Figure 5). Yet, the sensitivity of PAR2-AP stimulation of VWF release to protein kinase A inhibition suggests that PAR2-AP may increase local levels of cAMP. Evidence does exist that cAMP signals can be compartmentalized within a cellular domain rather than acting in a uniform and global manner.44,45 Expression of HUVEC-specific AKAPs or phosphodiesterases may mediate such local cAMP signaling.
Another alternative explanation for the reduced expression of P-selectin observed with PAR2-AP activation is that PAR2 activation causes preferential release of soluble P-selectin. However, this is unlikely because no difference was observed between the PAR agonists in their ability to stimulate release of soluble P-selectin as demonstrated in Figure 4A. Furthermore, to our knowledge, this is the first study to describe the regulated secretion of soluble P-selectin in human endothelial cells. Soluble P-selectin has been reported in the conditioned media of HUVECs,46 but the ability of agonists to stimulate acute release of soluble P-selectin has not been reported. This may be secondary to the low levels of expression of soluble P-selectin present in HUVECs. Even with the relative small amount of soluble P-selectin release, given the increasing realization of the role of soluble P-selectin in both platelet and leukocyte biology, the novel demonstration of regulated soluble P-selectin from human endothelial cells is of importance.
The time course of VWF release is delayed compared with P-selectin, which has been attributed to solubilization of VWF strings or ordered multimers of VWF. We have observed the delayed release of VWF compared with P-selectin irrespective of the agonist used (Figure 1). It has been proposed that VWF multimers bind to P-selectin on the endothelial surface and anchor this highly thrombogenic form of VWF.23 The delayed release of VWF observed with PAR2-AP and elevations of cAMP compared with thrombin, PAR1-AP, or histamine cannot be explained by an increase in VWF multimers binding to the cell membrane because the amount of VWF associated with endothelial surface is an order of magnitude less compared with the amount released (data not shown).
The relatively poor ability of PAR2-AP to stimulate cell-surface expression of P-selectin in HUVECs gives further insight into the role of PAR2 in inflammation. Depending on the tissue and the vasculature, PAR2 can assume either a protective (anti-inflammatory) or proinflammatory role.15,47 The administration of PAR2-AP in rats leads to increased leukocyte margination and infiltration in vivo.48,49 However, whether PAR2-induced leukocyte recruitment is secondary to leukocyte or endothelial activation is unknown. Indeed, it has been shown that PAR2 is strongly expressed in leukocytes.50 The present findings together with the results of Riewald et al,51 demonstrating that PAR2 does not lead to increased monocyte chemoattractant protein-1 expression and secretion, suggest that the proinflammatory effects of PAR2 activation with respect to leukocyte recruitment do not involve the endothelium. Further evidence that PAR2 produces less of an inflammatory effect in endothelial cells is derived from the observation that PAR2 leads to less vascular permeability changes than PAR1.32,52
Weibel-Palade bodies also contain premature von Willebrand factor, interleukin-8, endothelin-1, α1,3 fucosyltransferase, CD63,53,54 and possibly tissue plasminogen activator (t-PA).40,55 Our findings raise the question of whether the other contents of WPBs also display differential signaling. Endothelial t-PA is released in response to cAMP-raising agents, suggesting that t-PA secretion more closely resembles VWF than P-selectin. However, as mentioned in “Results,” it is controversial whether t-PA is colocalized with VWF in WPBs. Characterization of secretion of other vasoactive agents from WPBs in HUVECs is lacking.
The relative poor ability of cAMP agents to cause release of P-selectin also can be viewed in the context of cAMP's known ability to increase barrier function between endothelial cells. Agonists that increase cAMP in the endothelium would not only cause poor release of P-selectin and have a reduced ability to cause leukocyte adhesion, but also those leukocytes that did manage to adhere would face an increase in the endothelium barrier that would prevent infiltration. The relative inability of forskolin to cause cell-surface expression of P-selectin has pharmacologic consequences, for desmopressin is used in the treatment of certain variants of von Willebrand disease as a means to achieve hemostasis. One could extrapolate that activation of vasopressin-2 receptors on the endothelium, which couple to adenylyl cyclase and increase cAMP, cause a smaller release of P-selectin and subsequent inflammation.
In conclusion, we clearly demonstrate that VWF and P-selectin, both of which reside in the WPBs, can be differentially regulated by PAR2-AP and forskolin. Clearly, the endothelial secretory response is not binary and can be influenced by multiple factors, including the nature of the stimulus, receptors involved in downstream signaling, and packaging.
Acknowledgments
We thank Joe Covington for preparation of HUVECs and Sam Wells in the VUMC Cell Imaging Core Resource for assistance in the analysis of the confocal imaging.
Prepublished online as Blood First Edition Paper, December 6, 2005; DOI 10.1182/blood-2004-07-2698.
Supported by the American Heart Association (0325373) (J.H.C.) and by the National Institutes of Health (NIH) (HL60678) (H.E.H.). Confocal imaging was performed in part through the use of the VUMC Cell Imaging Core Resource (supported by NIH grants CA68485, DK20593, and DK58404).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407: 258-264. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson GA. Pathophysiology of platelet thrombin receptors. Thromb Haemost. 1997;78: 242-246. [PubMed] [Google Scholar]
- 3.Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. 2003;1: 1335-1342. [DOI] [PubMed] [Google Scholar]
- 4.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74: 541-554. [DOI] [PubMed] [Google Scholar]
- 5.Montalescot G, Philippe F, Ankri A, et al. Early increase of von Willebrand factor predicts adverse outcome in unstable coronary artery disease: beneficial effects of enoxaparin. French Investigators of the ESSENCE Trial. Circulation. 1998;98: 294-299. [DOI] [PubMed] [Google Scholar]
- 6.Collet JP, Montalescot G, Vicaut E, et al. Acute release of plasminogen activator inhibitor-1 in ST-segment elevation myocardial infarction predicts mortality. Circulation. 2003;108: 391-394. [DOI] [PubMed] [Google Scholar]
- 7.Dong ZM, Brown AA, Wagner DD. Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation. 2000;101: 2290-2295. [DOI] [PubMed] [Google Scholar]
- 8.Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191: 189-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blann AD, Lip GY. Hypothesis: is soluble P-selectin a new marker of platelet activation? Atherosclerosis. 1997;128: 135-138. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama S, Ikeda H, Haramaki N, Yasukawa H, Murohara T, Imaizumi T. Platelet P-selectin plays an important role in arterial thrombogenesis by forming large stable platelet-leukocyte aggregates. J Am Coll Cardiol. 2005;45: 1280-1286. [DOI] [PubMed] [Google Scholar]
- 11.Merten M, Thiagarajan P. P-selectin in arterial thrombosis. Z Kardiol. 2004;93: 855-863. [DOI] [PubMed] [Google Scholar]
- 12.Cambien B, Wagner DD. A new role in hemostasis for the adhesion receptor P-selectin. Trends Mol Med. 2004;10: 179-186. [DOI] [PubMed] [Google Scholar]
- 13.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24: 2166-2179. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt VA, Nierman WC, Maglott DR, et al. The human proteinase-activated receptor-3 (PAR-3) gene. Identification within a Par gene cluster and characterization in vascular endothelial cells and platelets. J Biol Chem. 1998;273: 15061-15068. [DOI] [PubMed] [Google Scholar]
- 15.Coughlin SR, Camerer E. PARticipation in inflammation. J Clin Invest. 2003;111: 25-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nystedt S, Ramakrishnan V, Sundelin J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J Biol Chem. 1996;271: 14910-14915. [DOI] [PubMed] [Google Scholar]
- 17.Blackhart BD, Emilsson K, Nguyen D, et al. Ligand cross-reactivity within the protease-activated receptor family. J Biol Chem. 1996;271: 16466-16471. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerman BJ, Paulson JC, Arrhenius TS, Gaeta FC, Granger DN. Thrombin receptor peptide-mediated leukocyte rolling in rat mesenteric venules: roles of P-selectin and sialyl Lewis X. Am J Physiol. 1994;267: H1049–1053. [DOI] [PubMed] [Google Scholar]
- 19.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53: 245-282. [PubMed] [Google Scholar]
- 20.McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84: 92-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hattori R, Hamilton KK, Fugate RD, McEver RP, Sims PJ. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989;264: 7768-7771. [PubMed] [Google Scholar]
- 22.Denis CV, Andre P, Saffaripour S, Wagner DD. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98: 4072-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padilla A, Moake JL, Bernardo A, et al. P-selectin anchors newly released ultralarge von Willebrand factor multimers to the endothelial cell surface. Blood. 2004;103: 2150-2156. [DOI] [PubMed] [Google Scholar]
- 24.Tranquille N, Emeis JJ. On the role of calcium in the acute release of tissue-type plasminogen activator and von Willebrand factor from the rat perfused hindleg region. Thromb Haemost. 1991;66: 479-483. [PubMed] [Google Scholar]
- 25.Vischer UM, Wollheim CB. Epinephrine induces von Willebrand factor release from cultured endothelial cells: involvement of cyclic AMP-dependent signalling in exocytosis. Thromb Haemost. 1997;77: 1182-1188. [PubMed] [Google Scholar]
- 26.Hegeman RJ, van den Eijnden-Schrauwen Y, Emeis JJ. Adenosine 3′:5′-cyclic monophosphate induces regulated secretion of tissue-type plasminogen activator and von Willebrand factor from cultured human endothelial cells. Thromb Haemost. 1998;79: 853-858. [PubMed] [Google Scholar]
- 27.Vischer UM, Wollheim CB. Purine nucleotides induce regulated secretion of von Willebrand factor: involvement of cytosolic Ca2+ and cyclic adenosine monophosphate-dependent signaling in endothelial exocytosis. Blood. 1998;91: 118-127. [PubMed] [Google Scholar]
- 28.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52: 2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boss V, Wang X, Koppelman LF, Xu K, Murphy TJ. Histamine induces nuclear factor of activated T cell-mediated transcription and cyclosporin A-sensitive interleukin-8 mRNA expression in human umbilical vein endothelial cells. Mol Pharmacol. 1998;54: 264-272. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn B, Schmid A, Harteneck C, Gudermann T, Schultz G. G proteins of the Gq family couple the H2 histamine receptor to phospholipase C. Mol Endocrinol. 1996;10: 1697-1707. [DOI] [PubMed] [Google Scholar]
- 31.Birch KA, Ewenstein BM, Golan DE, Pober JS. Prolonged peak elevations in cytoplasmic free calcium ions, derived from intracellular stores, correlate with the extent of thrombin-stimulated exocytosis in single human umbilical vein endothelial cells. J Cell Physiol. 1994;160: 545-554. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin JN, Shen L, Holinstat M, Brooks JD, Dibenedetto E, Hamm HE. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005;280: 25048-25059. [DOI] [PubMed] [Google Scholar]
- 33.Lafleur MA, Hollenberg MD, Atkinson SJ, Knauper V, Murphy G, Edwards DR. Activation of pro-(matrix metalloproteinase-2) (pro-MMP-2) by thrombin is membrane-type-MMP-dependent in human umbilical vein endothelial cells and generates a distinct 63 kDa active species. Biochem J. 2001;357: 107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishiwata N, Takio K, Katayama M, et al. Alternatively spliced isoform of P-selectin is present in vivo as a soluble molecule. J Biol Chem. 1994;269: 23708-23715. [PubMed] [Google Scholar]
- 35.Johnston GI, Bliss GA, Newman PJ, McEver RP. Structure of the human gene encoding granule membrane protein-140, a member of the selectin family of adhesion receptors for leukocytes. J Biol Chem. 1990;265: 21381-21385. [PubMed] [Google Scholar]
- 36.Scott G, Leopardi S, Parker L, Babiarz L, Seiberg M, Han R. The proteinase-activated receptor-2 mediates phagocytosis in a Rho-dependent manner in human keratinocytes. J Invest Dermatol. 2003;121: 529-541. [DOI] [PubMed] [Google Scholar]
- 37.van den Eijnden-Schrauwen Y, Atsma DE, Lupu F, de Vries RE, Kooistra T, Emeis JJ. Involvement of calcium and G proteins in the acute release of tissue-type plasminogen activator and von Willebrand factor from cultured human endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17: 2177-2187. [DOI] [PubMed] [Google Scholar]
- 38.Emeis JJ, van den Eijnden-Schrauwen Y, van den Hoogen CM, de Priester W, Westmuckett A, Lupu F. An endothelial storage granule for tissue-type plasminogen activator. J Cell Biol. 1997;139: 245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huber D, Cramer EM, Kaufmann JE, et al. Tissue-type plasminogen activator (t-PA) is stored in Weibel-Palade bodies in human endothelial cells both in vitro and in vivo. Blood. 2002;99: 3637-3645. [DOI] [PubMed] [Google Scholar]
- 40.Rosnoblet C, Vischer UM, Gerard RD, Irminger JC, Halban PA, Kruithof EK. Storage of tissue-type plasminogen activator in Weibel-Palade bodies of human endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19: 1796-1803. [DOI] [PubMed] [Google Scholar]
- 41.Datta YH, Youssoufian H, Marks PW, Ewenstein BM. Targeting of a heterologous protein to a regulated secretion pathway in cultured endothelial cells. Blood. 1999;94: 2696-2703. [PubMed] [Google Scholar]
- 42.Romani De Wit T, Rondaij MG, Hordijk PL, Voorberg J, Van Mourik JA. Real-time imaging of the dynamics and secretory behavior of weibel-palade bodies. Arterioscler Thromb Vasc Biol. 2003;23: 755-761. [DOI] [PubMed] [Google Scholar]
- 43.Vischer UM, Barth H, Wollheim CB. Regulated von Willebrand factor secretion is associated with agonist-specific patterns of cytoskeletal remodeling in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20: 883-891. [DOI] [PubMed] [Google Scholar]
- 44.Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci U S A. 2001;98: 13049-13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz JH. The many dimensions of cAMP signaling. Proc Natl Acad Sci U S A. 2001;98: 13482-13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semenov AV, Romanov YA, Loktionova SA, et al. Production of soluble P-selectin by platelets and endothelial cells. Biochemistry (Mosc). 1999;64: 1326-1335. [PubMed] [Google Scholar]
- 47.Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci. 2001;22: 146-152. [DOI] [PubMed] [Google Scholar]
- 48.Vergnolle N, Hollenberg MD, Sharkey KA, Wallace JL. Characterization of the inflammatory response to proteinase-activated receptor-2 (PAR2)-activating peptides in the rat paw. Br J Pharmacol. 1999;127: 1083-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vergnolle N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol. 1999;163: 5064-5069. [PubMed] [Google Scholar]
- 50.Howells GL, Macey MG, Chinni C, et al. Proteinase-activated receptor-2: expression by human neutrophils. J Cell Sci. 1997;110(Pt 7): 881-887. [DOI] [PubMed] [Google Scholar]
- 51.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296: 1880-1882. [DOI] [PubMed] [Google Scholar]
- 52.Klarenbach SW, Chipiuk A, Nelson RC, Hollenberg MD, Murray AG. Differential actions of PAR2 and PAR1 in stimulating human endothelial cell exocytosis and permeability: the role of Rho-GTPases. Circ Res. 2003;92: 272-278. [DOI] [PubMed] [Google Scholar]
- 53.Hannah MJ, Williams R, Kaur J, Hewlett LJ, Cutler DF. Biogenesis of Weibel-Palade bodies. Semin Cell Dev Biol. 2002;13: 313-324. [DOI] [PubMed] [Google Scholar]
- 54.van Mourik JA, Romani de Wit T, Voorberg J. Biogenesis and exocytosis of Weibel-Palade bodies. Histochem Cell Biol. 2002;117: 113-122. [DOI] [PubMed] [Google Scholar]
- 55.van den Eijnden-Schrauwen Y, Lakenberg N, Emeis JJ, de Knijff P. Alu-repeat polymorphism in the tissue-type plasminogen activator (tPA) gene does not affect basal endothelial tPA synthesis [letter]. Thromb Haemost. 1995;74: 1202. [PubMed] [Google Scholar]