Abstract
To improve production of functional fully γ-carboxylated recombinant human clotting factor IX (r-hFIX), cell lines stably overexpressing r-hFIX have been engineered to also overexpress proteins of the γ-carboxylation system. Here we demonstrate that siRNA silencing of calumenin, an inhibitor of the γ-carboxylation system, enhances production of functional r-hFIX produced by engineered BHK21 cells. The production yield of functional r-hFIX was 80% in engineered cells where calumenin had been silenced 78%. We propose that this high-yield expression system can easily be adapted to overproduce functional forms of all members of the vitamin K–dependent protein family.
Introduction
In the blood coagulation system, 7 proteins are dependent on the vitamin K–dependent γ-carboxylation modification,1,2 and fully modified and functional recombinant coagulation factors IX and VII and protein C have become important pharmaceuticals in treatment of hemophilia B, thrombosis, and sepsis.3-5 However, the yield of functional recombinant proteins recovered from media from cells overexpressing vitamin K–dependent coagulation factors has been reported to be less than 10%.6 With cloning of the γ-carboxylase,7,8 there was hope that enhanced expression of recombinant γ-carboxylase would increase synthesis and recovery of functional recombinant factor IX. On the contrary, cotransfection with a γ-carboxylase cDNA construct resulted, unexpectedly, in a significant decrease in recovery.9 We have reproduced this finding,10 but the mechanism of inhibition still remains unknown.
In this article, we report our work on engineering a eukaryotic cell with enhanced capacity to produce fully γ-carboxylated functional recombinant human factor IX (r-hFIX). Successful engineering was accomplished by overexpressing VKORC1 in BHK cells stably overexpressing r-hFIX. VKORC1 is proposed to be an 18-kDa subunit of the warfarin-sensitive enzyme complex vitamin K1 2,3-epoxide reductase (VKOR) embedded in the endoplasmic reticulum (ER) membrane.7,11,12 VKOR provides the γ-carboxylase with reduced vitamin K1 (VitK1H2) cofactor13 and is a rate-limiting step in vitamin K–dependent posttranslational γ-carboxylation.14-17 Previously, we have identified the ER chaperone protein calumenin as an inhibitor of vitamin K–dependent γ-carboxylation.12,17 In this paper, we show that siRNA silencing of calumenin in BHK cells engineered to overexpress VKORC1 and r-hFIX significantly increases production of functional r-hFIX.
Materials and methods
Engineering of BHK cell lines overexpressing r-hFIX and proteins of the vitamin K–dependent γ-carboxylation system
Cloning of VKORC1 and γ-carboxylase cDNAs into the dual promoter vector plasmid pBUDCE4.1 (Invitrogen, Carlsbad, CA) and construction of the pLXIN retroviral vector (Clontech, Palo, CA) containing a human FIX construct as well as selection of clones of cells stably overexpressing the recombinant proteins are published in recent articles from our laboratory. As documented in the published studies,10,17,18 it was found that BHK cells stably overexpressing r-hFIX and VKORC1 produced the highest amount of functional r-hFIX. These cells were selected for the studies described in this work.
Silencing of the γ-carboxylation inhibitor calumenin in BHK cells
Hamster calumenin was cloned by our laboratory17 using standard technology and the cDNA sequenced on both strands to eliminate cloning errors. The sequence has been deposited in GenBank under the accession no. gi:63148518. We provided Dharmacon RNA Technologies (Lafayette, CO) with the hamster calumenin cDNA sequence and ordered the siRNA SMART pool containing 50 nmol of a mixture of 4 oligonucleotides with potential for hamster calumenin mRNA destruction by RISC complexes. Transfection of the VKORC1 + r-hFIX–overproducing BHK cell line with the siRNA SMART pool oligonucleotides was carried out with Lipofectamine (Invitrogen) according to the protocol provided by the company. As recommended by Dharmacon RNA Technologies, siRNA against human GAPDH was used as a positive control, and a negative control consisted of a mixture of 4 scrambled siRNAs. Following transfection, cells were grown in DMEM containing 10% fetal bovine serum, 500 μg/mL G418, and 400 μg/mL Zeocin for 24 hours. The attached cells were then washed 2 times with PBS and continued growing in DMEM without serum but with the addition of 5 μg/mL vitamin K1 (AquaMEPHYTON; Merck, Whitehouse, NJ). After 24 hours in the serum-free medium, medium was collected for r-hFIX purification and cells were harvested for VKOR and γ-carboxylase activity measurements.
Purification of functional and nonfunctional r-hFIX from cell medium
Preparation of conformational-specific and nonconformational-specific immunoaffinity columns for purification of functional and nonfunctional r-hFIX, respectively, has been described recently in great detail by our laboratory for use in a tandem-designed chromatography system for isolation of the 2 different pools of r-hFIX.10 Purification of fully γ-carboxylated r-hFIX, with coagulation factor activity, is based upon the conformational change that the Gla region in the protein undergoes in the presence of Ca++ and the availability of antibodies that specifically recognize the Ca++-induced conformation.6,10 The nonfunctional r-hFIX proteins in the medium were purified on an anti–human factor IX affinity column that recognizes all forms of human factor IX.10 Cellular production of functional r-hFIX is reported as percentage of total r-hFIX produced by the cells.10 As described in our previous article,10 digitized images of immunoreactive protein bands of functional r-hFIX and nonfunctional r-hFIX on FUJI Medical X-Ray Film SuperRX (Fisher Scientific, Pittsburgh, PA) were analyzed with Kodak 1D software (Eastman Kodak, Rochester, NY) to determine the integrated areas representing the protein bands. For determination of r-hFIX protein content, standard curves of Western blots with known purified human FIX were established and compared with unknown samples. All measurements were adjusted to be in the linear range of the standard curve.
Immunoprecipitation, SDS-PAGE, and Western blotting
Cells were lysed on ice for 30 minutes in radioimmunoprecipitation (RIPA) buffer (10 mM Tris, 150 mM NaCl, 0.1% SDS, 1% Triton-X100, 1% deoxycholate, 5 mM EDTA, pH 7.2) containing 10 μg/mL of the Sigma Protease Inhibitor Cocktail for use with mammalian cell and tissue extracts (Protease Cocktail). After lysis, cell debris was removed by centrifugation at 12 000g for 30 minutes and the supernatant collected. Samples prepared for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) were boiled for 2 minutes in the presence of 5% mercaptoethanol (ME). Samples were loaded onto 8% to 16% CRITERION SDS-PAGE gels and electrophoresed proteins transferred to PVDF membranes for Western blotting.
Enzyme assays
Warfarin-sensitive VKOR activity was measured as described19 by estimating the percent conversion of vitamin K1 2,3-epoxide (VitK>O) to vitamin K1. γ-Carboxylase activity was assayed as described14 as 14CO2 incorporation into the synthetic peptide FLEEL (FLEEL γ-carboxylation). The reaction was triggered by adding chemically reduced VitK1H2 (100 μg/mL) to the assay mixture. Blood-clotting activities of purified r-hFIX and plasma hFIX were determined with the activated partial thromboplastin time (APTT) kit APTT-SP (liquid)-0020006300 from Instrumentation Laboratory (Lexington, MA). In order to determine the specific activity of r-hFIX, a standard curve of dilutions of purified plasma hFIX was made. One unit of hFIX activity was defined as the activity of hFIX in 1 mL pooled human plasma.
Materials
Affinity-purified anti–human factor IX conformational- and nonconformational-specific antibodies were prepared by our laboratory as described.10 Rabbit polyclonal affinity-purified anticalumenin antibodies were prepared by our laboratory as described.12 Bovine and human factor IX purified from plasma were purchased from Enzyme Research Laboratories (South Bend, IN). All other chemicals used were of highest quality.
Results and discussion
In a previous paper,10 r-hFIX–producing BHK cells were engineered to stably overexpress various proteins known to belong to the vitamin K–dependent γ-carboxylation system.20 Previously, we had also identified the ER chaperone protein calumenin as an inhibitor of chemically-reduced VitK1H2-triggered FLEEL γ-carboxylation in microsomes.17 Here, we set out to determine whether silencing of calumenin in our engineered BHK cells would improve production of functional r-hFIX.
siRNA SMART pool transfection
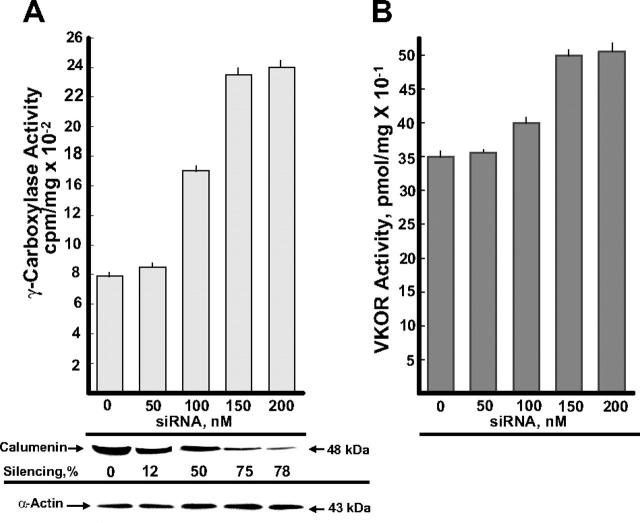
BHK cells stably overexpressing VKORC1 and r-hFIX were transfected with the various concentrations of the siRNA SMART pool shown in Figure 1. The effect of siRNA silencing of γ-carboxylase and VKOR activities is shown in Figure 1A and B, respectively, in addition to (1) Western blots of calumenin present in the different siRNA SMART pool–treated cells and (2) silencing of calumenin protein in percentage of the control (0 nM siRNA). In addition, blotting with an α-actin antibody is shown, which verified equal loading of protein in each lane. γ-Carboxylase activity measured as FLEEL γ-carboxylation increased 3-fold in cell cultures containing 150 to 200 nM of the siRNA SMART pool (Figure 1A). These concentrations of the siRNA SMART pool also had a smaller but significant activating effect on VKOR activity (Figure 1B). Transfection with siRNA against human GAPDH (positive control) and scrambled siR-NAs (negative control) (“Materials and methods”) provided the expected control data (not shown).
Figure 1.
Effect of calumenin siRNA silencing on enzyme activities of the vitamin K–dependent γ-carboxylation system. BHK cells engineered to overexpress r-hFIX and VKORC1 were transfected with the various concentrations of siRNA SMART pool against hamster calumenin shown in the figure (“Materials and methods”). After 48 hours, cells were harvested and tested for FLEEL γ-carboxylase activity triggered with chemically reduced VitK1H2 (A) and VKOR activity (B). Each bar represents the average activity of 3 parallel incubations, and standard deviations are shown on top of the bars. Western blots of calumenin (48 kDa) present in the various siRNA SMART pool–treated cells are shown as well as silencing of calumenin in percentage of the control with 0 nM siRNA. Western blotting of α-actin verifies equal protein loading in the various lanes.
siRNA SMART pool transfection and r-hFIX production
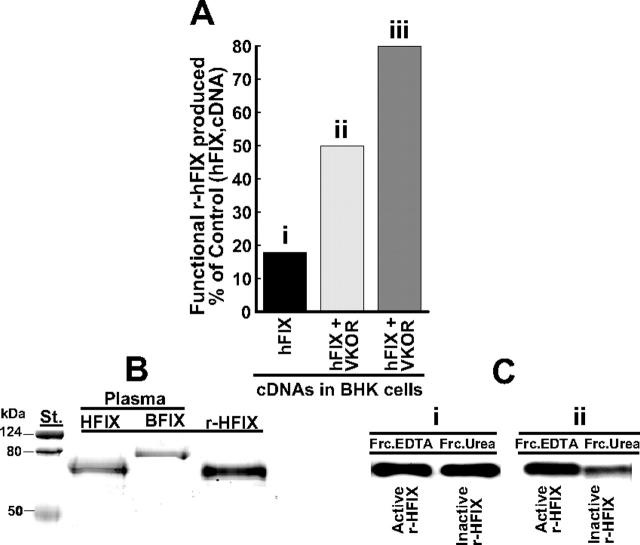
The new information we wanted from these experiments was to determine whether silencing of calumenin would enhance production of functional r-hFIX. Functional r-hFIX purified from the 200 nM calumenin siRNA SMART pool–containing medium had a specific activity of 147 units/mg, 12.1 ± 0.2 mol Gla/mol rhFIX, and the N-terminal sequence N-Tyr-Asn-Ser-Gly identical to human plasma factor IX. SDS-PAGE of the purified protein is shown in Figure 2B (r-HFIX), and factor IX purified from human and bovine plasmas is shown in Figure 2B, lanes HFIX and BFIX, respectively. The stained SDS-PAGE gel indicates that purified r-hFIX did not contain bovine FIX, which potentially could have been a contaminant from fetal bovine serum used in the early stages of culturing engineered BHK cells for r-hFIX production.10 Figure 2C shows Western blots of functional (FrcEDTA) and nonfunctional (FrcUrea) r-hFIX purified from r-hFIX + VKORC1–overexpressing BHK cells that had not been treated (Figure 2Ci) or had been treated (Figure 2Cii) with 200 nM of the siRNA SMART pool. The intensity of the blots shown in each lane is representative of the total isolated amount of protein present in the EDTA and urea fractions containing purified r-hFIX (“Materials and methods”). As can be gleaned from Figure 2C, significantly more functional r-hFIX (FrcEDTA) was produced by the r-hFIX + VKORC1–overexpressing and SMART pool–treated BHK cells (Figure 2Cii) than from the r-hFIX + VKORC1–overexpressing BHK cells where calumenin had not been silenced with the SMART pool (Figure 2Ci). Figure 2A shows functional r-hFIX produced in percentage of total r-hFIX produced by (1) r-hFIX–overexpressing (Figure 2Ai), (2) r-hFIX + VKORC1–overexpressing (Figure 2Aii), and (3) r-hFIX + VKORC1–overexpressing and SMART pool–treated (Figure 2Aiii) BHK cells. For panels i, ii, and iii shown as bar graphs in Figure 2A, the yields were 18%, 50%, and 80%, respectively. We conclude from these data that silencing of calumenin in the r-hFIX + VKORC1–overexpressing BHK cells significantly increased the cells' capacity to γ-carboxylate the pool of recombinant human factor IX appearing in the lumen of the ER. Consistent with previous results,10 we found that treating the cells with the siRNA SMART pool did not affect synthesis of r-hFIX. BHK cells stably overexpressing VKORC1 + r-hFIX and these cells treated with 200 nM of the siRNA SMART pool produced 17 ± 3 μg (n = 4) and 17 ± 2 μg (n = 4) r-hFIX/d/106 cells, respectively, which represent mixtures of nonfunctional and functional r-hFIX. Synthesis of functional r-hFIX by the VKORC1 + r-hFIX–overexpressing and siRNA SMART pool–treated BHK cells were estimated to be 13 μg/d per 106 cells in contrast 3 μg/d per 106 cells by BHK cells stably overexpressing only r-hFIX.
Figure 2.
Effect of calumenin siRNA silencing on production of functional r-hFIX by BHK cells engineered to overexpress r-hFIX and VKORC1. Functional and nonfunctional r-hFIX were purified from cell media obtained from BHK cells engineered to overexpress (1) r-hFIX, (2) r-hFIX + VKORC1, and (3) r-hFIX + VKORC1 that had been treated with 200 nM of the calumenin siRNA SMART pool (“Materials and methods”). (A) The production yield of functional r-hFIX in percentage of total r-hFIX produced by each of the 3 differently engineered cells. The x-axis identifies the various bars representing the differently engineered cells: (i) r-hFIX, (ii) r-hFIX + VKORC1, and (iii) r-hFIX + VKOC1 + siRNA (SMART pool). (B) Coomassie blue–stained factor IX proteins purified from (1) cell medium of BHK cells engineered to overexpress r-hFIX and VKORC1 and treated with 200 nM calumenin siRNA SMART pool (r-HFIX), (2) human plasma factor IX (HFIX), and (3) bovine plasma factor IX (BFIX). (C) Western blots of functional (FrcEDTA) and nonfunctional (FrcUrea) r-hFIX purified from (i) medium from BHK cells engineered to overexpress r-hFIX and VKORC1 and (ii) medium from the same engineered cells treated with 200 nM of the calumenin siRNA SMART pool. The blots were developed with a factor IX antibody that does not discriminate between active and inactive protein. Each purified protein was Western blotted such that each blot represents the total amount of protein isolated.
In summary, we demonstrate in this paper that BHK cells stably overexpressing VKORC1 and r-hFIX, when treated with 150 to 200 nM of the siRNA SMART pool against calumenin, produced significantly more functional r-hFIX coagulation factor as opposed to BHK cells relying on their endogenous vitamin K–dependent γ-carboxylation system for production. We conclude that we have created a recombinant eukaryotic cell system with high capacity for posttranslational vitamin K–dependent γ-carboxylation of proteins. Theoretically, all proteins of the vitamin K–dependent protein family20 could be highly produced as functional proteins by this cell system. As not pursued in this work, cell lines engineered to also stably overexpress calumenin siRNA could be productive cell systems for recombinant vitamin K–dependent proteins used as pharmaceuticals. Since gene deletions of members of the CREC family of proteins to which calumenin belongs have been shown to be lethal,21 cell lines deficient in the calumenin gene may not be functional.
Authorship
The authors declare no competing financial interests.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant RO1HL69331 (R.W.).
Prepublished online as Blood First Edition Paper, August 10, 2006; DOI 10.1182/blood-2006-02-004671.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
References
- 1.Furie B, Furie BC. The molecular basis of blood coagulation. Cell. 1988;53: 505-518. [DOI] [PubMed] [Google Scholar]
- 2.Forastiero RR, Martinuzzo ME, Lu J, Broze J. Autoimmune antiphospholipid antibodies impair the inhibition of activated factor X by protein Z/protein Z-dependent protease inhibitor. J Thromb Haemost. 2003;1: 1764-1770. [DOI] [PubMed] [Google Scholar]
- 3.Hedner U. Recombinant factor VIIa (Novoseven) as a hemostatic agent. Semin Hematol. 2001; 38(suppl 12): 43-47. [DOI] [PubMed] [Google Scholar]
- 4.Roth DA, Kessler CM, Pasi J, Rup B, Courter SG, Tubridy KL. Human recombinant factor IX: safety and efficacy studies in hemophilia B patients previously treated with plasma-derived factor IX concentrates. Blood. 2001;98: 3600-3606. [DOI] [PubMed] [Google Scholar]
- 5.Bernard GR, Vincent J-L, Laterre P-F, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001; 344: 699-709. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman RJ, Wasley LC, Furie BC, Furie B, Shoemaker CB. Expression, purification, and characterization of recombinant gamma-carboxylated factor IX synthesized in Chinese hamster ovary cells. J Biol Chem. 1986;261: 9622-9628. [PubMed] [Google Scholar]
- 7.Li T, Chang C-Y, Jin D-Y, Lin P-J, Khvorova A, Stafford D. Identification of the gene for vitamin K epoxide reductase. Nature. 2004;427: 541-544. [DOI] [PubMed] [Google Scholar]
- 8.Rost S, Fregin A, Ivaskevicius V, et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427: 537-541. [DOI] [PubMed] [Google Scholar]
- 9.Rehemtulla A, Roth DA, Wasley LC, et al. In vitro and in vivo functional characterization of bovine vitamin K-dependent gamma-carboxylase expressed in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1993;90: 4611-4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wajih N, Hutson SM, Owen J, Wallin R. Increased production of functional recombinant human clotting factor IX by baby hamster kidney cells engineered to overexpress VKORC1, the vitamin K 2,3-epoxide-reducing enzyme of the vitamin K cycle. J Biol Chem. 2005;280: 31603-31607. [DOI] [PubMed] [Google Scholar]
- 11.Goodstadt L, Ponting CP. Vitamin K epoxide reductase: homology, active site and catalytic mechanism. Trends Biochem Sci. 2004;29: 289-292. [DOI] [PubMed] [Google Scholar]
- 12.Wallin R, Hutson SM, Cain D, Sweatt A, Sane DC. A molecular mechanism for genetic warfarin resistance in the rat. FASEB J. 2001;15: 2542-2544. [DOI] [PubMed] [Google Scholar]
- 13.Wallin R, Hutson SM. Warfarin and the vitamin K-dependent gamma-carboxylation system. Trends Mol Med. 2004;10: 299-302. [DOI] [PubMed] [Google Scholar]
- 14.Wallin R, Sane DC, Hutson SM. Vitamin K 2,3-epoxide reductase and the vitamin K-dependent gamma-carboxylation system. Thromb Res. 2002;108: 221-226. [DOI] [PubMed] [Google Scholar]
- 15.Sun YM, Jin DY, Camire RM, Stafford DW. Vitamin K epoxide reductase significantly improves carboxylation in a cell line overexpressing factor X. Blood. 2005;106: 3811-3815. [DOI] [PubMed] [Google Scholar]
- 16.Hallgren KW, Qian W, Yakubenko AV, Ruge KV, Berkner KL. R-VKORC1 expression in factor IX BHK cells increases the extent of factor IX carboxylation but is limited by saturation of another carboxylation component or by a shift in the rate-limiting step. Biochemistry. 2006;45: 5587-5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wajih N, Sane DC, Hutson SM, Wallin R. The inhibitory effect of calumenin on the vitamin K-dependent gamma-carboxylation system: characterization of the system in normal and warfarin-resistant rats. J Biol Chem. 2004;279: 25276-25283. [DOI] [PubMed] [Google Scholar]
- 18.Wajih N, Sane DC, Hutson SM, Wallin RJ. Engineering of a recombinant vitamin K-dependent gamma-carboxylation system with enhanced gamma-carboxyglutamic acid forming capacity: evidence for a functional CXXC redox center in the system. J Biol Chem. 2005;280: 10540-10547. [DOI] [PubMed] [Google Scholar]
- 19.Wallin R, Martin LF. Vitamin K-dependent carboxylation and vitamin K metabolism in liver: effects of warfarin. J Clin Invest. 1985;76: 1879-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furie B, Furie BC. Molecular and cellular biology of blood coagulation. N Engl J Med. 1992;326: 800-806. [DOI] [PubMed] [Google Scholar]
- 21.Honore B, Vorum H. The CREC family, a novel family of multiple EF-hand, low-affinity Ca2+-binding proteins localized to the secretory pathway of mammalian cells. FEBS Lett. 2000;466: 11-18. [DOI] [PubMed] [Google Scholar]