Abstract
Outcome of acute lymphoblastic leukemia (ALL) in adults with central nervous system (CNS) disease at diagnosis is unclear. We treated 1508 de novo ALL patients with 2-phase induction and then high-dose methotrexate with l-asparaginase. Patients up to 50 years old in first remission (CR1) with a matched related donor (MRD) underwent an allogeneic stem cell transplantation (SCT); the remainder in CR1 were randomized to an autologous SCT or intensive consolidation followed by maintenance chemotherapy. Philadelphia chromosome (Ph)–positive patients were offered a matched unrelated donor (MUD) allogeneic SCT. Seventy-seven of 1508 (5%) patients a median age of 29 years had CNS leukemia at presentation; 13 of the 77 (17%) had Ph-positive ALL. Sixty-nine of 77 (90%) patients attained CR1. Thirty-six patients underwent transplantation in CR1 (25 MRD, 5 MUD, and 6 autografts). Eleven of 25 patients with MRD transplantation remain alive at 21 to 102 months, 2 of 5 with MUD at 42 and 71 months, and 1 of 6 with autologous SCT at 35 months. Seven of 27 treated with consolidation/maintenance remain in CR1 56 to 137 months after diagnosis. Overall survival at 5 years was 29% in those with CNS involvement at diagnosis versus 38% (P = .03) for those without. CNS leukemia in adult ALL is uncommon at diagnosis. Adult Ph-negative ALL patients, however, can attain long-term disease-free survival using SCT as well as conventional chemotherapy.
Introduction
Adult acute lymphoblastic leukemia (ALL) accounts for approximately 15% to 20% of all adult acute leukemias. Overall, adults with ALL have a 60% to 80% chance or more of attaining first complete remission (CR1) using combination chemotherapy.1-4 However, many patients eventually relapse, particularly those with high-risk features such as age at diagnosis more than 35 years; white blood cell (WBC) count at diagnosis in excess of 30 × 109/L (30 000/μL); non–T-cell phenotype; poor-risk cytogenetic abnormalities such as Philadelphia chromosome (Ph) positivity, t(4;11), or t(1;19); L2 or L3 phenotype; or failure to attain CR by induction day 35.5-12
Reports from a variety of studies indicate that less than 10% of adult ALL patients have central nervous system (CNS) involvement at presentation.3,13-15 Patient outcome for those individuals with CNS involvement at diagnosis is unclear because many patients are excluded from further analysis and specific patient characteristics and outcome are not reported. Because recent series of adult ALL patients routinely include CNS prophylaxis, the risk of developing disease in the absence of specific CNS treatment prevention when modern multiagent chemotherapy programs are used is unknown.
In 1993, we initiated an international trial (MRC UKALL XII/ECOG E2993) to evaluate the role of hematopoietic stem cell transplantation (HSCT) in newly diagnosed adult ALL patients. A total of 1508 patients were entered to October 2003 and data analyzed as of October 2004. At this time, the trial remains open. Seventy-seven patients were noted to have CNS involvement at diagnosis, and we report their outcome herein. The basic patient demographics, treatment, and early outcome have been reported recently.16
Patients, materials, and methods
Study eligibility
Patients age 15 to 60 years (Eastern Cooperative Oncology Group [ECOG] criteria) or 55 years (Medical Research Council [MRC] criteria) with newly diagnosed, previously untreated ALL (excluding mature B-cell ALL, ECOG criteria only, because this group has been shown to have a better outcome with alternative therapies such as high-dose alkylators and high-dose methotrexate therapy) and without prior malignancy were eligible for entry onto this study. Patients were not excluded if they had received corticosteroid therapy prior to study enrollment. The study was approved by the Institutional Review Board for Human Investigation (ECOG) or the Ethics Committee (MRC) of each participating center, and informed consent was obtained from each patient or parent/legal guardian if the patient was a minor.
Diagnostic procedures
Diagnosis was established by microscopic examination of bone marrow documenting more than 25% marrow lymphoblasts. Confirmation of the diagnosis of ALL by central morphology review was provided. Immunophenotyping and cytogenetic and molecular analyses on blood or marrow samples also were carried out at each institution. A lumbar puncture was not required prior to enrollment on protocol (it was prescribed between day 15 and 24). At some centers it was performed immediately before study entry as well.
Treatment
Therapy for all patients consisted of induction (phases I and II), intensification, and then either an HSCT or consolidation/maintenance.16 Induction consisted of phase I (weeks 1 to 4 after diagnosis) and phase II (weeks 5 to 8 after diagnosis). During phase I, patients were given weekly daunorubicin 60 mg/m2 intravenously and vincristine 1.4 mg/m2 (maximum 2 mg per dose) intravenously for 4 weeks along with prednisone 60 mg/m2 by mouth daily for 28 days. Initially, patients received l-asparaginase 10 000 U/m2 intramuscularly or intravenously on days 17 to 28, but the dose subsequently was modified to a flat dose of 10 000 units intramuscularly or intravenously. Daunorubicin doses were reduced 50% and 25% for serum bilirubin elevations of 34.2 to 51.3 μM (2 to 3 mg/dL) and more than 51.3 μM (3 mg/dL), respectively; vincristine doses were reduced 50% for moderate or severe neurologic dysfunction or serum bilirubin more than 51.3 μM (3 mg/dL).
Intrathecal methotrexate 12.5 mg was administered between days 15 and 24 followed 24 hours later by leucovorin 10 mg/m2 intravenously or by mouth every 6 hours for 4 doses. For those subjects in whom CNS leukemia was observed at diagnosis or at initial study lumbar puncture (days 15 to 24), intrathecal or intraventricular (via Ommaya reservoir) therapy was given up to thrice weekly until clearance. Protocol therapy permitted the addition of 2400 cGy cranial irradiation and 1200 cGy to the spinal cord administered concurrent with phase II at the discretion of the treating physician. Systemic chemotherapy was not delayed while intrathecal chemotherapy was given.
During induction phase II, patients were given cyclophosphamide 650 mg/m2 on days 1, 15, and 29; cytarabine 75 mg/m2 intravenously on days 1 to 4, 8 to 11, 15 to 18, and 22 to 25; along with 6-mercaptopurine 60 mg/m2 by mouth daily for 28 days and intrathecal methotrexate on days 1, 8, 15, and 22 (the latter followed each time 24 hours later by leucovorin 10 mg/m2 intravenously or by mouth every 6 hours for 4 doses). For patients with CNS leukemia at presentation who already were receiving intrathecal therapy during phase I, intrathecal methotrexate was omitted during phase II. No therapy was given weeks 8 to 11 while awaiting recovery of peripheral blood counts.
Intensification (weeks 12 to 16) was initiated 4 weeks from day 28 of phase II but was postponed until WBC count exceeded 3 × 109/L (3000/μL) and included high-dose methotrexate 3 g/m2 intravenously over 2 hours on days 1, 8, and 22 followed by l-asparaginase rescue 10 000 units intravenously on days 2, 9, and 23 and leucovorin rescue 10 mg/m2 intravenously or by mouth every 6 hours for 6 doses beginning 22 to 24 hours after completion of methotrexate therapy. For those patients not receiving an allogeneic or an autologous HSCT (who also did not present with CNS leukemia), 2400 cGy cranial irradiation and 1200 cGy to the spinal cord (12 fractions in 2 to 3 weeks) was given between intensification and the start of consolidation. Further, in such patients cytarabine 50 mg per dose is given by the intrathecal route weekly for 4 weeks during radiation therapy and also on 4 occasions 3 months apart for 1 year during maintenance therapy.
Those patients age 50 years or younger in CR after completion of phases I and II and intensification were assigned either an HLA-identical sibling HSCT or were encouraged to undergo a matched unrelated donor (MUD) HSCT if noted to be Ph positive. Those subjects without a sibling-matched donor, or older than age 50 years, or Ph positive and without an MUD donor were randomized to autologous HSCT or conventional consolidation and subsequently maintenance therapy. The conditioning regimens for sibling-matched allograft, MUD HSCT, and autologous HSCT consisted of fractionated total body irradiation (TBI) 1320 cGy given in 6 fractions twice daily (days –6 to –4) along with 400 cGy testicular boost in males and high-dose etoposide 60 mg/kg intravenously on day –3.17
Conventional consolidation and maintenance therapy was initiated when the WBC count exceeded 3 × 109/L (3000/μL) and platelet count exceeded 100 × 109/L (100 000/μL). This therapy included (consolidation cycle I) cytarabine 75 mg/m2 intravenously over 30 minutes and etoposide 100 mg/m2 intravenously over 1 hour on days 1 to 5; vincristine 1.4 mg/m2 (maximum 2 mg per dose) intravenously on days 1, 8, 15, and 22; and dexamethasone 10 mg/m2 by mouth daily for 28 days. Consolidation cycle II was initiated 4 weeks from initiating cycle I or when the WBC count exceeded 3 × 109/L (3000/μL) and included repeating cytarabine and etoposide as given in cycle I. Consolidation cycle III was begun 4 weeks from day 1 of cycle II or when the WBC count exceeded 3 × 109/L (3000/μL). Consolidation cycle III included daunorubicin 25 mg/m2 intravenous push on days 1, 8, 15, and 22; cyclophosphamide 650 mg/m2 intravenously over 30 minutes on day 29; cytarabine 75 mg/m2 intravenously over 30 minutes on days 31 to 34 and 38 to 41; and 6-thioguanine 60 mg/m2 by mouth on days 29 to 42. Finally, consolidation cycle IV was started 8 weeks after cycle III, or when the WBC count exceeded 3 × 109/L (3000/μL), and was identical to cycle II.
Maintenance therapy, to continue for 2.5 years after the start of intensification (ECOG) or 1.5 years after the start of consolidation (MRC), included 1 dose of vincristine 1.4 mg/m2 intravenously (maximum 2 mg) and prednisone 60 mg/m2 by mouth daily for 5 days every 3 months, oral 6-mercaptopurine 75 mg/m2 daily, and methotrexate 20 mg/m2 intravenously or by mouth once weekly. Ph-positive patients received interferon alfa 2b during maintenance at a dose of 3 × 106 units subcutaneously 3 times per week when the WBC count exceeded 3 × 109/L (3000/μL) and the platelet count exceeded 100 × 109/L (100 000/μL) and was continued for 15 months.
Granulocyte colony-stimulating factor (filgrastim; Amgen, Thousand Oaks, CA) therapy was permitted for neutropenia at any time during the study at the discretion of the treating physician.
Complete remission
Complete remission was defined as meeting all of the following criteria: peripheral blood counts showing more than 1000 × 109/L (1000 × 106/μL) neutrophils, more than 100 000 × 109/L (100 000 × 106/μL) platelets, and no circulating blasts; bone marrow cellularity of more than 20% with maturation of all cell lines and blasts up to 5%; and no evidence of extramedullary leukemia.
CNS leukemia
CNS leukemia was defined as unequivocal morphologic evidence of lymphoblasts in the cerebrospinal fluid (CSF) using a cytocentrifuged sample, or cranial nerve palsies, or significant neurologic dysfunction.18,19 In some patients a lumbar puncture was performed prior to study enrollment. In most patients, however, a lumbar puncture was performed soon after initiation of protocol therapy when the blood circulating blasts cleared or some time between days 15 to 24 of therapy. CNS leukemia at diagnosis or initiation of therapy was treated as described in “Treatment.”
Statistical analyses
Comparisons between groups at diagnosis used χ2 statistics for categoric variables and Wilcoxon rank sum tests for continuous variables. Overall survival and event-free survival were measured from the day of starting treatment. Survival analyses included death from any cause, with observation censored at the date of last contact for patients not known to have died. Event-free survival analyses included relapse at any site or death from any cause, with observation censored at the date of last contact for patients without report of relapse or death. Analyses are intention to treat, and remission failure was not counted as an event. Most patients who failed to achieve remission by the end of phase II eventually died, and for these their failure time is the time of death. Comparisons between groups used the log-rank test, with stratification used for examining effects within subgroups. All P values are 2 sided. Multivariate Cox regression analysis was also used to determine whether prognostics factors were independently associated with outcome.
Results
Patients entered up to the end of October 2003 are included, and follow-up is to the end of October 2004, so that all patients have a minimum of 1-year follow-up.
At total of 1519 patients were registered, of whom 11 have been excluded due to misdiagnosis.
Incidence of CNS involvement
Of the 1508 eligible enrollees, 77 (5%) patients had CNS involvement at diagnosis. Twenty-one of these 77 (27%) patients were not known to have CNS disease at registration but were diagnosed as having involvement when the initial study lumbar puncture was performed between days 15 and 24 Patient characteristics included 53 males and 24 females with a median age of 29 years (range, 16 to 56 years) (Table 1). Thirteen patients were Ph positive. More patients entered into the trial by ECOG centers had CNS disease (8.4%) than among those entered by MRC centers (3.2%) (P < .001). Patients with CNS disease were not different compared with those free of CNS involvement at diagnosis with regard to sex, age, Ph positivity, hepatomegaly, splenomegaly, or lymph node enlargement; however, they were more likely to have a mediastinal mass (21% versus 8%; P < .001). There was some difference in the proportion with CNS disease within different phenotype groups (common or pre-B: 42 of 952 [4.4%]; T: 25 of 261 [9.6%]; mature B: 2 of 29 [6.9%]; other: 4 of 122 [3.3%]; common or pre-B versus T, P < .001). Patients with CNS disease had a higher WBC count at diagnosis (Wilcoxon rank sum test, P < .001), with median value of 25.6 × 109/L (25.6 × 106/μL) compared with 13.5 × 109/L (13.5 × 106/μL).
Table 1.
Characteristics of patients with and without CNS leukemia at presentation
| Characteristic | With CNS disease | Without CNS disease | P |
|---|---|---|---|
| Group, no. (%) | < .001 | ||
| MRC | 31 (40) | 932 (65) | |
| ECOG | 46 (60) | 499 (35) | |
| Sex, no. (%) | .2 | ||
| Male | 53 (69) | 878 (61) | |
| Female | 24 (31) | 553 (39) | |
| Ph status, no. (%) | .6 | ||
| Positive | 13 (17) | 263 (18) | |
| Negative | 62 (80) | 1121 (78) | |
| Unknown | 2 (3) | 47 (3) | |
| Median age, y (range) | 29 (16-56) | 31 (14-60) | .8 |
| Median WBC count at diagnosis, × 103/μL (range) | 25.6 (2.7-541.0) | 13.5 (0.6-930.0) | < .001 |
| WBC count more than 30 000/μL at diagnosis, no. (%) | 34 (44) | 494 (35) | .08 |
| Median hemoglobin level at diagnosis, g/dL (range) | 9.8 (3.3-17.3) | 9.3 (2.6-18.2) | .2 |
| Immunophenotype, no. (%) | .001† | ||
| B lineage | 42 (55) | 921 (64) | |
| T cell | 25 (32) | 236 (16) | |
| Mature B cell | 2 (3) | 23 (2) | |
| Other* | 4 (5) | 118 (8) | |
| Unknown | 4 (5) | 133 (9) | |
| Hepatomegaly, no. (%) | 17 (22) | 241 (17) | .3 |
| Splenomegaly, no. (%) | 30 (39) | 410 (29) | .09 |
| Lymph nodes enlarged, no. (%) | 29 (38) | 443 (31) | .3 |
| Mediastinal mass, no. (%) | 16 (21) | 115 (8) | < .001 |
| Remission rates, no. (%) | NS | ||
| Death in induction | 4 (5.2) | 70 (4.9) | |
| Resistant disease | 3 (3.9) | 55 (3.9) | |
| Remitted during phase I | 51 (66.2) | 1019 (71.2) | |
| Remitted during phase II | 11 (14.3) | 157 (11) | |
| Remitted after induction | 7 (9.1) | 86 (6.0) | |
| Unknown | 1 (1.3) | 44 (3.1) |
To convert WBC count from × 103 cells per microliter to × 109 cells per liter, multiply × 103 cells per microliter by 1.
To convert hemoglobin level from grams per deciliter to grams per liter, multiply grams per deciliter by 10.
Ph indicates Philadelphia chromosome; NS, not significant.
Biphenotypic null cell, pro—B cell, or other rare type.
B lineage vs T.
Remission induction
One patient, taken off protocol during phase I induction due to a high level of blasts in the CSF, received a related-donor allogeneic HSCT without remission. After this therapy she relapsed in the CNS; there is considerable doubt as to whether remission was ever achieved. A further 7 patients never attained CR1 (4 died early during induction on days 4, 25, 32, and 36, while 3 subjects died from persistent ALL on days 78, 207, and 504). Of these 7 patients, 4 had pre–B-cell immunophenotype and an initial median WBC count of 251.8 × 109/L (251.8 × 106/μL) (range, 24.5× 109/L to 541 × 109/L [24.5× 106/μL to 541 × 106/μL]), while 3 had T-cell immunophenotype and an initial WBC count of 18.4× 109/L (18.4× 106/μL) (range, 4.5 × 109/L to 105 × 109/L [4.5 × 106/μL to 105 × 106/μL]). Sixty-nine (90%) patients attained CR1, 62 (81%) by the end of induction, a result that did not differ statistically from those who were CNS negative at diagnosis (90% and 85%, respectively).
Postremission therapy
Sixty-nine (90%) patients entered CR1 and 36 proceeded quickly to HSCT while 27 continued to receive chemotherapy only. Twenty-five patients received matched related donor (MRD) allografts, 5 received MUD allogeneic HSCT, and 6 underwent autologous HSCT in CR1. Six patients in CR1 relapsed before a transplantation could be performed, yet subsequently 3 each received an MRD and an MUD HSCT (Table 2). Protocol therapy for craniospinal irradiation in addition to intrathecal/intraventricular therapy was allowed at the discretion of the treating physician and was administered to 10 patients; 7 went on to receive an MRD allogeneic HSCT, 1 an MUD allogeneic HSCT, and 2 an autologous HSCT. Such additional therapy did not appear to be associated with heightened toxicity due to this treatment. Eleven of 25 patients receiving MRD allografts remain alive in CR1 at 29, 34, 38, 49, 52, 57, 65, 67, 71, 75, and 102 months from diagnosis; 13 died due to infection (n = 3), infection plus acute graft versus host disease (GVHD) (n = 3), bleed plus acute GVHD (n = 1), venoocclusive disease (n = 1), and further relapse (n = 5). Only 1 patient with autologous HSCT remains in CR1 at 35 months, while 4 relapsed and died and 2 succumbed to infection and pulmonary disease. Two of 5 patients with MUD allogeneic HSCT remain in CR1 at 42 and 71 months; 3 died: 1 of renal and pulmonary complications, a second of infection and acute GVHD, a third from relapse. Seven of the 27 patients treated with consolidation and maintenance chemotherapy alone remain in CR1 at 56, 59, 59, 91, 102, 130, and 137 months. Twenty patients died due to infection (n = 4), bleeding (n = 1), extramedullary toxicity (n = 1), and relapse (n = 14). Only 2 of 6 patients receiving an allograft beyond CR1 (n = 3 each receiving MUD and MRD) are alive: 1 with MRD allograft at 58 months and 1 with MUD at 46 months.
Table 2.
Patient outcomes
| Patient group | No. | Site of first relapse | Deaths | Survivors in CR, mo after diagnosis |
|---|---|---|---|---|
| Failure to attain CR1 | 8 | — | — | — |
| Induction (toxic) death | 4 | — | At 4 d, 25 d, 32 d, 36 d | — |
| Died due to resistant disease | 3 | — | At 78 d, 207 d, 504 d | — |
| MRD without remission | 1 | 1 CNS | 1 after relapse | — |
| Attained CR1 | 69 | — | — | — |
| MUD allograft, CR1 | 5 | 1 BM | 2 in CR1, 1 after relapse | 71, 42 |
| MRD allograft, CR1 | 25 | 1 CNS, 1 BM, 1 BM + CNS, 1 BM + ocular, 1 arm mass | 9 in CR1, 5 after relapse | 29, 34, 38, 49, 52, 57, 65, 67, 71, 75, 102 |
| Autograft, CR1 | 6 | 3 BM, 1 BM + CNS | 1 in CR1, 4 after relapse | 35 |
| Chemotherapy, CR1 | 27 | 11 BM, 1 BM + CNS, 1 testes, 1 skin | 6 in CR1, 14 after relapse | 56, 59, 59, 91, 102, 130, 137 |
| MRD allograft beyond CR1 | 3 | 3 BM | 2 after 2nd relapse | 58 |
| MUD allograft beyond CR1 | 3 | 1 CNS, 2 BM | 2 after 2nd relapse | 46 |
| Total | 77 | — | — | — |
CR1 indicates first complete remission; MUD, matched unrelated donor; MRD, matched related donor; —, not applicable.
Long-term outcome
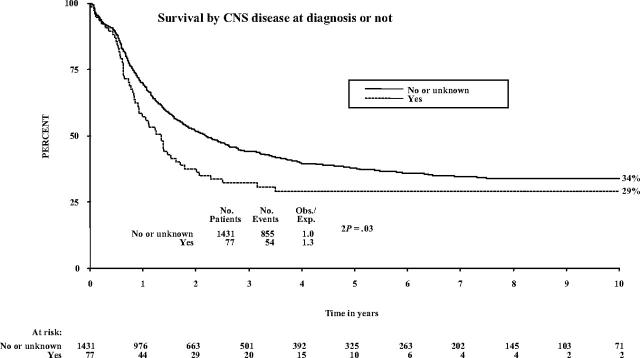
Twenty-three (30%) patients are alive: 2 Ph positive at 42 and 62 months and 21 Ph negative or unknown (2) in CR at a median 59 months (range, 29 to 137 months) after diagnosis. Event-free survival was somewhat but not statistically significantly lower for this group of patients (27%; 95% confidence interval [CI], 17% to 37% at 5 years) than for the remainder who did not have CNS involvement at diagnosis (34%; 95% CI, 31% to 36%; P = .06). Survival also was lower at 5 years (29% [95% CI, 19% to 39%] versus 38% [95% CI, 35% to 41%]; P = .03) (Figure 1). There was no difference in the proportion that failed to achieve remission (7 [9.1%] of 77 versus 125 [8.7%] of 1431) or in the rate of non-CNS relapse. Among those who achieved remission, isolated or combined CNS relapses were more frequent (11.9% [95% CI, 2.3% to 21.5%] versus 5.6% [95% CI, 4.0% to 7.2%]; log-rank, P = .04), and there were somewhat more deaths in remission (25.2% [95% CI, 21.9% to 28.5%] versus 35.0% [95% CI, 21.7% to 48.3%]; log-rank, P = .04).
Figure 1.
Patient survival by CNS involvement at diagnosis.
Overall survival rates at 5 years for Ph-negative patients were superior statistically in the CNS-negative group when compared with the CNS-positive group: 42% (95% CI, 39% to 45%) versus 29% (95% CI, 18% to 41%; P = .02), respectively. Outcome data at 5 years for Ph-positive patients showed better (but not significant) results in the CNS-negative patients (22%; 95% CI, 17% to 28%) when compared with those with CNS involvement at diagnosis (17%; 95% CI, 14% to 28%; P = .7). The heterogeneity test for the different effect of CNS disease between groups was not significant (P = .4). Finally, for those patients who underwent an MRD transplantation in CR1, subjects who did not have CNS involvement had a 5-year survival of 55% (95% CI, 50% to 61%) versus 44% (95% CI, 24% to 65%) in those patients with initial CNS leukemic involvement (P = .3).
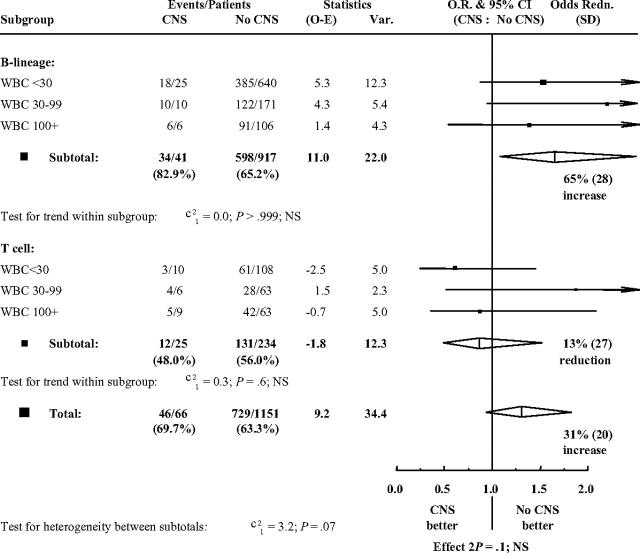
Multivariate analysis including the variables sex, age, log-(WBC+1), immunophenotype (B lineage or T), and mediastinal mass showed that age, WBC count, and immunophenotype were independently significant (each P < .001), while CNS involvement was of significance (P = .03) for survival but not for event-free survival (P = .07). There was no evidence that the effect of having CNS disease was different in any subgroup defined by immunophenotype (B lineage or T cell) and WBC count (below 30 × 109/L, 30 × 109/L to 99 × 109/L, and at least 100 × 109/L) with regard to event-free survival (Figure 2) or survival (not shown).
Figure 2.
Event-free survival difference within WBC-count groups.
Discussion
This study is the largest series of adult ALL patients ever reported (the trial is still recruiting), and so far there is a 5% incidence of (77 of 1508 eligible patients) CNS leukemia at presentation. We cannot exclude the possibility that investigators avoided enrollment of subjects due to suspected CNS involvement. Fière and coworkers14 of the French Group on Therapy for Adult Acute Lymphoblastic Leukemia reported that at diagnosis 38 of 572 (7%) adult ALL patients had CNS involvement confirmed by spinal fluid cytology. Kantarjian and coworkers3 similarly noted that 14 (7%) of 204 adult ALL patients had CNS leukemic involvement at presentation. In an earlier publication this group13 reported that 17 (4%) of 391 patients had evidence of CNS leukemia at diagnosis and were therefore excluded from analysis. Further, in a recent Southwest Oncology Group (SWOG) study that enrolled 404 patients (including 75 whose CNS status was unknown) leukemia was detected in 14 (5%) of 278 patients.2
B-cell ALL patients appear to have a higher incidence of CNS disease at diagnosis. Gururangan and colleagues20 reported a pediatric series (age below 19 years) in which 49 of 462 (10.6%) patients with disseminated small noncleaved-cell lymphoma and B-cell leukemia had CNS disease at diagnosis, defined as meningeal disease or CNS parenchymal masses with or without cranial neuropathies. Similarly, Thomas and associates21 reported a high incidence (11 of 42; 42%) of CNS involvement at diagnosis in Burkitt-type adult ALL. Six of these patients had cranial nerve palsies or other neurologic findings (1 with negative CSF cytology) whereas 5 were asymptomatic despite positive CSF cytopathology. Finally, 8 (12%) of 68 adult B-cell ALL patients were observed to have CNS involvement at diagnosis.22
Factors associated with CNS disease at presentation included only a higher WBC count at diagnosis (25.6 × 109/L versus 13.5 × 109/L [25.6 × 106/μL versus 13.5 × 106/μL]; P < .001), more frequent T-cell immunophenotype (P = .001), and presence of a mediastinal mass (22% versus 9%; P < .001); patients with CNS involvement did not differ significantly compared with those who did not have disease with respect to age, sex, Ph positivity, hepatomegaly, splenomegaly, or lymph node enlargement. One can speculate that the biologic behavior of a leukemia associated with mediastinal infiltration and hyperleukocytosis implies a predilection to involve the CNS as well.
In this study, CR rates for those presenting with CNS disease at diagnosis (90%) did not differ compared with rates for those who did not have involvement. In the smaller SWOG series reported by Petersdorf et al,2 however, only 4 (29%) of 14 of CNS-involved patients achieved CR compared with 166 (63%) of 264 CNS-negative patients and 48 (64%) of 75 patients with unknown CNS disease status (P = .013). The effect of CNS involvement on CR rates was not detailed in other series, including those involving B-cell ALL patients. The induction regimen we used appeared to be extremely effective to provide such excellent initial antileukemia therapy.
In our trial, 23 (30%) of the 77 patients with CNS disease at presentation attained long-term survival (median, 59 months; range, 29 to 137 months after diagnosis). Six patients, however, received MRD (n = 3) or MUD (n = 3) allografts beyond CR1, and 2 patients attained durable CR (at 46 and 58 months). The event-free survival result for the entire group of patients is nonsignificantly lower for this patient subset (27%; 95% CI, 17% to 37% at 5 years) than for those without CNS involvement at diagnosis (34%; 95% CI, 31% to 36%; P = .06). Overall survival at 5 years, however, was somewhat reduced: 29% (95% CI, 19% to 39%) versus 38% (95% CI, 35% to 41%; P = .03) for those without CNS disease at presentation. The SWOG investigators2 found no significant variation of survival according to CNS disease status (P = .14). For B-cell ALL patients, relapses were observed in 21 of 49 (43%) patients, predominantly in the CNS (71%) and bone marrow (52%).20 Three-year event-free survival for this patient subgroup was 45%, inferior to the 59% figure for those without evidence of CNS disease. In the B-cell ALL series published by Thomas et al21 that used hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) therapy and in work reported by Hoelzer and coworkers,22 however, CNS involvement at diagnosis did not adversely affect leukemia-free survival in B-cell ALL. Finally, in the French LALA-94 study, 48 patients had CNS disease and their outcome did not differ from the more than 700 remaining.23
Twenty-one of our patients (Ph negative or unknown) attained durable CR, while 2 Ph-positive ALL patients survive at 42 and 62 months. Eleven of the 25 ALL patients who received MRD allografts and 7 of 27 given chemotherapy alone remain in CR at 29 to 102 months and 56 to 137 months, respectively. Three of the 8 MUD allografts performed in CR1 or beyond remain in CR at 42, 46, and 71 months after diagnosis. Only 1 of 6 patients receiving autografts has experienced a durable CR (35 months); patient heterogeneity and the small sample size number preclude drawing conclusions as to why the autograft patients appear to fare worse compared with the chemotherapy group.
Clearly, the intensive induction and postremission chemoradiation therapy, with or without allogeneic HSCT, possesses sufficient antileukemic effect to eradicate disease and prevent relapse. Despite significant advances in supportive care including hematopoietic growth factors, potent antibiotics, and improved GVHD therapies, a number of patients died from therapy-related events. Because so many of the CNS-involved patients received myeloablative allogeneic transplantations, we are unable to draw conclusions regarding the competing causes of death due to allograft transplantation-related mortality versus CNS disease.
Efforts to decrease deaths due to toxicity should improve outcome for some of these patients. Equally important is to develop an improved understanding of the biology of adult ALL (ie, how to address the subgroup that presents with CNS involvement). One approach may use the strategy of risk-adapted therapy based on the biologic features of the patient's leukemia permitting appropriate, individualized therapy for a specific patient, as has been reported previously.24,25 In the LALA-94 multicenter trial, Thomas and coworkers23 recently reported examining risk-adapted postremission strategy in 922 ALL patients, including 48 who were considered high risk due to CNS disease at diagnosis (cranial nerve palsies or CSF leukemic blasts). Eighteen patients with a sibling-matched donor received an allograft, whereas 28 of 30 patients, without a donor and assigned an autograft, received the prescribed therapy. Five-year overall survival was 36% for the entire group; however, disease-free and overall survival rates did not differ significantly for the autograft versus the allograft groups. Thus, aggressive therapy using an allograft, an autograft, or intense conventional treatment was effective.
Adult Ph-negative ALL patients with CNS leukemia at diagnosis can attain long-term disease-free survival. CNS disease at presentation does not appear to be an independent poor-prognostic factor, because favorable outcome in such patients is a reflection of this aggressive systemic and CNS-directed therapy. Larger patient numbers are needed to exclude the possibility of a moderate effect additional to the consequence of WBC elevation. Overall survival, however, for those patients presenting with CNS disease is worse than those without involvement: 34% versus 29% at 10 years, respectively (P = .03). Practitioners should be encouraged to enroll such patients on standard ALL protocols. Both allogeneic HSCT as well as intensive, conventional chemotherapy can be used successfully to provide a better outcome in this patient group.
Acknowledgments
This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, MD). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Appendix
Participants in this study included the following people:
MRC UKALL XII doctors
United Kingdom. Dr J. E. Chandler, Dr D. Plews, and Dr A. C. Wood, James Cook University Hospital; Dr A. Cuthbert, Airedale General Hospital; Dr L. J. Newton, Dr L. A. Parapia, and Dr A. T. Williams, Bradford Royal Infirmary; Dr K. R. Speed, Diana Princess of Wales Hospital; Dr C. Carter, Huddersfield Royal Infirmary; Dr Sahra Ali, Dr R. D. Patmore, and Dr M. L. Shields, Hull Royal Infirmary; Dr S. M. Rajah, Seacroft Hospital; Prof J. A. Child, Dr P. Hillmen, Prof G. J. Morgan, Dr D. R. Norfolk, and Dr G. M. Smith, Leeds General Infirmary; Dr M. C. Galvin and Dr P. Hillmen, Pinderfields General Hospital; Dr D. L. Barnard, Dr S. E. Kinsey, and Dr B. A. McVerry, St. James's University Hospital; Dr L. R. Bond and Dr M. R. Howard, York Hospital; Dr A. J. Steed, Halifax General Hospital; Dr D. Wright, Pontefract General Infirmary; Dr Jim Cavet, Dr R. Chopra, and Dr Mike Dennis, Christie Hospital; Dr P. Carrington, Dr M. Garg, and Dr J. B. Houghton, Salford Royal Hospitals NHS Trust; Dr J. Burthem, Prof J. A. Liu Yin, and Dr G. S. Lucas, Manchester Royal Infirmary; Dr P. R. Kelsey, Victoria Hospital; Dr J. V. Clough and Dr E. Rhodes, Countess of Chester Hospital; Prof J. C. Cawley, Dr P. Chu, Prof R. E. Clark, and Dr A. R. Pettitt, Royal Liverpool University Hospital; Dr P. A. Stevenson, Walton Hospital; Dr G. Satchi and Dr J. Tappin, Whiston Hospital; Dr N. Butt, Dr T. J. Deeble, Dr D. W. Galvani, Arrowe Park Hospital; Dr Ranjit Dasgupta, Dr A. Olujohungbe, Dr W. Sadik, Dr B. E. Woodcock, University Hospital Aintree; Dr A. McKernan and Dr D. C. Mitchell, Derbyshire Royal Infirmary; Dr M. Auger and Dr E. C. L. Logan, King's Mill Hospital; Dr C. S. Chapman, Dr C. Haworth, Dr A. E. Hunter, Dr R. M. Hutchinson, and Dr J. K. Wood, Leicester Royal Infirmary; Dr M. A. Adelman, Dr D. R. Prangnell and Dr Cathy Williams, Lincoln County Hospital; Dr J. L. Byrne, Dr A. P. Haynes, Dr P. A. E. Jones, Dr A. McMillan, and Prof N. H. Russell, Nottingham City Hospital; Dr J. M. Davies and Dr G. Dolan, Nottingham University Hospital; Dr S. Sobolewski and Dr V. M. Tringham, Pilgrim Hospital; Dr D. C. Rees, Dr J. A. Snowden, Dr E. Vandenberghe, and Dr D. A. Winfield, Royal Hallamshire Hospital; Dr B. Paul, Bassetlaw Hospital; Dr H. F. Barker and Dr P. C. Taylor, Rotherham District General; Dr D. Chandra, Dr R. C. Chasty, Dr P. M. Chipping, and Dr R. M. Ibbotson, North Staffs Hospital Centre; Dr Oliver Chapman, Dr R. I. Harris, Dr Beth Harrison, Dr N. Jackson, Dr Juliet Mills, and Dr M. J. Strevens, University Hospitals Coventry & Walsgrave; Dr D. Bareford, Sandwell & West Birmingham Hospital; Dr C. Fegan, Dr M. J. Leyland, and Dr D. W. Milligan, Birmingham Heartlands Hospital; Dr M. Narayanan, George Eliot Hospital; Dr M. S. Hamilton, Dr S. M. Jobanputra, and Dr J. Tucker, Good Hope Hospital NHS Trust; Dr G. P. Galvin, Manor Hospital; Dr S. Basu, Dr S. I. Handa, Dr A. MacWhannell, and Dr A. M. Patel, The Royal Wolverhampton Hospital; Dr B. J. Boughton, Dr J. A. Holmes, Dr P. Mahendra, Dr J. A. Murray, Queen Elizabeth Hospital, Birmingham; Dr S. I. Handa and Dr P. J. Stableforth, Sandwell & West Birmingham Hospital; Dr J. A. Murray, Selly Oak Hospital; Dr T. A. S. Amos, Dr T. A. J. Phaure, and Dr P. Revell, Staffordshire General Hospital; Dr S. Basu and Dr P. E. Rose, University Hospitals Coventry; Dr N. Pemberton, Dr S. Shafeek, and Dr R. Stockley, Worcestershire Royal Hospital; Dr L. G. Robinson, Hereford County Hospital; Dr J. Neilson, Dr S. G. N. Richardson, and Dr Craig Taylor, Russells Hall Hospital; Dr J. I. O. Craig, Prof A. R. Green, and Dr R. Marcus, Addenbrooke's NHS Trust; Dr N. J. Dodd, Ipswich Hospital; Dr H. M. Daly and Dr T. R. Mitchell, James Paget Hospital; Dr A. M. Deane, Dr J. Leslie, Dr J. Parker, Dr G. E. Turner, and Dr J. Z. Wimperis, Norfolk & Norwich University Hospital N; Dr J. Z. Wimperis, Peterborough District Hospital; Dr P. Coates and Dr J. Keidan, Queen Elizabeth Hospital, King's Lynn; Dr P. Harper, West Suffolk Hospital; Dr I. J. Durrant, The Horton Hospital; Dr C. Bunch, Dr P. Emerson, Dr C. Hatton, and Dr T. J. Littlewood, Oxford Radcliffe Hospitals, The John Radcliffe Hospital; Dr D. J. Moir and Dr D. M. White, Milton Keynes General NHS Trust; Dr M. E. Haines, Dr J. R. Y. Ross, and Dr S. S. Swart, Northampton General Hospital; Dr F. Booth, Dr H. Grech, and Dr G. Morgenstern, Royal Berkshire Hospital; Dr A. M. O'Hea, Dr S. M. Sheerin, and Dr A. Watson, Stoke Mandeville Hospital; Dr R. Aitchison, Dr S. Kelly, and Dr J. K. Pattinson, Wycombe General Hospital; Dr D. L. Aston, Dr A. E. Milne, and Dr T. J. C. Nokes, The North Hampshire Hospital; Dr A. J. Bell and Dr F. Jack, Poole Hospital NHS Trust; Dr M. Ganczakowski, Queen Alexandra Hospital; Dr C. J. C. Knechtli, Dr C. R. J. Singer, and Prof J. G. Smith, Royal United Hospital NHS Trust, Bath; Dr A. J. Bell, Dr Rachel Hall, Prof T. J. Hamblin, Dr Sally Killick, and Dr H. Myint, Royal Bournemouth Hospital; Dr J. O. Cullis, Salisbury District Hospital; Dr A. Duncombe, Dr A. Duncombe, Dr J. Kohler, Dr K. Orchard, Dr D. Richardson, and Dr A. G. Smith, Southampton University Hospital Trust; Dr A. H. Moosa, Dorset County Hospital; Dr G. L. Scott, Bristol Royal Infirmary; Dr E. Blundell, Dr R. Lush, and Dr J. Ropner, Cheltenham General Hospital; Dr J. A. Copplestone, Dr M. D. Hamon, Dr A. Prentice, and Dr S. A. J. Rule, Derriford Hospital; Dr J. Ropner, Gloucestershire Royal Hospital; Dr B. Attock, North Devon District Hospital; Dr J. Blundell, Dr M. D. Creagh, Dr A. R. Kruger, and Dr R. Noble, Royal Cornwall Hospital (Treliske); Dr M. V. Joyner, Dr R. Lee, and Dr C. E. Rudin, Royal Devon and Exeter Hospital; Dr R. S. Evely and Prof J. Hows, Southmead Hospital; Dr S. Bolam, Dr S. V. Davies, Dr S. A. N. Johnson, and Dr S. Rule, Taunton & Somerset NHS Trust; Dr S. R. Smith and Dr D. L. Turner, Torbay Hospital; Dr J. M. Bird, Dr R. Evely, Prof J. Hows, and Dr D. Marks, Bristol Haematology and Oncolog; Dr A. Virchis, Barnet General Hospital; Dr K. Ryan, Central Middlesex Hospital; Dr M. Foadi, Charing Cross Hospital; Dr G. Abrahamson, Dr U. M. Hegde, and Dr N. J. Philpott, Ealing Hospital; Dr D. Harvey Edgware, General Hospital, Middlesex; Prof J. M. Goldman and Dr E. Kanfer, Hammersmith Hospital, Imperial College London; Dr R. Jan-Mohamed and Dr R. Kaczmarski, Hillingdon Hospital; Dr S. Allard, Dr T. J. Corbett, Dr N. Panoskaltsis, Dr C. D. L. Reid, and Dr P. Skacel, Northwick Park Hospital; Dr S. H. Abdalla, St. Mary's Hospital, London; Dr R. G. Hughes, West Middlesex University Hospital; Dr A. McMillan, Mount Vernon Hospital; Dr J. F. M. Harrison, Hemel Hempstead General Hospital; Dr J. VanDePette, Frimley Park Hospital NHS Trust; Dr C. Dearden and Dr Mark Ethell, Royal Marsden Hospital; Dr P. C. Bevan and Dr S. L. Janes, St. Richard's Hospital; Dr D. H. Bevan, Dr C. E. Dearden, Dr J. C. W. Marsh, Dr Marie Scully, and Dr Fenella Willis, St. George's Hospital NHS Trust; Dr J. Mercieca, St Helier Hospital; Dr F. Matthey, East Surrey Hospital; Dr A. S. Laurie, Ashford & St Peter's Hospital; Dr A. M. O'Driscoll, Dr C. L. Rist, and Dr A. W. W. Roques, Worthing Hospital; Dr I. D. C. Douglas and Dr G. Robbins, Royal Surrey County Hospital; Dr M. Treacy, Chase Farm Hospital; Dr J. K. Luckit, North Middlesex Hospital; Prof A. V. Hoffbrand, Dr A. B. Mehta, Dr M. N. Potter, Prof H. G. Prentice, Royal Free Hospital, London; Dr A. Eden, Southend General Hospital; Prof T. A. Lister and Prof A. Z. S. Rohatiner, St Bartholomew's Hospital; Dr K. Ardeshna, Dr S. Devereux, Prof A. H. Goldstone, Dr A. Khwaja, Prof D. C. Linch, Dr A. Nathwani, Dr K. G. Patterson, Dr J. B. Porter, and Dr K. Yong, University College Hospital, London; Dr C. C. Anderson and Dr C. DeSilva, Whipps Cross Hospital; Dr N. Akhtar, King George Hospital; Dr R. M. Ireland, Queen Elizabeth Hospital, London; Dr P. A. Gover and Dr R. J. Grace, Eastbourne District General; Dr C. F. M. De Lord, The Princess Royal University Hospital; Dr R. Carr, Dr C. C. Ozanne, and Dr S. A. Schey, Guy's Hospital; Dr K. Saied, Thanet Healthcare NHS Trust, Queen Elizabeth the Queen Mother Hospital; Dr C. F. E. Pocock, Kent & Canterbury Hospital; Prof G. Mufti, King's College Hospital; Dr M. L. Tillyer, University Hospital Lewisham; Dr D. S. Gillett and Dr C. Taylor, Pembury Hospital; Dr S. Bowcock, Dr S. Rassam, and Dr S. M. Ward, Queen Mary's Sidcup NHS Trust; Dr R. Gale, Dr V. Ratnayake, and Dr D. G. Wells, William Harvey Hospital; Dr M. Aldouri and Dr V. E. Andrews, Medway Maritime Hospital; Dr A. Stark, Dumfries & Galloway Royal Infirmary; Dr A. D. J. Birch, Falkirk District Royal Infirmary; Dr R. Chopra, Prof I. M. Franklin, Dr G. McQuaker, and Dr A. N. Parker, Glasgow Royal Infirmary; Dr J. D. Browning, Wishaw General Hospital; Dr R. C. Tait, Southern General Hospital; Dr R. B. Hogg, Stobhill NHS Trust; Dr R. A. Sharp and Dr P. J. Tansey, Victoria Infirmary; Dr E. J. Fitzsimons, Dr N. P. Lucie, and Dr R. Soutar, Western Infirmary; Dr E. Chalmers, Dr D. J. Culligan, Dr A. A. Dawson, and Dr J. Tighe, Aberdeen Royal Infirmary; Dr J. Tucker, Borders General Hospital; Dr E. J. Fitzsimons, Dr J. A. Murphy, Dr A. Raafat, and Dr W. Watson, Monklands District General; Dr D. T. Bowen, Dr P. Cachia, Dr K. Gelly, Dr A. Heppleston, and Prof M. J. Pippard, Dundee Teaching Hospitals NHS Trust; Dr C. J. Lush, Raigmore Hospital; Dr A. C. Parker, Royal Infirmary of Edinburgh; Dr S. Y. Rogers, Victoria Hospital; Dr J. M. Davies, Dr P. Ganly, Dr P. R. E. Johnson, Dr M. J. Mackie, and Dr P. H. Roddie, Western General Hospital; Dr D. R. Edwards, Dr M. Goodrick, Dr D. I. Gozzard, and Dr C. Hoyle, Glan Clwyd Hospital; Prof A. K. Burnett, Dr S. H. Lim, Dr C. Poynton, Dr C. Rowntree, and Dr J. A. Whittaker, University Hospital of Wales; Dr D. H. Parry and Dr J. R. C. Seale, Ysbyty Gwynedd; and Dr R. J. G. Cuthbert, Dr Z. R. Desai, Dr F. G. C. Jones, Dr M. F. McMullin, and Dr T. C. M. Morris, Belfast City Hospital.
Republic of Ireland. Dr J. R. O'Donnell, Beaumont Hospital; Prof E. L. Egan and Dr M. Murray, University College Hospital, Dublin; Dr D. McCarthy, St Vincent's Hospital; Dr P. V. Browne and Prof S. R. McCann, St James Hospital; and Dr H. Enright, Adelaide/Meath Hospitals.
New Zealand. Dr P. Ganly and Dr W. N. Patton, Canterbury Health Laboratories; Dr S. Gibbons, Prof D. N. J. Hart, Dr D. Heaton, and Dr R. L. Spearing, Christchurch Hospital; Dr C. H. Beresford and Dr C. Newhook, Dunedin Hospital; Dr B. Baker and Dr P. Harper, Palmerston North Hospital; Dr G. Corbett, Dr S. May, and Dr H. Pullon, Waikato Hospital; and Dr J. C. Carter, Dr J. Phillips, and Dr K. R. Romeril, Wellington Hospital.
Italy. Prof A. M. Carella, Azienda Ospedale San Martino; Dr M. Crugnola and Prof V. Rizzoli, Università degli Studi Di Parma; Prof U. Marini, Ospedale S. Carlo Borromeo; Dr DeCataldo and Dr Nosari, Ospedale Niguarda; and Dr A. Donelli, Servizio Di Ematologia, Modeno.
ECOG 2993 doctors
Dr J. A. Sparano, Albert Einstein College (NY); Dr M. B. Atkins, Beth Israel Deaconess Medical Center; Dr J. Aisner, Cancer Institute of New Jersey; Dr B. J. Averbook, Case Western-MetroHealth Medical Center; Dr J. L. Wade III, Central Illinois CCOP (EAPP); Dr S. S. Grubbs, Christiana Care CCOP (EAPP); Dr E. R. Pajon Jr, Colorado Cancer Research Program CCOP; Dr J. P. Kuebler, Columbus CCOP (EAPP); Dr W. C. Wood, Emory University; Dr G. Y. Locker, Evanston Hosp (CCOP); Dr L. J. Goldstein, Fox Chase Cancer Center; Dr P. J. Loehrer Sr, Indiana Univ Medical Center; Dr C. S. Vallejos, Instituto de Enfermedades Neoplasicas; Dr H. M. Lazarus, Ireland Cancer Center, University Hospitals of Cleveland; Dr A. A. Forastiere, Johns Hopkins University; Dr J. Gilbert, LSU Medical Center CCOP; Dr T. K. Banerjee, Marshfield Clinic; Dr T. M. Habermann, Mayo Clinic; Dr P. Flynn, Metro-Minnesota CCOP: Abbott-NW; Dr J. A. Kish, Moffitt Cancer Center; Dr H. Hochster, New York University Medical Center; Dr R. H. Ansari, Northern Indiana CRC CCOP; Dr A. B. Benson III, Northwestern University; Dr C. G. Kardinal, Ochsner Clinic; Dr P. Wiernik, Our Lady of Mercy Cancer Center; Dr W. B. Rybka, Penn State Cancer Institute; Dr D. G. Haller, University of Pennsylvania; Dr J. M. Kirkwood, University of Pittsburgh; Dr J. M. Lombard, University of Pretoria; Dr L. Baez, San Juan Minority Based; Dr L. Wong, Scott & White CCOP; Dr J. A. Ellerton, Southern NV Cancer Research Foundation; Dr G. K. Bayer, St Vincent Hospital Regional Cancer Center; Dr H. Pinto, Stanford University; Dr J. K. Erban, Tufts/New England Medical Center; Dr F.-C. Lee, University of New Mexico; Dr D. H. Johnson, Vanderbilt University; Dr P. S. Ritch, Medical College of Wisconsin; and Dr J. A. Stewart, University of Wisconsin.
Prepublished online as Blood First Edition Paper, March 23, 2006; DOI 10.1182/blood-2005-11-4666.
Supported in part by Public Health Service grants CA23318, CA66636, CA21115, CA14548 and from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services.
A complete list of the members of the United Kingdom Medical Research Council Acute Lymphoblastic Leukaemia XII/Eastern Cooperative Oncology Group E2993 (MRC UKALL XII/ECOG E2993) Trial appears in “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Hussein KK, Dahlberg S, Head D, et al. Treatment of acute lymphoblastic leukemia in adults with intensive induction, consolidation, and maintenance chemotherapy. Blood. 1989;73: 57-63. [PubMed] [Google Scholar]
- 2.Petersdorf SH, Kopecky KJ, Head DR, et al. Comparison of the L10M consolidation regimen to an alternative regimen including escalating methotrexate/L-asparaginase for adult acute lymphoblastic leukemia: a Southwest Oncology Group Study. Leukemia. 2001;15: 208-216. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian HM, O'Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18: 547-561. [DOI] [PubMed] [Google Scholar]
- 4.Linker C, Damon L, Ries C, Navarro W. Intensified and shortened cyclical chemotherapy for adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20: 2464-2471. [DOI] [PubMed] [Google Scholar]
- 5.Hoelzer D, Thiel E, Loffler H, et al. Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults. Blood. 1988;71: 123-131. [PubMed] [Google Scholar]
- 6.Linker CA, Levitt LJ, O'Donnell M, Forman SJ, Ries CA. Treatment of adult acute lymphoblastic leukemia with intensive cyclical chemotherapy: a follow-up report. Blood. 1991;78: 2814-2822. [PubMed] [Google Scholar]
- 7.Wetzler M, Dodge RK, Mrozek K, et al. Prospective karyotype analysis in adult acute lymphoblastic leukemia: the Cancer and Leukemia Group B experience. Blood. 1999;93: 3983-3993. [PubMed] [Google Scholar]
- 8.Cytogenetic abnormalities in adult acute lymphoblastic leukemia: correlations with hematologic findings outcome: a Collaborative Study of the Group Francais de Cytogenetique Hematologique. Blood. 1996;87: 3135-3142. [PubMed] [Google Scholar]
- 9.Gaynor J, Chapman D, Little C, et al. A cause-specific hazard rate analysis of prognostic factors among 199 adults with acute lymphoblastic leukemia: the Memorial Hospital experience since 1969. J Clin Oncol. 1988;6: 1014-1030. [DOI] [PubMed] [Google Scholar]
- 10.Hoelzer D. Prognostic factors in acute lymphoblastic leukemia. Leukemia. 1992;6(suppl 4): 49-51. [PubMed] [Google Scholar]
- 11.Kantarjian HM, O'Brien S, Smith T, et al. Acute lymphocytic leukaemia in the elderly: characteristics and outcome with the vincristine-adriamycin-dexamethasone (VAD) regimen. Br J Haematol. 1994;88: 94-100. [DOI] [PubMed] [Google Scholar]
- 12.Laport GF, Larson RA. Treatment of adult acute lymphoblastic leukemia. Semin Oncol. 1997;24: 70-82. [PubMed] [Google Scholar]
- 13.Cortes J, O'Brien SM, Pierce S, Keating MJ, Freireich EJ, Kantarjian HM. The value of high-dose systemic chemotherapy and intrathecal therapy for central nervous system prophylaxis in different risk groups of adult acute lymphoblastic leukemia. Blood. 1995;86: 2091-2097. [PubMed] [Google Scholar]
- 14.Fière D, Lepage E, Sebban C, et al. Adult acute lymphoblastic leukemia: a multicentric randomized trial testing bone marrow transplantation as postremission therapy. The French Group on Therapy for Adult Acute Lymphoblastic Leukemia. J Clin Oncol. 1993;11: 1990-2001. [DOI] [PubMed] [Google Scholar]
- 15.Larson RA, Dodge RK, Burns CP, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood. 1995;85: 2025-2037. [PubMed] [Google Scholar]
- 16.Rowe JM, Buck G, Burnett AK, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106: 3760-3767. [DOI] [PubMed] [Google Scholar]
- 17.Blume KG, Forman SJ, O'Donnell MR, et al. Total body irradiation and high-dose etoposide: a new preparatory regimen for bone marrow transplantation in patients with advanced hematologic malignancies. Blood. 1987;69: 1015-1020. [PubMed] [Google Scholar]
- 18.Mastrangelo R, Poplack D, Bleyer A, Riccardi R, Sather H, D'Angio G. Report and recommendations of the Rome workshop concerning poor-prognosis acute lymphoblastic leukemia in children: biologic bases for staging, stratification, and treatment. Med Pediatr Oncol. 1986;14: 191-194. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoud HH, Rivera GK, Hancock ML, et al. Low leukocyte counts with blast cells in cerebrospinal fluid of children with newly diagnosed acute lymphoblastic leukemia. N Engl J Med. 1993;329: 314-319. [DOI] [PubMed] [Google Scholar]
- 20.Gururangan S, Sposto R, Cairo MS, Meadows AT, Finlay JL. Outcome of CNS disease at diagnosis in disseminated small noncleaved-cell lymphoma and B-cell leukemia: a Children's Cancer Group study. J Clin Oncol. 2000;18: 2017-2025. [DOI] [PubMed] [Google Scholar]
- 21.Thomas DA, Cortes J, O'Brien S, et al. Hyper-CVAD program in Burkitt's-type adult acute lymphoblastic leukemia. J Clin Oncol. 1999;17: 2461-2470. [DOI] [PubMed] [Google Scholar]
- 22.Hoelzer D, Ludwig WD, Thiel E, et al. Improved outcome in adult B-cell acute lymphoblastic leukemia. Blood. 1996;87: 495-508. [PubMed] [Google Scholar]
- 23.Thomas X, Boiron J-M, Huguet F, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22: 4075-4086. [DOI] [PubMed] [Google Scholar]
- 24.Hoelzer D, Thiel E, Ludwig WD, et al. The German multi-center trials for treatment of acute lymphoblastic leukemia in adults. Leukemia. 1992;6: 175-177. [PubMed] [Google Scholar]
- 25.Lamanna N, Weiss M. Treatment options for newly diagnosed patients with adult acute lymphoblastic leukemia. Curr Hematol Rep. 2004;3: 40-46. [PubMed] [Google Scholar]