Abstract
Infant acute lymphoblastic leukemia (ALL) has a poor therapeutic outcome despite attempts to treat it based on prognostic factor–guided therapy. This is the first cooperative group trial characterizing all infants at the molecular level for MLL/11q23 rearrangement. All infants enrolled on Children's Cancer Group (CCG) 1953 were tested for MLL rearrangement by Southern blot and the 11q23 translocation partner was identified (4;11, 9;11, 11;19, or “other”) by reverse-transcriptase polymerase chain reaction (PCR). One hundred fifteen infants were enrolled; overall event-free survival (EFS) was 41.7% (SD = 9.2%) and overall survival (OS) was 44.8% at 5 years. Five-year EFS for MLL-rearranged cases was 33.6% and for MLL-nonrearranged cases was 60.3%. The difference in EFS between the 3 major MLL rearrangements did not reach statistical significance. Multivariate Cox regression analyses showed a rank order of significance for negative impact on prognosis of CD10 negativity, age younger than 6 months, and MLL rearrangement, in that order. Toxicity was the most frequent cause of death. Relapse as a first event in CCG 1953 was later (median, 295 days) compared with CCG 1883 historic control (median, 207 days). MLL/11q23 rearrangement, CD10 expression, and age are important prognostic factors in infant ALL, but molecular 11q23 translocation partners do not predict outcome.
Introduction
In the same treatment era that has seen the overall cure rate of childhood acute lymphoblastic leukemia (ALL) reach levels exceeding 70%, infants younger than 12 months of age with ALL continue to experience very low event-free survival (EFS). These infants comprise 2% to 5% of cases of childhood ALL.1 Published results show 22% to 54% 3- to 6-year EFS.1-6 Infants' high failure rate has been due to relapsed disease rather than toxicity.1,4,5 Therapeutic efforts in infant ALL center on intensification and determining relevant prognostic factors to identify infants needing the most aggressive therapy.
ALL in infancy has been associated with unfavorable features such as hyperleukocytosis, hepatosplenomegaly, and central nervous system (CNS) disease.7,8 The blast cells most commonly lack CD10 expression9 and frequently coexpress lymphoid and myeloid antigens.10,11 Cytogenetic 11q23 abnormalities, most commonly t(4;11)(q21;q23), and molecular genetic rearrangement of the MLL gene at chromosome band 11q23 have been associated with infant ALL.8,12
Correlation between various clinical and laboratory characteristics and outcomes has been studied in the context of a number of clinical trials. These factors are interrelated, making it difficult to identify independent prognostic factors. Higher white blood cell (WBC) count has correlated with worse outcomes, but the WBC categories for comparison have varied in the different reports.1,5,6 Even within the narrow one-year age range defined as infant ALL, the age in months at diagnosis shows a strong prognostic effect with increasingly poor prognosis for younger age. Infants younger than 6 months of age at diagnosis have had EFS from 8% to 40%, whereas those 6 to 12 months old at diagnosis have had EFS from 40% to 71%.1-3,5,6,13
Cytogenetic 11q23 rearrangement is found in 43% to 60% of infants with ALL12,14; MLL gene rearrangement is identified at the molecular level in 70% to 80% of all patients.13,15,16 This difference in the level of detection of MLL/11q23 abnormalities makes it difficult to interpret outcomes when comparing various trials. The published 3- to 6-year EFS for infants with MLL/11q23-rearranged leukemia ranges from 5% to 28%.1,5,13,15-18 Prior reports clearly suggest that infants with MLL translocation partner 4q21 do poorly.12,19 The outcome for infants with 11q23 translocation partners other than 4q21 has been less clear.12,20 The outcome for infants without MLL/11q23 rearrangement has been better, with published 3- to 6-year EFS from 46% to 91%.1,5,13,15-18
CD10 negativity correlates strongly but not completely with MLL/11q23 rearrangement. Infants without CD10 expression have a reported EFS of 21% to 40% at 4 to 6 years, with an EFS of 45% to 73% at 4 to 6 years in infants with CD10+ blasts.1-3,5,6,10 CD10 positivity is therefore correlated with better outcome.
Given the overall poor outcome of infants with ALL and the problem of early relapse on the historic controls Children's Cancer Group (CCG) 107 and CCG 1883,1 CCG 1953 was undertaken to evaluate intensive early therapy. The protocol was designed to assess the feasibility and outcome of induction/intensification followed by a reinduction, reintensification, and consolidation. The feasibility and outcome of bone marrow transplantation (BMT) using family or unrelated donors in infants with 11q23/MLL abnormality was also investigated. In order to identify prognostic factors, the trial was designed to include central analysis of Southern blot for detection of MLL rearrangement and reverse-transcriptase polymerase chain reaction (RT-PCR) to identify t(4;11)(q21;q23), t(11;19)(q23;p13), and t(9;11)(p22;q23).
Patients, materials, and methods
Patients
The protocol enrolled 115 infants (< 1 year of age) with newly diagnosed ALL from July 1996 to August 2000 onto CCG 1953. The diagnosis was based on morphology, cytochemistry, and immunophenotyping, all performed at individual CCG institutions. The protocol was approved by the National Cancer Institute and by Institutional Review Boards at CCG member institutions. Informed consent was obtained from parents according to the Code of Federal Regulations Guidelines and the Helsinki Protocol.
Treatment
Treatment for patients on CCG 1953 consisted of 4 courses of an induction/intensification, reinduction, reintensification, and consolidation schema followed by 3 cycles of an intensified maintenance and 4 cycles of routine maintenance (Table 1). The protocol included high-dose methotrexate and G-CSF to decrease the length of neutropenia and facilitate the delivery of chemotherapy in a dose-intensive fashion.
Table 1.
CCG 1953 therapy detail
| Phase | Treatment regimen |
|---|---|
| Induction, 4 wk | |
| CPM | 250 mg/m2 every 12 h × 4 doses, days 2, 3 |
| DXM | 10 mg/m2 orally 3 times daily, days 0-20, no taper |
| VCR | 0.05 mg/kg, days 0, 14; 0.03 mg/kg day 7 |
| DNM | 2 mg/kg/dose intravenously over 30 min for patients age ≤ 90 d |
| 2 mg/kg/d; 4 mg/kg over 48 h, age > 90 d to < 6 mo | |
| 2.5 mg/kg/d; 5 mg/kg over 48 h, age 6 to < 9 mo | |
| 3 mg/kg/d; 6 mg/kg over 48 h, age ≥ 9 mo, as a 48-h continuous infusion through central venous catheter, days 0, 1 | |
| L-ASP | 6000 IU/m2 × 8 doses, 3 times weekly |
| ITT | Intrathecal triple therapy (methotrexate 7.5 mg; hydrocortisone 7.5 mg; ARA-C 15 mg), days 0, 7, 14, 21, 28 |
| MTX | 4 g/m2, days 21, 28 |
| CF rescue | 10 mg/m2 orally or intravenously every 6 h × ≥ 5 doses, start 18 h after MTX completion |
| VCR | 0.05 mg/kg intravenous push |
| Intensification, 4 wk | |
| VP-16 | 100 mg/m2, days 36-40 |
| DXM | 10 mg/m2 orally 3 times daily, days 0-20, no taper |
| CPM | 300 mg/m2/day intravenously, days 36-40 |
| Reinduction, 4 wk | |
| CPM | 250 mg/m2 every 12 h × 4 doses, days 2, 3 |
| VCR | 0.05 mg/kg days 0, 14; 0.03 mg/kg day 7 |
| DNM | 45 mg/m2/d × 2 days |
| DXM | 10 mg/m2 divided 3 times daily, days 0-20, no taper |
| L-ASP | 6000 IU/m2 intramuscularly × 8 doses, 3 times daily, days 0-20 |
| ITT | Days 0, 14 |
| BMT for 11q23/MLL | |
| Reintensification, 6 wk | |
| VCR | 0.75 mg/m2 intravenously, days 0, 7 |
| VHMTX | 6000 mg/m2 in 1 h, followed by 1200 mg/m2/h × 23 hours × 2 doses, days 0, 14 |
| CF rescue | 200 mg/m2 intravenously over 1 h, then 12 mg/m2 intravenous push every 3 h × 6 doses, then 12 mg/m2 intravenously or orally every 6 h until plasma MTX level < 0.1 μM; start 12 h following MTX completion (36 h after start) |
| VP-16 | 100 mg/m2/d intravenously, days 28-32 |
| CPM | 300 mg/m2/d intravenously over 30 min, days 28-32 |
| IT ARA-C | 15 mg intrathecally, days 7, 21 |
| Consolidation, 9 wk | |
| VHMTX | 6000 mg/m2 intravenously in 1 h, followed by 1200 mg/m2/h intravenously for 23 h, days 28, 42 |
| CF rescue | 10 mg/m2 orally or intravenously every 6 h × ≥ 5 doses, start 18 h after MTX completion |
| IT ARA-C | 15 mg intrathecally, days 35, 49 |
| VCR | 0.75 mg/m2 intravenously, days 28, 42 |
| IV ARA-C | 3000 mg/m2 intravenous 3 h infusion every 12 h, days 0, 2, 7, 8 for total of 8 doses |
| l-ASP | 6000 IU/m2 intramuscularly 3 h after intravenous ARA-C on days 1, 8 |
| Intensified maintenance, 72 d × 3 | |
| VCR | 0.75 mg/m2 intravenously, days 0, 28 |
| DXM | 10 mg/m2 divided orally 3 times daily, days 1-4 and 28-32 |
| 6-MP | 75 mg/m2 orally, days 0-48 |
| MTX | 20 mg/m2 intramuscularly every week, days 0, 7, 14, 21, 28, 35, 42 |
| VP-16 | 100 mg/m2/d, days 49-53 |
| CPM | 300 mg/m2/d intravenously over 30 min, days 49-53 |
| IT ARA-C | 15 mg intrathecally, days 0, 28 |
| Routine maintenance, 4 cycles, 1 y | |
| VCR | 1.5 mg/m2 intravenously every 4 wk × 12 doses, days 0, 28, 56 |
| PRED | 40 mg/m2 orally divided in 3 doses for 5 d with each VCR dose |
| MTX | 20 mg/m2 orally weekly, days 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77 |
| 6-MP | 75 mg/m2 orally daily for 12 weeks per cycle |
| IT MTX | 8 mg age 12-23 mo, 10 mg age 24-35 mo, day 0 |
CPM indicates cyclophosphamide; DXM, dexamethasone; VCR, vincristine; DNM, daunomycin; l-ASP, l-asparaginase; ITT, intrathecal triple therapy (methotrexate; hydrocortisone; ARA-C); BMT, bone marrow transplantation; ARA-C, cytosine arabinoside; MTX, methotrexate; CF, citrovorum factor (leucovorin); VP-16, etoposide; VHMTX, very high dose methotrexate; IT, intrathecal; IV, intravenous; 6-MP, mercaptopurine; and PRED, prednisone.
Infants with 11q23/MLL abnormalities were allocated to BMT within 4 months if they had a protocol-defined 5-6/6 human leukocyte antigen matched related or unrelated donor. The protocol-specified preparative regimen included cytosine arabinoside 100 mg/kg/dose every 12 hours × 6 doses days –8, –7, –6; cyclophosphamide 45 mg/kg/dose intravenously daily × 2 doses days –7, –6; methylprednisolone 33 mg/kg/dose intravenous twice daily × 4 doses starting midnight day –2, –1; and total body irradiation (TBI) treatment dose was a total of 1200 cGy given in 150-cGy fractions (150 cGy twice daily × 8 doses days –3, –2, –1, 0) to the midline at the level of the umbilicus. Graft-versus-host disease (GVHD) prophylaxis consisted of single-agent cyclosporine 1.5 mg/kg intravenously every 12 hours starting day 1.
All infants received 7 doses of triple intrathecal therapy for CNS prophylaxis during the first 10 weeks of therapy, in order to maintain consistency with the concurrent Pediatric Oncology Group (POG) study 9407,21 in the pre-BMT phase of the trial. Infants not undergoing BMT received an additional 14 doses of intrathecal therapy with either single-agent cytosine arabinoside (reintensification, consolidation, and intensified maintenance) or methotrexate (maintenance). Additionally, infants not undergoing BMT received very high-dose methotrexate as given in the CCG 107 and 1883 studies1 in order to maintain the low (< 10%) CNS relapse rate attained in these studies. Patients with CNS disease at diagnosis received no additional therapy. CNS-directed radiation therapy was not administered, although patients receiving BMT received TBI.
The study opened for patient accrual in July of 1996. It was suspended in July of 1997 because of an induction mortality rate of 25% in the first 40 patients enrolled on both CCG 1953 and POG 9407, a POG study that used identical induction/intensification.21 The deaths in CCG 1953 were 3 (60%) of 5 in those younger than 3 months and 4 (20%) of 20 in those older than 3 months. These deaths were due to infections associated with severe breakdown of skin and oral/gastric mucosa associated with daunomycin. The study was first amended with a change in anthracycline dosing based on weight rather than body surface area, by a graded dose schedule calculated to deliver an equivalent dose of daunomycin to older infants and de-escalate the dose for younger infants to a dose that was previously tolerated by infants on the CCG 192P protocol. The 48-hour continuous-infusion daunomycin dose was changed to 4 mg/kg for infants younger than 6 months; 5 mg/kg for infants 6 to 9 months; and 6 mg/kg for infants 9 months or older. The study reopened in November 1997. The induction mortality on COG 1953 was reduced to 0 of 4 in those younger than 3 months and 1 (13%) of 8 in those older than 3 months, but unacceptable toxicity continued in those younger than 3 months on the POG 9407 study. As a result of the POG experience, a second modification was made in July of 1998, changing the daunomycin dosage to 2 mg/kg intravenous over 30 minutes daily for 2 days for infants younger than 90 days of age. Subsequent mortality was improved. A total of 115 patients were enrolled, and the study closed in August 2000.
The detailed results for infants receiving bone marrow transplantation will be described in a separate manuscript (P.A.D., J.M.H., H.S., D.V., N.A.H., R.M., F.O.S., W.G.W., W.L.S., H.S.J., Z.D., G.H.R., manuscript in preparation). This paper will include infants enrolled on CCG 1953 and the companion protocol POG 9407. A separate paper will report the long-term neuropsychologic outcomes of these infants (Thomas A. Kaleita, J.M.H., P.A.D., H.S., D.V., N.A.H., R.M., F.O.S., W.G.W., W.L.S., H.S.J., Z.D., G.H.R., manuscript in preparation).
Historic controls
A group of 135 patients entered on CCG 1883 was the historic control for this protocol. The treatment and outcomes have been reported in detail (Table 2; Figure 1).1 The historic controls and the CCG 1953 cohort were similar in presenting features with 2 main differences. First, there were slightly more infants younger than 90 days of age enrolled on CCG 1953 (20.9%) than on CCG 1883 (15.6%). Second, there were more CD10– infants on CCG 1953 (71.3% CD10– with all infants tested) than on CCG 1883 (57.7% CD10–, but no data are available for 38 of the 135 infants enrolled; Table 3). Thus, CCG 1953 had a population with poorer prognostic factors than the historic controls. Comparison of the frequency of MLL/11q23 gene rearrangement on these trials is problematic, as molecular and cytogenetic methods were employed on CCG 1953 but only cytogenetic methods were used on CCG 1883.
Table 2.
Cumulative dose comparison of key drugs
| Drug | CCG 1883 | CCG 1953 |
|---|---|---|
| Cyclophosphamide, g/m2 | 11 | 9.5 |
| Daunomycin*, mg/m2 | ||
| 3 mo or less | 87.5 | 160 |
| 4 to less than 6 mo | 125 | 166 |
| 6 to less than 9 mo | 125 | 193 |
| 9 to less than 12 mo | 125 | 218 |
| l-asparaginase, units/m2 | 132 000 | 108 000 |
| Etoposide, mg/m2 | 0 | 2 500 |
| Cytosine arabinoside, g/m2 | ||
| Less than 6 mo | 12 | 24 |
| 6 to less than 12 mo | 24 | 24 |
| Dexamethasone, mg/m2 | 0 | 700 |
| Prednisone, mg/m2 | 8 820 | 2 400 |
| Intravenous methotrexate, g/m2 | 134.6 | 142.4 |
CCG 1953 values are estimated conversions based on final protocol dosages.
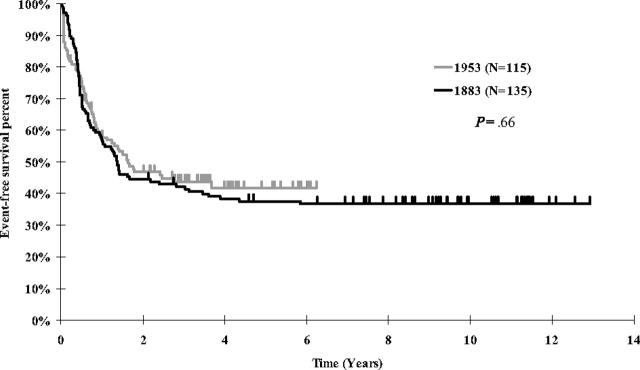
Figure 1.
Kaplan-Meier estimates of event-free survival for infant ALL patients treated on CCG 1953 and CCG 1883.
Table 3.
Presenting features of infants with ALL treated on CCG 1953 and 1883
| Variable and category* | CCG 1953, no. of patients (%) | CCG 1883, no. of patients (%) | P |
|---|---|---|---|
| Age | .224 | ||
| Less than 3 mo | 24 (20.9) | 21 (15.6) | |
| 3 to less than 6 mo | 37 (32.2) | 36 (26.7) | |
| At least 6 mo | 54 (47.0) | 78 (57.8) | |
| Race | .002 | ||
| White | 70 (62.5) | 96 (71.1) | |
| Hispanic | 34 (30.4) | 22 (16.3) | |
| Black | 0 (0.0) | 10 (7.4) | |
| Other/unknown | 8 (7.1) | 7 (5.2) | |
| Sex | .94 | ||
| Male | 54 (47.0) | 64 (47.4) | |
| Female | 61 (53.0) | 71 (52.6) | |
| WBC count | .58 | ||
| Less than 50 × 109/L | 37 (32.2) | 52 (38.5) | |
| 50 × 109/L to 199 × 109/L | 35 (30.4) | 37 (27.4) | |
| At least 200 × 109/L | 43 (37.4) | 46 (34.1) | |
| Liver | .91 | ||
| Normal | 25 (22.9) | 33 (24.4) | |
| Moderately enlarged | 70 (64.2) | 83 (61.5) | |
| Markedly enlarged | 14 (12.8) | 19 (14.1) | |
| Spleen | .66 | ||
| Normal | 22 (19.1) | 31 (23.0) | |
| Moderately enlarged | 65 (56.5) | 69 (51.1) | |
| Markedly enlarged | 28 (24.4) | 35 (25.9) | |
| CNS disease | .18 | ||
| Yes, CNS-3 | 18 (16.4) | 34 (25.4) | |
| Maybe, CNS-2 | 18 (16.4) | 24 (17.9) | |
| No, CNS-1 | 74 (63.7) | 76 (56.7) | |
| CD10 | .04 | ||
| CD10- | 82 (71.3) | 56 (57.7) | |
| CD10+ | 33 (28.7) | 41 (42.3) | |
| MLL/11q23 abnormality | .04 | ||
| Present | 79 (68.7) | 35 (53.0)† | |
| Absent | 36 (31.3) | 31 (47.0) |
For the CCG 1953 study, N = 115; for the CCG 1883 study, N = 135.
For some variables, some data were not submitted for individual patients (liver size, race, CNS, CD10), so totals do not add up to 115.
11q23 info for 1883 is based solely on karyotypes accepted by central review. Information was not complete on all 135 patients. Percentages were calculated excluding patients for whom data were unknown.
Molecular analysis
All molecular analyses were performed in a Clinical Laboratory Improvement Amendments (CLIA)–certified laboratory (University of Minnesota Molecular Diagnostics Lab). Detection of MLL gene rearrangement by Southern analysis and detection of t(4;11), t(11;19), or t(9;11) fusion transcripts by RT-PCR were performed as previously published.22-24
Cytogenetic analysis
Leukemic cell karyotype was determined by cytogenetic analysis at the individual institutions at the time of diagnosis. The protocol required both a direct preparation and a short-term unstimulated culture for bone marrow samples, with unstimulated peripheral blood as backup preparation. Complete analysis of 20 banded metaphases was required for each case. The definition of a clone was as follows: 2 or more metaphases with identical structural abnormalities or extra chromosomes, or 3 or more metaphases with identical missing chromosomes. Karyotypes were designated according to the ISCN 1995, An International System for Human Cytogenetic Nomenclature.25
Statistical methods
Life-table estimates were calculated by the Kaplan-Meier (KM) procedure26 and the standard deviation (SD) of the life-table estimate was obtained using Peto et al's estimate for the variance.27 The relative hazard rate (RHR) was estimated by the ratio of observed to expected events in the comparison groups.27,28 The primary end points examined were EFS and overall survival (OS) from study entry. EFS events included induction failure (nonresponse to therapy or death during induction), leukemic relapse at any site, death during remission, and second malignant neoplasm (SMN), whichever event occurred first. Disease-free survival (DFS) is also provided for some comparisons; this uses only the subset of patients who achieved an initial remission and includes the postinduction events (relapse, death, SMN). For all the life-table analyses, patients not experiencing the event of interest were censored in the analysis at the time of their last contact. Life-table comparisons of patient treatment groups or patient prognostic factors generally used the log-rank statistic29,30 to examine differences among the groups. Cox regression analysis was used to assess the relative importance of certain patient prognostic characteristics in a multivariate analysis for EFS outcome.31 Hypothesis testing in the regression analyses used likelihood ratio tests. Simple chi-square tests were used to assess similarity of patient characteristics when compared with the historic control study.
Results
Patient characteristics
The study population characteristics are detailed in Table 3. Twenty-four patients (20.9%) were younger than 3 months of age. Hepatomegaly and splenomegaly were present in 77.0% and 80.9% of patients, respectively, both defined as either enlarged but not below umbilicus (moderately enlarged) or below umbilicus (markedly enlarged). CNS disease (blasts present on cytospin examination of cerebrospinal fluid in which the WBC count was at least 0.005 × 109/L [5 cells/μL] or clinical signs of CNS leukemia) was present in 16.4%. There were 32.2% with a presenting WBC count under 50 × 109/L, 30.4% between 50 × 109/L and 199 × 109/L, and 37.4% had a WBC count of 200 × 109/L or higher. MLL/11q23 abnormality was present in 68.7%, and 71.3% of the infants had CD10– blasts.
Molecular and cytogenetic testing for MLL/11q23 rearrangement
Seventy-nine (68.7%) of 115 cases showed MLL gene rearrangement by Southern blot, RT-PCR, and/or cytogenetics (Table 4). There were 35 identified by cytogenetics and/or RT-PCR as having t(4;11)(q21;q23), 20 found to have t(11;19)(q23;p13), and 9 found to have t(9;11)(p22;q23). Fifteen cases were MLL rearranged but with 11q23 rearrangements other than these 3. This “other” 11q23 translocation partner was occult (MLL detected by Southern blot but not detected cytogenetically) in 9 patients. These 9 were MLL rearranged by Southern blot, with cytogenetics reported as either normal (4), inadequate (3), or with another karyotypic abnormality [one del(11)(p11.2p13) and one t(10;11)(p11;q13)]. The remaining 6 “other” MLL abnormalities/translocation partners were as follows: t(1,11)(p32;q23); t(4;1)(1;11)(q35;p13p32;q23); der(11)t(11;12)(q23;q15); cytogenetic del(11)(q23q25) and der(19)ins(19;11)(p13.1;q13q23) with MLL rearrangement by Southern blot; add(11q23); and t(10;11)(p13;q23).
Table 4.
MLL gene rearrangement and 5-year EFS
|
CCG 1953
|
||
|---|---|---|
| MLL/11q23 abnormality | No. of patients | 5-year EFS, % |
| t(4;11)(q21;q23) | 35 | 29.0 |
| t(11;19)(q23;p13) | 20 | 30.0 |
| t(9;11)(p22;q23) | 9 | 22.2 |
| Other 11q23 | 15 | 53.3 |
| Nonrearranged | 36 | 60.3 |
For the CCG 1953 study, N = 115.
Cases categorized as “BMT ineligible” were those with no MLL rearrangement or 11q23 abnormality identified by molecular and cytogenetic testing. In 12 cases, testing did not include the entire triad of cytogenetics, Southern blot, and RT-PCR. In these 12, the sample was inadequate for Southern blot testing, and cytogenetics were either inadequate (2) or normal (10). In 10 of these 12 cases, RT-PCR was completed and was negative for the 3 11q23 translocations for which testing was done.
Treatment outcomes
Remission induction. Induction was defined as the length of time it took to get through that phase, just before day 35. Data to assess remission induction were available for 103 of 115 patients entered. There were 10 patients who died prior to day 35. There were 85 patients who were M1 (< 5% blasts) at end of induction, although 3 of these died of complications incurred during induction. There were 3 patients with M2 (5%-25% blasts) marrows at the end of induction, and one with M3 (> 25% blasts). The complete remission (CR) rate was 82.5% (85/103). The remission induction rate for the historic control was 94%.
There were 12 patients who were not evaluable for remission induction because a marrow aspiration was not done on day 35. Ten of these 12 patients had M1 marrows on either day 7 or day 14 or both. Of these 10, 8 patients recovered counts and received further study therapy. One patient received 16 days of systemic therapy and was taken off study and subsequently died of disseminated fungal disease with evidence of peripheral blasts 71 days from study entry. One patient died 54 days from study entry of pulmonary actinomycosis and mucormycosis with an empty marrow at autopsy. One patient with an M2 marrow aspirate on day 7 was removed from study on day 19 of induction; a marrow aspirate was done on 25 days after study enrollment and this marrow aspirate documented a remission. One patient had no evaluable marrow aspirates done during induction; this patient recovered counts and received further study therapy.
First remission transplantation. Patients with MLL rearrangement identified by central review were assigned to BMT if a related or unrelated matched or 1-antigen–mismatched donor was identified, and transplantation could be scheduled by 4 months of study entry.
There were 79 patients with MLL rearrangements and 37 of these received a transplant in first remission. One patient without central identification of MLL rearrangement underwent transplantation in first remission. Compliance with the protocol-specified BMT regimen was poor. Sixteen patients were treated according to the protocol, and there is a group of 22 MLL-rearranged chemotherapy patients that made it out to the median time to transplantation (125 days) and serve as the “control” group for this comparison. In comparing the EFS outcome subsequent to transplantation for the group that received MLL transplants per protocol to the post–125-day outcome in the MLL control, the 4-year EFS is 58.7% (SE = 12.6%) for the chemotherapy control and 25.0% (SE = 10.8%) for the BMT group, with a log rank P value of .006. Nineteen patients received transplants with non–protocol-specified preparative regimens (to be reported in detail in a separate BMT manuscript [P.A.D., J.M.H., H.S., D.V., N.A.H., R.M., F.O.S., W.G.W., W.L.S., H.S.J., Z.D., G.H.R., manuscript in preparation]).
Study outcome and comparisons to the historic control. EFS for the 115 infants was 41.7% (SD = 9.2%) and OS was 44.8% (SD = 5.6%) at 5 years. EFS for CCG 1883 at 5 years was 37.6% (Figure 1; P = .66, RHR = 1.07 for CCG 1883 vs CCG 1953). Comparing EFS for the infants younger than 90 days of age, the current study had a much better EFS at 5 years of 41.7% versus 9.5% for CCG 1883 (P = .06, RHR = 1.89 for CCG 1883). Outcomes within CCG 1953 were examined for cohort 1, who received the original daunomycin dosing, versus cohort 2, who received a modified daunomycin dosing (including those treated after both the first and second modification). EFS outcome was not significantly improved for cohort 2 versus cohort 1 at 5 years, 42.3% versus 36.0%, respectively (P = .37, RHR = 1.30 for cohort 1). Reasons for failure were as follows: cohort 1, relapse in 7 (28%) of 25 and toxic death in 9 (36%) of 25; cohort 2, relapse in 17 (19%) of 90 and toxic death in 31 (34%) of 90. The patterns of failure were not statistically significantly different (P = .52, chi square).
For those alive at the end of induction, 5-year DFS was 49.2% (38.5% for those infants in CCG 1883, P = .09). The difference in DFS was especially dramatic for infants younger than 90 days of age at diagnosis. The 5-year DFS for infants younger than 90 days of age at diagnosis on CCG 1953 was 56.3% and on CCG 1883 was 11.1% (P = .009). There was no difference for the infants older than 90 days of age at diagnosis, with 5-year DFS for infants in remission at end induction of 43.0% on CCG 1953 and 47.6% on CCG 1883 (P = .40).
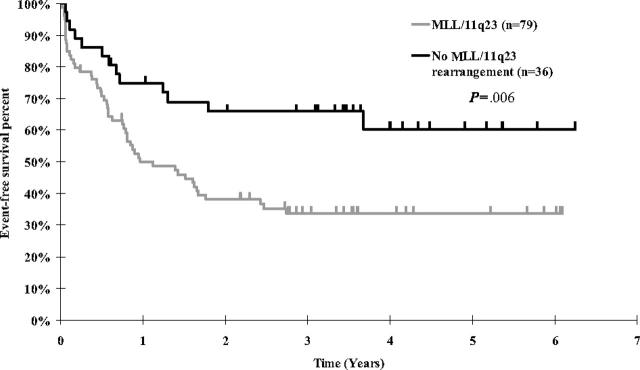
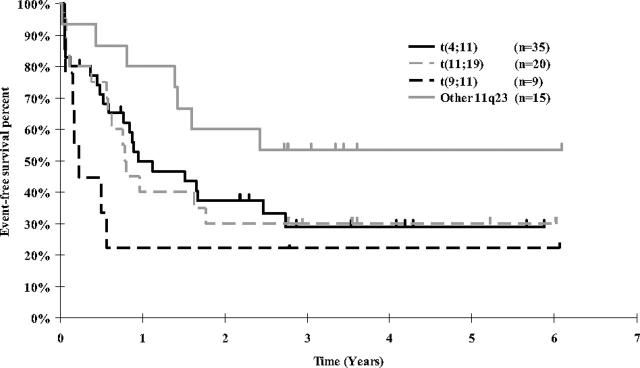
Event-free survival by individual prognostic factors. The 5-year EFS for MLL-rearranged cases was 33.6% and for MLL-nonrearranged cases was 60.3% (Figure 2; P = .006, RHR = 2.29 for MLL rearranged). The difference in EFS between the different MLL rearrangements using a global log rank test (used to compare all 4 groups simultaneously) did not reach a conventional statistical significance level (Figure 3; P = .12). The 5-year EFS for t(4;11) was 29.0%, t(11;19) 30.0%, t(9;11) 22.2%, and other 11q23 53.3%. Outcomes for the t(4;11), t(11;19), and t(9;11) groups were similar (P = .77). When the remaining MLL/11q23 partners (“other 11q23”) were compared with the combination of the t(4;11), t(11;19), and t(9;11) MLL groups, there was a better outcome for the “other 11q23” group (P = .03).
Figure 2.
Kaplan-Meier estimates of event-free survival for CCG 1953 patients by MLL status.
Figure 3.
Kaplan-Meier estimates of event-free survival for CCG 1953 patients in MLL-rearranged subgroups. Comparison of t(4;11), t(11;19), and t(9;11) groups, P = .77. Comparison of “other 11q23” group versus combination of t(4;11), t(11;19), and t(11;19), and t(9;11) groups, P = .03.
Outcomes by age and WBC count are presented with CD10 and MLL data in Table 5. Outcome by CD10 expression was very similar to the MLL data. Five-year EFS for CD10– infants was 31.4% and for CD10+ infants was 66.7% (P < .001). Examining age, the 5-year EFS for infants 3 months of age or younger was 41.7% but for those 3 to less than 6 months was 23.4% and for those 6 months or older was 53.9% (P = .01). Five-year EFS by WBC count was as follows: 54.0% for infants with WBC count less than 50 × 109/L, 37.2% for WBC count 50 × 109/L to 199 × 109/L, and 31.1% for WBC count of at least 200 × 109/L (global P = .19; P = .09 for ordered trend).
Table 5.
CCG 1953 and CCG 1883: five-year EFS according to prognostic factors
|
CCG 1953
|
CCG 1883
|
|||||
|---|---|---|---|---|---|---|
| Variable | No. of patients | 5-year EFS, % | P | No. of patients | 5-year EFS, % | P |
| Age | .012 | < .001 | ||||
| Less than 3 mo | 24 | 41.7 | 21 | 9.5 | ||
| 3 to less than 6 mo | 37 | 23.4 | 36 | 27.8 | ||
| At least 6 mo | 54 | 53.9 | 78 | 49.7 | ||
| CD10 | < .001 | .002 | ||||
| CD10+ | 33 | 66.7 | 41 | 55.3 | ||
| CD10- | 82 | 31.4 | 56 | 28.6 | ||
| WBC count | .191 | < .001 | ||||
| Less than 50 × 109/L | 37 | 54.0 | 52 | 57.6 | ||
| 50 × 109/L to 199 × 109/L | 35 | 37.2 | 37 | 34.8 | ||
| At least 200 × 109/L | 43 | 31.1 | 46 | 17.4 | ||
For the CCG 1953 study, N = 115; for the CCG 1883 study, N = 135.
Early marrow response. Most patients had a rapid early response (RER) status by day 7 (82 [82%] of 101 with results available compared with 60% in the historic control). The effect of early marrow response on outcome was examined separately for MLL/11q23-rearranged versus -nonrearranged patients who were in remission at the end of induction. For the infants with MLL/11q23 rearrangement, rapid early responders (n = 32) had a 5-year DFS of 42.9%; the DFS of intermediate responders (M2/3 day 7, M1 day 14) was 44%. There were only 2 slow responders, both of whom had very early disease events at 7 and 9 months. Among the MLL/11q23-nonrearranged group, rapid early responders had a 5-year DFS of 65.8%, the intermediate responders had 50% DFS (P = .4%), and there were no slow responders.
Multivariate Cox regression analyses were used to determine the relative prognostic influence of MLL/11q23 status, CD10 expression, age at diagnosis, and WBC count. In a univariate Cox regression for each of these factors individually, the rank order of significance would be (1) CD10 expression, P = .001 (RHR of 3.10 for CD10– compared with CD10+); (2) age, P = .012 (RHR of 1.99 for age 6 months and older versus age 0-5 months); (3) presence or absence of MLL/11q23 abnormality, P = .013 (RHR of 2.25 for patients with MLL/11q23 present versus those with MLL/11q23 absent); and (4) WBC count, P = .18 (RHR of 1.51 for WBC count ≥ 50 000 × 109/L versus WBC count < 50 000 × 109/L). Most patients that were CD10– also had MLL/11q23 present, and patients that were CD10+ usually had MLL11q23 absent. Thus, once one of these factors is included in the regression model, the second factor would have little statistical significance on outcome after adjustment for the first. Hence, with CD10 present in the regression model, MLL/11q23 did not retain sufficient additional prognostic effect to meet a typical significance criterion (P = .38). Age had a marginally significant effect (P = .07) after adjusting for CD10 expression, but WBC count failed to demonstrate a significant effect (P = .83) after adjusting for CD10 expression. Thus, in this particular infant study, only CD10 expression maintained a strong prognostic effect in multivariate analysis (P = .004), although age did also show some effect on outcome.
In a stratified life-table analysis, data were analyzed for outcomes within the 2 MLL groups (rearranged and nonrearranged) varying by WBC count, age, and CD10 expression. Outcomes were looked at only for those groups with reasonable numbers of patients. The group that was MLL rearranged had only CD10– infants in significant numbers and among those, WBC count and age did not significantly influence outcome. Among the group without a detected MLL/11q23 abnormality, CD10– infants had poor outcomes (n = 14), with 5-year EFS of 37% compared with CD10+ infants (n = 22) having a 5-year EFS of 73.6%.
Patterns of relapse and events. First events on CCG 1953 were death in 40, isolated marrow relapse in 19, CNS relapse in 1, isolated testicular relapse in 2, concurrent marrow plus CNS relapse in 1, and concurrent marrow plus testicular relapse in 1. Among the 40 patients who experienced death as a first event, the median time to death was 137 days (approximately 20 weeks); range, 9 to 901 days. For the historic control CCG 1883, 8 (of 135) patients experienced death as a first event; the median time to death was 170 days (approximately 24 weeks); range, 3 to 1026 days. Among the 24 patients who experienced relapse as a first event in CCG 1953, the median time to relapse was 295 days (approximately 42 weeks); range, 63 to 1343 days. For CCG 1883, 74 patients experienced relapse as a first event; the median time to relapse was 207 days (approximately 30 weeks); range, 49 to 2138 days.
In MLL/11q23-rearranged patients with events, first event was relapse in 33% and death in 67%. For MLL/11q23-nonrearranged infants with events, first event was death in 46%, relapse in 46%, and induction failure in 8% (1 patient). The median time to death was 110 days for MLL-rearranged babies and was 320 days for MLL-nonrearranged patients. The median time to relapse was 291 days. Forty-seven percent of relapses in MLL-rearranged patients had occurred by day 300 (median 319 days); all relapses in MLL-nonrearranged infants occurred by this day (median 230 days). (These figures are not available for the historic control, as MLL testing was not broadly performed.)
Toxicities
The median number of hospital days through all phases was 87, with the majority during induction/intensification. With the modification of therapy, decreasing induction anthracycline from body surface area to weight dosing, a decrease in induction hospital days was not seen (median 48 days for age < 90 days and 49.5 days for age ≥ 90 days before the amendments compared with 59.5 days for age < 90 days and 49 days for age ≥ 90 days after the amendments). Grades 3 and 4 nonhematologic toxicities are shown in Table 6.
Table 6.
Grades 3 and 4 nonhematologic toxicities by phase of therapy
| Induction/intensification, no. of patients | Reinduction, no. of patients | Reintensification, no. of patients | Consolidation, no. of patients | Intensified maintenance, no. of patient exposures | Maintenance, no. of patient exposures | |
|---|---|---|---|---|---|---|
| Total | 115 | 91 | 66 | 53 | 120 | 126 |
| Cardiac | ||||||
| Cardiac function | 2 | 0 | 0 | 0 | 0 | 1 |
| Hypertension | 12 | 2 | 1 | 0 | 0 | 1 |
| Hypotension | 9 | 0 | 0 | 0 | 1 | 1 |
| Coagulation | ||||||
| Fibrinogen | 8 | 2 | 0 | 0 | 0 | 1 |
| PT | 7 | 0 | 0 | 2 | 0 | 1 |
| PTT | 10 | 1 | 0 | 1 | 0 | 0 |
| Hemorrhage | 6 | 1 | 0 | 0 | 0 | 0 |
| Gastrointestinal | ||||||
| Stomatitis | 54 | 3 | 10 | 0 | 1 | 4 |
| Abdominal pain | 5 | 1 | 0 | 0 | 0 | 3 |
| Diarrhea | 24 | 9 | 5 | 7 | 9 | 9 |
| Nausea/vomiting | 11 | 1 | 6 | 1 | 2 | 5 |
| Liver | ||||||
| AST | 18 | 1 | 13 | 10 | 3 | 2 |
| ALT | 25 | 4 | 12 | 11 | 8 | 8 |
| Bilirubin | 18 | 2 | 8 | 6 | 1 | 2 |
| Nervous system | ||||||
| Peripheral | 8 | 0 | 0 | 0 | 1 | 4 |
| Central | 5 | 1 | 3 | 0 | 3 | 5 |
| Pancreas | ||||||
| Glucose | 17 | 2 | 2 | 0 | 1 | 2 |
| Pulmonary | ||||||
| PaO2 | 8 | 0 | 0 | 0 | 0 | 0 |
| Functional | 28 | 2 | 2 | 2 | 3 | 2 |
| Clinical | 16 | 0 | 0 | 0 | 1 | 1 |
| Renal | ||||||
| BUN | 6 | 1 | 0 | 0 | 0 | 0 |
| Creatinine clearance | 2 | 1 | 1 | 0 | 0 | 0 |
PT indicates prothrombin time; PTT, partial thromboplastin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PaO2, oxygen saturation; and BUN, blood urea nitrogen.
Overall, 495 infectious complications occurred among 109 patients. Bacterial infections occurred most frequently (74%), followed by viral (13%), fungal (11%), and protozoan (1%). The most common sites of infection were blood (57%), cutaneous (12%), stool (9%), and lower respiratory (8%). Infectious complications continued through all phases: induction/intensification (84%), reinduction (35%), reintensification (41%), consolidation (72%), intensified maintenance (32%), and maintenance (28%). Despite the high rate of infectious complications, infection-related mortality was greatly reduced after induction/intensification: induction/intensification (16.5%), reinduction (0.9%), reintensification (0%), consolidation (0.9%), intensified maintenance (1.7%), and maintenance (3.5%). Significantly, reinduction, which included both continuous infusion daunomycin and dexamethasone, was not associated with the infection-related mortality of induction/intensification. In addition, although infectious complications were high during consolidation (very high-dose methotrexate and high-dose cytosine arabinoside), infection-related mortality was acceptable.
There were 19 infection-related deaths (8 bacterial, 5 fungal, and 6 viral) during induction/intensification among the 115 patients (16.5%). There were 7 deaths in induction/intensification among 25 patients (28%) in cohort 1 and 12 deaths among 90 patients (13%) treated in cohort 2. Despite the decrease in mortality, infectious-related complications did not change significantly in numeric incidence after induction treatment modifications.
Twenty-nine percent (n = 6) of infants younger than 3 months of age at diagnosis died of infection-related causes during induction/intensification compared with 14% (n = 13) of infants 3 months of age or older at diagnosis (Table 7). Corresponding with the daunomycin dosage amendments, a decrease in mortality was seen across all age groups. However, no clear correlation with rates of grades 3 and 4 mucositis or skin breakdown was seen (Table 8) nor was there a relationship to any other recorded grades 3 and 4 toxicities.
Table 7.
Infection-associated mortality during phase 1 by age at diagnosis
|
Age less than 3 mo
|
Age 3 to less than 6 mo
|
Age at least 6 mo
|
||||
|---|---|---|---|---|---|---|
| % | No. out of total | % | No. out of total | % | No. out of total | |
| Before 11/97 amendment | 50 | 2/4 | 30 | 3/10 | 18 | 2/11 |
| After 11/97 amendment | 0 | 0/4 | 11 | 3/28 | 11 | 5/45 |
| After 7/98 amendment | 31 | 4/13 | NA | NA | NA | NA |
| Total | 29 | 6/21 | 16 | 6/38 | 13 | 7/56 |
Phase 1 indicates induction/intensification; NA, not applicable.
Table 8.
Grades 3 and 4 mucositis and skin breakdown during phase 1
|
Age less than 3 mo
|
Age 3 to less than 6 mo
|
Age at least 6 mo
|
||||
|---|---|---|---|---|---|---|
| % | No. out of total | % | No. out of total | % | No. out of total | |
| Mucositis | ||||||
| Before 11/97 amendment | 50 | 2/4 | 30 | 3/10 | 27 | 3/11 |
| After 11/97 amendment | 50 | 2/4 | NA | NA | NA | NA |
| After 7/98 amendment | 31 | 4/13 | 57 | 16/28 | 5 | 24/45 |
| Skin breakdown | ||||||
| Before 11/97 amendment | 50 | 2/4 | 10 | 1/10 | 9 | 1/11 |
| After 11/97 amendment | 25 | 1/4 | 43 | 12/28 | 31 | 14/45 |
| After 7/98 amendment | 69 | 9/13 | NA | NA | NA | NA |
Phase 1 indicates induction/intensification; NA, not applicable.
Discussion
Poor outcomes for infants with ALL led to the development of protocols that simultaneously intensified therapy and attempted to define prognostic factors. In the current report, we present data on overall disease outcome and prognostic factor analysis for the largest series of infants in a single clinical trial studied at the molecular level for MLL gene rearrangement.
The CCG clinical trials preceding this one (CCG 107 and CCG 1883) reported 5-year EFS for infants of 33% and 38%, respectively.1 The EFS for CCG 1953 was 41.6% at 5 years. Thus, while there are fewer relapses and relapse occurs later (207 days on CCH 1883 vs 295 days on CCG 1953), ultimate outcome has not improved to a level of statistical significance despite intensified therapy. With the remission induction rate inferior to the historic control due to early toxicity and the 5-year EFS higher, the benefit of intensified therapy is lost in early toxicity. The prognostic factor analysis must be taken in the context of the treatment failure patterns observed. In CCG 107 and 1883, the major cause of treatment failure was early marrow relapse.1 CCG 107 and 1883 reported 2 (2.0%) and 5 (3.7%) deaths in induction, and 65% and 72% of relapses were within the first year after induction.1
The CCG 1953 protocol was thus developed to address early relapse and to test the hypothesis that intensification of early therapy would improve outcome for these patients. The induction chemotherapy of the protocol was modeled on the CCG 192P protocol, which used intensive multi-agent chemotherapy. Infants treated on this protocol had an EFS of 36% at 4 years, with minimal treatment-related morbidity.32 Dosing of chemotherapy for infants in previous protocols was determined by weight not body surface area. Using weight rather than body surface area results in significantly lower doses of drugs for these patients. Therefore, to intensify the protocol therapy, body surface area was used to calculate doses of all agents except vincristine. Dexamethasone at 10 mg/m2 was chosen as the steroid during the first year of therapy, based on reports of improved outcome using this steroid and particularly better CNS disease control. Additional consideration in the development of the protocol was that MLL-rearranged ALL in infants may have a biology similar to MLL-rearranged acute myeloid leukemia (AML). High-dose cytosine arabinoside is an active agent in the consolidation of AML, including AML in infants with MLL rearrangements. This biology also contributed to the rationale for BMT for infants with MLL rearrangement. The CCG 107 and 1883 studies used very high-dose methotrexate as CNS prophylaxis and these studies demonstrated that this approach led to acceptable CNS prophylaxis with the toxicity of central nervous prophylaxis.
The early relapse pattern is reversed with the intensified therapy of CCG 1953, with 19 (16.5%) deaths related to induction, and less early relapse. The early toxicity seen in CCG 1953 resulted in amendments decreasing anthracycline intensity. The subsequent cohorts had decreased induction deaths but EFS only marginally increased. The concurrent POG 9407 trial was similarly amended, and remains open (COG P9407), and was further amended with the removal of dexamethasone in induction replaced by prednisone.
In CCG 107 and 1883, age, WBC count, French-American-British morphology, hepatosplenomegaly, CD10 expression, and t(4;11) status (as opposed to any 11q23 rearrangement) were all significant prognostic factors.1 However, it must be emphasized that 11q23 status was determined strictly by cytogenetics and results were available to report on only 38 of 99 patients on CCG 107 and on 66 of 135 patients on CCG 18831 (additional cases reviewed since publication). Thus the impact of particular 11q23 partners on outcome was not determined for CCG 1883 (N.A.H., unpublished data, December 1988–August 1993).
For the CCG 1953 cohort, paired molecular and cytogenetic determination of MLL/11q23 status yielded the expected outcomes: the rate of MLL rearrangement (69%), that most of these are t(4;11), and that a significant number of t(11;19) are detected with this methodology. MLL/11q23 abnormality was an important predictor of outcome; the 5-year EFS for MLL-rearranged infants was significantly less (33.6%) than that of MLL-nonrearranged cases (60.3%). With molecular testing completed on the great majority of cases, we showed that the individual MLL rearrangements t(4;11), t(11;19), and t(9;11) do not have different effects on prognosis. Specifically, t(4;11) did not have outcomes worse than t(11;19) and t(9;11). The group of infants with t(11;19) (n = 20) having outcomes equivalent to those with t(4;11) stands in contrast to data from the previous CCG trials12,19 but is in agreement with data from Rubnitz et al.20
The outcomes of infants for whom MLL rearrangement was indeterminate but CD10 was negative were as poor as those of MLL-rearranged infants. This suggests that CD10– infants without sufficient material for molecular testing, who have inadequate or normal cytogenetics, either had undetected MLL/11q23 rearrangement or, as suggested by multivariate analysis, CD10 negativity stands alone as a negative prognostic factor. Such infants should be treated as MLL-rearranged infants in protocols using MLL/11q23 status to dictate therapy.
Age and CD10 expression remain individual adverse prognostic factors, but WBC count at diagnosis did not emerge as prognostically significant (Table 5). The 5-year EFS for infants younger than 90 days of age (n = 24; 41.7% in CCG 1953) is a significant improvement from the 9.5% 5-year EFS seen in that age group in CCG 1883 (n = 21). It may be that for those infants younger than 90 days of age surviving induction, the increase in intensity of CCG 1953 compared with CCG 1883 was sufficient to overcome late relapse.
CD10 expression and MLL/11q23 status correspond so closely in infants with ALL that it is difficult to assess their independent prognostic significance. In multivariate analysis, age had reduced prognostic significance after adjustment for either CD10 status or MLL/11q23 status with the close association between age under 6 months, CD10 negativity, and MLL/11q23 rearrangement making it difficult to discern the relative impact of these factors on disease outcome. Future clinical trials should continue to assess these factors.
Evaluation of the impact of early marrow response shows that while early response status is important, it does not override the impact of MLL/11q23 rearrangement. As was seen in CCG 1883,1 the majority of infants were rapid early responders. MLL/11q23-rearranged early responders had an EFS of 46% compared with the overall 37% EFS of this group. Slow early response in MLL/11q23-rearranged infants was particularly ominous. Interestingly, rapid early marrow response was a better prognostic sign in CCG 1953 than CCG 1883, where there were still poor outcomes seen in MLL/11q23-rearranged infants even if the marrow was in early response. Specifically, infants in CCG 1883 with cytogenetically detected 11q23 rearrangements and rapid early response had a 5-year EFS of 29% (n = 24). Thus, for those infants surviving induction, the intensified CCG 1953 therapy improved outcomes for MLL/11q23-rearranged infants. While this reflects the fact that poor-risk patients were able to tolerate the less intense CCG 1883 induction and survive ultimately to relapse, it also points out that intensified therapy can change the natural history of MLL disease.
There was only one isolated CNS relapse as a first event in this group of infants (0.9%) and only 4 isolated CNS relapses (3%) in CCG 1883.1 The CNS-directed therapy in these protocols was nearly identical and consisted of intrathecal chemotherapy (21 total doses in CCG 1953, 18-22 in CCG 1883), high-dose methotrexate (delivered 4 times in each protocol), high-dose Ara-C (8 doses in CCG 1953, 4 in CCG 1883), and l-asparaginase therapy (18 doses each protocol) without CNS radiotherapy. It is intriguing to note the similar low CNS relapse rate in 2 protocols that have had such dissimilar toxicity/relapse patterns. The current open protocol for infant ALL in the COG P9407 study21 includes these treatments but does not include “very high-dose” methotrexate (33.6 g/m2).
The intensity of therapy has resulted in regimen-related toxicity in a pattern that is predominantly infectious. In many cases the infections seen were the type of infection expected in immune incompetent patients, such as pneumocystis and viral infections. These were felt to be due to dexamethasone in induction. Consequently, the currently open successor protocol substitutes the high-dose induction dexamethasone with standard doses of prednisone to determine whether disease control will be maintained with less-prolonged immune suppression and less infection-related mortality. This remains an outcome to be examined in the ongoing study COG P9407.
With the design of future protocols for infant ALL will come the opportunity to evaluate innovative therapies. Traditionally such therapies are ethically reserved for those with EFS less than 50%.33 MLL-rearranged patients on this study had a 5-year EFS of 34%, clearly challenging us to come up with new therapy plans. With CCG 1953 therapy, MLL-nonrearranged infants have an EFS of 60.3%. While not “excellent,” these patients too present us with the challenge of improving outcomes while decreasing toxicity. Stratification of infants using multiple prognostic factors may help to better select subsets of patients requiring more intensive therapy. At the same time, even if MLL/11q23-rearranged infants were to achieve outcomes better than an EFS of 50%, the prospect of less toxic therapy with innovative or targeted therapies challenges us to look beyond the traditional paradigms of therapy design. In light of this, there is a great need to increase the availability of preclinical data to guide the selection of new agents for infants with ALL.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and are not to be construed as the official policy or position of the US government, the Department of Defense, or the Department of the Air Force. The authors wish to thank all investigators who enrolled their patients and the physicians and nurses who cared for them. We gratefully acknowledge Dr William Carroll's review of the article and his excellent suggestions.
Appendix
Principal investigators for the Children's Oncology Group Study 1953 and their participating institutions are as follows: Martha Greenwood, A. B. Chandler Medical Center, University of Kentucky, Lexington; Jennifer Pearce, Albany Medical Center, NY; Mansoor Haq, Allan Blair Cancer Centre, Regina, SK, Canada; Hazem Mahmoud, Atlantic Health System, Morristown, NJ; Tribhawan Vats, Backus Children's Hospital at Memorial Health University Medical Center, Savannah, GA; Philippa Sprinz, Baystate Medical Center, Springfield, MA; Bellin Memorial Hospital, Green Bay, WI; Mason Bond, British Columbia's Children's Hospital, Vancouver, Canada; Raymond Hutchinson, C. S. Mott Children's Hospital, Ann Arbor, MI; Andrew Pendleton, Cabell Huntington Hospital, Huntington, WV; Rochelle Yanofsky, CancerCare Manitoba, Winnipeg, Canada; Carole Hurvitz, Cedars-Sinai Medical Center, Los Angeles, CA; Children's Healthcare of Atlanta at Scottish Rite, GA; Howard Katzenstein, Children's Healthcare of Atlanta, Emory University, GA; John Iacuone, Children's Hem/Onc Team at Covenant Children's Hospital, Lubbock, TX; Joanne Hilden, The Children's Hospital at The Cleveland Clinic, OH; Children's Hospital at the Medical Center of Central Georgia, Macon; Douglas Hawkins, Children's Hospital and Regional Medical Center, Seattle, WA; Vonda Crouse, Children's Hospital Central California, Madera; John Perentesis, Children's Hospital Medical Center Cincinnati, OH; Steven Kuerbitz, Children's Hospital Medical Center–Akron, OH; James Feusner, Children's Hospital Oakland, CA; Bruce Bostrom, Children's Hospital of Minnesota, Minneapolis; Violet Shen, Children's Hospital of Orange County, Orange, CA; Richard Womer, Children's Hospital of Philadelphia, PA; Rebecca Byrd, Children's Hospital–King's Daughters, Norfolk, VA; Children's Hospitals and Clinics-St Paul, St Paul, MN; Emmett Broxson, Children's Medical Center Dayton, OH; Minnie Abromowitch, Children's Memorial Hospital of Omaha, NE; Maxine Hetherington, The Children's Mercy Hospital, Kansas City, MO; Nita Seibel, Children's National Medical Center–DC, Washington; Gregory Griffin, Christiana Care Health Services/A. I. duPont Institute, Wilmington, DE; Judith Sato, City of Hope National Medical Center, Duarte, CA; Clarian Health, Indianapolis, IN; Linda Granowetter, Columbia Presbyterian College of Physicians & Surgeons, New York, NY; Arnold Altman, Connecticut Children's Medical Center, Hartford; Cooper Hospital/University Medical Center, Camden, NJ; Dakota Midwest Cancer Institute, Sioux Falls, SD; David Grant USAF Medical Center, Travis Air Force Base, CA; Deaconess Medical Center, Spokane, WA; David Freyer, DeVos Children's Hospital, Grand Rapids, MI; Linda Stork, Doernbecher Children's Hospital–Oregon Health & Science University, Portland; Ray Pais, East Tennessee Children's Hospital, Knoxville; Jeffrey Taylor, Geisinger Medical Center, Danville, PA; Aziza Shad, Georgetown University Medical Center, Washington, DC; Group Health Cooperative, Seattle, WA; Gundersen Lutheran, La Crosse, WI; Henry Ford Hospital, Detroit, MI; Robert Fallon, Indiana University–Riley Children's Hospital, Indianapolis; Margaret Yhap, Izaak Walton Killam (IWK) Health Centre, Halifax, NS, Canada; John (Jack) Hand, Janeway Child Health Center, St John's, NF, Canada; Vincent Kiley, Kaiser Permanente Medical Group, Northern California, Sacramento; Leonard Mattano Jr, Kalamazoo Center for Medical Studies, MI; Salvatore Bertolone, Kosair Children's Hospital, Louisville, KY; Antranik Bedros, Loma Linda University Medical Center, Loma Linda, CA; Jong-Hyo Kwon, Lutheran General Children's Medical Center, Park Ridge, IL; Joann Ater, M. D. Anderson Cancer Center, Houston, TX; Ludovico Guarini, Maimonides Medical Center, Brooklyn, NY; Michael McManus, Marshfield Clinic, WI; Ronald Louie, Mary Bridge Hospital, Tacoma, WA; Carola Arndt, Mayo Clinic and Foundation, Rochester, MN; Roger Vega, Medical College of Georgia Children's Medical Center, Augusta, GA; Memorial Miller Children's Hospital at Long Beach Memorial Medical Center, CA; Rama Jasty, Mercy Children's Hospital, Toledo, OH; Nathan Kobrinsky, MeritCare Medical Group doing business as (DBA) Roger Maris Cancer Center, Fargo, ND; Donna Wall, Methodist Children's Hospital of South Texas, San Antonio; MetroHealth Medical Center, Cleveland, OH; Renuka Gera, Michigan State University, East Lansing; W. Roberts, Miller Children's Hospital/Harbor–University of California at Los Angeles (UCLA), Long Beach; Adam Levy, Montefiore Medical Center, Bronx, NY; Morristown Memorial Hospital, NJ; Jonathan Bernstein, Nevada Cancer Research Foundation–Community Clinical Oncology Program, Las Vegas; Fevzi Ozkaynak, New York Medical College, Valhalla; Elizabeth Raetz, New York University Medical Center, NY; Peri Kamalakar, Newark Beth Israel Medical Center, NJ; Patricia Shearer, Ochsner Clinic, New Orleans, LA; John Neely, Penn State Children's Hospital, Hershey Medical Center, PA; Stephen Palmer, Presbyterian/St Luke's Medical Center and Childhood Hematology Oncology Associates, Denver, CO; Phillip Barnette, Primary Children's Medical Center, Salt Lake City, UT; David Baker, Princess Margaret Hospital for Children, Perth, WA, Australia; Susan Wiersma, Rainbow Babies and Children's Hospital, Cleveland, OH; Daniel Greenfield, Santa Barbara Cottage Children's Hospital, CA; Arlene Redner, Schneider Children's Hospital, New Hyde Park, NY; Schneider Children's Hospital at North Shore, Manhasset, NY; Joseph Wiley, Sinai Hospital of Baltimore, MD; Linda Stout, Sioux Valley Children's Specialty Clinics, Sioux Falls, SD; Ronnie Neuberg, South Carolina Cancer Center, Columbia; Robert Cooper, Southern California Permanente Medical Group, Downey; Mary Ann Bonilla, St Joseph's Hospital and Medical Center, Paterson, NJ; Randy Hock, St Vincent Children's Hospital–Indiana, Indianapolis; Draga Barbaric, Sydney Children's Hospital, Randwick, NSW, Australia; Curtis Turner, Texas Tech University Health Sciences Center–Amarillo; Ayman Saleh, Tod Children's Hospital–Forum Health, Youngstown, OH; Dagmar Stein, Toledo Children's Hospital, OH; Theodore Moore, UCLA School of Medicine, Los Angeles; Stanley Calderwood, University of California, Irvine, Orange; James Nachman, The University of Chicago Comer Children's Hospital, IL; Mary Schmidt, University of Illinois, Chicago; University of Illinois–Rockford; Richard Drachtman, University of Medicine and Dentistry of New Jersey, New Brunswick; Joseph Neglia, University of Minnesota Cancer Center, Minneapolis; Peter Coccia, University of Nebraska Medical Center, Omaha; Stuart Gold, University of North Carolina at Chapel Hill; Yousif (Joe) Matloub, University of Wisconsin–Children's Hospital Madison; James Whitlock, Vanderbilt Children's Hospital, Nashville, TN; Charles Main, William Beaumont Hospital, Royal Oak, MI; Mark Weinblatt, Winthrop University Hospital, Mineola, NY.
Note: Institutions listed without a principal investigator may have served as treatment facilities collaborating with a principal investigator at a nearby institution or are no longer members of the COG.
Prepublished online as Blood First Edition Paper, March 23, 2006; DOI 10.1182/blood-2005-07-3011.
A complete list of the participating members of the Children's Oncology Group appears in “Appendix.”
Supported by grants from the National Institutes of Health (grants CA13539 and CA98543). A complete listing of grant support for research conducted by Children's Cancer Group (CCG) and Pediatric Oncology Group (POG) before initiation of the COG grant in 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm.
P.A.D., F.O.S., W.G.W., Z.D., and G.H.R. designed the protocol; P.A.D., J.M.H., and H.S.J. ran the clinical trial; J.M.H., S.O.M., and P.A.D. wrote the manuscript; H.S. and D.V. performed the statistical analyses; N.A.H. reviewed the cytogenetics; R.M. performed the molecular analyses; and W.L.S. reviewed and summarized toxicity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Reaman GH, Sposto R, Sensel MG, et al. Treatment outcome and prognostic factors for infants with acute lymphoblastic leukemia treated on two consecutive trials of the Children's Cancer Group. J Clin Oncol. 1999;17: 445-455. [DOI] [PubMed] [Google Scholar]
- 2.Ferster A, Bertrand Y, Benoit Y, et al. Improved survival for acute lymphoblastic leukemia in infancy: the experience of EORTC-Childhood Leukaemia Cooperative Group. Br J Haematol. 1994;86: 284-290. [DOI] [PubMed] [Google Scholar]
- 3.Chessels JM, Harrison CJ, Watson SL, Vora AJ, Richards SM. Treatment of infants with lymphoblastic leukaemia: results of the UK Infant Protocols 1987-1999. Br J Haematol. 2002;117: 306-314. [DOI] [PubMed] [Google Scholar]
- 4.Frankel LS, Ochs J, Shuster JJ, et al. Therapeutic trial for infant acute lymphoblastic leukemia: the Pediatric Oncology Group experience (POG 8493). J Pediatr Hematol Oncol. 1997;19: 35-42. [DOI] [PubMed] [Google Scholar]
- 5.Dördelmann M, Reiter A, Borkhardt A, et al. Prednisone response is the strongest predictor of treatment outcome in infant acute lymphoblastic leukemia. Blood. 1999;94: 1209-1217. [PubMed] [Google Scholar]
- 6.Silverman SB, McLean TW, Gelber RD, et al. Intensified therapy for infants with acute lymphoblastic leukemia: results from the Dana-Farber cancer institute consortium. Cancer. 1997;80: 2285-2295. [PubMed] [Google Scholar]
- 7.Reaman GH, Zeltzer P, Bleyer WA, et al. Acute lymphoblastic leukemia in infants less than one year of age: a cumulative experience of the Children's Cancer Group. J Clin Oncol. 1985;3: 1513-1521. [DOI] [PubMed] [Google Scholar]
- 8.Pui C-H, Evans WE. Editorial: acute lymphoblastic leukemia in infants [editorial]. J Clin Oncol. 1999;17: 438-440. [DOI] [PubMed] [Google Scholar]
- 9.Dinndorf PA, Reaman GR. Acute lymphoblastic leukemia in infants: evidence for B cell origin of disease by use of monoclonal antibody phenotyping. Blood. 1986;68: 975-978. [PubMed] [Google Scholar]
- 10.Basso G, Putti C, Cantu-Rajnoldi A, et al. The immunophenotype in infant acute lymphoblastic leukemia: correlation with clinical outcome: an Italian multicentre study (AIEOP). Br J Haematol. 1992;81: 184-191. [DOI] [PubMed] [Google Scholar]
- 11.Pui C-H, Kane JR, Crist WM. Biology and treatment of infant leukemias. Leukemia. 1995;9: 762-769. [PubMed] [Google Scholar]
- 12.Heerema NA, Arthur DC, Sather H, et al. Cytogenetic features of infants less than 12 months of age at diagnosis of acute lymphoblastic leukemia: impact of the 11q23 breakpoint on outcome: a report of the Children's Cancer Group. Blood. 1994;83: 2274-2284. [PubMed] [Google Scholar]
- 13.Pui C-H, Ribeiro RC, Campana D, et al. Prognostic factors in the acute lymphoid and myeloid leukemias of infants. Leukemia. 1996;10: 952-956. [PubMed] [Google Scholar]
- 14.Raimondi SC, Peiper SC, Kitchingman GR, Behm FG, Williams DL. Childhood acute lymphoblastic leukemia with chromosomal breakpoints at 11q23. Blood. 1989;73: 1627-1634. [PubMed] [Google Scholar]
- 15.Hilden JM, Frestedt JL, Moore RO, et al. Molecular analysis of infant acute lymphoblastic leukemia: MLL gene rearrangement and reverse-transcriptase polymerase chain reaction for t(4; 11)(q21;q23). Blood. 1995;86: 3876-3882. [PubMed] [Google Scholar]
- 16.Rubnitz JE, Link MP, Shuster JJ, et al. Frequency and prognostic significance of HRX rearrangements in infant acute lymphoblastic leukemia: a Pediatric Oncology Group study. Blood. 1994;84: 570-573. [PubMed] [Google Scholar]
- 17.Cimino G, Rapanotti MC, Ribolta A, et al. Prognostic relevance of ALL-1 gene rearrangement in infant acute leukemia. Leukemia. 1995;9: 391-395. [PubMed] [Google Scholar]
- 18.Taki T, Ida K, Bessho F, et al. Frequency and clinical significance of the MLL gene rearrangements in infant acute leukemia. Leukemia. 1996;10: 1303-1307. [PubMed] [Google Scholar]
- 19.Heerema NA, Sather HN, Ge J, et al. Cytogenetic studies of infant acute lymphoblastic leukemia: poor prognosis of infants with t(4;11): a report of the Children's Cancer Group. Leukemia. 1999;13: 679-686. [DOI] [PubMed] [Google Scholar]
- 20.Rubnitz JE, Camitta BM, Mahmoud H, et al. Childhood acute lymphoblastic leukemia with the MLL-ENL fusion and t(11;19)(q23;p13.3). J Clin Oncol. 1999;17: 191-196. [DOI] [PubMed] [Google Scholar]
- 21.Dreyer ZE, Steuber CP, Bowman WP, et al. High risk infant ALL-Improved survival with intensive chemotherapy [abstract]. Proc Am Soc Clin Oncol. 1998;17: 529a. [Google Scholar]
- 22.Chen CS, Sorensen PH, Domer PH, et al. Molecular rearrangements on chromosome 11q23 predominate in infant acute lymphoblastic leukemia and are associated with specific biologic variables and poor outcome. Blood. 1993;81: 2386-2393. [PubMed] [Google Scholar]
- 23.Repp R, Borkhardt A, Haupt E, et al. Detection of four different 11q23 chromosomal abnormalities by multiplex-PCR and fluorescence-based automatic DNA-fragment analysis. Leukemia. 1995;9: 210-215. [PubMed] [Google Scholar]
- 24.Hilden JM, Chen CS, Moore R, Frestedt J, Kersey JH. Heterogeneity in MLL/AF-4 fusion messenger RNA detected by the polymerase chain reaction in t(4;11) acute leukemia. Cancer Res. 1993;53: 3853-3856. [PubMed] [Google Scholar]
- 25.Mitelman F, ed. ISCN: An International System for Human Cytogenetic Nomenclature. Basel, Switzerland: S. Karger; 1995.
- 26.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53: 457-481. [Google Scholar]
- 27.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient, II: analysis and examples. Br J Cancer. 1977;35: 1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernstein L, Anderson J, Pike M. Estimation of the proportional hazard in two-treatment group clinical trials. Biometrics. 1981;37: 513-519. [PubMed] [Google Scholar]
- 29.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50: 163-170. [PubMed] [Google Scholar]
- 30.Breslow N. Analysis of survival data under the proportional hazards model. Int Stat Rev. 1975;43: 45-58. [Google Scholar]
- 31.Cox DR. Regression models and life tables. J Royal Stat Soc B. 1972;34: 187-220. [Google Scholar]
- 32.Reaman GH, Steinherz PG, Gaynon PS, et al. Improved survival of infants less than 1 year of age with acute lymphoblastic leukemia treated with intensive multiagent chemotherapy. Cancer Treat Rep. 1987;71: 1033-1038. [PubMed] [Google Scholar]
- 33.Beauchamp T, Lukens J, Sallan S, Pentz R, Ellenberg S, Weiner S. Phase II Window Studies in Pediatric Oncology Meeting Report. http://ctep.cancer.gov/resources/phase2.html. Accessed August 2005.