Abstract
Human embryonic stem cells are a promising tool to study events associated with the earliest ontogenetic stages of hematopoiesis. We describe the generation of erythroid cells from hES (H1) by subsequent processing of cells present at early and late stages of embryoid body (EB) differentiation. Kinetics of hematopoietic marker emergence suggest that CD45+ hematopoiesis peaks at late D14EB differentiation stages, although low-level CD45- erythroid differentiation can be seen before that stage. By morphologic criteria, hES-derived erythroid cells were of definitive type, but these cells both at mRNA and protein levels coexpressed high levels of embryonic (ε) and fetal (γ) globins, with little or no adult globin (β). This globin expression pattern was not altered by the presence or absence of fetal bovine serum, vascular endothelial growth factor, Flt3-L, or coculture with OP-9 during erythroid differentiation and was not culture time dependent. The coexpression of both embryonic and fetal globins by definitive-type erythroid cells does not faithfully mimic either yolk sac embryonic or their fetal liver counterparts. Nevertheless, the high frequency of erythroid cells coexpressing embryonic and fetal globin generated from embryonic stem cells can serve as an invaluable tool to further explore molecular mechanisms.
Introduction
During human development, hematopoietic cells sequentially recruit new anatomic sites for their development, from the yolk sac, to the fetal liver, to the bone marrow (BM) in adults. Erythroid cells developing at these sites are distinguished morphologically, and they display distinct transcriptional factor and growth factor requirements, proliferative kinetics, and globin patterns.1 Thus, erythroid cells maturing in yolk sac (primitive erythroid cells) have a characteristic morphology: they remain mostly nucleated at terminal maturation and synthesize mainly embryonic globins (ε, ζ, and α). Fetal cells have a macrocytic cell morphology and synthesize more than 80% fetal globins (α2γ2), in contrast to adult cells that synthesize more than 90% adult globins (α2β2). Fetal and adult cells in circulation are enucleated, and both are considered of definitive type.
Due to the transient nature of primitive erythropoiesis and because of ethical concerns in conducting experiments in human embryos, the regulation of primitive erythropoiesis has remained inadequately explored. Extensive research with murine embryonic stem (ES) cells differentiated through embryoid body (EB) formation and directed hematopoietic differentiation has shown that it recapitulates the earliest stages of murine hematopoietic development, as the appearance of primitive hematopoietic cells was followed by the emergence of definitive cells expressing the appropriate globin phenotypes.2,3 Similar studies with human ES cells have been conducted only recently.4-11 However, there are discrepancies among the studies published regarding the kinetics as well as the morphology and globin patterns of erythroid cells generated from human ES cells.5-7,9,11 Thus, a clear, reproducible picture is lacking, precluding any definitive conclusions regarding the patterns of hematopoietic/erythroid differentiation from human ES cells. For example, while Kaufman and colleagues demonstrated by reverse transcriptase-polymerase chain reaction (RT-PCR) that ES-derived erythroid cells express adult globins (α, β, and δ) but not fetal or embryonic globins,5 subsequent publications have shown expression of both embryonic and fetal globins by ES-derived erythroid cells.6,9,11 Zambidis and colleagues9 recently have marshaled the notion that indeed human ES cells recapitulate in their in vitro differentiation the earliest events of human hematopoietic ontogeny. However, several questions about the concordance of morphological appearance and globin phenotypes are raised by this study, and none of the prior studies have shown conclusively that the same cells synthesize all globin species identified by biochemical/molecular analyses.
In the present study, we describe the generation of erythroid cells from human ES cell encompassing a span of 15- to 56-day total culture time. At all time points tested, erythroid cells were of fetallike appearance and, invariably, coexpressed mainly embryonic globins (ε and ζ) and fetal globin (γ), with very little adult β globin, at both the protein and RNA levels. Our data are discussed in the context of previous in vivo studies of human hematopoietic ontogeny as well as of recent studies with hES cells.
Materials and methods
Expansion of hESCs
Five National Institutes of Health (NIH)-approved hES lines—H112 (NIH code WA01, WiCell Research Institute, Madison, WI), hSF6 (NIH code UC06, University of California at San Francisco, San Francisco, CA), BG01, BG02, and BG03 (NIH code BG01, BG02, and BG03, respectively, all from BresaGen, Masons, GA)—were tested initially for their hematopoietic differentiation. As H1 line showed the most prominent erythroid differentiation,13 it was used in this study. For propagation of hESC in undifferentiated state, H1 was cocultured with mouse embryonic fibroblasts that had been exposed to 3000 rads γ-irradiation as described elsewhere.14 For work reported in this study, H1 hES cells from passage 50 to 65 were used. ES medium consisted of Dulbecco modified Eagle medium/F12 supplemented with 15% knock-out serum replacement, 1 mM sodium pyruvate (all 3 from Invitrogen, Carlsbad, CA), 0.1 mM beta-mercaptoethanol (Sigma, St Louis, MO), 0.1 mM minimum essential media (MEM) nonessential amino acids (Mediatech, Herndon, VA), 1 × penicillin/streptomycin (Mediatech), and 2 ng/mL basic fibroblast growth factor (bFGF) (R&D Systems, Minneapolis, MN).
Hematopoietic induction of hES cells
The protocol for generating high frequency of erythroid progenitors from hES cells was adapted from an established method by Carotta et al15 and is summarized in Figure 1.
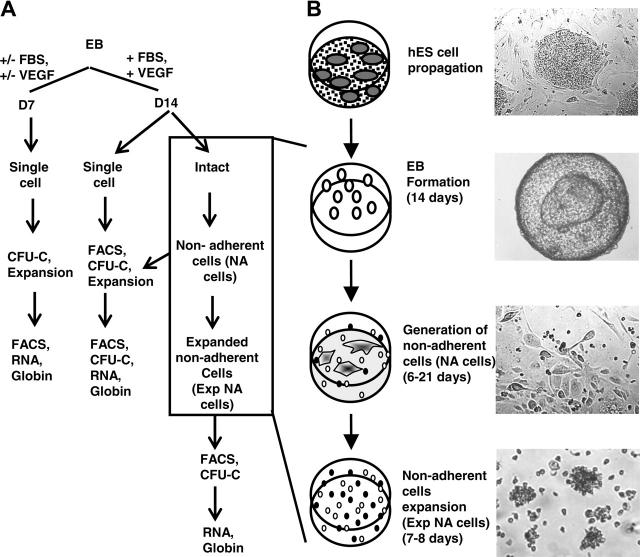
Figure 1.
Outline of successive culture steps for erythroid differentiation by hES cells. (A) A flow chart of sample collection and analyses performed. (B) Clumps of undifferentiated ES cells were cultured in EB medium for 14 days in suspension, which gave rise to cystic embryoid bodies (EBs). EBs then were plated on matrigel-coated plates in the GEM medium for up to 21 days, during which time nonadherent cells were generated. Nonadherent cells then were further expanded as described in “Materials and methods.” Images were obtained using a Leica DMIL inverted microscope (Leica, Heidelberg, Germany) with a Leica C PLAN 4×/0.10 numeric aperture (NA) (top 2 panels) or 10×/0.22 NA (bottom 2 panels) objective and a PixeLINK megapixel FireWire camera (model PL-A662; PixeLINK, Ottawa, ON, Canada) with PixeLINK Capture software version 1.0.
Embryoid body formation. On the day of passage, hES colonies were inspected. Only hES cultures containing colonies with well-defined boundaries and minimum differentiation were used. Undifferentiated hES were treated with 1.2 U/mL dispase (Invitrogen) dissolved in calcium- and magnesium-free phosphate-buffered saline (PBS; Invitrogen or Mediatech) supplemented with 10% ES-qualified fetal bovine serum (FBS) (Invitrogen) at 37°C until the hES colonies were almost lifted from the plates. Colonies then were washed off the plates, washed twice in ES medium without bFGF, and resuspended in EB medium consisted of Iscove MEM (IMDM) (Mediatech), 15% ES-qualified FBS, 10% protein-free hybridoma medium II (PFHM-II) (Invitrogen), 300 μg/mL iron-saturated human transferrin (Sigma), 50 μg/mL ascorbic acid (Sigma), 0.1 mM β-mercaptoethanol, 1 × penicillin/streptomycin, 2 mM L-glutamine (Mediatech), 4 ng/mL bFGF, and 1 ng/mL vascular endothelial growth factor (VEGF-165) (Affinity BioReagents, Golden, CO) unless stated otherwise. Approximately 1 × 106 to 1.5 × 106 hES cells in clusters were seeded onto each well of 6-well ultralow attachment plates (Corning, Acton, MA) and cultured at 37°C, 5% CO2 for 7 or 14 days in a humidified incubator until large, cystic embryoid bodies formed (Figure 1B). At the end of the culture, EBs either were kept intact for the generation of nonadherent cells or dissociated into single cells for phenotypic analysis, clonogenic analysis, or expansion culture as described in each respective section later (Figure 1A). To prepare single cell suspension from EBs, EBs were treated with 0.1% collagenase IA (Sigma) supplemented with 30% FBS. EBs were treated for 1 hour at 37°C, passed gently through an 18G needle, and incubated for 30 minutes further at 37°C. At the end of the incubation, EBs were passed through a 20G needle, washed twice with PBS supplemented with 0.1% bovine serum albumin (PBS-BSA), centrifuged at 450g for 5 minutes, filtered through a 41-μM nylon filter, and resuspended in 500 μL of PBS-BSA.
Generation and expansion of nonadherent cells. To generate nonadherent cells, day-14 EBs (D14EBs) were used. On day 13 of EB culture, 35 mm TC plates (BD Falcon) were coated with 0.5 mL of matrigel (BD Biosciences, San Jose, CA) diluted 1:20 in IMDM overnight at 37°C. Plates were rinsed gently once with PBS prior to the addition of EBs. EBs from each well of the 6-well ultralow attachment plates were resuspended in 9 mL of hematopoietic growth and expansion medium (GEM). GEM consisted of Stemline hematopoietic stem cell growth and expansion medium (Sigma) supplemented with 2 mM l-glutamine, 1 × penicillin/streptomycin, 0.1 mM MEM nonessential amino acids, 0.1 mM β-mercaptoethanol, 0.1 μM dexamethoson (Sigma), 3 U/mL erythropoietin (EPO), 200 ng/mL stem cell factor (SCF) (both from Amgen, Thousand Oaks, CA), 20 or 100 ng/mL Fms-like tyrosine kinase 3 ligand (Flt3-L) as indicated, 10 ng/mL interleukin-3 (IL-3), 10 ng/mL interleukin-6 (IL-6), 50 ng/mL granulocyte colony-stimulating factor (G-CSF) (all from PeproTech, Rocky Hill, NJ), 96 U/mL thrombopoietin (a kind gift from Dr Kenneth Kaushansky, Department of Medicine, Division of Hematology/Oncology, University of California, San Diego), 4 ng/mL bFGF, 5 ng/mL VEGF-165, 300 μg/mL iron-saturated transferrin, and 15% ES-qualified FBS. An aliquot of 1.5 mL of EBs was dispensed per 35-mm matrigel-coated TC plate. After 3 days, most EBs flattened and attached to the plates. Nonattached EBs were removed by aspiration. The nonadherent cells were collected every 2 to 3 days for up to 21 days by gently flushing the plates several times with PBS-BSA and were subjected to phenotypic analysis, clonogenic analysis, or further expansion (Figure 1A). To expand nonadherent cells, the latter were cultured in GEM or serum-free (SF) conditions, as described in the following section, at a density of 80 × 103 cells/mL for 7 to 8 days. At the end of the incubation, cells were collected and made into single cell suspension by repeatedly pipetting for phenotypic and clonogenic analysis (Figure 1A).
SF culture of ES-derived cells. Dissociated EBs and collected nonadherent cells were cultured at a density of 80 × 103 cells/mL for 8 days in an SF medium, adapted from the protocol of Zambidis et al,9 consisting of IMDM supplemented with 10% BSA, 2 mM l-glutamine, 0.1 mM β-mercaptoethanol, 50 ng/mL SCF, 50 ng/mL granulocyte-macrophage colony-stimulating factor, 50 ng/mL G-CSF, 20 ng/mL IL-3, 20 ng/mL IL-6, 3 U/mL EPO, 150 μg/mL iron-saturated human transferrin, 0.5 × insulin-transferrin-selenium (Invitrogen), 0.5% EX-CYTE (Serologicals Proteins, Kankakee, IL), and 5% PFHM-II, with or without the addition of serum or Flt3-L, or coculture with confluent irradiated OP-9 murine stromal cells (a generous gift from Dr James Douglas Engel, Department of Cell and Developmental Biology, University of Michigan Medical School, Ann Arbor). At the end of culture, single cell suspensions were made by repeated pipetting, and cells were used for phenotypic, clonogenic, and globin analysis.
Phenotypic analysis of ES-derived cells
Immunophenotype of ES-derived cells was examined using flow cytometry analysis. Antibodies used included biotinylated mouse antihuman CD133 (Miltenyi Biotech, Auburn, CA), glycophorin-A-PE (DAKO, Glostrup, Denmark), CD34-PE, CD45-APC, CD31-PE, CD41-fluorescein isothiocyanate (FITC), CD71-FITC, CD117-PE, CD49d-APC (α-4 integrin), streptavidin-APC (all from BD Biosciences), and EP1-FITC.16 Cells were washed once with PBS-BSA and then incubated with primary antibodies for 30 minutes at 4°C, washed, then stained with secondary antibody as required. Cells were then washed twice and resuspended in PBS-BSA containing 100 ng/mL propidium iodide (Sigma) for dead cell exclusion. Cells were acquired using FACScalibur (BD Biosciences), and results were analyzed with Cell Quest Pro (BD Biosciences). Gatings were set according to isotype controls.
Clonogenic growth of ES-derived cells
Aliquots of 10 × 103 to 100 × 103 cells were plated per 1 mL of semisolid methylcellulose (CFU-lite with Epo, Miltenyi Biotech, or complete human methycellulose medium, Stem Cell Technologies, Vancouver, BC, Canada). Frequency of colony-forming units-culture (CFU-Cs) was scored morphologically after culturing for 10 to 14 days at 37°C, 5% CO2, in a humidified incubator.
Hemoglobin analysis
Benzidine staining. Smears from single cell suspension of erythroid cells derived from EBs or CFU-Cs were prepared by using a Shanton Elliot cytocentrifuge. They were air dried, fixed with absolute methanol for 5 minutes, air dried, and immersed in 1% 3, 3′ dimethoxy-benzidine (Eastman Chemicals, Kingsport, TN) in absolute methanol for 5 minutes, followed by a 2-minute incubation in 2.5% H2O2 solution (Sigma) in 70% ethanol. Slides were then washed 3 times in water for 5 minutes each. Harris Modified Hematoxylin (Fisher Chemicals, Hampton, NH) was used for counterstain.
Immunofluorescent staining of globin chains. Smears of erythroid cells were fixed for 25 minutes in absolute methanol (Fisher Chemicals) at room temperature then dried quickly and rinsed for 2 minutes in PBS, followed by 2 minutes in distilled water, then fan dried. Buffer consisting of PBS supplemented with 0.1% BSA and 0.02% sodium azide (Sigma) was used to dilute monospecific antibodies.17 Anti-β globin ascitic fluid was prepared in 1:1000 dilution; anti-γ globin ascitic fluid was prepared in 1:500 dilution; and anti-ε globin and anti-ζ globin (kind gifts from Dr David Chui, Hemoglobin Diagnostic Reference Laboratory, Boston Medical Center, MA) were from culture supernatants and were used without dilution. Slides were incubated at 37°C in a humidified chamber for 35 minutes with the primary antibodies. At the end of the incubation, slides were rinsed for 2 minutes in PBS, followed by another 2-minute rinse in distilled water. Slides were fan dried then incubated with goat antimouse FITC-conjugated antibody (Sigma) diluted 1:30 with staining buffer. Slides were rinsed with PBS and water and fan dried.
Measurement of globin mRNA synthesis. RNA was prepared using RNeasy Micro Kit and RNeasy Mini kit (both from Qiagen, Valencia, CA) per manufacturer's instructions. Human α, ζ, γ, β, and ε globin mRNA were quantitated as described by Navas et al.18 Human ζ probe was a generous gift from Dr Douglas R. Higgs, Medical Research Council Molecular Haemabiology Unit, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, Oxford, United Kingdom.
Real-time PCR analysis
RNA was prepared from erythroid cells derived from adult BM CD34+ cells, peripheral blood mononuclear cells, fetal liver cells, or hES culture as described in “Measurement of globin mRNA synthesis” with additional RNase-free DNase (Qiagen) treatment. First-strand cDNA was reversed transcribed with oligo-dT priming using M-MLV reverse transcriptase (both from Invitrogen). Primers for Kruppel-like factor-1 (KLF1), GATA1, and MYB were designed based on those reported by Zambidis et al.9 Primers for KLF2, GATA2, and β-actin were designed using Universal ProbeLibrary Software (Roche Applied Science, Indianapolis, IN). Primer sets are summarized in Table 1. Real-time polymerase chain reaction (PCR) was performed using SYBR-Green PCR master mix reagent in the 7300 real-time PCR system and analyzed with 7300 system SDS software (all from Applied Biosystems, Foster City, CA).
Table 1.
Real time polymerase chain reaction primers
| Genes | Primer sequences | Amplicon size, bp |
|---|---|---|
| KLF1 | 5′-cgg aca cac agg atg act tc-3′ forward | 115 |
| 5′-ggc tgg tcc tca gac ttc ac-3′ reverse | ||
| KLF2 | 5′-cat ctg aag gcg cat ctg-3′ forward | 120 |
| 5′-cgt gtg ctt tcg gta gtg g-3′ reverse | ||
| GATA1 | 5′-ggg atc aca ctg agc ttg c-3′ forward | 176 |
| 5′-acc cct gat tct ggt gtg g-3′ reverse | ||
| GATA2 | 5′-aag gct cgt tcc tgt tca ga-3′ forward | 102 |
| 5′-ggc att gca cag gta gtg g-3′ reverse | ||
| MYB | 5′-gtc aca aat tga ctg tta caa cac cat-3′ forward | 212 |
| 5′-ttc tac tag atg aga ggg tgt ctg agg-3′ reverse | ||
| β-actin | 5′-cca acc gcg aga aga tga-3′ forward | 97 |
| 5′-cca gag gcg tac agg gat ag-3′ reverse |
Statistical analysis
Statistical differences among samples were tested by Student t test. A P value less than .05 was considered statistically significant.
Results
Sequential emergence of hematopoietic markers during the differentiation of human ES cells
Prior to differentiation, hES cells expressed high level of CD133 and moderate levels of CD117, and CD71 with very low-to-undetectable levels of CD41, CD31, CD34, CD45, glycophorin-A, and α-4 integrin (Figure 2). Following a 14-day incubation of EB culture, the percentage of D14EB cells expressing CD133, CD117, and CD71 remained in levels comparable to that of undifferentiated ES cells. However, a significant increase in the proportion of cells expressing hematopoietic and endothelial markers—CD34, CD45 (increased from undetectable to 3.51% ± 0.76%), CD31, glycophorin-A, CD41, and adhesion molecule α-4 integrin—was observed. The commitment of ES-derived cells toward the hematopoietic lineage was most evident in the nonadherent cell population, expressing CD45, CD31, and α-4 integrin, emerging from the culture of intact EBs on matrigel-coated plates with a cocktail of hematopoietic growth factors in GEM (100 ng/mL Flt3-L). It is of interest that these nonadherent cells were generated from adherent cells highly expressing CD31 (data not shown), suggesting the development of hematoendothelial progenitors in the adherent population. In the nonadherent population, there was a decrease in the proportion of cells expressing CD133, CD34, CD117, and glycophorin-A compared to that of D14EB cells. Following further expansion of the nonadherent cells in GEM (100 ng/mL Flt3-L), the proportion of cells expressing CD45 and CD31 remained high with a small yet significant increase in the expression of CD117. Furthermore, the proportion of cells expressing CD133, CD34, CD41, and α-4 integrin decreased significantly compared to that of nonadherent cells prior to expansion. The expansion of nonadherent cells did not alter the proportion of cells expressing glycophorin-A.
Figure 2.
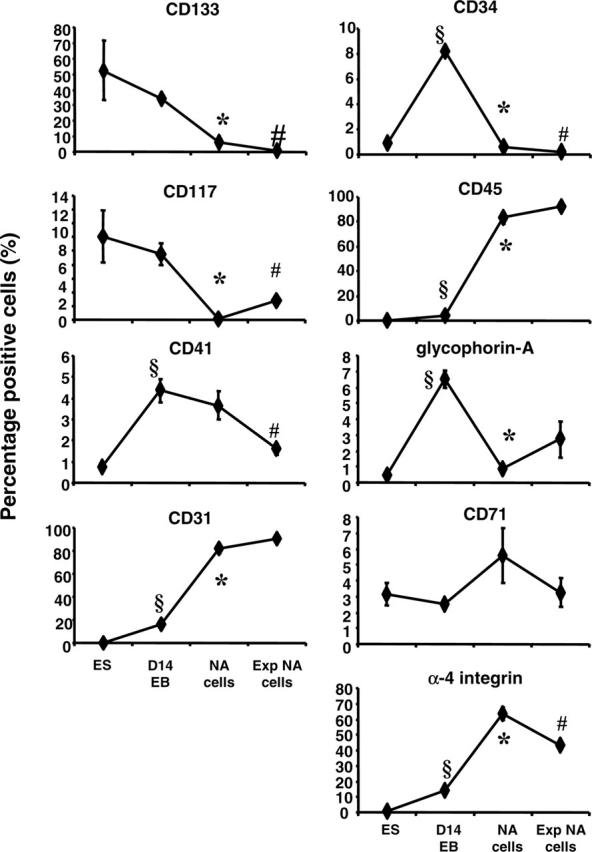
Phenotypic analysis of ES-derived cells before and after EB differentiation. Single cell suspensions were prepared from undifferentiated ES cells, from disassociated D14EBs, from nonadherent cells, or from expanded nonadherent cells. Nonadherent cells (NA cells) and expanded nonadherent cells (Exp NA cells) were derived in medium supplemented with 100 ng/mL Flt3-L. Surface marker expression was analyzed by flow cytometry. Error bars indicate SEM. § indicates that the marker expression in the cells of D14EBs was significantly different from that of undifferentiated ES cells (P < .05). * indicates that the marker expression in the nonadherent cells was significantly different from that in cells of D14EBs (P < .05). # indicates that the marker expression in the expanded nonadherent cells was significantly different from that in the nonadherent cells prior to expansion (P < .05).
Expansion of nonadherent cells from D14EBs led to a robust increase in the frequency of erythroid burst-forming unit (BFU-E)
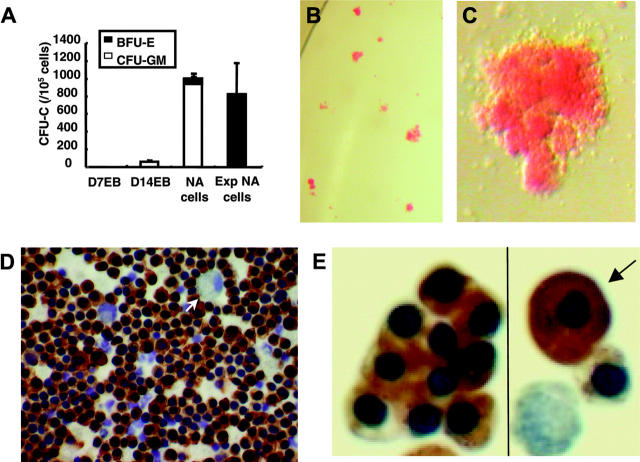
Despite having the highest proportion of cells expressing CD34, dissociated D14EB cells had low clonogenic hematopoietic potential, with the myeloid colonies predominating among CFU-Cs, suggesting that many of the ES-derived CD34+ cells may be of endothelial lineage rather than hematopoietic (Figure 3A). Culturing of intact D14EBs on matrigel-coated plates in GEM (100 ng/mL Flt3-L) led to the generation of adherent and nonadherent cells. Among the latter, the frequency of CFU-Cs, mainly of myeloid lineage, was increased approximately 20-fold compared to that of dissociated D14EB cells. Interestingly, however, further expansion of nonadherent cells in GEM (100 ng/mL Flt3-L) significantly shifted the type of colonies from myeloid to erythroid lineage. As shown in Figure 3B, most of the colonies derived from expanded nonadherent cells were of erythroid lineage with few myeloid colonies. The morphology of the erythroid colonies (Figure 3C) and erythroid cells (Figure 3D,E) suggested they were mainly fetallike definitive erythroblasts with only rare embryonic-like erythroblasts (< 2%) among them.
Figure 3.
Clonogenic potential of ES-derived cells during differentiation and morphology of BFU-E-derived erythroblasts. (A) Single cell suspension was prepared from D7EB, D14EB, nonadherent cells (NA cells), or expanded nonadherent cells (Exp NAcells) and cultured to detect the clonogenic potential of hematopoietic progenitors present. The D7EB data were from 3 experiments. The D14EB data were pooled from 6 experiments. The nonadherent and expanded nonadherent cells data were pooled from 2 experiments. (B) Most of the colonies from expanded nonadherent cells were of erythroid lineage. (C) A single BFU-E colony from the CFU-C culture of expanded nonadherent cells. (D) Smears prepared from picked BFU-E colonies of expanded nonadherent cells were stained with benzidine and counterstained with hematoxylin to reveal the nuclei. Most of the cells in the BFU-E colonies stained positive for hemoglobin with the presence of some macrophages (arrow). (A,B,D) Error bars indicate SEM. (E) While most of the hemoglobin-positive erythroblasts had the morphology of fetallike definitive erythroblasts, few erythroblasts (< 2%) resembled embryonic erythroblasts, with large cytoplasm-to-nucleus ratio (arrow). Panels B and C were taken under a Leica MZ6 dissecting microscope (Heidelberg, Germany) with 1.6 × and 4 × original magnification using a Nikon coolpix995 camera (Melville, NY). The picture in panel C has been enlarged 20 ×. Panel D was taken under an Olympus BH2 microscope (Melville, NY) with a 40 ×/0.70 NA Olympus objective lens using a Nikon coolpix995 camera. Panel E was taken under a Leica DMLB microscope with a 40 ×/0.70 NA PL Fluotar objective lens using an RT Slider Spot camera using spot version 4.0.9 (Diagnostic Instruments, Sterling Heights, MI). The picture has been enlarged 94 ×.
Reduced Flt3-L and SF conditions promote the generation of glycophorin-A+ erythroid cells
To explore the effects of culture conditions on the generation of glycophorin-A+ cells by differentiating D14EB cells, we decreased the level of Flt3-L in GEM from 100 ng/mL to 20 ng/mL. Under the reduced-Flt3-L conditions, the proportion of nonadherent cells expressing glycophorin-A was increased by 9-fold (Figure 4A). An increased proportion of glycophorin-A+ cells also was observed when the nonadherent cells were further expanded in GEM supplemented with a low level of Flt3-L as compared to those expanded in GEM supplemented with a high level of Flt3-L. Next, dissociated D14EBs cells were cultured in GEM supplemented with 20 ng/mL Flt3-L in an SF medium without FBS, or in an SF medium supplemented with FBS for 8 days. The SF medium without FBS led to a higher frequency of CD45-glycophorin-A+ cells, whereas the GEM led to a higher frequency of CD45+glycophorin-A- cells (Figure 4B). Presence of FBS promoted the generation of CD45+glycophorin-A- cells and decreased the proportion of CD45-glycophorin-A+ cells. Similar to dissociated D14EBs, when the nonadherent cells were expanded in the SF medium without FBS, they gave rise to a higher frequency of glycophorin-A+ cells than those expanded in the GEM supplemented with a low level of Flt3-L (Figure 4C). Interestingly, while expanding the nonadherent cells in the SF medium led to a higher proportion of glycophorin-A+ cells, the erythroid progenitor frequency among the cells expanded in the SF medium was significantly reduced in comparison to those expanded in the GEM (Figure 4D), suggesting enhancement of maturation under the SF conditions.
Figure 4.
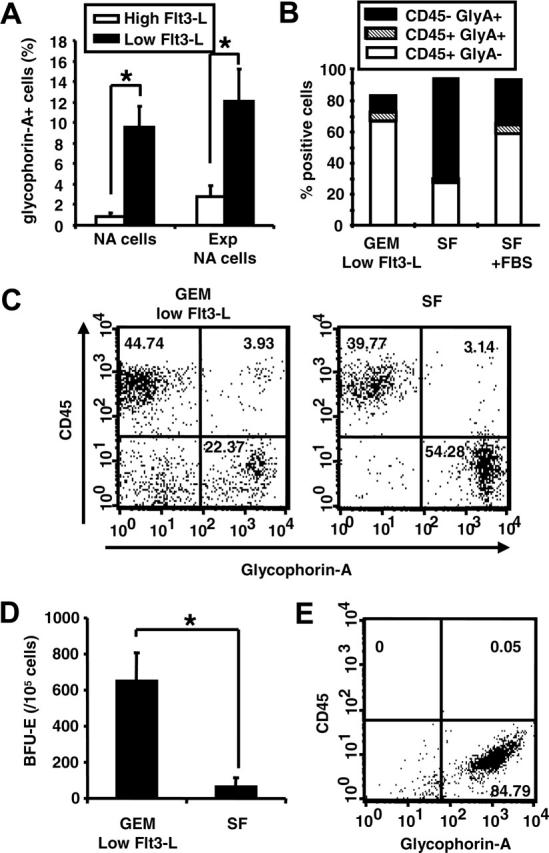
Low Flt3-L and SF enhance the differentiation of erythroid cells. (A) Nonadherent cells derived from intact D14EBs prior to (NA cells) or after (Exp NA cells) expansion were analyzed for the expression of glycophorin-A. (B) Dissociated D14EBs were expanded for 8 days in GEM supplemented with 20 ng/mL of Flt3-L (GEM-low Flt3-L) or in the SF medium without FBS (SF) or with 15% FBS (SF + FBS) and analyzed for the expression of CD45 and glycophorin-A. (C) Nonadherent cells generated from intact D14EBs were expanded for 8 days in suspensions in GEM-low Flt3-L or in the SF medium and examined for the expression of glycophorin-A and CD45. (D) The erythroid colony formation by expanded nonadherent cells as described in panel C. (E) Dissociated D7EBs were expanded for 8 days in the SF medium and analyzed for the expression of CD45 and glycophorin-A. * indicates that the 2 sets of data are significantly different (P < .05). (A,D) Error bars indicate SEM.
As the SF culture was demonstrated to be optimal for promoting the generation of glycophorin-A+ cells, we next tested whether glycophorin-A+ cells could be generated prior to D14EBs. D7EBs were dissociated and cultured either in the semisolid methylcellulose medium or in the suspension SF medium for 8 days. While no clonogenic growth was detected in the dissociated D7EB cells (Figure 3A), suspension SF culture of dissociated D7EB cells gave rise almost exclusively to glycophorin-A+ cells (Figure 4E).
Erythroid cells derived from ES cells expressed high levels of both embryonic and fetal globins with little or no adult β-locus globin
Erythroid cells generated under the SF conditions had a morphology resembling that of definitive erythroid cells (fetal or adult) as revealed by benzidine or Giemsa staining. We next examined the globin pattern of the erythroid cells generated from our cultures by immunofluorescent staining of specific globin chains using monospecific antibodies17 (Figure 5). Although ES-derived erythroid cells morphologically resembled fetallike definitive erythroblasts (Figure 3), they coexpressed (> 85%) embryonic globins (ε and ζ) and fetal globin (γ) (Figure 5, Figure 6A). There were only rare ES-derived erythroid cells expressing adult globin (β/δ). The pattern of globin expression was stable throughout all the culture time points tested.
Figure 5.
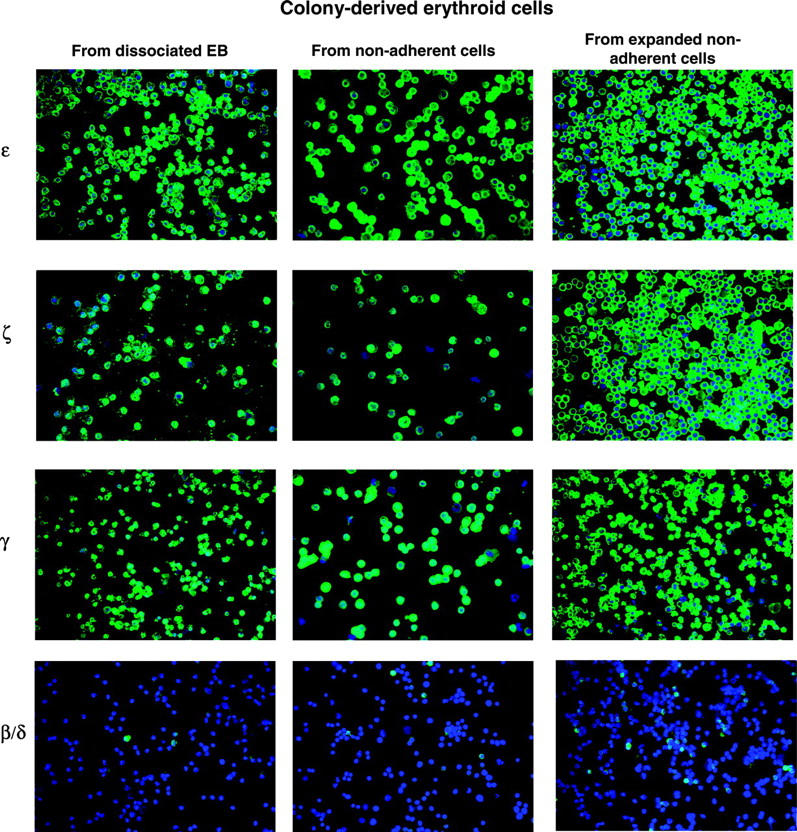
Globin protein expression by ES-derived erythroid cells. Expression of ε, ζ, γ, and β/δ globin proteins was detected in the colony-derived erythroid cells from D14EBs, from nonadherent cells, or from expanded nonadherent cells by staining with specific globin antibodies followed by an FITC-conjugated anti-mouse antibody and counterstained with DAPI (4′6-diamidino-2-phenylindole 2HCl). Pictures were taken under a Leica DMLB microscope with a 40 ×/0.70 NA PL Fluotar objective lens using an RT Slider Spot camera (Diagnostic Instruments, Sterling Heights, MI) with spot software version 4.0.9.
Figure 6.
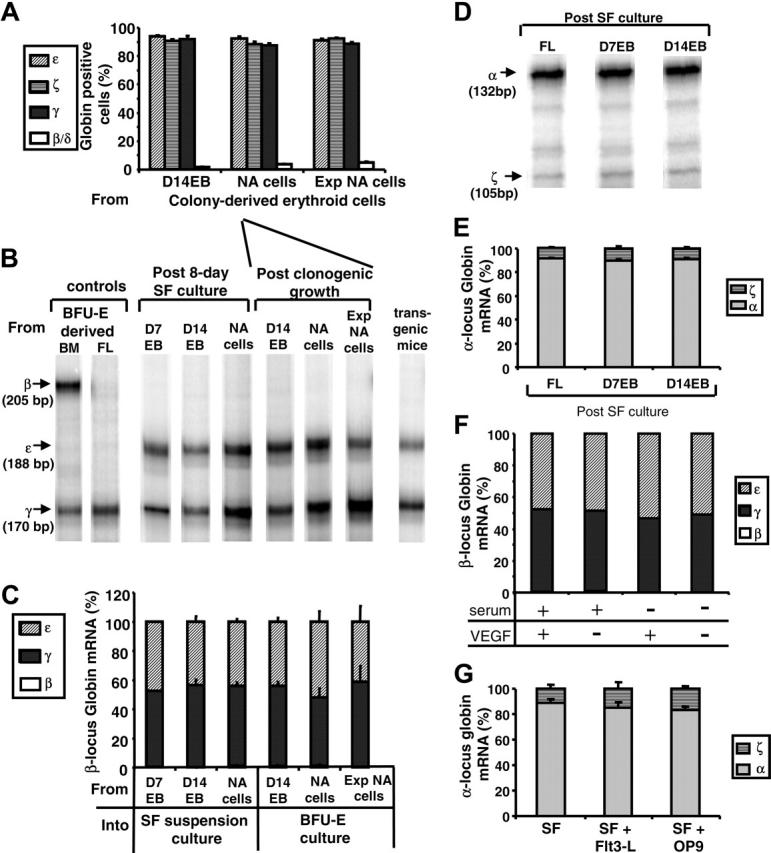
Globin protein and mRNA expression by ES-derived erythroid cells. (A) Frequencies of ε, ζ, γ, or β/δ globin-expressing cells were determined using monospecific antibodies among the erythroid colony-derived cells from dissociated D14EBs, from nonadherent cells (NA cells), or from expanded nonadherent cells (Exp NA cells). (B) RNase protection assays were performed on samples collected from post-SF culture of cells from D7EB, D14EB, nonadherent cells (NA cells), or BFU-E cultures. Erythroid cells derived from human bone marrow (BM) or fetal liver (FL) cultures were used as controls for β- and γ-globin expression. Embryonic blood of a transgenic mouse carrying the human β-globin locus yeast artificial chromosome was used as a control for ε- and γ-globin expression. A total of 47 samples were tested, and representative samples are shown. (C) The amount of specific β-locus globin: ε, γ, and β mRNA relative to the total amount of β-locus globin mRNA was computed and compared in the groups described in panel B. (D) RNase protection assays were performed on samples collected from post-SF culture of FL, D7EB, or D14EB for the expression of α-locus globin (α and ζ). A total of 10 samples were analyzed, and representative samples are shown. (E) The amount of specific α-locus globin: relative expression of α and ζ mRNA was computed and compared in the groups described in panel D. (F) EBs were generated in the EB medium supplemented with or without fetal bovine serum and with or without VEGF-165 for 7 days. D7EBs then were dissociated and cultured in suspension in the SF medium for 8 days. β-locus mRNA expression was analyzed with RNase protection assays. (G) EBs were generated in the EB medium supplemented with fetal calf serum and VEGF-165. After 7 days, EBs were dissociated and cultured in the SF medium (SF) or SF medium supplemented with 100 ng/mL Flt3-L (SF + Flt3-L) or cocultured with confluent irradiated OP-9 cells (SF + OP9) for 8 days. α-locus mRNA expression was analyzed with RNase protection assays. β-locus mRNA expression also was analyzed, and there were no significant differences among groups (data not shown). (A, C, E, G) Error bars indicate SEM.
Consistent with the results of the immunofluorescent staining of specific globins at the RNA level, these erythroid cells expressed approximately equal levels of ε and γ globin mRNA, whereas the β globin mRNA was almost undetectable, regardless of the timing of the sampling or culture method (Figure 6B,C), distinguishing them from erythroid cells derived either from the culture of adult BM cells expressing both fetal (γ) and adult (β) globin mRNA or from the culture of fetal liver cells, expressing mainly fetal globin (γ) mRNA (Figure 6B).
In contrast to the β-locus globin mRNA expression pattern, erythroid cells derived from hES cells had an α-locus globin mRNA expression pattern similar to that of erythroid cells derived from fetal liver cells, with α-globin predominating over ζ-globin (Figure 6D,E). While lower levels of ζ-globin mRNA were present compared to α-globin mRNA, there was a uniform distribution of ζ-globin protein among the ES-derived erythroid population in contrast to β-globin (Figures 5,6A). This pattern remained constant whether the erythroid cells were derived from D7EBs after SF expansion or from D14EBs after SF expansion (Figure 6E).
Culture conditions do not affect globin expression
In the experiments described above, the EB medium was supplemented with 1 ng/mL of VEGF-165, which has been implicated in up-regulating the embryonic globin synthesis in human EB cells.6 To explore the potential factors contributing to the high level of β-locus embryonic (ε) globin expression by fetallike definitive erythroid cells, we evaluated the effect of VEGF-165 on the β-locus globin mRNA expression. EBs were generated in EB medium with or without FBS and with or without VEGF-165 for 7 days. The D7EBs were then dissociated and expanded in the SF condition for 8 days and processed for globin mRNA analysis. Our results suggested that during the early stage of EB development, that is, from days 0 to 7, the addition of FBS or VEGF-165 did not significantly alter the β-locus globin mRNA expression in the subsequently developed erythroid cells (Figure 6F).
Similarly, when dissociated D7EBs cells were cultured in the SF medium supplemented with or without Flt3-L, or cocultured with OP-9 murine stromal cells, erythroid cells derived from these culture conditions had similar β-locus (data not shown) as well as α-locus globin mRNA expression (Figure 6G).
Nonglobin surface marker expression and transcriptional profiles of erythroid cells expanded from EBs
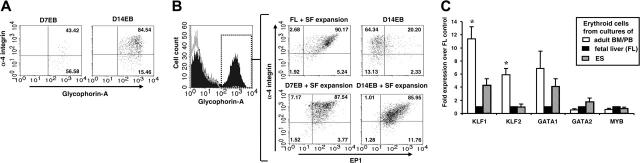
To further characterize the ES-derived erythroid cells, the expression of surface markers was examined. Interestingly, prolonged EB culture increased, among glycophorin-A+ cells, the expression of α-4 integrin (Figure 7A), which has been linked to the development of definitive erythroid cells in murine ontogenesis.19 On the other hand, while glycophorin-A+ cells within D14EBs expressed higher levels of α-4 integrin, only about 22% of these cells expressed EP1 (Figure 7B), an antigen previously demonstrated to be expressed by definitive erythroid cells only but not by embryonic erythroblasts in humans.16 However, corresponding to their fetallike definitive morphology, most erythroid cells derived from SF expansion cultures initiated with cells from dissociated D7EBs or dissociated D14EBs coexpressed α-4 integrin and EP1, a pattern displayed similarly by erythroid cells derived from fetal liver culture (Figure 7B).
Figure 7.
Phenotypic and transcriptional analysis of ES-derived erythroid cells. (A) Dissociated D7EB or D14EB cells were double-stained for α-4 integrin and glycophorin-A expression. Only viable glycophorin-A+ cells are shown. (B) Dissociated D14EBs and erythroid cells derived from SF expansion of fetal liver cells (FL), dissociated D7EBs, or dissociated D14EBs were triple-stained for glycophorin-A, α-4 integrin, and EP1 expression. Gate was set on viable glycophorin-A+ cells, as the example shown on the left. Quadrants of α-4 integrin and EP1 were set according to isotype controls of individual samples. (C) Real-time PCR analysis was performed on erythroid cells derived from the cultures of human adult bone marrow (BM) CD34+ cells (n = 2), peripheral blood (PB) mononuclear cells (n = 1), fetal liver (FL) (n = 3), or ES cells (n = 4-5). Duplicates/triplicates were analyzed for each sample. Expression levels of specific transcription factors were normalized to the expression level of β-actin of individual samples. Data from adult BM and PB were pooled together. Data are expressed as fold expression over FL-derived erythroid cells. Error bars indicate SEM. * indicates that only data of adult erythroid cells are significantly different from those of both FL- and ES-derived erythroid cells (P < .05).
In spite of displaying similar morphology and surface marker phenotype to the definitive erythroid cells, hES-derived erythroid cells had a transcriptional profile unlike that of adult definitive erythroid cells (Figure 7C). ES-derived erythroid cells expressed significantly lower levels of KLF1 and KLF2 mRNA compared to adult erythroid cells, while the expression levels of GATA1, GATA2, and MYB mRNA were not significantly different between these 2 groups of erythroid cells. In contrast, ES-derived erythroid cells had similar levels of KLF2, GATA2, and MYB mRNA expression compared to those of fetal liver-derived cells. While ES-derived erythroid cells appeared to have increased expression of KLF1 and GATA1 mRNA compared to fetal liver-derived erythroid cells, the increases were not statistically significant (P = .052 and .078, respectively), possibly due to the small sample size.
Discussion
In the present study we characterized several aspects of hematopoietic differentiation initiated from hES-derived EBs. The salient features of our study are as follows: CD45+ hematopoiesis emerged late during the EB differentiation (D14), and the peak of CD45+ cell development was preceded by a peak of CD34+ and CD41+ cells but was closely related to the emergence of CD31 and of α-4 integrin positivity (Figure 2). However, at the earliest stage of EB differentiation (ie, D7), erythroid differentiation was documented largely in the absence of CD45 expression (Figure 4E). This early CD45- hematopoiesis is consistent with recent murine studies showing CD45- macrophages in addition to erythroid cells present in yolk sac.20 The frequency of maturing glycophorin-A+ cells was particularly enhanced in the absence of FBS and at low levels of Flt3-L (Figure 4A-C). Since Flt3-L receptors (Flt3R) are not present in erythroid progenitors in contrast to nonerythroid progenitors,21,22 it is likely that higher levels of Flt3-L reduced the frequency of erythroid cells indirectly by enhancing the differentiation/maturation of nonerythroid cells. Alternatively, whether ES-derived hematopoietic cells respond to Flt3-L differently from BM cells is not clear, but the finding of lower Flt-3 message by ES-derived CD34+/CD38- cells compared to BM cells may be of relevance.7
Of particular interest in our studies was the morphologic appearance and the globin or other phenotypic markers of hES-derived erythroid cells. The bulk of erythroid cells generated resembled morphologically definitive (fetal or adult) cells, and only rare cells had embryonic-like appearance (Figure 3). When studied at a cellular level, the hES-derived erythroid cells coexpressed, in a near homogeneous fashion, high levels of embryonic (ε) and fetal globins (γ), with very little evidence of adult β-globin (Figure 5). This phenotype also was confirmed by mRNA studies and did not change by increased passage number of H1 cells, by prolongation of differentiation culture time (15-56 days), the presence or absence of FBS, the presence or absence of VEGF-165, or coculture on OP-9 cells (Figure 6). Therefore, based on morphology and on globin phenotype, the hES-derived erythroid cells neither faithfully mimicked primitive yolk sac cells (expressing mainly ε and ζ globin) nor the stable fetal liver phenotype (with expression of fetal globin and low levels of other globins).1,23 Is this somewhat-altered phenotype due to in vitro culture conditions? By altering several parameters of culture conditions, we did not obtain any evidence that in vitro conditions dictated this unusual phenotype. Although the degree of maturation can influence the relative levels of different globins within definitive cells, that is, fetal versus adult globin in BM cells,24 or primitive βH1 to εy globin in murine embryonic erythroid cells,25 this occurs without changing the morphologic characteristics of cells. Alternatively, is the globin phenotype intrinsically controlled? Previous studies using fetal cells did not find that their phenotype was influenced by culture conditions in vitro26 or after their transplantation in adult environment.27 If indeed the globin phenotype seen in our studies is intrinsically controlled, as we favor, it may indicate that we have captured in vitro a narrow window of early stages of fetal differentiation with higher levels of embryonic globin (ε) before the fetal phenotype (predominant γ) is stabilized.
Our data on globin phenotype of hES-derived erythroid cells agree with findings of several previous studies on mRNA expression by hES-derived cells.7,9,11 However, despite similarities in phenotypes, previous studies have assigned the cells either exclusively to primitive erythroid lineage11 or have supported a transition from primitive to definitive phenotype because of increased levels of β-globin,9 despite the continuation of high levels of embryonic and fetal globin in these cells.9 Of interest, 2 studies deviate further from all previous ones, since an exclusive or predominant expression of β-globin later in culture is emphasized.5,6 How can these discrepancies be explained? Although maturation levels of hES-derived erythroid cells can vary among these different studies, it is unlikely that they explain the complete adult phenotype (ie, high β-globin) claimed by these studies. It is also unlikely that a complete fetal-to-adult switch could occur within days in vitro or by changing culture conditions, as fetal cells maintain a stable phenotype for several months in vivo. Technical differences in estimating the level of adult globin in relevance to other globins in these studies or other as-yet-undetermined reasons could account for these discrepancies. Furthermore, in relevance to previous studies, it is notable that with one exception,10 all published studies on hES-derived erythroid cells have used the H-lines.5-7,9,11 Since we have documented that under the same protocols, erythroid differentiation did not occur or only occurred in low level in 4 other ES lines (hSF6, BG01, BG02, and BG03),13 the use of H-lines may not be a mere coincidence.
In our studies, in addition to morphology, we examined other globin-independent parameters that distinguish primitive from definitive cells. Thus, 2 markers, α-4 integrin and EP-1, highly expressed by fetal liver cells but absent in yolk sac cells,16,19 were present in hES-derived erythroid cells, rendering further support to the fetal phenotype we advocate. Furthermore, a limited transcriptional profile of these cells studied here did not significantly deviate from that of fetal cells (Figure 7C). Nevertheless, it is to be pointed out that the first small wave of erythropoiesis that we have documented from EB differentiation lacks CD45, α-4 integrin, and EP-1 (Figure 7A,B) may be embryonic in nature. However, it was very transient: no clonogenic progenitors were present in this population (Figure 3A), and cells with this phenotype could not be expanded further to allow additional studies.
In summary, in our studies we provide evidence that a high frequency of erythroid cells can be generated from hES cells. The first wave of erythropoiesis is from CD45-, followed by a peak of CD45+ hematopoietic cells. Erythroid cells generated by progenitors present in differentiating EBs appear to have a definitive cell morphology and coexpress high levels of embryonic and fetal globins with very few adult globin-expressing cells. We speculate that this pattern is likely intrinsically controlled and captures an early window of fetal hematopoiesis.
Acknowledgments
The authors acknowledge the expert advice of Patrick Navas for RNase protection studies and Zhi-jun Duan for real-time PCR studies.
Prepublished online as Blood First Edition Paper, April 27, 2006; DOI 10.1182/blood-2005-11-011874.
Supported by National Institutes of Health grants DK055820, 1P20GM69983, and T32-HL007093 (K.-H.C.).
K.-H.C. designed and performed research, analyzed data, and wrote the manuscript; A.M.N. performed the expansion of undifferentiated ES culture and helped in EB formation; H.C. was responsible for globin mRNA analysis; L.W. was responsible for real-time PCR analysis; B.N. performed the progenitor culture and immunofluorescent staining; C.B.W. supervised expansion of undifferentiated ES culture; and T.P. designed the research and wrote the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Migliaccio AR, Papayannopoulou T. Erythropoiesis. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, eds. Disorders of Hemoglobin. Stanford, CA: Cambridge; 2001: 52-71.
- 2.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13: 473-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19: 1129-1155. [DOI] [PubMed] [Google Scholar]
- 4.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105: 617-626. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98: 10716-10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerdan C, Rouleau A, Bhatia M. VEGF-A165 augments erythropoietic development from human embryonic stem cells. Blood. 2004;103: 2504-2512. [DOI] [PubMed] [Google Scholar]
- 7.Lu SJ, Li F, Vida L, Honig GR. CD34+CD38- hematopoietic precursors derived from human embryonic stem cells exhibit an embryonic gene expression pattern. Blood. 2004;103: 4134-4141. [DOI] [PubMed] [Google Scholar]
- 8.Zhan X, Dravid G, Ye Z, et al. Functional antigen-presenting leucocytes derived from human embryonic stem cells in vitro. Lancet. 2004;364: 163-171. [DOI] [PubMed] [Google Scholar]
- 9.Zambidis ET, Peault B, Park TS, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106: 860-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106: 1601-1603. [DOI] [PubMed] [Google Scholar]
- 11.Qiu C, Hanson E, Olivier E, et al. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp Hematol. 2005;33: 1450-1458. [DOI] [PubMed] [Google Scholar]
- 12.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282: 1145-1147. [DOI] [PubMed] [Google Scholar]
- 13.Chang K-H, Nelson AM, Ware CB, Papayannopoulou T. Wide variation in hematopoietic induction potential among five human ES lines and synthesis of unique globin patterns by ES-derived erythroid cells. Paper presented at the third Annual Meeting of the International Society of Stem Cell Research, June 23, 2005, San Francisco, CA. Abstract 204.
- 14.Ware CB, Nelson AM, Blau CA. Controlled-rate freezing of human ES cells. Biotechniques. 2005; 38: 879-880, 882-883. [DOI] [PubMed] [Google Scholar]
- 15.Carotta S, Pilat S, Mairhofer A, et al. Directed differentiation and mass cultivation of pure erythroid progenitors from mouse embryonic stem cells. Blood. 2004;104: 1873-1880. [DOI] [PubMed] [Google Scholar]
- 16.Yokochi T, Brice M, Rabinovitch PS, Papayannopoulou T, Stamatoyannopoulos G. Monoclonal antibodies detecting antigenic determinants with restricted expression on erythroid cells: from the erythroid committed progenitor level to the mature erythroblast. Blood;1984:63: 1376-1384. [PubMed] [Google Scholar]
- 17.Stamatoyannopoulos G, Farquhar M, Lindsley D, Brice M, Papayannopoulou T, Nute PE. Monoclonal antibodies specific for globin chains. Blood. 1983;61: 530-539. [PubMed] [Google Scholar]
- 18.Navas PA, Peterson KR, Li Q, et al. Developmental specificity of the interaction between the locus control region and embryonic or fetal globin genes in transgenic mice with an HS3 core deletion. Mol Cell Biol. 1998;18: 4188-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papayannopoulou T. Very late activation/β1 integrins in hematopoiesis. In: Zon LI, ed. Hematopoiesis: A Developmental Approach. New York, NY: Oxford; 2001: 337-342.
- 20.Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A, Godin I. Three pathways to mature macrophages in the early mouse yolk sac. Blood. 2005;106: 3004-3011. [DOI] [PubMed] [Google Scholar]
- 21.Adolfsson J, Mansson R, Buza-Vidas N, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121: 295-306. [DOI] [PubMed] [Google Scholar]
- 22.Lyman SD, Jacobsen SE. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood. 1998;91: 1101-1134. [PubMed] [Google Scholar]
- 23.Papayannopoulou T, Shepard TH, Stamatoyannopoulos G. Studies of hemoglobin expression in erythroid cells of early human fetuses using antigamma- and anti-beta-globin chain fluorescent antibodies. Prog Clin Biol Res. 1983;134: 421-430. [PubMed] [Google Scholar]
- 24.Papayannopoulou T, Kalmantis T, Stamatoyannopoulos G. Cellular regulation of hemoglobin switching: evidence for inverse relationship between fetal hemoglobin synthesis and degree of maturity of human erythroid cells. Proc Natl Acad Sci U S A. 1979;76: 6420-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kingsley PD, Malik J, Emerson RL, et al. “Maturational” globin switching in primary primitive erythroid cells. Blood. 2006;107: 1665-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papayannopoulou T, Kurachi S, Nakamoto B, Zanjani ED, Stamatoyannopoulos G. Hemoglobin switching in culture: evidence for a humoral factor that induces switching in adult and neonatal but not fetal erythroid cells. Proc Natl Acad Sci U S A. 1982;79: 6579-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papayannopoulou T, Nakamoto B, Agostinelli F, Manna M, Lucarelli G, Stamatoyannopoulos G. Fetal to adult hemopoietic cell transplantation in humans: insights into hemoglobin switching. Blood. 1986;67: 99-104. [PubMed] [Google Scholar]