Abstract
In 87 patients with aplastic anemia who failed to respond to immunosuppressive treatment, we determined the minimal dose of total body irradiation (TBI) required when added to antithymocyte globulin (ATG, 30 mg/kg × 3) plus cyclophosphamide (CY, 50 mg/kg × 4) to achieve engraftment of unrelated donor marrow. TBI was started at 3 × 200 cGy, to be escalated or deescalated in steps of 200 cGy depending on graft failure or toxicity. Patients were aged 1.3 to 53.5 years (median, 18.6 years). The interval from diagnosis to transplantation was 3 to 328 months (median, 14.6 months). Donors were HLA-A, -B, -C, -DR, and -DQ identical for 62 patients, and nonidentical for 1 to 3 HLA loci at the antigen or allele level for 25. The dose-limiting toxicity was diffuse pulmonary injury. The optimum TBI dose was 1 × 200 cGy. Nine patients did not tolerate ATG and were prepared with CY + TBI. Graft failure occurred in 5% of patients. With a median follow-up of 7 years, 38 (61%) of 62 HLA-identical, and 10 (40%) of 25 HLA-nonidentical transplant recipients are surviving. The highest survival rate with HLA-identical transplants was observed at 200 cGy TBI. Thus, low-dose TBI + CY + ATG conditioning resulted in excellent outcome of unrelated transplants in patients with aplastic anemia who had received multiple transfusions.
Introduction
Approximately 85% to 90% of patients with aplastic anemia who undergo transplantation with marrow from HLA genotypically identical siblings are cured.1 Patients without suitably HLA-matched related donors generally receive immunosuppressive therapy (IST) first and receive transplants only if IST fails. Results with transplantation from unrelated volunteer donors in these patients have been inferior to those achieved with HLA-identical siblings.2-4 Causes of failure have included a higher frequency of graft rejection, regimen-related toxicity (because of intensification of the conditioning regimens aimed at preventing rejection), and acute graft-versus-host disease (GVHD) than observed with HLA-identical sibling transplants.2,3,5
We have reported early results of a trial in which patients who underwent marrow transplantation from unrelated donors were conditioned with antithymocyte globulin (ATG) and cyclophosphamide (CY), as used for sibling transplantations,1 with the addition of deescalating doses of total body irradiation (TBI). Best results were achieved with the addition of 200 cGy TBI.6 Here, we update results on the patients included in the original report and extend our experience to a larger number of patients who received a transplant from HLA-identical or HLA-mismatched donors.
Patients, materials, and methods
Patients
The study population consisted of 87 patients with severe aplastic anemia (excluding patients with Fanconi anemia or other constitutional causes of marrow failure) who, between February 1994 and April 2004, received a transplant with unrelated donor marrow at 1 of 17 centers in the United States, Germany, or the United Kingdom; 75% of the patients received a transplant at the 6 largest centers. Included were patients with aplastic anemia who had failed to respond to the best available immunosuppressive treatment within 75 days of initiation of therapy or who, following initial responses, became aplastic again. Patients did not have suitable HLA-identical family donors and were aged 55 years or younger. Eighty-two donors were recruited through the National Marrow Donor Program (NMDP) (and were registered with the NMDP) and 5 through other registries. All patients were registered with the study coordinator at the Fred Hutchinson Cancer Research Center in Seattle, WA, for TBI dose assignment. Patient characteristics are summarized in Table 1. Patients were aged 1.3 to 53.4 years (median, 18.6 years); 61 were white, 13 were Hispanic, 6 were Asian, 5 were African American, and 2 were of unknown ethnicity. All patients had received transfusions with either red blood cells or platelet preparations or both, and all had received IST, growth factors, and other modalities of treatment for 1 to 11 courses (median, 3 courses). Patients with recurrent marrow failure after IST were eligible for this protocol even if hematologic parameters did not satisfy the criteria for severe aplastic anemia at the time of transplantation.
Table 1.
Patient and donor characteristics
| Characteristic | Data |
|---|---|
| Age, y, median (range) | 18.6 (1.3-53.4) |
| No. patients younger than 20 y | 47 |
| No. patients 20 y or older | 40 |
| No. male/no. female | 50/37 |
| Ethnic origin, no. patients | |
| White | 61 |
| Hispanic | 13 |
| African American | 5 |
| Asian | 6 |
| Unknown | 2 |
| Disease duration, mo, median (range) | 14.6 (3-328) |
| Pretransplantation therapy | |
| Transfusions, no. patients | 87 |
| Immunosuppression, no. patients | 87 |
| No. courses, median (range) | 3 (1-11)* |
| Donor age, y, median (range) | 36 (18-58) |
| Donor sex, no. male/no. female† | 51/34 |
| Donor†/patient sex combination, no. | |
| Female → female | 21 |
| Female → male | 13 |
| Male → male | 35 |
| Male → female | 16 |
| CMV status, no. patients | |
| D+–P+ | 21 |
| D+–P– | 32 |
| D––P– | 15 |
| D––P+ | 5 |
| Other‡ | 14 |
| Donor/patient HLA compatibility, no. patients | |
| Identical | 62 |
| Nonidentical | 25 |
| Marrow cell dose, × 108/kg, median (range) | 3.1 (0.006-15.6) |
N = 87 patients.
P indicates patient; D, donor
Single agent or combination regimens, with or without hemopoietic growth factors
Unavailable in 2 donors
In 3 patients the test was indeterminate; in the remaining 11 patients the data were not available
Donor selection and marrow collection
Donor characteristics are listed in Table 1. Donors were unrelated volunteers aged 18 to 58 years (median, 36 years); 34 were women and 51 were men (in 2 cases sex information was not available). Donors were selected on the basis of the highest level of HLA typing available at the time of enrollment into the study. Additional typing was carried out retrospectively by using cryopreserved cells from patients and donors. Those data were incorporated in the results as reported here. Sixty-two patients were HLA-A, -B, -C, -DR, and -DQ identical with their donors based on oligonucleotide typing or DNA sequencing, and 25 patients were considered HLA-nonidentical with their donors. Nine patients showed single class I mismatches (antigen level in 6, allele level in 3), 3 had 2 class I mismatches (antigen level in 3, allele level in 1), 6 patients had isolated allele level class II mismatches, 4 had class I + II mismatches (antigen level in 2 and allele level in 1), and 1 patient was HLA-DR and -DQ mismatched at the allele level. In 2 patients, HLA typing was incomplete (HLA-C and -DQ data missing), and they were considered HLA-nonidentical. HLA-identical and -nonidentical patients were considered separately for TBI dose finding. One patient who had received 1 × 200 cGy TBI was reassigned to the HLA-identical cohort on the basis of retrospective typing. Marrows were harvested according to the policies of the NMDP and brought to the transplantation center by courier.
Transplantation regimen
The protocol prescribed 4 doses of CY 50 mg/kg per day intravenously on 4 consecutive days, and 3 infusions of equine ATG (ATGAM) at 30 mg/kg per day. CY and ATG were followed by TBI at a starting dose of 3 × 200 cGy (2 fractions on 1 day, and 1 fraction on the next day), followed by the infusion of donor marrow cells.6 Criteria for TBI dose escalation or deescalation are described in “Study design.” Patients who could not complete the ATG regimen, either because of anaphylactic or other severe adverse reactions to the initial dose of ATG, received alternatively CY 60 mg/kg per day for 2 consecutive days (if the first dose of CY of 50 mg/kg had been given, the second CY dose consisted of 70 mg/kg), followed by TBI 200 cGy twice a day for 3 consecutive days for cumulative doses of 1200 cGy, and donor marrow infusion (n = 9, 7 received a transplant from HLA-identical and 2 from HLA-nonidentical donors).
Engraftment
The day of engraftment was defined as the first of 3 consecutive days on which the neutrophil count exceeded 0.5 × 109/L.7 Patients who did not reach neutrophil counts of greater than 0.5 × 109/L for 3 consecutive days after transplantation were considered as having primary graft failure. Patients with initial engraftment in whom absolute neutrophil counts declined to less than 0.5 × 109/L subsequently were considered to have secondary graft failure (if they had survived for at least 21 days). None of the 6 patients who survived for fewer than 21 days had developed GVHD, and none were considered evaluable for engraftment. Marrow and peripheral blood cells were tested sequentially for chimerism using Y chromosome-specific cDNA probes in patients with donors of the opposite sex or probes specific for polymorphic DNA sequences (eg, variable number tandem repeats [VNTRs]).
GVHD
For GVHD prophylaxis, patients received methotrexate 10 mg/m2 on days 1, 3, 6, and 11 after the marrow infusion, and cyclosporine (CSP; Sandimmune), starting on day -1 at doses of 5 mg/kg per day intravenously as continuous infusion, decreased to 3 mg/kg per day intravenously on day +4, and switched to 12 mg/kg per day orally in 2 divided doses once oral medications were tolerated. CSP was continued daily and, beginning on day 50, given at gradually reduced doses until day 180 if the patient was free of GVHD. In patients with evidence of GVHD, CSP was continued as deemed necessary. Acute GVHD was graded based on findings in skin, liver, and intestinal tract according to the modified Glucksberg criteria as described by Martin et al.8,9 Chronic GVHD was assessed as limited (mild skin involvement only) or extensive disease as described previously.10
Data collection
Data were collected on forms designed specifically for this study, in addition to the standardized forms provided by the NMDP. Data collection included pretransplantation information, peritransplantation events, and follow-up at 6- to 12-month intervals. Data were analyzed as of July 1, 2005.
Study design
This study was designed to develop and evaluate conditioning regimens for patients with severe aplastic anemia given marrow transplants from unrelated donors. The goal was to achieve sustained engraftment without encountering prohibitive toxicity. The probability of graft failure (and possibly treatment-related toxicity) was expected to be different among patients given HLA-identical or HLA-nonidentical grafts; hence, the TBI dose finding was pursued separately for the 2 groups.
Patients were to be treated in groups of 6, with TBI starting at 3 × 200 cGy. If more than one patient was registered at a given time, and endpoints of toxicity or efficacy were not yet evaluable in preceding patients, the actual number of patients in a dose cohort could be greater than 6. Possible doses for subsequent patients were 4 × 200, 5 × 200, 6 × 200, or alternatively, 2 × 200 or 1 × 200 cGy. Guidelines were as follows: if 2 or more patients had unacceptable toxicity at a given dose, the next lower dose would be chosen as the final dose for testing. Details of the dose finding scheme have been described before.6 The properties of this scheme were evaluated by Monte Carlo simulation using various assumptions for the probabilities of prohibitive toxicity and engraftment failure. For the scenarios evaluated, this design had a high probability of choosing an acceptable dose level when one existed.
As reported previously, a single TBI fraction of 200 cGy was identified as the optimum dose for patients with HLA-matched donors.6 At this point, the protocol was modified to allow for a phase 2 extension for accrual of additional patients who received transplants from HLA-identical donors; dose finding for HLA-nonidentical transplants continued as per the original design. The protocol modification was approved by the Institutional Review Boards of all participating institutions.
Survival probabilities were estimated by the Kaplan-Meier method. Likelihood ratio tests from proportional hazards regression models were used to compare survival among groups of patients. The frequencies of grades 3 or greater toxicities and fatal regimen-related toxicities in TBI dose cohorts were compared using a t test for trend.
Results
Identification of donors
Sixty-two patients received transplants from HLA-identical donors and 25 from HLA-nonidentical donors (Table 1). The search for an unrelated donor was initiated at the discretion of the referring physicians. The time from initiation of the search to the start of donor work-up was 5 to 4088 days (median, 77 days); the time to transplantation was 48 to 4110 days (median, 135 days). In 13 patients, the search time extended beyond 1 year.
Conditioning regimen
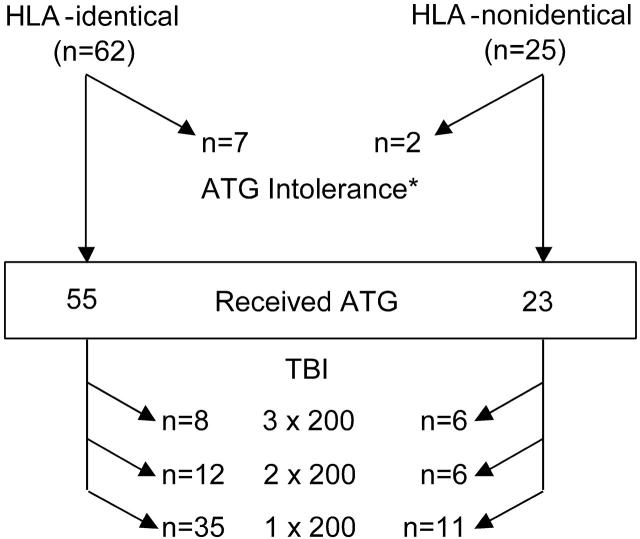
The conditioning regimens given to patients are summarized in Figure 1. Nine patients, 7 who received a transplant from HLA-identical and 2 from HLA-nonidentical donors, could not receive ATG and were conditioned with CY 120 mg/kg over 2 days and TBI 6 × 200 cGy administered over 3 days.
Figure 1.
Actual conditioning regimen given. Among the 87 patients who received a transplant, 78 (55 from HLA-identical and 23 from HLA-nonidentical donors) were conditioned with CY + ATG + TBI at the doses shown. *Nine patients (7 from HLA-identical and 2 from HLA-nonidentical donors) were unable to receive ATG and were conditioned with CY (120 mg/kg) and TBI (6 × 200 over 3 days) only.
Outcome
Outcomes are summarized in Table 2 separately for patients who received a transplant from HLA-identical and HLA-nonidentical donors and for patients who were conditioned without ATG. The follow-up was 1.2 to 10.2 years (median, 7 years) for both HLA-identical and HLA-nonidentical transplants.
Table 2.
Transplantation outcome
|
No. patients affected/no. evaluable patients (%)
|
||
|---|---|---|
| Parameter | HLA-identical | HLA-nonidentical |
| Early deaths, before day 21 | 5/62 (8) | 1/25 (4) |
| Sustained engraftment | 56/57 (99) | 22/25 (88) |
| GVHD | ||
| Acute, grades II-IV | 40/57 (70) | 18/24 (75) |
| Chronic* | 25/48 (52) | 12/21 (57) |
| Overall survival | 38/62 (61) | 11/25 (44) |
| Survival by regimen | ||
| CY + ATG + TBI | ||
| –3 × 200 cGy | 4/8 (50) | 2/6 (33) |
| –2 × 200 cGy | 6/12 (50) | 3/6 (50) |
| –1 × 200 cGy | 23/35 (66) | 5/11 (45) |
| CY + TBI (6 × 200) | 5/7 (71) | 0/2 (0) |
Median follow-up was 7 years (range, 1.2-10.2 years)
Chronic GVHD requiring therapy
Engraftment. Six patients, 5 who received a transplant from HLA-identical and 1 from HLA-nonidentical donors, died before day 21 and were considered not evaluable for engraftment. Among the remaining 81 patients, 2 conditioned with ATG, CY, and 200 cGy TBI and received a transplant from HLA-identical and nonidentical donors, respectively, failed to achieve sustained engraftment. Two additional patients, both conditioned with ATG, CY, and 200 cGy TBI and who received a transplant from HLA-nonidentical donors developed secondary graft failure in association with systemic CMV infections. The remaining 77 evaluable patients (95%) had sustained engraftment. There was no apparent difference in the tempo of hematologic recovery between patients who had received different doses of TBI.
Toxicity. Toxicities of grades 3 or greater thought to be conditioning-related are summarized in Table 3. Clinically significant toxicity occurred in 18 (21%) of the 87 patients enrolled. There was no apparent difference between HLA-identical and HLA-nonidentical transplants. However, the incidence of grades 3 or greater toxicities and fatal outcome decreased with deescalation of the TBI dose (P = .02 and P = .002, respectively). Most frequently affected were the lungs, the most striking finding being diffuse alveolar damage, generally manifest at about 3 weeks after transplantation. This type of toxicity at the low doses of TBI administered was unexpected. The histology, described previously,6 was remarkable for hyaline membranes in the terminal bronchioli. Overall, 9 of 18 of grades 3 or greater toxicities involved the lungs (Table 3), and 6 of these proved fatal (Table 4). Some patients experienced toxicity in more than one organ, most frequently kidneys, lungs, and liver. Clinically relevant mucositis and bladder toxicity were infrequent.
Table 3.
Toxicity by conditioning regimen
|
No. patients with grade 3 or greater toxicity
|
|||||
|---|---|---|---|---|---|
|
ATG (3 × 30) + CY (4 × 50) + TBI
| |||||
| Toxicity* | 3 × 200 | 2 × 200 | 1 × 200 | CY (2 × 60) + TBI 6 × 200 | All patients |
| Overall† | 5 (5)/14 | 6 (5)/18 | 5 (2)/46 | 2 (2)/9 | 18 (14)/87 |
| Pulmonary | 2 | 4 | 1 | 2 | 9 |
| Renal | 2 | 0 | 1 | 0 | 3 |
| Oral mucosa | 2 | 0 | 0 | 1 | 2 |
| Hepatic | 0 | 0 | 1 | 0 | 1 |
| Cardiac | 1 | 0 | 0 | 0 | 1 |
| CNS | 0 | 0 | 1 | 0 | 1 |
| Multiorgan failure | 1 | 2 | 1 | 0 | 4 |
| Urinary tract | 0 | 0 | 1 | 0 | 1 |
| Hematologic (TTP) | 0 | 0 | 2 | 0 | 2 |
The frequency of toxicities in each column do not add up to the total (overall) because an individual patient may have had more than one organ toxicity.
ATG indicates antithymocyte globulin; CY, cyclophosphamide; TBI, total body irradiation, 3, 2, or 1 × 200 cGy when combined with ATG/CY, or 6 × 200 cGy when combined with CY only; CNS, central nervous system; TTP, thrombotic thrombocytopenic purpura
Severity grade 4 in any organ system or at least grade 3 for pulmonary, oral mucosa (stomatitis), renal, or hepatic toxicity41
Data for the overall row are number of patients with toxicity of grade 3 or greater versus the total number of patients. The numbers of patients with toxicity of grade 3 or greater who died are shown in parentheses
Table 4.
Causes of death
|
No. patients
|
||
|---|---|---|
| HLA-identical | HLA-nonidentical | |
| Diffuse alveolar damage | 4 | 2 |
| Systemic viral infections | 0 | 3 |
| Systemic fungal infections | 2 | 0 |
| Multiorgan failure | 4 | 4 |
| TTP | 2 | 0 |
| Acute GVHD and associated problems | 4 | 2† |
| Chronic GVHD | 5 | 1 |
| Complications from second transplantation | 1 | 1 |
| Miscellaneous* | 2 | 2 |
| Total (%) | 24 (39) | 15 (60) |
CNS indicates central nervous system; GVHD, graft-versus-host disease; TTP, thrombotic, thrombocytopenic purpura
One each from asphyxia as a result of aspiration, CNS hemorrhage, secondary graft failure, and suicide
Both patients developed posttransplantation lymphoproliferative disorders following extensive treatment for acute GVHD
GVHD. Acute GVHD of grades II to IV occurred in 69% of patients with HLA-identical and in 77% of patients with HLA-nonidentical donors. Chronic GVHD developed in 52% of evaluable patients who received a transplant from HLA-identical donors, and in 57% of patients who received a transplant from HLA-nonidentical donors. In 8 patients who received a transplant from HLA-identical donors, acute or chronic GVHD was considered the primary cause of death. Among patients who received a transplant from HLA-nonidentical donors, 2 died of GVHD, and 2 additional patients with steroid-refractory GVHD died of Epstein-Barr virus-associated posttransplantation lymphoproliferative disorders (PTLD).
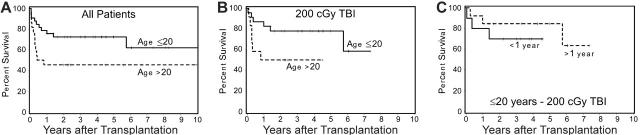
Survival. As of July 2005, 48 (55%) of 87 patients were surviving, including 38 (61%) of 62 patients who received a transplant from HLA-identical and 10 (40%) of 25 patients who received a transplant from HLA-nonidentical donors. Differences in survival between cohorts of patients conditioned with different doses of TBI were not significant. There was a suggestion, however, that the probability of survival among patients who received the CY + ATG + TBI regimen and who received a transplant from HLA-identical donors increased as the TBI dose was decreased (Figure 2). Although 5-year survival was 62% for patients who received a transplant within 1 year of diagnosis and 54% for patients with a longer interval from diagnosis to transplantation, this difference was not significant (P = .67). Younger patients (≤ 20 years) had a higher probability of survival than older patients (> 20 years; P = .05) (Figure 3A). This was particularly true for patients given TBI doses of 1 × 200 cGy (Figure 3B), regardless of the time interval since diagnosis (Figure 3B). Finally, among 9 patients who did not receive ATG and were conditioned with lower doses of CY (120 mg/kg) and higher doses of TBI (6 × 200 cGy), 5 are surviving. Results were not appreciably affected by donor or patient CMV status or sex combination. All long-term surviving patients have normal hematologic parameters.
Figure 2.
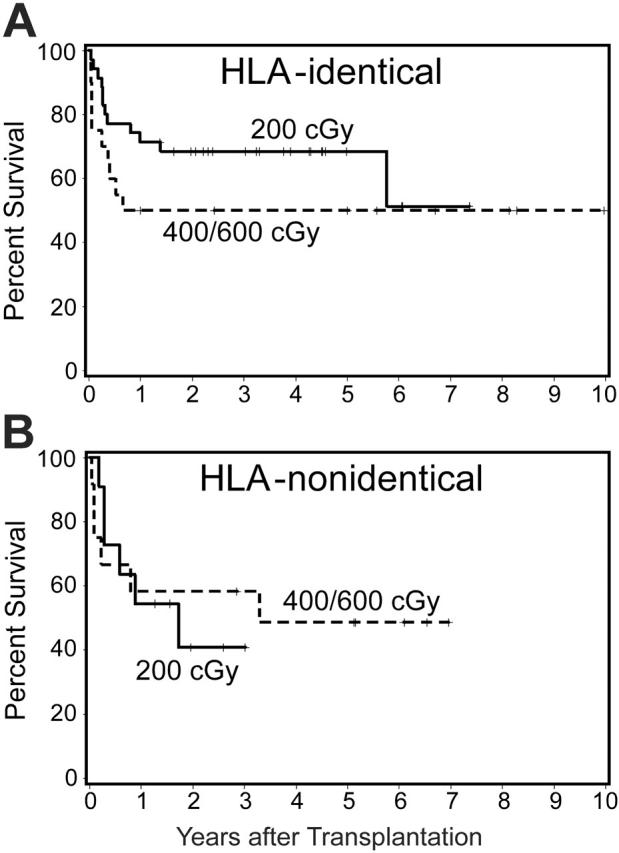
Survival by TBI dose in patients who were able to receive ATG. Shown separately are patients with HLA-identical (A) and HLA-nonidentical donors (B). Results with TBI doses of 400 cGy and 600 cGy were pooled.
Figure 3.
Effect of patient age on survival among patients conditioned with CY/ATG/TBI and who received a transplant from HLA-identical donors. (A) Five-year survival among 33 patients 20 years old or younger was 73%, compared with 46% among 22 patients older than 20 years (P = .05). (B) Among patients conditioned with 200 cGy TBI, the survival figures were 78% and 50% for patients 20 years old or younger and for patients older than 20 years, respectively. (C) Survival among 23 patients aged 20 years or younger, conditioned with 200 cGy TBI, with disease duration of 1 year or less (70%) or longer than 1 year (85%).
Causes of death. Thirty-nine patients have died (Table 4). The mortality rate was higher after HLA-nonidentical transplants (60%) than after HLA-identical transplants (39%); however, the causes of death were similar, the most frequent being organ toxicity and GVHD. The one late death (> 5 years) was related to chronic GVHD and pulmonary insufficiency.
Discussion
Survival in patients with aplastic anemia who receive a transplant from unrelated donors has been inferior to that achieved with HLA-identical sibling donors.4-6,11-13 Longer time from diagnosis to transplantation, the use of IST as initial therapy, and greater genetic differences between donors and patients may all contribute. One cause of failure with unrelated donors has been lack of sustained engraftment with conditioning regimens that have been successful with sibling transplants.1,14 High-dose TBI regimens, however, have been associated with severe acute toxicity5,15 and the risk of secondary malignancies.16,17 Here, we show in a prospective trial that low doses of TBI added to a CY plus ATG regimen that has been successful in patients with aplastic anemia who receive transplants from HLA-identical sibling donors is effective in achieving engraftment of unrelated donor marrow and in improving long-term survival to a level that, at least in young patients, approaches that of HLA-identical sibling transplants.
The primary objective of this prospective study, reduction of regimen-related toxicity and mortality without jeopardizing engraftment, was met for both HLA-identical and partially HLA-mismatched transplants. Engraftment was achieved with the lowest TBI dose of 200 cGy. Four (5%) of 81 evaluable patients experienced graft failure, one (2%) with HLA-identical and 3 (11%) with HLA-nonidentical transplants. Among the latter, however, only one patient had primary rejection, whereas 2 patients manifested graft failure in the context of systemic CMV infections and ganciclovir treatment, without evidence of immunologic graft rejection by recipient immune cells. Although all graft failures occurred at the lowest TBI dose used, it is unlikely that CMV-associated graft failure was affected by the TBI dose. Nevertheless, results overall suggest that a dose of 200 cGy in combination with ATG plus CY was the minimum dose that allowed for sustained engraftment of unrelated marrow.14 A graft failure rate of 2% for HLA-identical unrelated donor transplants compares favorably with other reports12 and is, in fact, not different from that observed with HLA-identical sibling transplants.1
Although TBI dose deescalation did not significantly increase the risk of graft failure and reduced the incidence of severe organ toxicity, differences between TBI dose levels were not significant, and the effect on survival was not as decisive as anticipated. One of the rationales for the design of the present TBI dose-finding trial had been earlier observations, which suggested that high-dose TBI (1200 cGy) was poorly tolerated in patients with aplastic anemia.15 Because, on the one hand, a combination of ATG and CY provided insufficient immunosuppression for sustained engraftment of marrow from unrelated donors,14 we speculated that the addition of low doses of TBI might, first, allow for sustained engraftment, and, second, not cause unacceptable clinical toxicity.18 Still, pulmonary toxicity occurred in the present trial even at TBI doses as low as 400 or 200 cGy given in combination with ATG and CY. It is not clear which component of the regimen was responsible for the adverse pulmonary effects. Interactions between TBI and ATG are unlikely to blame in view of the fact that ATG is well tolerated in conjunction with high-dose TBI (and CY at doses of 120 mg/kg) in patients who received a transplant for malignant disorders.19 On the other hand, interactions between high-dose CY (200 mg/kg) and TBI or ATG may have to be considered. Circumstantial support for such a possibility comes from results in the 9 patients conditioned with CY at doses of 120 mg/kg and high-dose TBI (6 × 200 cGy) without ATG who did not experience prohibitive acute pulmonary toxicity. In fact, 5 of 9 patients so treated survived, suggesting that the 10% survival observed historically (with HLA-identical sibling transplants for aplastic anemia) may have been due to the poor overall condition of those earlier patients.18 Nevertheless, potential late effects of high-dose irradiation, including the development of new malignancies,16,17 remains a concern.
Others have suggested that unusual cytokine profiles in patients with aplastic anemia, in particular, high levels of proinflammatory cytokines such as interferon-γ and tumor necrosis factor α (TNF-α)20,21 and high levels of IL-1022,23 may contribute to transplantation-related toxicity. However, considering the extensive transplantation experience with HLA-identical sibling transplants in patients with aplastic anemia, it would seem surprising that the described adverse effects should become manifest with increased frequency only in the present trial with unrelated donors.
The incidence of both acute and chronic GVHD in the present study was in the same range as in other recent trials,24,25 and, although patient numbers may be too small for a definitive assessment, there was no indication that the GVHD incidence declined with decreasing TBI doses.26 New approaches to GVHD prevention and therapy are needed. Several trials in patients with aplastic anemia have used the anti-CD52 antibody alemtuzumab27 or rabbit ATG (Thymoglobulin)28 rather than equine ATG or in vitro T-cell depletion with TIOB9 or OKT3,29 and reported reductions in the incidence of acute GVHD to as low as 11%, albeit generally at the expense of an increased graft failure rate. It is possible, however, that the timing of ATG administration was important. Although in the present study ATG was given on days -5 to -3, we30 and others31 have given rabbit ATG in other trials on days -3 to -1. As a result, high concentrations of antibody were present in patient plasma at the time of donor cell infusion, conceivably sufficient to inactivate donor T lymphocytes, thereby leading to less GVHD.
Despite problems with GVHD, however, long-term survival in the present trial was comparable to that reported by Kojima et al12 in Japanese patients with aplastic anemia, in whom the frequency of GVHD has been lower than in North American patients.12,13,32,33 We observed an overall survival at 5 years of 61% among patients who received a transplant from HLA-identical donors and 40% among those who received a transplant from HLA-nonidentical donors, reemphasizing the importance of selecting donors on the basis of the highest resolution of HLA typing possible, even though the GVHD incidence remained high.34 The survival rate of 61% with HLA-identical donors was comparable to that reported by Ades et al24 for HLA-identical sibling transplants following conditioning with CY and thoracoabdominal irradiation.24 Patient age distribution in that study (55% were < 20 years of age) was virtually identical to that in the present trial. As in the trial reported by Ades et al,24 survival in the present study was age dependent, showing a 5-year probability of approximately 80% among patients aged 20 years or younger, who were conditioned with the optimum TBI dose of 200 cGy. Younger age has also been shown to be a favorable factor for transplantation outcome in earlier trials.4,12,35
In these young patients, in contradistinction to earlier reports,4,6 disease duration did not have a significant impact on transplantation outcome. This observation may be related to earlier referral of patients with growing awareness of the success rate of transplantation.
The lowest TBI dose used here was identical to that currently used in so-called non-myeloablative conditioning regimens.36,37 Those regimens often incorporate agents other than CY, for example, fludarabine, and are well tolerated as far as acute toxicity is concerned.38-40 Whether replacement of CY by fludarabine in the conditioning regimen, as proposed in a new trial for patients with aplastic anemia (BMT CTN protocol 0301) will reduce toxicity, remains to be determined.
In summary, the present TBI dose-finding study has provided encouraging results in patients with aplastic anemia who had not responded to IST and who received transplants from unrelated donors. TBI dose deescalation was effective in reducing transplant-related toxicity without jeopardizing engraftment. In young patients for whom HLA-identical donors were available, long-term survival approached that in patients who received a transplant from HLA-identical siblings. Conceivably, such patients should receive transplants even earlier in their disease course, without having received IST, to further improve transplantation outcome. However, future studies must be aimed at providing more effective GVHD prophylaxis.
Acknowledgments
The following transplantation centers enrolled patients in this trial: Baylor University, Dallas, TX; Cincinnati Children's Hospital, Cincinnati, OH; City of Hope National Medical Center, Duarte, CA; Emory University, Atlanta, GA; Fred Hutchinson Cancer Research Center, Seattle, WA; Heinrich-Heine University, Düsseldorf, Germany; Ohio State University, Columbus, OH; Oregon Health & Science University, Portland, OR; Royal Hospital for Sick Children, University of Bristol, United Kingdom; Rush-Presbyterian Medical Center, Chicago, IL; Stanford University and Packard Children's Hospital, Stanford, CA; Texas Children's Hospital, Houston, TX; University of California, Los Angeles, CA (Pediatric and Adult Transplantation Units); University of Colorado, Denver, CO; University of Minnesota, Minneapolis, MN; University of Utah, Salt Lake City, UT; Veterans Administration Hospital, Seattle, WA.
We thank Deborah Monroe, Sarah Ryan, Jennifer Freese, and John Sedgwick for data coordination; Dennis Confer, MD, and Marie Matlack at NMDP for providing information on donor searches and marrow cell dose; Effie Petersdorf, MD, for her suggestions regarding HLA-typing; Rainer Storb, MD, for critical comments regarding the manuscript; and Margaret Pepe, PhD, and Beth Muller, PhD, for their help with the original study design.
We thank Bonnie Larson and Helen Crawford for typing the manuscript.
Prepublished online as Blood First Edition Paper, May 9, 2006; DOI 10.1182/blood-2006-03-005041.
Supported by the National Institutes of Health, Department of Health and Human Services (DHHS), Bethesda, MD (grants HL36444 and CA15704) and by the National Marrow Donor Program.
H.J.D. codesigned the study, wrote the protocol, coordinated patient accrual, analyzed results, and wrote the manuscript. M.O'D. provided critique for the protocol, enrolled patients, and provided constructive comments on the manuscript. J.T. enrolled patients and provided comments on the manuscript. R.A. enrolled patients and provided comments on the manuscript. R.E.H. provided critique for the protocol, enrolled patients, and provided constructive comments on the manuscript. M.C.T. provided critique for the protocol, enrolled patients, and provided constructive comments on the manuscript. S.A.F. provided critique for the protocol, enrolled patients, and provided constructive comments on the manuscript. R.H.C. provided critique for the protocol, enrolled patients, and provided constructive comments on the manuscript. P.A.M. enrolled patients and provided comments on the manuscript. E.A.C. enrolled patients and provided comments on the manuscript. S.P.K. enrolled patients and provided comments on the manuscript. A.W. was responsible for the donor search at the Fred Hutchinson Cancer Research Center (FHCRC), verified all HLA data and the degree of matching, and offered comments on the manuscript. B.S. was responsible for protocol design and modifications, carried out all analyses, and provided comments on the manuscript.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Storb R, Blume KG, O'Donnell MR, et al. Cyclophosphamide and antithymocyte globulin to condition patients with aplastic anemia for allogeneic marrow transplantations: the experience in four centers. Biol Blood Marrow Transplant. 2001;7: 39-44. [DOI] [PubMed] [Google Scholar]
- 2.Hows JM, Yin JL, Marsh J, et al. Histocompatible unrelated volunteer donors compared with HLA nonidentical family donors in marrow transplantation for aplastic anemia and leukemia. Blood. 1986;68: 1322-1328. [PubMed] [Google Scholar]
- 3.Kernan NA, Bartsch G, Ash RC, et al. Analysis of 462 transplantations from unrelated donors facilitated by The National Marrow Donor Program. N Engl J Med. 1993;328: 593-602. [DOI] [PubMed] [Google Scholar]
- 4.Bacigalupo A, Oneto R, Bruno B, et al. Current results of bone marrow transplantation in patients with acquired severe aplastic anemia. Report of the European Group for Blood and Marrow Transplantation. On behalf of the Working Party on Severe Aplastic Anemia of the European Group for Blood and Marrow Transplantation. Acta Haematol. 2000;103: 19-25. [DOI] [PubMed] [Google Scholar]
- 5.Gajewski JL, Chattopadhyay A. Treatment of aplastic anemia with bone marrow transplants from closely matched unrelated donors. In: Champlin RE, Gale RP, eds. New Strategies in Bone Marrow Transplantation. New York, NY: Wiley-Liss; 1991: 101-108.
- 6.Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7: 208-215. [DOI] [PubMed] [Google Scholar]
- 7.Petersdorf EW, Longton GM, Anasetti C, et al. Association of HLA-C disparity with graft failure after marrow transplantation from unrelated donors. Blood. 1997;89: 1818-1823. [PubMed] [Google Scholar]
- 8.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18: 295-304. [DOI] [PubMed] [Google Scholar]
- 9.Martin PJ, Schoch G, Gooley T, et al. Methods for assessment of graft-versus-host disease [letter]. Blood. 1998;92: 3479-3481. [PubMed] [Google Scholar]
- 10.Sullivan KM. Graft-versus-host disease. In: Thomas ED, Blume KG, Forman SJ, eds. Hematopoietic Cell Transplantation. Malden, MA: Blackwell Sciences, Inc; 1999: 515-536.
- 11.Deeg HJ, Seidel K, Casper J, et al. Marrow transplantation from unrelated donors for patients with severe aplastic anemia who have failed immunosuppressive therapy. Biol Blood Marrow Transplant. 1999;5: 243-252. [DOI] [PubMed] [Google Scholar]
- 12.Kojima S, Horibe K, Inaba J, et al. Long-term outcome of acquired aplastic anaemia in children: comparison between immunosuppressive therapy and bone marrow transplantation. Br J Haematol. 2000;111: 321-328. [DOI] [PubMed] [Google Scholar]
- 13.Kodera Y, Morishima Y, Kato S, et al. Analysis of 500 bone marrow transplants from unrelated donors (UR-BMT) facilitated by the Japan Marrow Donor Program: confirmation of UR-BMT as a standard therapy for patients with leukemia and aplastic anemia. Bone Marrow Transplant. 1999; 24: 995-1003. [DOI] [PubMed] [Google Scholar]
- 14.Deeg HJ, Anasetti C, Petersdorf E, et al. Cyclophosphamide plus ATG conditioning is insufficient for sustained hematopoietic reconstitution in patients with severe aplastic anemia transplanted with marrow from HLA-A, B, DRB matched unrelated donors [letter]. Blood. 1994;83: 3417-3418. [PubMed] [Google Scholar]
- 15.Storb R, Anasetti C, Appelbaum F, et al. Marrow transplantation for severe aplastic anemia and thalassemia major. Semin Hematol. 1991;28: 235-239. [PubMed] [Google Scholar]
- 16.Socié G, Henry-Amar M, Bacigalupo A, et al. Malignant tumors occurring after treatment of aplastic anemia. N Engl J Med. 1993;329: 1152-1157. [DOI] [PubMed] [Google Scholar]
- 17.Deeg HJ, Socié G, Schoch G, et al. Malignancies after marrow transplantation for aplastic anemia and Fanconi anemia: a joint Seattle and Paris analysis of results in 700 patients. Blood. 1996; 87: 386-392. [PubMed] [Google Scholar]
- 18.Storb R, Thomas ED, Buckner CD, et al. Marrow transplantation for aplastic anemia. Semin Hematol. 1984;21: 27-35. [PubMed] [Google Scholar]
- 19.Aversa F, Terenzi A, Carotti A, et al. Improved outcome with T-cell-depleted bone marrow transplantation for acute leukemia. J Clin Oncol. 1999; 17: 1545-1550. [DOI] [PubMed] [Google Scholar]
- 20.Hsu HC, Tsai WH, Chen LY, et al. Overproduction of inhibitory hematopoietic cytokines by lipopoly-saccharide-activated peripheral blood mononuclear cells in patients with aplastic anemia. Ann Hematol. 1995;71: 281-286. [DOI] [PubMed] [Google Scholar]
- 21.Holler E, Kolb HJ, Möller A, et al. Increased serum levels of tumor necrosis factor α precede major complications of bone marrow transplantation. Blood. 1990;75: 1011-1016. [PubMed] [Google Scholar]
- 22.Dirksen U, Asadi-Moghaddam K, Fuhrer M, Burdach S. Defunct hematopoietic progenitor growth and heterogeneous immunological phenotypes in acquired aplastic anemia of childhood: identification of subsets with decreased hematopoietic progenitors and increased IL15 or IL10 production. Klin Padiatr. 1998;210: 167-172. [DOI] [PubMed] [Google Scholar]
- 23.Hempel L, Korholz D, Nussbaum P, Bonig H, Burdach S, Zintl F. High interleukin-10 serum levels are associated with fatal outcome in patients after bone marrow transplantation. Bone Marrow Transplant. 1997;20: 365-368. [DOI] [PubMed] [Google Scholar]
- 24.Ades L, Mary JY, Robin M, et al. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood. 2004;103: 2490-2497. [DOI] [PubMed] [Google Scholar]
- 25.Deeg HJ, Storer B, Slattery JT, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100: 1201-1207. [DOI] [PubMed] [Google Scholar]
- 26.Deeg HJ, Spitzer TR, Cottler-Fox M, Cahill R, Pickle LW. Conditioning-related toxicity and acute graft-versus-host disease in patients given methotrexate/cyclosporine prophylaxis. Bone Marrow Transplant. 1991;7: 193-198. [PubMed] [Google Scholar]
- 27.Gupta V, Ball SE, Yi QL, et al. Favorable effect on acute and chronic graft-versus-host disease with cyclophosphamide and in vivo anti-CD52 monoclonal antibodies for marrow transplantation from HLA-identical sibling donors for acquired aplastic anemia. Biol Blood Marrow Transplant. 2004;10: 867-876. [DOI] [PubMed] [Google Scholar]
- 28.Bacigalupo A, Locatelli F, Lanino E, et al. Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transplant. 2005;36: 947-950. [DOI] [PubMed] [Google Scholar]
- 29.Bunin N, Aplenc R, Iannone R, et al. Unrelated donor bone marrow transplantation for children with severe aplastic anemia: minimal GVHD and durable engraftment with partial T cell depletion. Bone Marrow Transplant. 2005;35: 369-373. [DOI] [PubMed] [Google Scholar]
- 30.Deeg HJ, Storer BE, Boeckh M, et al. Reduced incidence of acute and chronic graft-versus-host disease with the addition of thymoglobulin to a targeted busulfan/cyclophosphamide regimen. Biol Blood Marrow Transplant. 2006;12: 573-584. [DOI] [PubMed] [Google Scholar]
- 31.Bacigalupo A, Lamparelli T, Bruzzi P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98: 2942-2947. [DOI] [PubMed] [Google Scholar]
- 32.Hansen JA, Gooley TA, Martin PJ, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338: 962-968. [DOI] [PubMed] [Google Scholar]
- 33.Anasetti C, Petersdorf EW, Martin PJ, Woolfrey A, Hansen JA. Improving availability and safety of unrelated donor transplants. Curr Opin Oncol. 2000;12: 121-126. [DOI] [PubMed] [Google Scholar]
- 34.Petersdorf EW, Malkki M. Human leukocyte antigen matching in unrelated donor hematopoietic cell transplantation. Semin Hematol. 2005;42: 76-84. [DOI] [PubMed] [Google Scholar]
- 35.Doney K, Leisenring W, Storb R, Appelbaum FR, for the Seattle Bone Marrow Transplant Team. Primary treatment of acquired aplastic anemia: outcomes with bone marrow transplantation and immunosuppressive therapy. Ann Intern Med. 1997;126: 107-115. [DOI] [PubMed] [Google Scholar]
- 36.Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104: 3535-3542. [DOI] [PubMed] [Google Scholar]
- 37.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97: 3390-3400. [DOI] [PubMed] [Google Scholar]
- 38.Childs RW, Clave E, Tisdale J, Plante M, Hensel N, Barrett J. Successful treatment of metastatic renal cell carcinoma with a nonmyeloablative allogeneic peripheral-blood progenitor-cell transplant: evidence for a graft-versus-tumor effect. J Clin Oncol. 1999;17: 2044-2049. [DOI] [PubMed] [Google Scholar]
- 39.Khouri IF, Keating M, Körbling M, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16: 2817-2824. [DOI] [PubMed] [Google Scholar]
- 40.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102: 2021-2030. [DOI] [PubMed] [Google Scholar]
- 41.Bearman SI, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988; 6: 1562-1568. [DOI] [PubMed] [Google Scholar]