Abstract
Mesenchymal stem cells (MSCs) are multipotent progenitor cells that have emerged as a promising tool for clinical application. Further clinical interest has been raised by the observation that MSCs are immunoprivileged and, more important, display immunosuppressive capacities. These properties may be of therapeutic value in allogeneic transplantation to prevent graft rejection and to prevent and treat graft-versus-host disease. In the present study, we examined the in vivo immunomodulatory properties of MSCs in murine models of allogeneic bone marrow (BM) transplantation. Sublethally irradiated recipients received allogeneic BM with or without host or donor MSCs. The addition of host MSCs significantly enhanced the long-term engraftment associated with tolerance to host and donor antigens. However, the infusion of donor MSCs was associated with significantly increased rejection of allogeneic donor BM cells. Moreover, we showed that the injection of merely allogeneic donor MSCs in naive mice was sufficient to induce a memory T-cell response. Although the observed engraftment-promoting effects of host MSCs in vivo support the therapeutic potential of MSCs, our results also indicate that allogeneic MSCs are not intrinsically immunoprivileged and that under appropriate conditions, allogeneic MSCs induce a memory T-cell response resulting in rejection of an allogeneic stem cell graft.
Introduction
Human bone marrow stromal cells, also referred to as mesenchymal stem cells (MSCs), are able to differentiate along multiple lineages such as chondrocytes, osteoblasts, adipocytes, myocytes, and astrocytes.1 MSCs, rare residents in the bone marrow, can be rapidly expanded ex vivo without loss of their multilineage differentiation potential. Because of their ability to migrate to sites of tissue injury,2,3 MSCs have emerged as a promising therapeutic modality for tissue regeneration and repair. Several studies in animal models have demonstrated that MSCs are capable of long-term engraftment and in vivo differentiation, and encouraging results have been reported in clinical use.4-6
MSCs are known to secrete a number of cytokines and regulatory molecules implicated in different aspects of hematopoiesis.7 These characteristics have generated clinical interest to use MSCs to enhance hematopoietic stem cell engraftment. Although animal models provide experimental evidence that MSCs facilitate engraftment,8,9 no conclusive evidence has yet been presented in humans.6 In addition to providing critical growth factors, MSCs display immunosuppressive properties that might facilitate engraftment. In vitro studies with human, baboon, and murine MSCs demonstrated that MSCs suppress the proliferation of T cells induced by alloantigens or mitogens.10-12 Furthermore, MSCs have been reported to induce T-cell division arrest,13 to inhibit the differentiation and maturation of dendritic cells,14,33 and to decrease the production of inflammatory cytokines by various immune cell populations.15 Controversy exists regarding their effect on cytotoxic T cells and NK cells.16,17 Animal studies indicate that, in line with their immunosuppressive capacities in vitro, MSCs also display immunosuppressive capacities in vivo; allogeneic MSCs may prolong skin allograft survival in immunocompetent baboons10 and may prevent the rejection of allogeneic tumor cells in immunocompetent mice.18 The mechanisms underlying these effects of MSCs have not been clearly identified. Although conflicting results have been reported, most studies agree that soluble factors are involved.11,18-20 The therapeutic application of the immunosuppressive properties of MSCs has already been exploited in the clinical setting for the treatment of acute graft-versus-host disease after allogeneic stem cell transplantation.21
The immunophenotype of MSCs, the low expression of human leukocyte antigen (HLA) major histocompatibility complex (MHC) class I, and the absence of costimulatory molecules, together with the observation that MSCs do not elicit a proliferative response from allogeneic lymphocytes, suggest that MSCs are of inherently low immunogenicity.11,20 These properties might open attractive possibilities to use universal donor MSCs for different therapeutic applications.
The aim of this study was to examine whether MSCs display immunosuppressive properties in vivo in murine allogeneic bone marrow transplantation models. The transplantation of host murine MSCs into sublethally conditioned recipients resulted in decreased rejection of donor BM cells. In contrast, cotransplantation with allogeneic donor MSCs significantly decreased engraftment of allogeneic BM. Moreover, the infusion of allogeneic MSCs led to the development of a cellular immune response in nonimmunocompromised mice. These results indicate that MSCs can have potent immunosuppressive capacities in vivo, but, importantly, MSCs are not intrinsically immunoprivileged and may trigger immune responses in vivo resulting in graft rejection.
Materials and methods
Mice
Female BALB/c, C57BL/6 (B6), and C3H mice (8-12 weeks old) were obtained from Charles River Laboratories (Maastricht, The Netherlands) and were kept under standard animal housing setting. Congenic C57BL/6-Ly5.1 and BALB/b mice were bred at our own facilities and were used at 8 to 12 weeks of age. Mice used in experiments were sex and age matched.
Bone marrow transplantation
BALB/c and C57BL6/-Ly5.1 recipients were conditioned with a sublethal 6-Gy or 5-Gy dose of total body irradiation, respectively, 1 day before transplantation. Donor bone marrow (BM) cells from B6 or BALB/b mice were flushed from tibiae, femurs, and humeri using RPMI 1640 (BioWhittaker, Verviers, Belgium) supplemented with 2% heat-inactivated fetal calf serum (FCS; BioWhittaker) and heparin (24 IU/mL; Hospital Pharmacy, Leiden University Medical Center, Leiden, The Netherlands). BM cells were filtered through a sterile nylon mesh, resuspended in sterile phosphate-buffered saline (PBS; Hospital Pharmacy), and injected intravenously with or without murine mesenchymal stem cells from different origins. In vivo depletion of donor CD4+ and CD8+ T cells was accomplished by using 100 μg anti-CD4 mAb (GK1.5) and anti-CD8 mAb 2.43 intraperitoneally on day –5 and day –1.
Culture of murine MSCs
Murine MSCs were obtained from bone marrow aspirates, as described previously,22 and were cultured in α-modified minimum essential medium (α-MEM) supplemented with 10% FCS, 100 IU/mL penicillin, 100 μg/mL streptomycin (Gibco Life Technologies), and 2 mM L-glutamine (BioWhittaker). Before experimental use, MSCs were tested for their ability to differentiate into adipocytes, osteoblasts, and chondrocytes. To induce adipogenic differentiation, MSCs were cultured with 10–8 M dexamethasone and 5 μg/mL insulin, and Oil-red-O was used to for lipid droplet staining. Osteogenic differentiation was induced by 10–8 M dexamethasone, 10 mM β-glycerol-phosphate, and 50 μg/mL ascorbic acid. Osteoblasts were identified by Alizarine Red S staining. For chondrocyte differentiation, a pellet culture system was used. Chondrogenic differentiation medium consisted of high-glucose DMEM (Gibco Laboratories, Grand Island, NY) supplemented with 40 μg/mL proline, 50 mg/mL ITS + premix, 100 μg/mL sodium pyruvate, glutamax, 50 μg/mL ascorbate-2-phosphate, 10 ng/mL TGF-β3, 10–7 M dexamethasone, and 500 ng/mL BMP-6. Chondrogenic differentiation was visualized by Toluidine blue staining. Murine MSCs expanded in vitro showed positive surface staining for CD106 and lacked hematopoietic (CD45, CD14) and endothelial (CD31) markers.
Mixed-lymphocyte reactions
Splenocytes were isolated by mechanical dissociation through sterile nylon mesh (100 μM; BD Biosciences, Franklin Lakes, NJ), followed by red blood cell lysis (incubation for 10 minutes at 4°C with NH4Cl (8.4 g/L)/KHCO3 (1 g/L) buffer). Responder splenocytes from BALB/c mice (3 × 105/well in 96-well round-bottom plates) were added to irradiated (30 Gy) stimulator splenocytes in Iscove modified Dulbecco medium (IMDM; BioWhittaker) supplemented with 10% FCS, PS, and 50 μM β-mercaptoethanol. In mitogen proliferative assays, responder splenocytes were incubated with 5 μg/mL concanavalin A (ConA; Sigma). MSCs (60 Gy irradiated) were added in graded doses to the mixed-leukocyte reaction (MLR). After 5 days of incubation, cells were pulsed with 3H-thymidine (1 μCi (0.037 MBq)/well) for the last 18 hours, harvested, and counted with a Topcount NXT (Canberra Packard, Meriden, CT). Results are expressed in counts per minute and are presented as means (± SD) obtained from triplicate cultures.
Chimerism analysis
Blood was collected at various time points after bone marrow transplantation (BMT), and red blood cells were removed. Multilineage chimerism was determined by flow cytometry (FACSCalibur) using fluorescein isothiocyanate (FITC)–conjugated anti–H-2Kb (clone AF6-88.5) or phycoerythrin (PE)–conjugated anti-CD45.1 mAbs (clone A20) specific for the donor and PE-conjugated anti–H-2Kd (clone SF1-1.1) or FITC-conjugated anti-CD45.2 mAbs (clone 104) specific for the recipient in combination with allophycocyanin (APC)–conjugated anti-CD3e (clone 145-2C11), anti-B220 (clone RA3-6B2), and anti–GR-1 (clone RB6-8C5) mAbs. All antibodies were obtained from PharMingen (Alphen aan de Rijn, The Netherlands). Chimerism was expressed by percentage donor granulocytes, and engraftment was considered positive if at least 10% of recipient granulocytes had the donor BM Ly5 or H-2 phenotype.
Immunologic analyses
Specificity of the induced tolerance was determined by performing MLR and cell-mediated lympholysis (CML) assays. MLRs were performed by obtaining spleen cells from recipient BALB/c and B6-Ly5.1 mice 4 months after transplantation and then were used as responders (3 × 105/well in round-bottom 96-well plates) against γ-irradiated (30 Gy) BALB/c, B6, or DBA splenocytes stimulated for 48 hours with 50 μg/mL LPS. After 5 days of incubation, 3H-thymidine (1 μCi [0.037 MBq]/well) was added overnight, and thymidine incorporation was measured. CML assays were performed by incubating recipient spleen cells (3 × 106/well in 24-well plates) with irradiated D1 cells (104/well). After 6 days of culture, effector cells were collected and incubated for 4 hours with 103 chromium 51 (51Cr)–labeled target cells expressing H-2Kb (EL4) or H-2Kd (p815) MHC molecules. Specific lysis was defined as 100 × (experimental release – spontaneous release)/(detergent release – spontaneous release).
In vivo immunization of mice
MSCs derived from different origins were injected (0.5 × 106 cells) intravenously into BALB/c (MSCs from B6 and BALB/c) or B6 (MSCs from BALB/b and B6) mice. Four weeks later, in vivo cytotoxicity was determined as described previously.24 In brief, allogeneic and syngeneic splenocytes (107/mL in PBS) were differentially labeled for 10 minutes at 37°C with 5 or 0.5 μM carboxy fluorescein diacetate succimidyl ester (CFDA-SE; Molecular Probes, Leiden, The Netherlands), respectively. Labeled cells (10 × 106) were injected intravenously in a 1:1 ratio, and after various time points the ratio of carboxy fluorescein succinimidyl ester (CFSE)high/CFSElow cells was determined by flow cytometry.
Statistical analysis
Statistical analysis was performed by Student 2-tailed unpaired t test or 2-way analysis of variance (ANOVA) using GraphPadPRISM (GraphPad Software, San Diego, CA). Differences were considered statistically significant when P was below .05.
Results
MSCs inhibit the proliferation of T cells
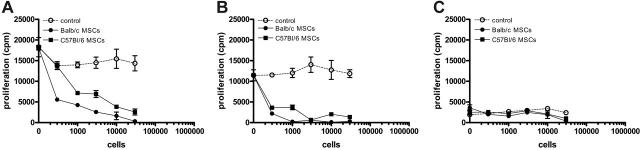
To confirm the in vitro immunomodulatory effect of murine MSCs, graded doses of BALB/c or B6 MSCs were added to mixed lymphocyte cultures of BALB/c and B6 splenocytes. Murine MSCs inhibited the proliferation of allogeneic responder splenocytes in a dose-dependent manner (Figure 1A). Similarly, murine MSCs inhibited the proliferation of responder splenocytes induced by ConA (Figure 1B), whereas murine MSCs themselves did not elicit a proliferative response of allogeneic splenocytes (Figure 1C).
Figure 1.
Murine MSCs inhibit the response of allogeneic T cells in a dose-dependent manner. Responder BALB/c splenocytes were stimulated for 4 days with either C57Bl/6 splenocytes (A), 5 μg/mL ConA (B), or BALB/c splenocytes (C) with or without graded doses of BALB/c MSCs, C57Bl/6 MSCs, or BALB/c splenocytes. Results are expressed as the mean (± SD) cpm obtained from triplicate cultures and are representative of 3 independent experiments.
Optimization of BM engraftment in sublethally irradiated recipient mice
Initial experiments were performed to determine the dose of sublethal irradiation that resulted in graft rejection in 50% of recipient mice. Engraftment of donor cells in peripheral blood was examined by flow cytometry at various time points after transplantation. All BALB/c recipients conditioned with 7 Gy showed long-term multilineage chimerism, whereas after the exposure to 6.5 Gy or 6 Gy, only 80% or 30%, respectively, of the BALB/c mice showed long-term multilineage engraftment (Table 1). In the multiple minor mismatched transplantations, long-term chimeras were observed in all recipients exposed to 6 Gy, in 50% of the recipients exposed to 5 Gy, and in none of the recipients exposed to 4 Gy (Table 1).
Table 1.
Effect of irradiation dose on the level of chimerism
|
Donor chimerism in chimeric mice 12-16 weeks after Tx, %
|
||||
|---|---|---|---|---|
| Donor → recipient, irradiation dose, Gy | Long-term donor chimerism (%) | GR-1 | B220 | CD3 |
| B6 → BALB/c | ||||
| 6 | 3 of 10 (30) | 47.6 ± 47.9 | 59.3 ± 38.3 | 22.3 ± 11.9 |
| 6.5 | 4 of 5 (80) | 99.8 ± 0.2 | 99.9 ± 0.1 | 68.0 ± 8.1 |
| 7 | 5 of 5 (100) | 99.4 ± 0.5 | 99.0 ± 0.9 | 89.1 ± 8.4 |
| BALB/b → B6 | ||||
| 4 | 0 of 6 (0) | ND | ND | ND |
| 5 | 3 of 6 (50) | 85.6 ± 11.4 | 60.1 ± 33.6 | 13.7 ± 14.0 |
| 6 | 5 of 5 (100) | 89.3 ± 22.2 | 96.8 ± 1.3 | 76.4 ± 3.1 |
BALB/c mice underwent irradiation with 6, 6.5, or 7 Gy (day 1) and underwent transplantation with 10 × 106 B6-depleted BM cells (day 0). B6 recipient mice were exposed to 4, 5, or 6 Gy (day 1) and underwent transplantation with 10 × 106 BALB/b BM cells (day 0). Donor chimerism was determined by flow cytometry 12 to 16 weeks after transplantation.
Tx indicates transplantation; ND, not determined.
Syngeneic MSCs enhance long-term donor chimerism
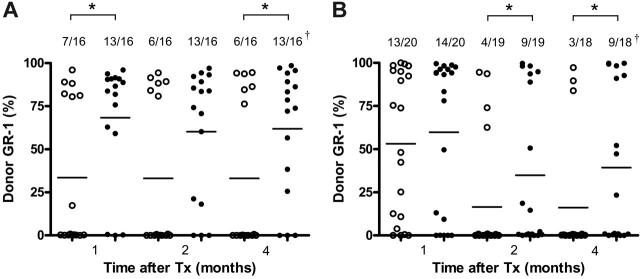
To determine the ability of MSCs to prevent the rejection of multiple minor histocompatibility antigen mismatched BM, B6 recipient mice were exposed to sublethal irradiation (5 Gy) and underwent transplantation with T-cell–depleted BALB/b BM cells (10 × 106). B6 MSCs (0.25 × 106) were infused on days 0, 4, 7, 10, and 14. Peripheral blood samples were collected at different time points after transplantation and examined for the presence of donor cells by flow cytometry. The addition of B6 MSCs resulted in a significant increase in the percentage of engrafted mice (44% vs 82%; P < .05; Figure 2A). Furthermore, MSC-facilitated engraftment was still evident at 4 months after transplantation, and donor chimerism included lymphoid (CD3+, B220+) and myeloid (GR-1+) lineages (data not shown).
Figure 2.
Syngeneic MSCs support long-term engraftment. (A) T-cell–depleted BM from BALB/b mice (10 × 106) was transplanted alone or together with B6 MSCs (0.25 × 106) into 5 Gy-irradiated B6 recipients. (B) BALB/c mice were irradiated with 6 Gy and engrafted with T-cell–depleted BM (10 × 106) from B6 mice with or without BALB/c MSCs (0.25 × 106). MSCs were infused 5 times at days 0, 3, 7, 10, and 14 after BM transplantation. Peripheral blood was harvested and analyzed by flow cytometry to determine chimerism 1, 2, and 4 months after BM transplantation by the expression of Ly5.1 (A) or MHC class I (B). Data presented are pooled from 2 experiments. Horizontal bar indicates the average percentage of positive staining of donor cells for each group. †Number of engrafted mice/number of analyzed mice. *P < .05.
To investigate whether syngeneic MSCs could display the same graft-facilitating effect in a major MHC mismatch transplantation, irradiated BALB/c recipient mice (6 Gy) were transplanted with T-cell–depleted B6 BM cells (10 × 106) with or without multiple doses of BALB/c MSCs. One month after transplantation, the percentage of recipient mice that experienced engraftment after transplantation of BM cells alone was comparable to the engraftment in mice that received BM cells and MSCs (65% vs 70%, respectively) (Figure 2B). In contrast, longer-term assessment in peripheral blood indicated that syngeneic MSCs were capable of increasing the engraftment rate in recipient mice (47% and 50% engraftment vs 20% and 17% in the absence of MSCs, 2 and 4 months after transplantation, respectively, P < .05) (Figure 2B).
Recipients of syngeneic MSCs are tolerant of donor and host antigens
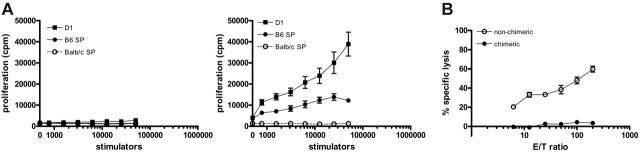
To examine whether chimerism was associated with tolerance to donor and host antigens, MLR and cytotoxic T-cell responses were assessed against B6 (donor) and BALB/c (host) antigens. Splenocytes from chimeric BALB/c recipients of B6 BM and BALB/c MSCs did not generate proliferative responses to B6 (D1 cells or B6 spleen cells) or BALB/c stimulators in mixed lymphocyte responses (Figure 3A; left). In contrast, BALB/c recipients of B6 BM cells alone that rejected their grafts exhibited a high proliferative response to donor B6 antigens (Figure 3A; right). Moreover, splenocytes from chimeric recipients failed to induce CTLs against targets of H-2b haplotype (RMA) or H-2d haplotype (p815), whereas nonchimeric recipients generated allogeneic donor CTLs (Figure 3B).
Figure 3.
Chimeric recipients of allogeneic BM and syngeneic MSCs do not respond to donor and host antigens. (A) Splenocytes from chimeric BALB/c recipients of B6 BM and BALB/c MSCs (n = 5; left) and BALB/c recipients of B6 BM only who rejected allogeneic BM (n = 5; right) were isolated 4 months after transplantation. Recipient splenocytes were stimulated with D1 cells, B6 splenocytes, or BALB/c splenocytes for 4 days. Results are expressed as the mean (± SD) obtained from triplicate cultures. (B) CTL responses were generated by incubation of recipient splenocytes (3 × 106) with D1 cells (104) for 6 days. CTL activity was measured in a cytotoxicity assay using targets of H-2Kb haplotype (RMA). Values represent means (± SD) of triplicates.
Allogeneic MSCs decrease engraftment after allogeneic BMT
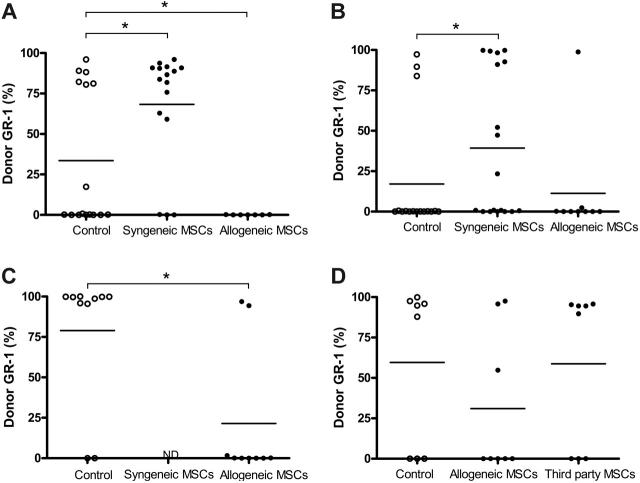
Previous studies have suggested that MSCs are immunoprivileged and that the immunosuppressive effect of MSCs on T cells appeared to be independent of MHC matching between MSCs and T cells.25 To examine whether MSCs had to be MHC matched to recipient mice to exert their graft-facilitating effect, recipient mice underwent transplantation with allogeneic BM in the presence or absence of syngeneic or allogeneic donor MSCs. As shown in Figure 4, none of the recipient B6 mice that received transplanted multiple minor antigen–mismatched BALB/b BM and BALB/b MSCs experienced engraftment, whereas 44% of recipient mice did so after the administration of BALB/b BM cells alone (P < .05). In contrast, the addition of syngeneic MSCs increased the proportion of engraftment in mice (82%), as presented in Figure 2A.
Figure 4.
Allogeneic MSCs decrease engraftment of allogeneic BM. B6 recipients (irradiated with 5 Gy) underwent engraftment with T-cell–depleted BM from BALB/b mice (10 × 106) with or without B6 MSCs or BALB/b MSCs (A). Alternatively, T-cell–depleted BM from B6 mice (10 × 106) was transplanted in the presence or absence of BALB/c or B6 MSCs into BALB/c recipients (irradiated with 6 Gy) (B). MSCs (0.25 × 106) were infused 5 times at days 0, 3, 7, 10, and 14 after BM transplantation. Peripheral blood was harvested 4 months after BM transplantation and was analyzed by flow cytometry. Data presented are pooled from 2 experiments. (C) BALB/c recipients were irradiated with 6.5 Gy and underwent transplantation with T-cell–depleted BM from B6 mice with or without a single infusion of B6 MSCs (0.25 × 106). (D) BM from B6 mice was transplanted into BALB/c recipients (irradiated with 6 Gy) with or without 5 infusions of B6 or C3H MSCs (0.25 × 106). Four months after transplantation, peripheral blood was harvested and examined for chimerism by expression of MHC class I. Horizontal bar indicates the average percentage of positive staining of donor cells for each group. ND indicates not determined. *P < .05.
The reduced engraftment with allogeneic donor MSCs was also observed in the major MHC mismatch transplantation model (B6 BM transplanted into BALB/c recipients) (13% engraftment vs 50% with syngeneic MSCs) (Figure 4B). To examine whether a single dose of allogeneic MSCs could hamper the engraftment of allogeneic BM cells, BALB/c recipients were irradiated with a higher dose (6.5 Gy) to allow a higher rate of engrafted recipient mice after the administration of B6 BM cells only. Under these conditions, 80% of recipient mice experienced engraftment. In contrast, only 22% of recipient BALB/c mice did so after the administration of B6 BM cells and a single dose of B6 MSCs (Figure 4C). Furthermore, infusion of a single dose of allogeneic MSCs in the multiple minor mismatched transplantation model also significantly reduced the number of chimeric mice (0% vs 38% in the presence of allogeneic MSCs). In contrast, no difference in the number of chimeric mice was observed after infusion of a single dose of syngeneic MSCs in both models at the time of BM transplantation (data not shown).
To examine the effect of third-party MSCs on the engraftment of allogeneic BM, recipient BALB/c mice underwent transplantation with allogeneic B6 BM cells with or without B6 or C3H MSCs. Engraftment level was not affected by cotransplantation with third-party MSCs (63% in the absence and presence of C3H MSCs), whereas the infusion of B6 MCSs decreased the number of chimeric mice (25% vs 63% in the absence of MSCs) (Figure 4D). These results suggest that the infusion of syngeneic, allogeneic, and third-party MSCs have contrasting effects, whereas syngeneic MSCs can promote long-term chimerism, allogeneic MSCs appear to hamper the engraftment of allogeneic BM, and third-party MSCs seem to have a neutral effect on engraftment.
Allogeneic MSCs are immunogenic
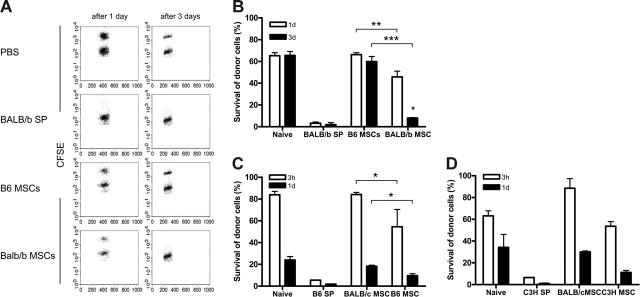
The decreased engraftment of allogeneic BM observed after the injection of allogeneic MSCs suggests that MSCs may trigger an alloresponse. To examine whether the infusion of allogeneic MSCs can induce T-cell responses, naive immunocompetent B6 recipient mice were infused with PBS, 0.5 × 106 B6 MSCs, 0.5 × 106 BALB/b MSCs, or 0.5 × 106 BALB/b SP cells, and, 4 weeks later, an in vivo cytotoxicity assay was performed. B6 mice that had received BALB/b SP cells eliminated almost all CFSE-labeled BALB/b splenocytes within 1 day, indicating that the infusion of 0.5 × 106 was sufficient to trigger a memory T-cell response (Figure 5A-B). The elimination kinetics of allogeneic BALB/b splenocytes in B6 that received syngeneic B6 MSCs resembled that of PBS-treated mice (60% survival after 3 days). In contrast, B6 mice treated with BALB/b MSCs eliminated almost all CFSE-labeled allogeneic BALB/c cells within 3 days (Figure 5A-B), indicating that the infusion of allogeneic MSCs can trigger a memory T-cell response.
Figure 5.
Allogeneic MSCs trigger the onset of a memory T-cell response. (A-B) B6 mice were infused with PBS, BALB/b splenocytes (0.5 × 106), BALB/b MSCs (0.5 × 106), or B6 MSCs (0.5 × 106). (C) BALB/c mice were infused with PBS, B6 splenocytes (0.5 × 106), B6 MSCs (0.5 × 106), or BALB/c MSCs (0.5 × 106). (D) Balb/c mice were injected with PBS, C3H splenocytes (0.5 × 106), BALB/c MSCs (0.5 × 106), or C3H MSCs (0.5 × 106). After 4 weeks, in vivo cytotoxicity assay was performed and peripheral blood was harvested after 1 and 3 days (A-B) or after 3 hours and 1 day (C-D). Representative dot plots are displayed (A). Results are expressed as the mean survival (± SD) of allogeneic donor cells of 2 independent experiments (B-D). *P < .05; **P < .001; ***P < .001 compared with syngeneic MSCs.
Next, the immunogenicity of major MHC-mismatched MSCs was examined. Although NK cells and T cells play a role in the elimination of allogeneic cells,24 treatment with B6 MSCs or C3H resulted in decreased survival of CFSE-labeled allogeneic B6 SP cells (55% and 9% after 3 hours and 1 day, respectively) or C3H SP cells (54% and 11% after 3 hours and 1 day, respectively) compared with treatment with BALB/c MSCs (84% and 18% after 3 hours and 1 day, respectively), suggesting the involvement of memory T cells (Figure 5C-D).
Discussion
MSCs have gained interest for their use in regenerative medicine because of their ability to differentiate into several mesenchymal tissues and their ability to be easily expanded ex vivo. Further clinical interest has been gained by the observations that MSCs may be immunoprivileged and may display immunosuppressive properties. Although considerable amounts of in vitro data support the nonimmunogenicity and immunomodulatory effects of MSCs, relatively little information is available on the immunogenicity and immunomodulatory capacities of MSCs in vivo. In the present study, we examined whether MSCs display immunosuppressive properties in murine allogeneic bone marrow transplantation models. The infusion of host MSCs into sublethally irradiated recipients promoted engraftment of allogeneic BM that was associated with tolerance to donor and recipient antigens. Conversely, cotransplantation of allogeneic donor MSCs hampered allogeneic BM engraftment, associated with a strong proliferative response of host spleen cells against donor cells. Moreover, reduced survival of allogeneic splenocytes was observed in naive immunocompetent mice after the injection of allogeneic MSCs, showing that MSCs had triggered the onset of a memory T-cell response. These data strongly suggest that MSCs are not intrinsically immunoprivileged.
The graft-promoting effect induced by the infusion of syngeneic MSCs is in accordance with results obtained from studies conducted in animal models.10,18 The mechanisms underlying these properties of MSCs are still unclear. However, 2 hypotheses have been proposed, the first related to the immunomodulatory properties of MSCs. The immunosuppressive effects of MSCs have been generally ascribed to the inhibitory effect on T cells, but recently it was also shown that MSCs have inhibitory effects on other immune cells in vitro, including dendritic cells.15,26 In the major MHC mismatch transplantation, T cells and NK cells can contribute to the rejection of allografts. However, predominantly T cells and not NK cells play a role in the rejection of multiple minor antigen–mismatched allografts, suggesting that the putative immunomodulatory effect of syngeneic MSCs that promotes engraftment is mediated by the suppression of host T cells. Although the mechanism underlying the MSC-mediated suppression of T-cell activation in vitro is not completely understood and is surrounded by controversy, most studies agree that soluble factors are involved. The graft-promoting effect by systemic administration of MSCs also suggests that MSCs might exert immunoregulatory effects through the secretion of soluble immunoregulatory mediators. MSCs can produce transforming growth factor β,11 prostaglandin E2,15 and indoleamine 2,3-dioxygenase (IDO),19 which can exert immunosuppressive effects on T-cell responses.
Another possibility involves the concept of providing stromal support of donor hematopoietic stem cells that may enhance hematopoiesis after transplantation. Although there is minimal evidence that donor stromal cells can engraft after systemic administration in the bone marrow of recipients, experimental evidence indicates that MSCs can enhance the engraftment of hematopoietic stem cells in nonobese diabetic–severe compromised immunodeficient (NOD-SCID) mice and fetal sheep.8,9 The mechanism of enhancement is not understood and may not require homing of MSCs to BM. Support could be mediated by hematopoietic growth factors produced by MSCs, including stem cell factor (SCF), macrophage–colony-stimulating factor (M-CSF), granulocyte macrophage–colony-stimulating factor (GM-CSF), interleukin-6 (IL-6), and IL-11.27,28
In the present study, the infusion of allogeneic MSCs can prime allogeneic T cells in naive immunocompetent mice, indicating that MSCs are immunogenic under these conditions. Several studies have described that fetal calf serum present in culture media can influence immune responses against cultured cells.29,30 However, it is not very likely that the infusion of allogeneic cultured MSCs triggers an FCS response because CFSE-labeled allogeneic spleen cells that had not been exposed to FCS were specifically eliminated. Furthermore, no memory response was observed after the infusion of syngeneic cultured MSCs. In support of these observations are recent findings demonstrating that allogeneic MSCs can be immunogenic in immunocompetent animals.31,32 Injection of adult MSCs in rat myocardium was associated with significant infiltration primarily of macrophages and with rejection, whereas persistent engraftment of adult human MSCs was observed in immunologic-incompetent rats. Furthermore, peripheral blood lymphocytes of rats injected with adult MSCs proliferated after restimulation with adult MSCs in vitro, suggesting cellular immunization. Moreover, another study32 demonstrated that implanted murine MSCs were rejected in allogeneic recipient mice and that splenocytes of mice implanted with allogeneic MSCs were activated upon restimulation with allogeneic MSCs in vitro. The immune response elicited by the infusion of allogeneic donor MSCs may enhance the response of the host against donor antigens, resulting in increased rejection of allogeneic donor BM. In contrast, though the infusion of third-party MSCs induces a memory T-cell response, it does not affect the engraftment of unrelated allogeneic BM. Moreover, the immune response provoked by the infusion of third-party MSCs may result in rapid rejection of the MSCs. Therefore, no engraftment-enhancing effects are observed after cotransplantation with third-party MSCs.
In conclusion, our results show that MSCs are capable of modulating immune responses in vivo and that these responses are affected by MHC antigen matching between donors and recipients. In addition, MSCs are not intrinsically immunoprivileged and are capable of inducing a memory T-cell response after injection in vivo in immunocompetent hosts, in striking contrast to the general notion that MSCs are nonimmunogenic. After cotransplantation in an immunocompromised host that has received sublethal irradiation, allogeneic donor MSCs still induce an allogeneic response that results in graft rejection. These results suggest that the immunogenic properties of allogeneic MSCs are not fully prevented by the conditioning regimen. Although it is still unclear whether the immunogenicity of allogeneic MSCs is preserved after a full myeloablative conditioning regimen, the possibility that allogeneic or third-party MSCs are immunogenic should be taken into account in designing clinical studies in the setting of allogeneic stem cell transplantation.
Acknowledgments
We thank Darwin Prockop (Tulane University Health Sciences Center, New Orleans, LA) for providing valuable reagents.
Prepublished online as Blood First Edition Paper, May 11, 2006; DOI 10.1182/blood-2005-11-011650.
Supported by research grant 03-3014 from the Dutch Cancer Society, by the EuroCord Nederland Foundation, and by a grant from the National Center for Research Resources of the National Institutes of Health (P40 RR 017447).
A.J.N. designed and performed the research, analyzed the data, and wrote the paper; G.W. designed and performed the research; A.B.K. performed the research and analyzed the data; E.G.A.L. performed the research; R.W. designed the research; and W.E.F. designed the research, analyzed the data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284: 143-147. [DOI] [PubMed] [Google Scholar]
- 2.Wu GD, Nolta JA, Jin YS, et al. Migration of mesenchymal stem cells to heart allografts during chronic rejection. Transplantation. 2003;75: 679-685. [DOI] [PubMed] [Google Scholar]
- 3.Pereira RF, O'Hara MD, Laptev AV, et al. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998;95: 1142-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99: 8932-8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koc ON, Day J, Nieder M, et al. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant. 2002;30: 215-222. [DOI] [PubMed] [Google Scholar]
- 6.Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18: 307-316. [DOI] [PubMed] [Google Scholar]
- 7.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28: 875-884. [DOI] [PubMed] [Google Scholar]
- 8.Almeida-Porada G, Porada CD, Tran N, Zanjani ED. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000;95: 3620-3627. [PubMed] [Google Scholar]
- 9.Noort WA, Kruisselbrink AB, in't Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30: 870-878. [DOI] [PubMed] [Google Scholar]
- 10.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30: 42-48. [DOI] [PubMed] [Google Scholar]
- 11.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or non-specific mitogenic stimuli. Blood. 2002;99: 3838-3843. [DOI] [PubMed] [Google Scholar]
- 12.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101: 3722-3729. [DOI] [PubMed] [Google Scholar]
- 13.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105: 2821-2827. [DOI] [PubMed] [Google Scholar]
- 14.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105: 4120-4126. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105: 1815-1822. [DOI] [PubMed] [Google Scholar]
- 16.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171: 3426-3434. [DOI] [PubMed] [Google Scholar]
- 17.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76: 1208-1213. [DOI] [PubMed] [Google Scholar]
- 18.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102: 3837-3844. [DOI] [PubMed] [Google Scholar]
- 19.Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103: 4619-4621. [DOI] [PubMed] [Google Scholar]
- 20.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75: 389-397. [DOI] [PubMed] [Google Scholar]
- 21.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363: 1439-1441. [DOI] [PubMed] [Google Scholar]
- 22.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103: 1662-1668. [DOI] [PubMed] [Google Scholar]
- 23.Schuurhuis DH, Laban S, Toes RE, et al. Immature dendritic cells acquire CD8(+) cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J Exp Med. 2000;192: 145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westerhuis G, Maas WG, Willemze R, Toes RE, Fibbe WE. Long-term mixed chimerism after immunological conditioning and MHC-mismatched stem cell transplantation is dependent on NK cell tolerance. Blood. 2005;106: 2215-2220. [DOI] [PubMed] [Google Scholar]
- 25.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57: 11-20. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13: 263-271. [DOI] [PubMed] [Google Scholar]
- 27.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176: 57-66. [DOI] [PubMed] [Google Scholar]
- 28.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166: 585-592. [DOI] [PubMed] [Google Scholar]
- 29.MacDermott RP, Bragdon MJ. Fetal calf serum augmentation during cell separation procedures accounts for the majority of human autologous mixed leukocyte reactivity. Behring Inst Mitt. 1983; 122-128. [PubMed]
- 30.Kievits F, Boerenkamp WJ, Ivanyi P. H-2-dependent binding of xenogeneic beta 2-microglobulin from culture media. J Immunol. 1988;140: 4253-4255. [PubMed] [Google Scholar]
- 31.Grinnemo KH, Mansson A, Dellgren G, et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004;127: 1293-1300. [DOI] [PubMed] [Google Scholar]
- 32.Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I and II mismatched recipient mice. Blood. 2005;106: 4057-4065. [DOI] [PubMed] [Google Scholar]
- 33.Nauta AJ, Kruisselbrink AB, Lurvink EGA, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of CD34+-derived and monocyte-derived dendritic cells. J Immunol. In press. [DOI] [PubMed]