Abstract
Reduced insulin sensitivity following chronic alcohol consumption may contribute to alcohol-induced brain damage although the underlying mechanism(s) has not been elucidated. This study was designed to examine the effect of chronic alcohol intake on insulin signaling in mouse cerebral cortex. FVB mice were fed with a 4% alcohol diet for 16 weeks. Insulin receptor substrates (IRS-1, IRS-2) and post-receptor signaling molecules Akt, mammalian target of rapamycin (mTOR), ribosomal p70s6 kinase (p70s6k) and the eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) as well as the apoptotic marker caspase-3 were evaluated using Western blot analysis. Chronic alcohol intake significantly dampened whole body glucose tolerance, enhanced expression of caspase-3 and mTOR, reduced p70s6k and 4E-BP1 with little effect on Akt signaling in alcohol consuming mice. These data suggest that chronic alcohol intake may contribute to cerebral cortex dysfunction through mechanisms related, at least in part, to dampened post insulin receptor signaling at the levels of mTOR, p70s6k and 4E-BP1.
Keywords: alcohol, cerebral cortex, Akt, mTOR, p70s6k, 4E-BP1
INTRODUCTION
Uncomplicated alcoholics with little prior neurological problems display signs of brain damage and cognitive deficit following chronic alcohol consumption (Harper and Matsumoto 2005). This is further confirmed by a broad array of neurological lesions found in alcoholics characterized by impaired neuronal survival, growth, neurotransmitter function and intracellular adhesion (Harper and Matsumoto 2005). Although several hypotheses have been postulated for alcohol-induced brain tissue damage including toxicity of alcohol or its metabolite acetaldehyde, accumulation of reactive oxygen species and fatty acid ethyl esters, modifications of lipoprotein and apolipoprotein particles, metabolic and excitotoxic changes as well as genetic predisposition (Hannuksela et al. 2002; Kucera et al. 2002; Patel et al. 1997; Zhang et al. 2004; Zimatkin et al. 2006), none of the scenarios received full validation from clinical and experimental data. Recent evidence suggests a role of insulin sensitization in neurocognitive recovery and psychosocial adaptation in chronic alcoholics (Esler et al. 2001), which is in line with the notion that alcohol intake may alter insulin secretion and autonomic activity (Flanagan et al. 2002). Nonetheless, the relationship between alcohol intake and insulin sensitivity has been controversial for the last several decades. Although light to moderate alcohol intake may reduce cerebrovascular risk via increased high density lipoprotein-cholesterol (HDL-C) and insulin sensitivity, chronic heavy alcohol intake is closely correlated with onset of insulin resistance syndrome (Vernay et al. 2004). When cells fail to receive sufficient trophic input from insulin or insulin-like growth factors under insulin resistant state, oxidative injury and apoptosis occur (Connor and Dragunow 1998; Ebadi et al. 1997). However, alteration in insulin signaling following alcohol intake is still rather vague especially in brains. Thus the aim of the present study was to examine the impact of chronic alcohol ingestion on insulin signaling cascade with an emphasis on Akt, mammalian target of rapamycin (mTOR) and ribosomal p70s6 kinase in cerebral cortex.
MATERIALS AND METHODS
Experimental animals and chronic alcohol administration
The animal procedures used in this study were approved by our institutional Animal Use and Care Committee. All experimental animals were housed in individual cages under temperature- and circadian (12hr/12hr-light/dark) control with free access to tap water. Three month-old adult male FVB mice were introduced to a nutritionally complete liquid diet (Shake & Pour Bioserv Inc., Frenchtown, NJ, USA) for a one-week acclimation period. The use of a liquid diet is largely based on the notion that ethanol self-administration should not induce nutritional deficiency and stress compared with forced-feeding, intravenous injection and aerosolized inhalation (Keane and Leonard 1989). Upon completion of the 1-week acclimation period, half of the FVB mice were maintained on the regular liquid diet (without ethanol), and the remaining half began a 16-week period of isocaloric 4% (vol/vol) ethanol diet using a pair-feeding regimen. Body weight was monitored at every 4 weeks (Hintz et al. 2003). Levels of blood ethanol were determined by gas chromatography.
Intraperitoneal glucose tolerance test (IPGTT)
Following 16 weeks of ethanol or control diet feeding, mice were fasted for 12 hrs before an intraperitoneal injection of glucose (2 g/kg body weight). Blood glucose levels were determined by clipping the mouse tail immediately before glucose challenge, as well as at 15, 30, 60 and 120 min thereafter. Blood glucose levels were determined using an ACCU-CHEK Advantage Glucose Analyzer (Roche Diagnostics Corporation, IN, USA) (Fang et al. 2005).
Tissue collection and western blot analysis
Following ketamine/xylazine sedation (3:1, 1.32 mg/kg, i.p.), mouse brains were removed (mice were euthanized by cardioectomy). To collect cerebral cortex, a pair of sturdy dissecting scissors was used to cut open the scalp along the longitudinal fissure. After the brain was carefully opened with the membranous tissues removed, cerebellum located at the posterior dorsal region of brain was gently lifted to isolate the two hemispheres of cerebral cortex. Membrane proteins from cerebral cortex were extracted as previously described (Fang et al. 2005). In brief, tissues were homogenized and lysed in a RIPA lysis buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 0.1% SDS, 20 mM NaF, 2 mM Na3VO4 and 1% protease inhibitor cocktail. Samples were then sonicated for 15 sec and centrifuged at 4,500× g for 20 min at 4°C. The protein concentration of the supernatant was evaluated using the Protein Assay Reagent (Bio-Rad, Hercules, CA, USA). Equal amount (50 μg protein/lane) protein and prestained molecular weight marker (Gibco-BRL, Gaithersburg, MD, USA) were loaded onto 10% or 7% SDS-polyacrylamide gels in a minigel apparatus (Mini-PROTEAN II, Bio-Rad), separated and transferred to nitrocellulose membranes (0.2 μm pore size). Membranes were incubated for 1 hr in a blocking solution containing 5% non-fat milk in Tris-buffered saline (TBS) before being washed in TBS and incubated overnight at 4°C with anti-Akt (1:1,000), anti-phospho-Akt (pAkt, Thr308, 1:1,000), anti-mTOR (1:1,000), anti-phospho-mTOR (pmTOR, Ser2448, 1:1,000), anti-p70s6k (1:1,000), anti-phospho-p70s6k (pp70s6k, Thr389, 1:1,000), anti-p4E-BP1 (Thr70, 1:1,000), anti-Caspase-3 (1:1000), anti-IRS-1 (1:500), and anti-IRS-2 (1:1,000) antibodies. Polyclonal antibodies to IRS-1 and IRS-2 were obtained from BD Biosciences (Mississauga, ON, Canada) and Upstate (Charlottesville, VA, USA), the rest antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). Following incubation with the primary antibodies, blots were incubated with either anti-mouse or anti-rabbit IgG HRP-linked antibodies at a dilution of 1:5,000 for 60 min at room temperature. Immunoreactive bands were detected using the Super Signal West Dura Extended Duration Substrate (Pierce, Milwaukee, WI, USA). The intensity of bands was measured with a scanning densitometer (model GS-800; Bio-Rad) coupled with Bio-Rad PC analysis software (Ren et al. 2003). For all western blot analysis, βactin (1:5,000) was used as the loading control.
Data Analysis
Data were presented as Mean ± SEM. Statistical significance (p < 0.05) for each variable was estimated by two-way analysis of variance (ANOVA) or t-test, where appropriate. A Dunnett’s test was used for post hoc analysis when required.
RESULTS
General features of FVB mice following alcohol administration
Chronic alcohol ingestion did not elicit any notable effects on body, heart, liver or kidney weights as well as organ size (normalized to body weight) (Table 1). Following acute intraperitoneal glucose challenge (2 g/kg body weight), the plasma glucose levels in non-alcohol consuming FVB mice started to decline after peaking at 15 min. The plasma blood glucose levels returned to near baseline value after 120 min in non-alcohol consuming mice. In alcohol consuming FVB mice, however, the post-challenge plasma glucose levels continued to rise and peaked at 30 min. The plasma glucose levels remained at much higher levels between 15 and 120 min in alcohol-consuming FVB mice (Fig. 1), indicating glucose intolerance in alcohol consuming FVB mice.
Table 1.
General features of FVB mice with or without 16 weeks of ethanol (ETOH) feeding
| Parameter | FVB (10) | FVB-ETOH (11) |
|---|---|---|
| Body Weight (g) | 29.17 ± 0.82 | 29.37 ± 1.10 |
| Heart Weight (mg) | 190 ± 19 | 191 ± 15 |
| Heart/Body Weight (mg/g) | 6.44 ± 0.54 | 6.48 ± 0.43 |
| Liver Weight (g) | 1.43 ± 0.06 | 1.51 ± 0.06 |
| Liver/Body Weight (mg/g) | 49.25 ± 2.38 | 51.55 ± 1.58 |
| Kidney Weight (mg) | 412 ± 17 | 408 ± 22 |
| Kidney/Body Weight (mg/g) | 14.09 ± 0.35 | 13.84 ± 0.38 |
| Blood Alcohol Level (mM) | 0.00 ± 0.00 | 2.74 ± 0.25* |
Mean ± SEM,
p < 0.05 vs. FVB group, number of mice per group is given in parentheses.
Fig. 1.
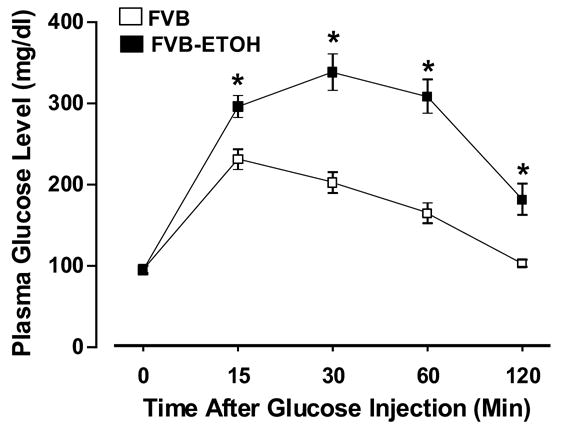
Intraperitoneal glucose tolerance test (IPGTT) displaying plasma glucose concentrations in response to intraperitoneal glucose challenge (2 g glucose/kg body weight) in FVB mice with or without alcohol consumption. The mice were fasted for 12-hr before IPGTT was conducted. Mean ± SEM, n = 6 (FVB) and 16 (FVB-ETOH), * p < 0.05 vs. FVB group.
Protein expression of caspase-3, IRS-1, IRS-2, Akt, mTOR, p70s6k and 4E-BP1
Western blot analysis displayed that chronic alcohol intake enhanced caspase-3 protein expression without affecting expression of IRS-1 and IRS-2 in cerebral cortex (Fig. 2). While total Akt and Akt phosphorylation (pAkt or pAkt/Akt ratio) were not altered by chronic alcohol intake. Expression of the key insulin signaling molecules downstream of Akt - mTOR (Asnaghi et al. 2004) was upregulated without any change in mTOR phosphorylation, which results in a significantly decreased pmTOR-to-mTOR ratio. Our further study revealed that chronic alcohol intake reduced p70s6k phosphorylation without affecting expression of total p70s6k. This is also reflected in the significantly reduced pp70s6k-to-p70s6k ratio in alcohol-fed FVB mouse cerebral cortex (Fig. 3). Last but not the least, the eukaryotic translation initiation factor 4E-BP1, a signaling molecule downstream of mTOR (Aoki et al. 2001), was slightly but significantly reduced in chronic alcohol fed cortex (Fig. 2D).
Fig. 2.
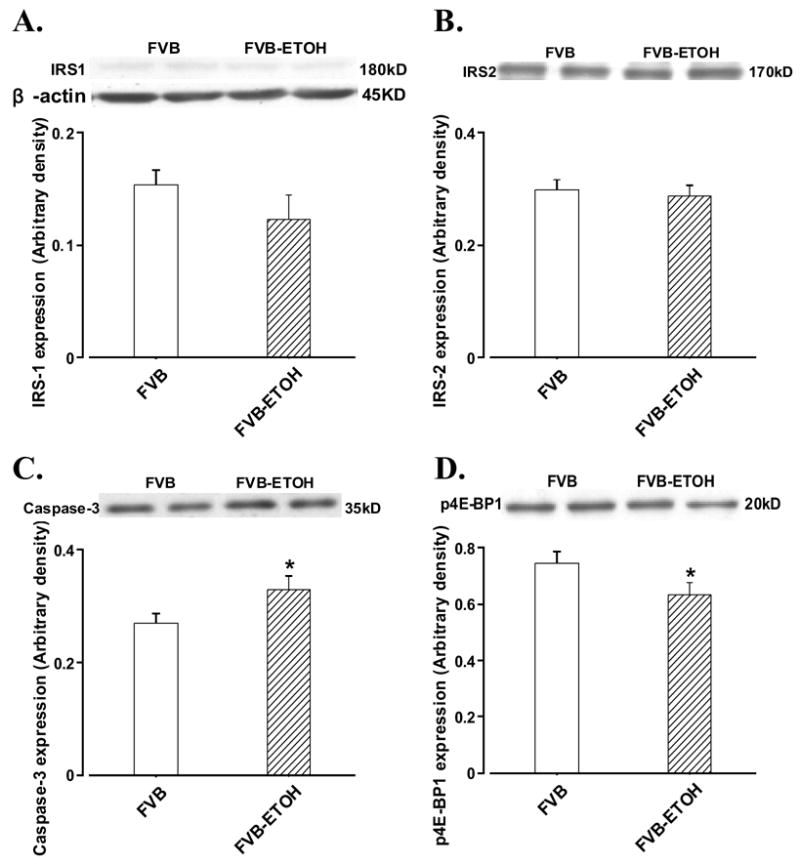
Protein expression of IRS-1, IRS-2, caspase-3 and phosphorylated 4E-BP1 in cerebral cortex from FVB mice with or without alcohol consumption. Panel A: IRS-1; Panel B: IRS-2; Panel C: caspase-3; and Panel D: p4E-BP1. Insets: representative immunoblots of IRS-1, IRS-2, caspase-3, p4E-BP1 and β-actin (loading control) using specific anti-IRS-1, anti-IRS-2, anti-caspase-3, anti-p4E-BP1 and anti-β-actin antibodies; Mean ± SEM. n = 5, * p < 0.05 vs. FVB group.
Fig. 3.
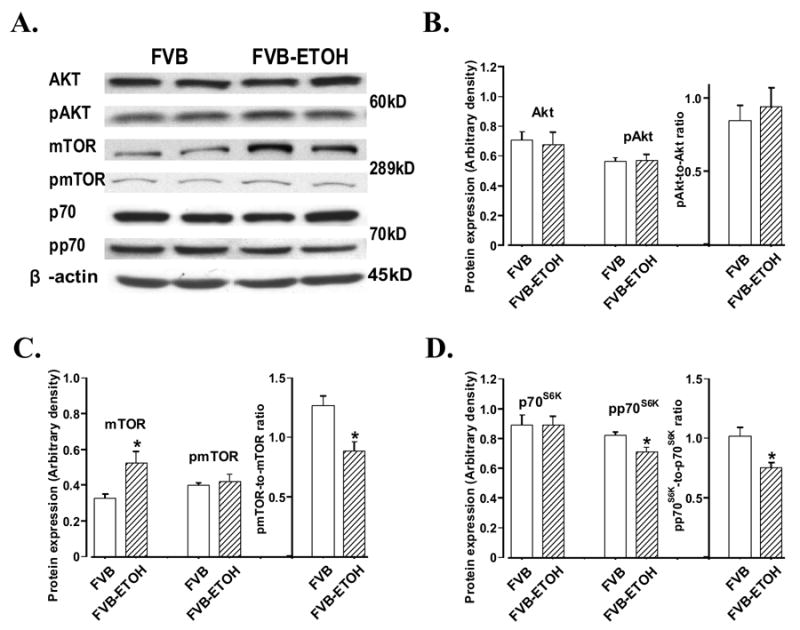
Western blot analysis of total Akt, total mTOR, total p70s6k and their phosphorylation (pAkt, pmTOR and pp70s6k) in cerebral cortex from mice with or without alcohol consumption. Panel A: representative immunoblots of Akt, pAkt, mTOR, pmTOR, p70s6k and pp70s6k using specific antibodies. β-actin was used as the loading control; Panel B: total Akt, pAkt and pAkt-toAkt ratio; Panel C: total mTOR, pmTOR and pmTOR-to-mTOR ratio; and Panel D: total p70s6k, pp70s6k and pp70s6k-to-p70s6k ratio. Mean ± SEM. n = 7 – 9, * p < 0.05 vs. FVB group.
DISCUSSION
The salient findings from our current study include compromised glucose tolerance, enhanced apoptosis and altered post-receptor insulin signaling including mTOR, ribosomal p70s6k and 4E-BP1 in cerebral cortex following chronic alcohol intake. These results support the notion that alcoholism leads to altered insulin sensitivity and brain injury.
Data from our study revealed compromised whole body glucose tolerance, elevated apoptosis seen as caspase-3 expression and altered mTOR/p70s6k/4E-BP1 signaling in cerebral cortex following chronic drinking. Insulin resistance itself has been known to lead to brain injury associated with impaired post-receptor insulin signaling mechanism (Buijs and Kreier 2006). In fact, dampened insulin sensitivity has been implicated as a key early life risk factor for the ultimate development of clinical expression of neurological disorders including Alzheimer disease (Borenstein et al. 2006). Our current experimental data supported that impaired insulin sensitivity and insulin signaling may underscore chronic alcohol intake-elicited cerebral injury. mTOR, a central regulator of ribosome biogenesis, protein synthesis and cell growth, has been speculated to regulate translation machinery in response to amino acids and growth factors, via activation of p70s6k and inhibition of eIF-4E binding protein 4E-BP1 (Asnaghi et al. 2004). All three molecules Akt, mTOR and p70s6k may be activated by insulin, nutrients and growth factors and participate in cell signaling related to cell function, growth and survival (Asnaghi et al. 2004; Fang et al. 2005; Rota et al. 2005). Chronic alcohol intake reduced phosphorylation of mTOR, p70s6k and 4E-BP1, suggesting disrupted insulin signaling at multiple levels following chronic drinking. Our data did not favor an involvement of IRS-1, IRS-2 and Akt in altered insulin signaling mechanism following alcohol intake although contribution from other alternative pathway(s) to alcohol-induced insulin response cannot be ruled out at this time. For example, protein kinase C, which may be activated by ethanol and acetaldehyde (Wyatt et al. 2000), could subsequently activate p70s6k (Ghosh et al. 2004). Mitogen-activated protein kinase (MAPK) pathways may also be turned on by ethanol and exerts an indirect effect on cerebral insulin sensitivity and impair cerebral function (Zhang et al. 2004).
In summary, our present study provided convincing evidence that chronic alcohol intake is associated with impaired glucose tolerance, apoptosis and disrupted mTOR/p70s6k/4E-BP1 signaling. Although data from our current experimental setting did not favor involvement of IRS-1, IRS-2 and Akt in alcohol-induced alteration in mTOR/p70s6k/4E-BP1 signaling, further study is warranted to better understand the cellular mechanisms underlying alcohol-induced loss of insulin sensitivity and the precise contribution of insulin resistance to the pathogenesis of alcoholic neurological damage.
Acknowledgments
This work was supported in part by grants from NIH/NIAAA 1R15AA/HL13575-01 and 1R01 AA013412-01A2 to JR. Neither author declares conflict of interest related to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki M, Blazek E, Vogt PK. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc Natl Acad Sci U S A. 2001;98:136–141. doi: 10.1073/pnas.011528498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnaghi L, Bruno P, Priulla M, Nicolin A. mTOR: a protein kinase switching between life and death. Pharmacol Res. 2004;50:545–549. doi: 10.1016/j.phrs.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:63–72. doi: 10.1097/01.wad.0000201854.62116.d7. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Kreier F. The metabolic syndrome: a brain disease? J Neuroendocrinol. 2006;18:715–716. doi: 10.1111/j.1365-2826.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- Connor B, Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res Brain Res Rev. 1998;27:1–39. doi: 10.1016/s0165-0173(98)00004-6. [DOI] [PubMed] [Google Scholar]
- Ebadi M, Bashir RM, Heidrick ML, Hamada FM, Refaey HE, Hamed A, Helal G, Baxi MD, Cerutis DR, Lassi NK. Neurotrophins and their receptors in nerve injury and repair. Neurochem Int. 1997;30:347–374. doi: 10.1016/s0197-0186(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens. 2001;14:304S–309S. doi: 10.1016/s0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- Fang CX, Dong F, Ren BH, Epstein PN, Ren J. Metallothionein alleviates cardiac contractile dysfunction induced by insulin resistance: role of Akt phosphorylation, PTB1B, PPARgamma and c-Jun. Diabetologia. 2005;48:2412–2421. doi: 10.1007/s00125-005-1940-y. [DOI] [PubMed] [Google Scholar]
- Flanagan DE, Pratt E, Murphy J, Vaile JC, Petley GW, Godsland IF, Kerr D. Alcohol consumption alters insulin secretion and cardiac autonomic activity. Eur J Clin Invest. 2002;32:187–192. doi: 10.1046/j.1365-2362.2002.00970.x. [DOI] [PubMed] [Google Scholar]
- Ghosh PM, Bedolla R, Thomas CA, Kreisberg JI. Role of protein kinase C in arginine vasopressin-stimulated ERK and p70S6 kinase phosphorylation. J Cell Biochem. 2004;91:1109–1129. doi: 10.1002/jcb.10789. [DOI] [PubMed] [Google Scholar]
- Hannuksela ML, Liisanantti MK, Savolainen MJ. Effect of alcohol on lipids and lipoproteins in relation to atherosclerosis. Crit Rev Clin Lab Sci. 2002;39:225–283. doi: 10.1080/10408360290795529. [DOI] [PubMed] [Google Scholar]
- Harper C, Matsumoto I. Ethanol and brain damage. Curr Opin Pharmacol. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Hintz KK, Relling DP, Saari JT, Borgerding AJ, Duan J, Ren BH, Kato K, Epstein PN, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates cardiac contractile dysfunction, lipid peroxidation, and protein damage after chronic ethanol ingestion. Alcohol Clin Exp Res. 2003;27:1090–1098. doi: 10.1097/01.ALC.0000075823.73536.DD. [DOI] [PubMed] [Google Scholar]
- Keane B, Leonard BE. Rodent models of alcoholism: a review. Alcohol Alcohol. 1989;24:299–309. doi: 10.1093/oxfordjournals.alcalc.a044916. [DOI] [PubMed] [Google Scholar]
- Kucera P, Balaz M, Varsik P, Kurca E. Pathogenesis of alcoholic neuropathy. Bratisl Lek Listy. 2002;103:26–29. [PubMed] [Google Scholar]
- Patel VB, Why HJ, Richardson PJ, Preedy VR. The effects of alcohol on the heart. Adverse Drug React Toxicol Rev. 1997;16:15–43. [PubMed] [Google Scholar]
- Ren J, Duan J, Hintz KK, Ren BH. High glucose induces cardiac insulin-like growth factor I resistance in ventricular myocytes: role of Akt and ERK activation. Cardiovasc Res. 2003;57:738–748. doi: 10.1016/s0008-6363(02)00788-5. [DOI] [PubMed] [Google Scholar]
- Rota M, Boni A, Urbanek K, Padin-Iruegas ME, Kajstura TJ, Fiore G, Kubo H, Sonnenblick EH, Musso E, Houser SR, Leri A, Sussman MA, Anversa P. Nuclear targeting of Akt enhances ventricular function and myocyte contractility. Circ Res. 2005;97:1332–1341. doi: 10.1161/01.RES.0000196568.11624.ae. [DOI] [PubMed] [Google Scholar]
- Vernay M, Balkau B, Moreau JG, Sigalas J, Chesnier MC, Ducimetiere P. Alcohol consumption and insulin resistance syndrome parameters: associations and evolutions in a longitudinal analysis of the French DESIR cohort. Ann Epidemiol. 2004;14:209–214. doi: 10.1016/S1047-2797(03)00131-5. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Schmidt SC, Rennard SI, Tuma DJ, Sisson JH. Acetaldehyde-stimulated PKC activity in airway epithelial cells treated with smoke extract from normal and smokeless cigarettes. Proc Soc Exp Biol Med. 2000;225:91–97. doi: 10.1046/j.1525-1373.2000.22511.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li SY, Brown RA, Ren J. Ethanol and acetaldehyde in alcoholic cardiomyopathy: from bad to ugly en route to oxidative stress. Alcohol. 2004;32:175–186. doi: 10.1016/j.alcohol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Pronko SP, Vasiliou V, Gonzalez FJ, Deitrich RA. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin Exp Res. 2006;30:1500–1505. doi: 10.1111/j.1530-0277.2006.00181.x. [DOI] [PubMed] [Google Scholar]


