Abstract
Hematopoietic stem cells (HSCs) can be isolated from murine bone marrow by their ability to efflux the Hoechst 33342 dye. This method defines an extremely small and hematopoietically potent subset of cells known as the side population (SP). Recent studies suggest that transplanted single SP cells are capable of lymphohematopoietic repopulation at near absolute efficiencies. Here, we carefully reevaluate the hematopoietic potential of individual SP cells and find substantially lower rates of reconstitution. Our strategy involved the cotransplantation of single SP cells along with different populations of competitor cells that varied in their self-renewal capacity. Even with minimized HSC competition, SP cells were only able to reconstitute up to 35% of recipient mice. Furthermore, through immunophenotyping and clonal in vitro assays we find that SP cells are virtually homogeneous. Isolation of HSCs on the basis of Hoechst exclusion and a single cell-surface marker allows enrichment levels similar to that obtained with complex multicolor strategies. Altogether, our results indicate that even an extremely homogeneous HSC population, based on phenotype and dye efflux, cannot reconstitute mice at absolute efficiencies.
Introduction
All mature blood cell lineages in the hematopoietic system derive from hematopoietic stem cells (HSCs). Decades of work in the hematopoietic field have established that bone marrow (BM)–derived HSCs can self-renew and differentiate into multiple hematopoietic lineages at the clonal level.1-3 Thus, the study of HSCs has proven to be central not only in the understanding of basic hematopoietic development but also in the clinical translation of these studies. Both basic and translational research would be greatly benefited by the identification, purification, and characterization of a homogeneous transplantable HSC population.
In the last few years, a great deal of effort has been dedicated toward the development of protocols for the prospective isolation of HSCs using cell-surface markers. In the mouse, the vast majority of hematopoietic activity resides within the rare c-Kit+Sca-1+Lin– (KSL) BM population.4,5 Particularly, selection of a small fraction of KSL cells that have low to negative expression of the CD34 glycoprotein allows for the enrichment of an extremely small and homogeneous population of long-term (LT)–HSCs.3 In a remarkable feat, when single KSL-CD34–/low cells were transplanted into lethally irradiated mice, approximately 25% of the recipients showed long-term multilineage clonal reconstitution.3 Thus, the KSL-CD34–/low hematopoietic subset, with a BM frequency of approximately 0.004%, has been considered a near-to-homogeneous HSC population since this report was published.
We have previously described a method for isolating HSCs based on their differential efflux of the fluorescent dye Hoechst 33342.6 Murine BM cells isolated using this approach—also known as side population (SP) cells—are highly enriched for long-term reconstituting activity in competitive repopulation assays.6 Hoechst dye efflux has also been used to purify primitive stem cells from different organisms, tissues, and developmental states.7-9 Thus, in contrast to cell-surface markers, Hoechst efflux seems to be a physical property that correlates with a general state of “stemness” and could potentially mark the HSC with the most potent self-renewal activity. However, the actual HSC content on a per-cell basis of the SP is still unknown. Furthermore, the relationship between SP cells and HSCs isolated on the basis of cell-surface markers3-5 has not been thoroughly described. A further understanding of the hierarchical relationships among HSCs purified by different groups is necessary in order to establish a definitive protocol for the isolation and study of the most primitive LT HSCs.
Recently, Matsuzaki et al10 reported that a combination of dye-efflux and cell-surface markers could be used to purify an even more homogenous HSC population. Remarkably, it was reported that 96% of mice given transplants of a single CD34–KSL-SP cell exhibited long-term multilineage hematopoietic engraftment. These results were interpreted as reflecting an unexpected absolute efficiency in the homing ability of purified HSCs.10 These data, however, contradict previous engraftment results obtained after transplantation of single HSCs isolated by very comparable parameters to those used by Matsuzaki and colleagues.3,11-13 In all of these reports, the engraftment rates achieved never exceeded 30%. Moreover, the probability of any individual HSC to reach and “seed” the bone marrow, when injected intravenously, has previously been calculated to be at best 10% to 20%,14-16 which is greatly exceeded by the Matsuzaki report.
Here, we perform a detailed analysis of SP cells by multicolor flow cytometry and single-cell transplantation. We demonstrate that the Hoechst-effluxing ability of HSCs is directly correlated to their capacity to self-renew and that all the long-term blood reconstituting ability resides within the SP compartment. Additionally, we show that the SP cells with the highest dye-efflux activity (SPlow) contain the highest LT-HSC activity and are remarkably homogeneous for expression of surface receptors, including the CD34 antigen. Nevertheless, after injection of single SPlow cells, no more than 36% of the recipient mice showed stable hematopoietic engraftment. Our results challenge the notion that HSCs, even homogeneous for both cell-surface markers and dye efflux, may seed marrow with absolute efficiency. Importantly, by showing that HSC homing is not absolute, our work suggests that this capacity can be experimentally enhanced.
Materials and methods
Mouse strains
C57Bl/6-(CD45.2), C57B/6-(CD45.1), and C57Bl/6-(CD45.1/45.2) heterozygous mouse strains were bred and maintained at the animal care facility at Baylor College of Medicine. We are grateful to Dr Irving Weissman of Stanford University School of Medicine for providing C57Bl6/Ka-Thy1.1 mice. Cd34–/– mice (on a C57Bl/6 background) were kindly provided by Dr T. W. Mak (Ontario Cancer Institute, Toronto, ON, Canada).17 For primary transplantation studies donor mice were 7 to 10 weeks of age. Irradiated recipient mice were 5 to 8 weeks old. All mice were maintained on acidified water.
Antibodies
The antibodies used for immunofluorescence detection included 104FITC (anti-CD45.2), A20PE (anti-CD45.1), 2B8APC (anti–c-kit), E13PE (anti–Sca-1), 19XE5FITC (anti-Thy1.1; kindly provided by Dr Irving Weissman), LG.3A10PE (anti-CD27), MEC13.3bio (anti–PECAM-1), RAM34FITC (anti-CD34), A2F10.1PE (anti–Flk-2), AA4.1FITC (anti-AA4), 2B11bio (anti-CXCR4), and HA2/5bio (anti–B1-integrin). All antibodies are from Pharmingen (San Diego, CA) except were noted, and were typically used at concentrations of 0.1 μg to 0.2 μg per million BM cells. Lineage markers included 17A2 (anti-CD3), GK1.5 (anti-CD4), 53-6.7 (anti-CD8), M1/70 (anti–Mac-1), RA3-6B2 (anti-B220), RB6-8C5 (anti–Gr-1), PK136 (anti-NK1.1), and TER-119 (anti–erythrocyte-specific antigen). All lineage antibodies were directly conjugated to Cychrome (eBioscience, San Diego, CA). For the Mac-1/CD4 fractionation studies, we used anti–Mac-1PE and anti-CD4bio. Biotin-conjugated antibodies were detected by subsequent staining with either streptavidinPE or streptavidinAPC (Molecular Probes, Eugene, OR).
Bone marrow preparation and SP staining
Marrow was flushed from the tibias and femurs of donor mice. Cells were suspended at 106 cells/mL in Dulbecco modified Eagle medium (DMEM) supplemented with 2% fetal calf serum (FCS)/10 mM HEPES (HyClone, Logan, UT; and Life Technologies, Carlsbad, CA; respectively) and stained with 5 μg/mL Hoechst 33342 (Sigma-Aldrich, St Louis, MO) for 90 minutes at 37°C, as described previously.6 The cells were then resuspended in cold Hanks balanced salt solution (HBSS) containing 2% FCS and 2 μg/mL propidium iodide. Sorting and analysis of SP cells were performed on a triple-laser instrument (MoFlow; Cytomation, Fort Collins, CO). SP cells, as isolated, consistently accounted for 0.04% to 0.05% of whole bone marrow–nucleated cells. For characterization studies, antibody staining always followed Hoechst incubation.
Cell sorting and transplantation
Single SP Sca-1+ cells were sorted directly into individual wells of a flat-bottom 96-well plate containing 150 μL of StemPro buffer (Gibco BRL, Gaithersburg, MD) by a single-cell deposition unit (Cytomation). A preliminary experiment was undertaken to verify the accuracy of single-cell sorting. Careful examination of more than 300 wells sorted with single fluorescent cells demonstrated that no well contained more than 1 cell. Approximately 30% of the wells contained no cells, whereas the other 70% to 75% contained individual single cells. For single-cell transplantations, the contents of every sorted well were transplanted without previous visualization of cells inside the well. Thus, approximately 25% to 30% of the single-cell recipients were actually given transplants with no cells. For transplantations with higher numbers of cells, donor cells were directly sorted into separate wells of a 96-well plate containing 300 μL of buffer and the corresponding number of competitor cells (105 CD45.1 whole bonemarrow cells). For sorting of KLS-SP, KLS-nonSP, and Mac-1 subfraction cells were double sorted to obtain precise number of cells that were more than 97% pure for the parameters analyzed.
Transplantation assays and peripheral blood analyses
Congenic recipient mice were lethally irradiated (11 Gy) with γ irradiation in a split dose, with 3 hours between doses. The reconstituting cells were injected retro-orbitally within 24 hours of irradiation. In order to maximize the recovery of cells from the single-cell–sorted wells, we first aspirated the contents of each well and then washed the same well with 150 μL buffer containing the corresponding number of competitor cells. The volume in the well is then re-aspirated and injected. For transplantation into secondary recipients, bone marrow from primary recipients was harvested and stained with the antibody against CD45.2. Sorted 2 × 105 CD45.2+ cells were then mixed with 2 × 105 freshly isolated CD45.1 cells and injected into a lethally irradiated CD45.1/CD45.2 recipient. At least 4 different secondary hosts were given transplants of clonally derived marrow from individual single-cell–recipient mice.
Peripheral blood analysis was performed as previously described.18 At various time points after transplantation, 200 μL peripheral blood was collected from the retroorbital plexus of anesthetized transplant recipients. Nucleated cells were simultaneously stained with both CD45.1 and CD45.2 antibodies. Very low background signals (< 0.03%) were observed when using this double-staining procedure in negative control mice. Mice were considered positive for engraftment when they showed greater than 0.1% chimerism. For lineage analysis, samples were costained with anti-CD45.2 and the antibodies against B220 or CD3, or Gr-1 and Mac-1. Stained blood samples were then analyzed by flow cytometry by using a 2-laser instrument, FACScan (Becton Dickinson, Mountain View, CA).
Methylcellulose cultures
Single SPlow Sca-1+ cells were sorted in individual wells of a flat-bottom 96-well plate containing 100 μL Methocult m3434 (StemCell Technologies, Vancouver, BC, Canada). Colony number was assessed 12 days after sorting.
Real-time PCR analysis
1250 SP-CD34– or SP-CD34+ cells obtained after staining BM with 2.5 μg RAM34/106 cells were sorted into 50 μL lysis buffer. The lysis buffer consisted of 152 μL nuclease-free water, 40 μL 5 × strand buffer, 2 μL Prime RNase inhibitor (Eppendorf, Hamburg, Germany), 2 μL RNA Guard inhibitor (Amersham, Bucks, United Kingdom), 1 μL NP-40, and 3 μL diluted random primer mix. Superscript II (2.5 μL; Invitrogen, Carlsbad, CA) was added to the lysis buffer followed by incubation at 42°C for 1 hour. Cells were assayed for gene expression immediately after sorting. Following reverse transcription, multiplexed real-time polymerase chain reactions (PCRs) were performed containing 25 μL TaqMan universal PCR master mix, 2.5 μL 18S rRNA endogenous control (VIC/MGB), 2.5 μL CD34 TaqMan gene expression assay (FAM-MGB), and 16 μL nuclease-free water. The final PCR reaction contained 100 cell equivalents of DNA in 4 μL of lysis buffer. Fold changes were calculated using the ΔΔCt method according to the manufacturer's instructions (Applied Biosystems, Foster City, CA).
Statistical analysis of results
All comparisons were performed with a 2-sided Student t test unless otherwise specified.
Results
Dye efflux as a marker for long-term stem cells
We first examined whether all LT-HSCs in the mouse BM were contained in the Hoechst-effluxing subset. In order to do this, we focused on the overlap of the highly characterized KSL population and the SP cells. Previous studies have shown that KSL cells contain most, if not all, of the hematopoietic activity in murine BM.4,5 As shown in Figure 1A, the KSL compartment (comprising about 0.1% nucleated BM cells) can be further subdivided into KSL-SP and KSL non-SP fractions. The KSL-SP subset usually comprised approximately 0.02% to 0.03% of the BM, whereas the KSL-nonSP made up 0.07% to .08% of nucleated marrow cells. We analyzed the reconstituting activity of these cell compartments by competitive transplantation assays using limiting cell numbers. Cells were sorted from CD45.2 mice and transplanted along with 1 × 105 whole (W) BM cells from congenic CD45.1 mice into lethally irradiated CD45.1 recipients. When 10- and 20-cell doses of both subpopulations were transplanted, we were only able to detect significant engraftment in animals transplanted with KSL-SP cells (Figure 1B). Engraftment by KSL–non-SP cells was always lower than background levels (< 0.03%). Even larger numbers (60) of non-SP cells produced only very low levels of engraftment that tended to decline with time, indicating that this population is composed of only short-term progenitors (Figure 1B). These results strongly suggest that within a population highly purified on the basis of surface markers, only cells that actively efflux Hoechst dye are capable of long-term self-renewal. Therefore, SP cells are the active subset of the widely used KSL BM population.
Figure 1.
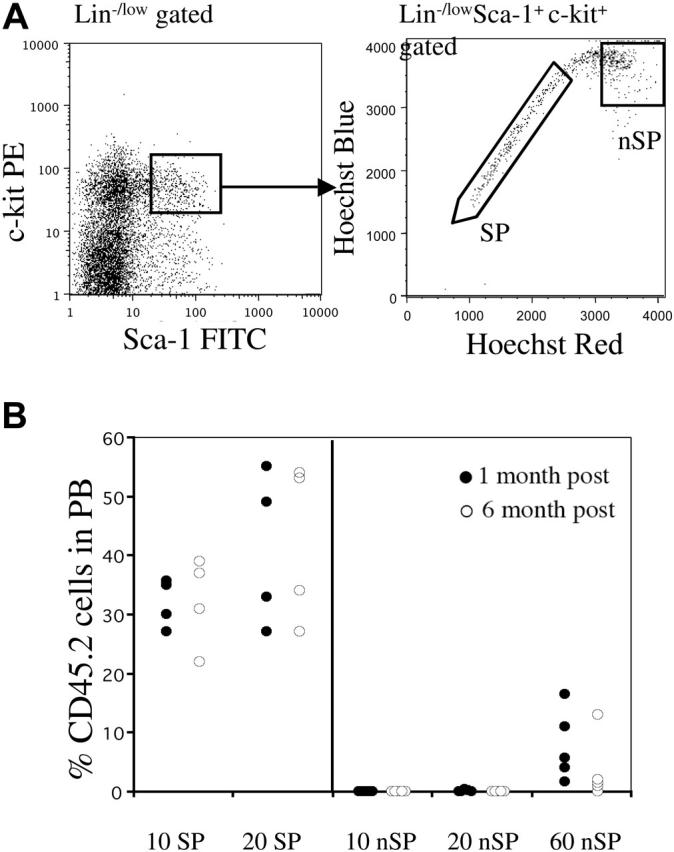
Only SP cells within the KSL population contain LT-HSCs. (A) Whole bone marrow was stained with antibodies against c-Kit, Sca-1, and lineages. The KSL population represented approximately 0.1% of nucleated cells. Right panel shows Hoechst profile of KSL-gated cells. Approximately 25% to 35% of cells fell into the SP gate. Regions marked as SP and non-SP (nSP) were sorted and then re-sorted for transplantation experiments. (B) Reconstitution profiles of mice that received transplants of limited cell numbers of KSL-SP or KSL non-SP cells along with a radioprotective dose of 1 × 105 host-type whole bone marrow cells. Peripheral blood chimerism is shown at 1 month (•), or 6 months (○) after transplantation.
Dye efflux directly correlates with extent of self-renewal
We showed that the vast majority of murine LT-HSCs have an SP phenotype; we next wanted to determine if all SP cells were in turn LT-HSCs. It was previously demonstrated that the SP cells with higher levels of Hoechst efflux (lower SP) had greater long-term reconstituting potential than cells in the upper SP.7 These observations suggested that the extent of dye efflux could be directly correlated with the ability of HSCs to self-renew in vivo. Therefore, we evaluated SP cells for the expression of 2 lineage markers, Mac-1 and CD4, which have previously been associated with a reduction of self-renewal capacity in vivo.4 Interestingly, we found that Mac-1 and CD4 expression in SP cells varies along the Hoechst gradient (Figure 2A). In the region of highest dye efflux, 75% to 85% of the cells were Mac-1 negative (R1, Figure 2A), with the remainder Mac-1low. Conversely, only 25% to 33% of SP cells in the region of lowest dye efflux were Mac-1–negative cells (R3, Figure 2A). CD4 expression, although less dramatic, also tended to increase with decreased Hoechst efflux (Figure 2A). Furthermore, in order to determine whether there were functional differences between the Mac-1low and Mac-1– subsets within the SP population, we tested their in vivo long-term repopulating ability in lethally irradiated congenic recipients. All Sca-1+ SP cells from CD45.2 donor mice were gated and the expression of Mac-1 was used to sort for Mac-1low and Mac-1– populations. Low numbers of donor cells (20 or 60) were transplanted along with a radioprotective dose (1 × 105 cells) of host-type CD45.1 BM and peripheral blood (PB) was analyzed periodically for CD45.2 chimerism. As shown in Figure 2B, the majority of lethally irradiated mice that received transplants of 20 Mac-1– SPlow (high efflux) cells showed high levels of hematopoietic engraftment (mean 43.8% at 18 weeks, n = 8). This repopulation was stable, as donor-derived chimerism could be detected more than 5 months after transplantation. However, reconstitution by Mac-1low SPhigh (low efflux) cells was largely short term and disappeared by 2 months after injection in the majority of recipients (mean, 6.4% at 18 weeks, n = 10) (Figure 2B). These findings indicate that both long- and short-term repopulating cells are contained within the SP population and their extent of self-renewal correlates with their dye efflux activity.
Figure 2.
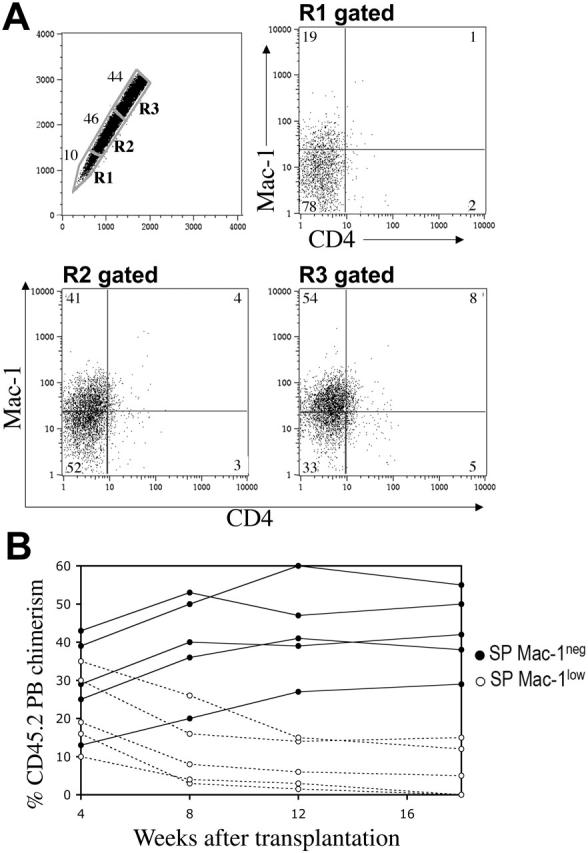
Dye efflux directly correlates with self-renewal. SP cells from Sca-1–enriched bone marrow (A) were stained with anti–Mac-1PE and anti-CD4APC antibodies. When the SP is subdivided into the geometrically similar regions R1 to R3, the percentage of cells in R1 is lowest, as indicated. Mac-1 and CD4 expression on the gated subpopulations is shown. (B) All CD45.2 Sca-1+ SP cells, independent of their R gate status, were sorted on the basis of Mac-1 expression and transplanted into CD45.1 lethally irradiated recipients along with 1 × 105 CD45.1 WBM cells. A representative analysis of 2 different experiments is shown from animals transplanted with 20 Mac-1– (•) or 20 Mac-1low (○) SP cells. Engraftment values from the 2 different populations are statistically different (P < .005), except for the 4-week time point (P < .3, t test).
Phenotype and in vitro clonogenicity of SPlow cells
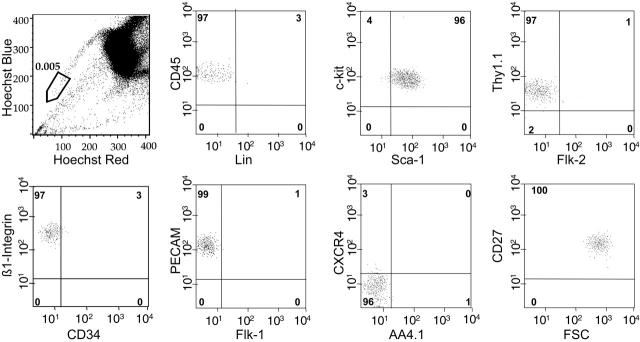
The studies described indicated that the SP cells with the highest dye efflux (R1 in Figure 2; SPlow) were highly enriched for LT-HSC activity. SPlow cells compromise approximately 10% of the entire SP and account for a frequency in whole BM of approximately 0.005%. Whereas the whole SP has been shown to be phenotypically a fairly homogeneous population7 (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article), SPlow cells are nearly 100% homogeneous for all relevant cell-surface markers tested so far (Figure 3). Furthermore, as well as having the KLS phenotype, SPlow cells are Thy1.1low, CD34–/low, and Flk2–, indicating that the phenotype of this population is highly overlapping with HSCs purified exclusively on the basis of multiple cell-surface markers.3,4,19,20
Figure 3.
SPlow cells are phenotypically a homogeneous population. SPlow cells as gated in the top left panel usually represented the lowest 10% of SP cells in a given sort, with a bone marrow frequency of approximately 0.005% to 0.007%. Cell-surface marker expression on more than 500 SPlow cells was analyzed, with the percentages shown in the relevant quadrants. A representative analysis, out of at least 3 performed for each marker, is exhibited.
In order to determine whether homogeneity in phenotype translated to functional homogeneity, we analyzed the hematopoietic potential of individual SPlow cells in vitro. We compared directly the clonogenic potential of single SPlow cells with that of KLS CD34–/low cells, as this represents the most homogeneous isolatable HSC population to date. Single SPlow or KLS34 cells were sorted into wells of a 96-well plate containing methylcellulose supplemented with interleukin-3 (IL-3), IL-6, and steel factor. As shown in Table 1, around 67% and 74% of wells sorted with single SPlow or KLS34 cells, respectively, contained large multilineage colonies (> 103 cells) containing granulocytes, macrophages, and sometimes erythroid cells. Interestingly, when we sorted KLS34 cells that had also been stained with Hoechst, around 65% of the wells were clonogenic, indicating that Hoechst dye is slightly toxic to HSCs. These results demonstrate that cells isolated simply on the basis of Hoechst efflux and a single marker, Sca-1, have comparable clonogenic potency to a highly purified cell subset isolated on the basis of more than 9 cell-surface markers. Thus, isolating SPlow cells with the addition of Sca-1 is a very practical and simple method for HSC purification.
Table 1.
Clonogencitiy of SPlow and KLS34- cells
| Sorted cell type | No. wells sorted | Clonogenicity, % |
|---|---|---|
| SPlow | 194 | 67 |
| KLS CD34- | 90 | 74 |
| KLS CD34- (+ Hoechst) | 56 | 65 |
Single SPlow Sca-1+ cells were sorted in individual wells of a flat bottom 96-well plate containing 100 μL Methocult m3434. Colony number was assessed 12 days after sorting. Two-tailed P values with Fisher exact test: SPlow compared with KLS34- (P < .2); KLS34- compared with Hoechst (P < .7); KLS34- compared with KLS34- with Hoechst (P < .2).
Long-term multilineage hematopoietic reconstitution by a single SPlow cell
We next wanted to test the homogeneity of SPlow cells at a functional level in vivo. The gold standard for purity at this level is single-cell transplantation. In this assay, single HSCs are isolated by FACS and individually injected into lethally irradiated mice. In order to guarantee the survival of the hosts during the lag phase between the injection of the donor HSCs and the development of single-cell–derived mature blood cells, a carrier population is co-injected with the single cell. We used different carrier populations that were genetically distinguishable from the donor HSCs. In the first experiment, 2 × 105 CD45.1 BM cells were transplanted along with a single SPlow CD45.2 cell into lethally irradiated CD45.1 mice. We observed long-term reconstitution (LTR) in about 5% (5 of 95) of the recipient mice (Figure 4A). LTR was defined as stable donor-derived chimerism in PB for more than 4 months. The degrees of hematopoietic contribution by a single SP cell are shown in Figure 4. For the second experiment, we used a carrier population depleted of Sca-1+c-kit+ hematopoietic progenitors. The frequency of donor-derived reconstitution increased notably, with 11 (15.7%) of 70 recipient mice showing detectable LTR (Figure 4A). The overall levels of hematopoietic chimerism in this group of mice also increased, averaging 14% at 6 months after transplantation (Figure 4). In experiment 3, single CD45.2 SPlow cells were transferred with 600 to 1000 Lin–/lowSca-1+c-kit+CD34+ CD45.1 cells. This population has been previously shown to contain short-term hematopoietic progenitors with limited self-renewal capacity,3 thus alleviating the competition of the single donor cell with other LT-HSCs. Using this approach, we obtained LTR in approximately 25% (13 of 53) of recipient mice (Figure 4A) with the majority of recipients in this cohort displaying high levels of hematopoietic engraftment (average, 29.8%). Long-term follow-up of these mice as represented in Figure 4B demonstrates the stability of the hematopoietic clones. In addition, all of these mice showed clonal reconstitution in the myeloid, B-, and T-cell compartments that maintained a constant level for more than 9 months (Figure 4C). Secondary and tertiary transfers of single SP-derived cells resulted in repopulation of lethally irradiated congenic hosts (data not shown).
Figure 4.
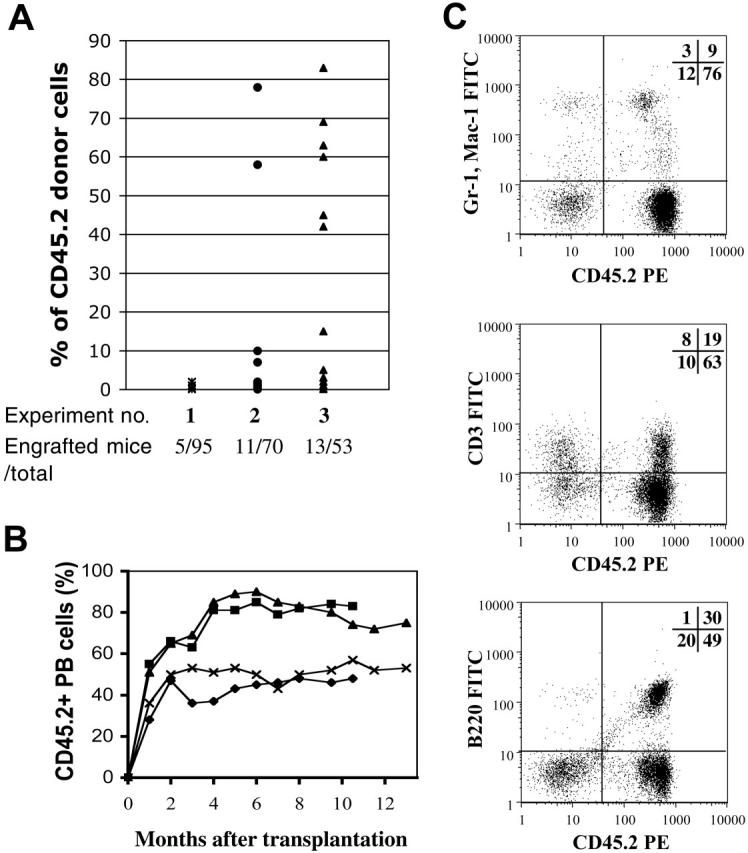
Long-term multilineage reconstitution by a single SP. Single SPlow cells as shown in Figure 3, also selected for Sca-1 expression, were sorted into wells of a 96-well plate and transplanted into lethally irradiated recipients. (A) Carrier cells for the different transplantation experiments were as follows: (1) 2 × 105 WBM; (2) 1-3 × 105 Sca-1+ c-Kit+ depleted; and (3) 600 or 1000 Lin–Sca-1+ c-Kit+ CD34+. Donor chimerism was determined as the percentage of CD45.2+ cells of the total number of CD45+ peripheral blood cells. Dots represent engrafted mice at 6 months after single-cell transfer. (B) Representative long-term follow up of 4 single HSC transplant recipients. Chimerism in peripheral blood was assessed by CD45.2 staining. Each line represents an individual mouse. (C) Lineage analysis in 1 representative chimera with 85% PB contribution 4 months after one HSC transplantation demonstrates contribution to the myeloid (top), T-cell (middle), and B-cell (bottom) lineages. Numbers represent the percentage of cells in each quadrant.
Given that approximately 30% of the wells “sorted” with a single cell are empty (“Materials and methods”), and the contents of every single well were transplanted without previous visualization, we could argue that only 70% of the recipient mice received a viable SPlow cell. Therefore, under optimal conditions (Figure 4A, experiment 3) single SPlow cells are able to engraft long term in approximately one third (35%) of lethally irradiated recipients. Although these frequencies compare favorably with the 20% obtained by Weissman and colleagues,13 the 22% by the Nakauchi group,3 and the 33% of Uchida et al21, they are nowhere near the 96% obtained by Matsuzaki et al.10
SP cells are homogeneous for the expression of CD34
We originally had reported that SP cells were essentially negative or very low for the expression of the CD34 antigen. However, Matsuzaki et al,10 using CD34 antibody concentrations 5 to 10 times higher than what used in our study, reported that SPlow cells could be fractionated into distinct CD34– and CD34+ populations. Furthermore, they showed that these cells were functionally distinct in that CD34– cells gave near quantum reconstitution of recipients. Therefore, we decided to reevaluate the expression of CD34 by our SP cells by 2 different strategies.
In the first experiment, we used different concentrations of the anti-CD34 antibody (clone RAM34) in order to see if higher antibody (Ab) titers allowed us to distinguish a rare CD34+ population. As shown in Figure 5A, only Ab concentrations of 2.5 μg/106 cells produced a positive fluorescent shift in the SP cells. Ab concentrations of 1 μg or 0.2 μg per million cells, determined to be the correct antibody titer by staining 3T3 cells which express CD34 at high levels (data not shown), did not produce the CD34+ population observed. The 2.5 μg/106 cells staining Ab concentration needed to observe the CD34+ population is 25-fold higher than the one routinely used in our lab of approximately 0.1 to 0.2 μg/106 cells for all other antigens expressed in BM cells. Therefore, in order to test whether the appearance of this population was due to nonspecific binding of the RAM34 antibody at elevated concentrations, we decided to stain SP cells from CD34-deficient mice. Staining of Cd34–/– SP cells with RAM34 at high concentrations, 2.5 μg/106 cells, produced a small but significant positive shift compared to the isotype, 1 μg or 0.2 μg/106 cells antibody controls (Figure 5A). Even though the shift was not as dramatic as that obtained with normal SP cells, the fold increase in the ratio of median fluorescence intensity (MFI) between the 2.5 μg and 1 μg samples was similar: 2.23 (range, 1.9-2.7, n = 4) versus 1.85 (range, 1.7-2.0, n = 2) for wild type and Cd34–/– SP cells, respectively (Figure 5A). Since the appearance of the “pseudo”-positive CD34 population seemed to be an artifact of elevated antibody concentrations, we thus expected that there would not be any difference in expression of CD34 mRNA between the 2 populations defined by RAM34. In order to test this, we analyzed by real-time quantitative PCR the CD34 mRNA expression levels of sorted KSL-SP CD34– and KSL-SP CD34+ subfractions of wild-type BM stained with 2.5 μg/106 cells RAM34. SP cells were sorted according to the gates depicted in Figure 5B. Re-analysis of the sorted subfractions revealed populations enriched for either CD34– or CD34+ cells (Figure 5C). Quantitative PCR analysis on these 2 populations revealed no significance difference in the expression of CD34 mRNA, compared with a ubiquitously expressed 18S RNA, in 3 different experiments (Figure 5D). Altogether, these results indicate that SP cells express low levels of CD34, and that the CD34+ SP cells observed by others, likely represent a staining artifact
Figure 5.
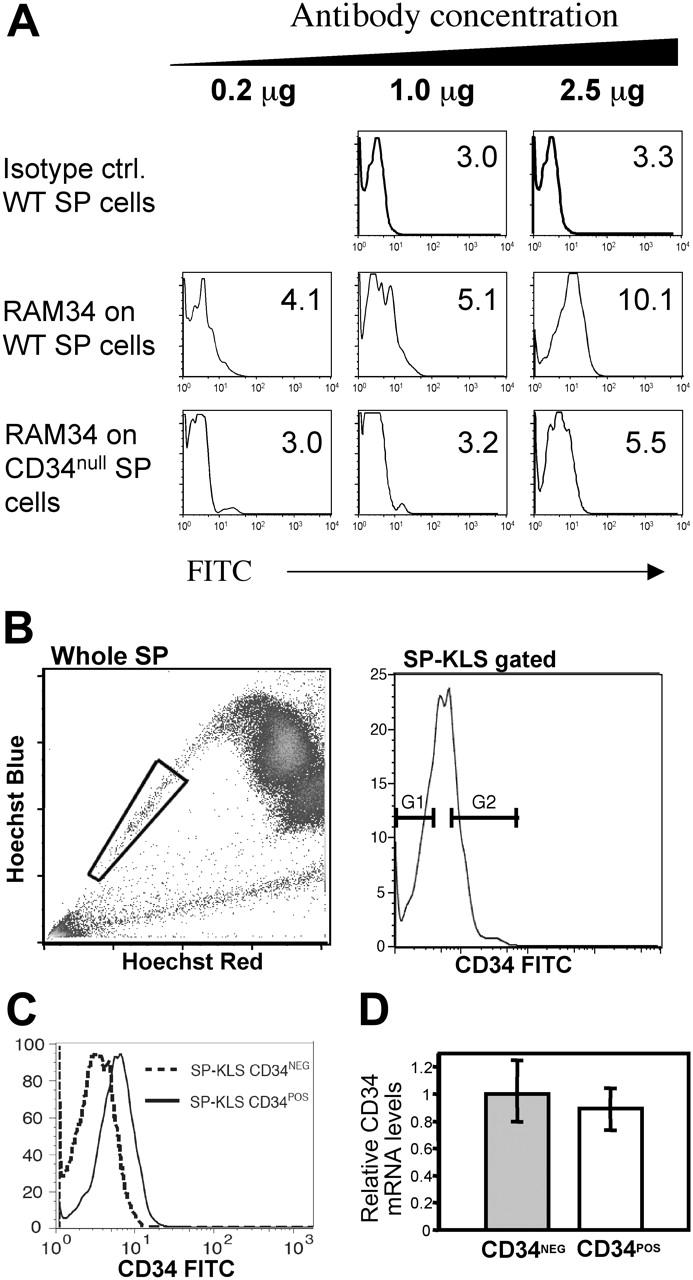
SP cells are homogeneous for CD34 expression. (A) Nonspecific staining at high concentrations of RAM34 antibody. Wild-type (WT) and CD34-null BM cells were stained with Hoechst and subsequently stained with standard concentrations (0.2 μg) of anti-lineage, Sca-1, c-kit, and the indicated concentrations of RAM34-FITC. Histograms shown are gated on KLS-SP cells. Numbers within each panel represent the mean fluorescent intensity (MFI) for the FITC channel. (B) No difference in CD34 mRNA expression within SP cells. Whole bone marrow from wild-type mice (top left) was stained with Hoechst and antibodies against c-kit, Sca-1 lineage markers, and RAM34-FITC at 2.5μg/106 cells. SP cells (gated as in left panel) that were lineage–, c-kit+, and Sca-1+ were displayed for CD34 and sorted with the gates shown (top right). (C) Representative reanalysis of sorted CD34– and CD34+ cells. (D) CD34 mRNA levels of sorted populations were analyzed by real-time PCR. Error bars represent SD. P < .5 (t test).
Discussion
The HSC literature abounds with different purification protocols and suggestions of incremental advantages by the additional use of a wide variety of markers.3,22-24 A neophyte could find it difficult to distinguish between these “best methods,” all assayed under slightly different conditions. Here, we have directly examined many of these markers on SP cells and show that these purification strategies are highly overlapping. Our studies demonstrate that SP cells are phenotypically Lin–/lowSca-1+c-kit+Thy1.1low CD34–/lowFlk2– and thus are, in essence, the same HSC population isolated by other groups.3-5,22 Furthermore, our data suggest that isolating SPlow cells with the addition of a single marker such as Sca-1 would be a very practical and simple method leading to the purification of a very homogenous population that would carry all of the markers associated with HSCs. In addition, our results demonstrating the existence of Mac-1low ST-HSCs within the upper SP are in direct agreement with previous data demonstrating that up-regulation of Mac-1 in the KLS compartment marked loss of self-renewal capacity and thus defined HSCs with a short-term in vivo life span.4 The comparable bone marrow frequencies of the SP Mac-1low and KTLS Mac-1low populations (0.02%-0.03% and 0.04%, respectively) and their levels and patterns of in vivo reconstitution strongly suggest that these populations are also functionally equivalent.4 In summary, the murine BM-SP population contains phenotypically identifiable short- and long-term hematopoietic stem cells that bear the same antigens described by others. In addition, these data, along with our original report,7 suggest that dye efflux is a property that directly correlates with the ability of given stem cells to self renew.
Two very recent reports have also analyzed the phenotypic and functional relationships between the SP and other well-characterized antigen-defined stem cell subsets.10,25 Whereas the report by Bonnet and colleagues25 demonstrates that the vast majority of SP cells possessed the primitive stem cell phenotype described here, data by Matsuzaki et al10 indicate that SP cells are largely CD34+. The reason for the increased frequency of CD34+ cells in the Matsuzaki report might be explained by the unusually high concentration of anti-CD34 antibody used in this study, which is 5 to 10 times higher than that used by our lab and others. Our staining data here using CD34-deficient cells indicates that high concentrations of the RAM34 antibody may be partly responsible for this discrepancy, potentially indicating cross-reactivity with another antigen. This is corroborated by our quantitative PCR results. Furthermore, data from several other studies evaluating the expression of CD34 in murine BM cells support our claim that the SP cells are negative to low for the expression of CD34.7,25,26
Our present study shows that SPlow bone marrow cells are both antigenically and functionally homogeneous. Since only 10% to 20% of injected stem cells home to bone marrow,14-16 our work indicating that approximately 35% of single-transplanted SPlow cells generate stable lymphohematopoietic grafts strongly suggests that all cells within this population are capable of engraftment, provided successful migration to the marrow. Using a similar single-cell transplantation strategy, Matsusaki and colleagues have recently reported an unprecedented 96% engraftment rate with an average chimerism of 50% in animals given transplants of single SPlow KLS CD34– stem cells.10,25 The very high levels of engraftment in these experiments are particularly surprising given that the number of carrier cells used, 2 × 105 WBM, should have contained at least 10 equally potent HSCs (assuming a 0.005% BM frequency of their SPlow CD34–KLS). Thus, the expected levels of single-cell–derived engraftment should in theory be 10%, much less than the chimerism reported. Additionally, it is equally puzzling that in the same study, transplantation of 100 CD34–Sca-1+SP (which should contain at least 20 SPlow cells) achieved mean engraftment values of only approximately 35%, significantly less than the average chimerism obtained from single cells. Another recent study using single SP cell transplants found that only 33% of mice that received transplants of a single SP Lin– Rhodaminelow were reconstituted long term.21 Even though these studies cannot directly be compared due to the different sorting parameters for SP cells and the use of the more sensitive c-kitW41 mice as recipients, the data by Uchida et al21 and others3,13 further support our claim that purified HSCs do not reconstitute mice at absolute efficiencies.
Hematopoietic engraftment after stem cell transplantation constitutes a multifactorial process that includes homing, extravasation, anchorage, proliferation, and differentiation. In this context, Benveniste and colleagues recently reported that although single HSCs produce short-term grafts at absolute efficiencies,23 only 33% of cells sustain permanent engraftment. Even though these results produced similar long-term reconstitution ratios as what is described in our report, the authors concluded that HSC engraftment is absolute and that initially engrafted HSCs fail to sustain self-renewal.23 Even though we did not observe transient reconstitution by any of our HSC clones, as all clones detected at 4 to 6 weeks persisted for more than 9 months, we can still not rule out this possibility. We did not measure erythroid grafts and we did not use the extra sensitive c-Kit41W strain as recipients as done by Benveniste et al.23 We consider instead that bone marrow homing and colonization might be the limiting factors in the engraftment process after intravenous injection at least in lethally irradiated hosts. This view is supported by the enhanced engrafting ability of human HSCs transplanted directly in the BM of murine recipients27,28 and the dramatic homing improvement obtained after abolishment of CD26 activity in HSCs.29 Additional studies involving intrafemoral transplantation of single HSCs or clonal studies using CD26-deficient cells will help elucidate which specific aspect of hematopoietic engraftment limits single HSC reconstitution. These studies will pave the way for therapeutic manipulations aimed at enhancing the repopulating ability of human marrow grafts.
Supplementary Material
Prepublished online as Blood First Edition Paper, October 4, 2005; DOI 10.1182/blood-2005-02-0655.
Supported by National Institutes of Health (NIH) grant nos. DK58192 and CA81179 (M.A.G.) and Canadian Institutes of Health Research (CIHR) operating grant no. MOP-64278 (K.M.M). M.A.G. is a scholar of the Leukemia and Lymphoma Society, and K.M.M. is a Michael Smith Foundation for Health Research and a CIHR Scholar.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Jordan CT, Lemischka IR. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990;4: 220-232. [DOI] [PubMed] [Google Scholar]
- 2.Lemischka IR, Raulet DH, Mulligan RC. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986;45: 917-927. [DOI] [PubMed] [Google Scholar]
- 3.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273: 242-245. [DOI] [PubMed] [Google Scholar]
- 4.Morrison S, Weissman I. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1: 661-673. [DOI] [PubMed] [Google Scholar]
- 5.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80: 3044-3050. [PubMed] [Google Scholar]
- 6.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183: 1797-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3: 1337-1345. [DOI] [PubMed] [Google Scholar]
- 8.Nadin BM, Goodell MA, Hirschi KK. Phenotype and hematopoietic potential of side population cells throughout embryonic development. Blood. 2003;102: 2436-2443. [DOI] [PubMed] [Google Scholar]
- 9.Uchida N, Fujisaki T, Eaves AC, Eaves CJ. Transplantable hematopoietic stem cells in human fetal liver have a CD34(+) side population (SP) phenotype. J Clin Invest. 2001;108: 1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuzaki Y, Kinjo K, Mulligan RC, Okano H. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity. 2004;20: 87-93. [DOI] [PubMed] [Google Scholar]
- 11.Camargo FD, Green R, Capetenaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med. 2003;9: 1520-1527. [DOI] [PubMed] [Google Scholar]
- 12.Corbel SY, Lee A, Yi L, et al. Contribution of hematopoietic stem cells to skeletal muscle. Nat Med. 2003;9: 1528-1532. [DOI] [PubMed] [Google Scholar]
- 13.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297: 2256-2259. [DOI] [PubMed] [Google Scholar]
- 14.Hendrikx PJ, Martens CM, Hagenbeek A, Keij JF, Visser JW. Homing of fluorescently labeled murine hematopoietic stem cells. Exp Hematol. 1996;24: 129-140. [PubMed] [Google Scholar]
- 15.Lanzkron SM, Collector MI, Sharkis SJ. Hematopoietic stem cell tracking in vivo: a comparison of short-term and long-term repopulating cells. Blood. 1999;93: 1916-1921. [PubMed] [Google Scholar]
- 16.van der Loo JC, Ploemacher RE. Marrow- and spleen-seeding efficiencies of all murine hematopoietic stem cell subsets are decreased by preincubation with hematopoietic growth factors. Blood. 1995;85: 2598-2606. [PubMed] [Google Scholar]
- 17.Suzuki A, Andrew DP, Gonzalo JA, et al. CD34-deficient mice have reduced eosinophil accumulation after allergen exposure and show a novel crossreactive 90-kD protein. Blood. 1996;87: 3550-3562. [PubMed] [Google Scholar]
- 18.Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999;96: 14482-14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98: 14541-14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells [published erratum appears in Science. 1989;244:1030]. Science. 1988;241: 58-62. [DOI] [PubMed] [Google Scholar]
- 21.Uchida N, Dykstra B, Lyons KJ, Leung FY, Eaves CJ. Different in vivo repopulating activities of purified hematopoietic stem cells before and after being stimulated to divide in vitro with the same kinetics. Exp Hematol. 2003;31: 1338-1347. [DOI] [PubMed] [Google Scholar]
- 22.Adolfsson J, Borge OJ, Bryder D, et al. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15: 659-669. [DOI] [PubMed] [Google Scholar]
- 23.Benveniste P, Cantin C, Hyam D, Iscove NN. Hematopoietic stem cells engraft in mice with absolute efficiency. Nat Immunol. 2003;4: 708-713. [DOI] [PubMed] [Google Scholar]
- 24.Chen CZ, Li M, de Graaf D, et al. Identification of endoglin as a functional marker that defines long-term repopulating hematopoietic stem cells. Proc Natl Acad Sci U S A. 2002;99: 15468-15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearce DJ, Ridler CM, Simpson C, Bonnet D. Multiparameter analysis of murine bone marrow side population cells. Blood. 2004;103: 2541-2546. [DOI] [PubMed] [Google Scholar]
- 26.Haruta H, Nagata Y, Todokoro K. Role of Flk-1 in mouse hematopoietic stem cells. FEBS Lett. 2001;507: 45-48. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Kimura T, Asada R, et al. SCID-repopulating cell activity of human cord blood-derived CD34-cells assured by intra-bone marrow injection. Blood. 2003;101: 2924-2931. [DOI] [PubMed] [Google Scholar]
- 28.Yahata T, Ando K, Sato T, et al. A highly sensitive strategy for SCID-repopulating cell assay by direct injection of primitive human hematopoietic cells into NOD/SCID mice bone marrow. Blood. 2003;101: 2905-2913. [DOI] [PubMed] [Google Scholar]
- 29.Christopherson KW 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305: 1000-1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.