Abstract
Platelet endothelial cell adhesion molecule-1 (PECAM-1) is a cell surface glycoprotein receptor expressed on a range of blood cells, including platelets, and on vascular endothelial cells. PECAM-1 possesses adhesive and signaling properties, the latter being mediated by immunoreceptor tyrosine-based inhibitory motifs present on the cytoplasmic tail of the protein. Recent studies in vitro have demonstrated that PECAM-1 signaling inhibits the aggregation of platelets. In the present study we have used PECAM-1–deficient mice and radiation chimeras to investigate the function of this receptor in the regulation of thrombus formation. Using intravital microscopy and laser-induced injury to cremaster muscle arterioles, we show that thrombi formed in PECAM-1–deficient mice were larger, formed more rapidly than in control mice, and were more stable. Larger thrombi were also formed in control mice that received transplants of PECAM-1–deficient bone marrow, in comparison to mice that received control transplants. A ferric chloride model of thrombosis was used to investigate thrombus formation in carotid arteries. In PECAM-1–deficient mice the time to 75% vessel occlusion was significantly shorter than in control mice. These data provide evidence for the involvement of platelet PECAM-1 in the negative regulation of thrombus formation.
Introduction
Platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) is a 130-kDa membrane glycoprotein that is expressed on a range of blood cells including platelets, monocytes, neutrophils, B lymphocytes, some T lymphocyte subsets, and also on vascular endothelial cells.1-4 This member of the immunoglobulin superfamily has been reported to be associated with a wide range of functions, depending on the cell of interest. These include transendothelial migration of leukocytes,5-7 integrin regulation,8-16 modulation of T- and B-lymphocyte antigen receptor signaling,17,18 B-lymphocyte development,19 vasculogenesis,20 apoptosis,21,22 and protection against endotoxic shock.23
Several lines of investigation have recently determined that PECAM-1 is involved in the negative regulation of platelet function in vitro. The activation of PECAM-1 prior to the stimulation of platelets results in the inhibition of platelet aggregation and the inhibition of activatory signaling mechanisms.24,25 Of particular note, therefore, are the observations that mouse platelets deficient in PECAM-1 are hyperresponsive to stimulation with collagen and demonstrate enhanced aggregation, secretion, and adhesion to this agonist.26 Platelets from PECAM-1–deficient mice have also been shown to form larger thrombi in vitro under physiologic flow conditions.25
PECAM-1 participates in homophilic ligand-binding interactions27-29; indeed, such interactions between PECAM-1 molecules on the same cell and between cells are believed to underlie most of its identified functions. Additional potential ligand-binding interactions have been reported, such as with integrin αvβ3 and CD38, but the functional significance of these is not known.30,31 Signal transduction through this receptor is mediated through 2 immunoreceptor tyrosine-based inhibitory motifs (ITIMs),32 conserved signaling sequences defined by the consensus sequence L/I/V/S/T-x-Y-x-x-L/V, that it shares with a family of inhibitory receptors of the immune system including FcγRIIB and killer inhibitory receptors. The ligand-induced clustering of platelet PECAM-1,24,25 platelet activation by collagen and thrombin,33 and fibrinogen-mediated platelet aggregation25 results in the tyrosine phosphorylation of the cytoplasmic ITIMs.34 This leads to the recruitment of signaling proteins such as the tyrosine phosphatases SHP-1 and SHP-2, which bind to the phosphotyrosine residues via src-homology 2 (SH2) domains.35,36 Little is known, particularly in platelets, of what follows or the identity of substrates for recruited phosphatases, but this leads to the inhibition of activatory signaling mechanisms.
The role of PECAM-1 signaling in hemostasis is uncertain. Using a photochemical model of thrombosis in mice, Rosenblum et al37,38 demonstrated that intravenous injection of anti–PECAM-1 antibodies prolonged time to the first detectable intravascular adhesion of platelet aggregates, which was attributed to blockade or inhibition of adhesion to, or activation by, PECAM-1 exposed on injured endothelium. In contrast, however, Vollmar et al39 have reported no difference, in comparison to controls, in the time to photochemically induced platelet adhesion and the rate of thrombus formation in PECAM-1–deficient mice.
The aim of this study was therefore to examine in more detail the potential role of PECAM-1 in the regulation of thrombus formation. We have used mice that lack the systemic expression of PECAM-1 and chimeric PECAM-1–deficient mice to explore the respective roles of platelet and endothelial PECAM-1 in thrombus formation in vivo. Two distinct in vivo assays of thrombosis were used: a high-speed intravital microscopy system that allows real-time analysis of several parameters of laser-induced thrombus formation, and a ferric chloride model of thrombosis with measurement of blood flow using a Doppler flow probe. Our data indicate the involvement of platelet PECAM-1 in the negative regulation of platelet function in vivo.
Materials and methods
Materials
Antibodies. Rat anti–mouse CD41 was purchased from BD (Palo Alto, CA) and mouse anti–human fibrin antibody (NYBT2G1) was from Accurate Chemical and Scientific Corporation (Westbury, NY).
Mice. Transgenic PECAM-1–deficient mice were obtained from Dr Tak Mak (Amgen Institute, Toronto, ON, Canada) and bred on a C57BL/6J background at the Biomedical Resource Center at the Medical College of Wisconsin. Control animals were age- and sex-matched C57BL/6J mice.
Creation of radiation chimeras
Bone marrow cells were harvested from the femurs and tibias of wild-type or PECAM-1–deficient C57BL/6J mice into X-vivo 15 media (Atlanta Biologicals, Norcross, GA). Small mononuclear cells were further isolated by gradient centrifugation through Fico/Lite-LM (density 1.086 g/L; Atlanta Biologicals) and resuspended in X-Vivo 15, and 2 × 106 cells were injected retro-orbitally into each recipient mouse 24 hours following lethal irradiation (11 Gy, Shepherd Mark I cesium irradiator; J. L. Shepherd, San Fernando, CA). Recipient mice were phenotyped by assessing the level of PECAM-1 expression on lymphocytes by flow cytometry 4 weeks following transplantation. All chimeric mice demonstrated the transplanted phenotype on greater than 90% of lymphocytes by this time after transplantation.
Intravital microscopy
The design and use of a novel intravital microscopy system to analyze thrombus formation in the mouse has been described recently.40 This model has been shown to stimulate thrombus formation without causing exposure of subendothelial collagens.41 Briefly, antibodies to murine CD41 were labeled with Alexa-660 or -647 using the Alexa Fluor Protein Labeling kit (Molecular Probes, Eugene, OR) in accordance with the manufacturer's instructions. Prior to labeling, Fab fragments of antibodies were prepared using papain (Pierce, Rockford, IL). Similarly, whole antifibrin antibodies were conjugated with Alexa-488.
Mice were anesthetized with intraperitoneal injection of 125 mg/kg ketamine, 12.5 mg/kg xylazine, and 250 μg/kg atropine sulfate. Anesthesia was maintained with 5 mg/kg pentobarbital as required through a jugular vein cannula. The cremaster muscle was exteriorized, connective tissue was removed, and an incision was made to allow the muscle to be affixed as a single sheet over a glass slide. During preparation and throughout the experiment the muscle preparation was hydrated with buffer (135 mM NaCl, 4.7 mM KCl, 2.7 mM CaCl2, 18 mM NaHCO3, pH 7.4).
Labeled anti-CD41 antibodies (0.1 μg/g bodyweight) were infused through the jugular cannula 10 minutes before beginning induction of the first thrombus. Between 1 and 3 arterioles were visible in each cremaster muscle preparation. Arterioles with undisrupted flow were chosen (estimated shear rate between 800 and 1600 s–1) and a number of thrombi were studied tracking up each vessel against the direction of blood flow. Endothelial injury was induced using a pulsed nitrogen dye laser at 440 nm that was focused onto the blood vessel wall through the microscope optics. Widefield fluorescence (660 nm excitation wavelength, 60 ms) and brightfield (40 ms) images were collected alternately for up to 3 minutes after injury formation. For some experiments the accumulation of fibrin and platelets was measured simultaneously. This was achieved by coinfusion of fluorescently labeled anti-CD41 and antifibrin (0.5 μg/g bodyweight) antibodies. Thrombi were visualized using an Olympus AX-70 fluorescence microscope (Olympus, Melville, NY) with a 60× water immersion objective lens (numeric aperture 0.9) and recorded using a Cooke SensiCam digital camera (Cooke, Auburn Hills, MI). Images were taken in rapid-repeating sequence to visualize platelets (excitation wavelength 660 nm, 20 ms) and fibrin (excitation wavelength 488 nm, 15 ms) followed by a brightfield image (20 ms).
Data were collected and analyzed using the SlideBook imaging software (Intelligent Imaging Innovations, Denver, CO), and some graphical analysis was performed using SigmaPlot (SPSS, Chicago, IL). Specifically, thrombus area and integrated thrombus fluorescence were measured over time.
Ferric chloride–induced model of thrombosis
Ferric chloride–induced models of thrombosis have been shown to cause substantial damage to the endothelium and exposure of underlying collagens.41,42 The following method was developed by Kurz et al43 for use in rats and has been subsequently applied to mouse studies.44,45 Adult mice (> 8 weeks old, 22-32 g) were anesthetized by intraperitoneal injection of pentobarbital (120 mg/kg). The carotid artery was surgically exposed and sodium chloride solution (0.9%) was placed in the surgical wound. A miniature ultrasound Doppler flow probe (Model:0.5VB; Transonic, Ithaca, NY) was placed under the artery to record the baseline blood flow using a Transonic Model T106 flow meter. Afterward, the sodium chloride was removed from the wound and filter paper (Whatman no. 4; Florham Park, NJ) saturated with 10% ferric chloride was applied for 3 minutes on the adventitial surface of the artery. After 3 minutes, the filter paper was removed, the wound was rinsed and saturated with saline solution, and the carotid blood flow was monitored. The blood flow readings were continuous. Time to thrombotic occlusion was defined as the time for the blood flow to drop to 25% of the baseline values.
Statistical analysis
Data were checked for normal distribution using the Shapiro-Wilk normality test and statistical significance was determined using the Student t test. P values of .05 or less were taken to indicate significant differences.
Results
Thrombi are larger in PECAM-1–deficient mice
The role of PECAM-1 in the inhibition of platelet aggregation and signaling in vitro has been recently documented.24,25 In addition, platelets from PECAM-1–deficient mice have been shown to be hyperresponsive to collagen and to form larger thrombi when flowed over collagen surfaces in vitro, further implicating PECAM-1 in the negative regulation of platelet function.25,26 In the present study, intravital microscopy of cremaster muscle arterioles was used to study the process of thrombus formation in real-time in normal and PECAM-1–deficient mice.
Thrombus formation was stimulated by laser-induced injury in arterioles in control and PECAM-1–deficient mice and studied in real time by assessing the accumulation of platelets fluorescently labeled with Alexa-660–conjugated anti-CD41 antibodies into the developing thrombus. With both control and PECAM-1–deficient mice, the degree of correlation between the time of initial thrombus formation and either the amount of time elapsed since the laser was fired or the duration of laser firing was variable. Similarly, the rate of thrombus formation and the size and shape of the thrombus formed was variable in mice of different genotypes. Multiple thrombi in up to 6 separate mice were therefore analyzed for quantitative differences between control and PECAM-1–deficient mice to be assessed.
Thrombus size was assessed in real time by measuring the area occupied by the thrombus and integrated thrombus fluorescence. Since thrombus area measurements may be affected by the position of the focal plane during data collection and the orientation of the thrombus within the blood vessel, integrated thrombus fluorescence was incorporated since this may be expected to produce more precise quantitative data. This is a measure of total fluorescence and is proportional to the number of platelets present in a thrombus at a given time.
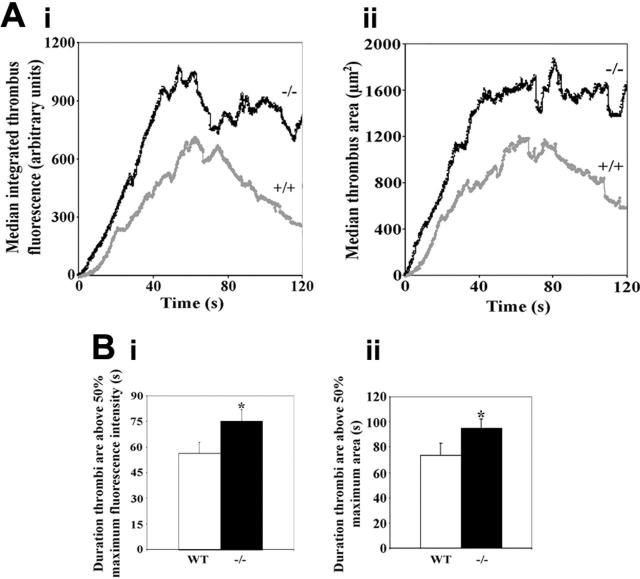
Blinded analysis of the area occupied by thrombi and integrated thrombus fluorescence over time was performed using SlideBook image analysis software. Thrombus sizes were analyzed for up to 160 seconds following the initiation of thrombus formation and expressed as plots of thrombus area (μm2) or arbitrary fluorescence levels against time. Median thrombus area and integrated thrombus fluorescence are displayed over a period of 120 seconds following thrombus initiation in Figure 1A. Thrombus area and integrated fluorescence levels were observed to be larger in the PECAM-1–deficient mice at all time points studied following initiation of thrombus formation (control: n = 14 thrombi; PECAM-1–deficient: n = 20 thrombi). The profiles of median thrombus area and integrated thrombus fluorescence levels over this time period were similar. As an alternative method of analysis, thrombus area or fluorescence levels were plotted over time and areas under curves calculated. Analysis of mean data indicated that thrombi were significantly larger in PECAM-1–deficient mice (control: n = 11 thrombi; PECAM-1–deficient: n = 12 thrombi; P < .05).
Figure 1.
Thrombi are larger in PECAM-1–deficient mice. (A) Median thrombus integrated fluorescence (anti-CD41) (Ai) and area (μm2) (Aii) were calculated for thrombi formed in control (+/+) and PECAM-1–deficient (–/–) mice and are plotted against time for a duration of 120 seconds following thrombus initiation (control: n = 14 thrombi; PECAM-1–deficient: n = 20 thrombi). (B) As a measure of thrombus stability, the durations for which thrombi remained above 50% of maximal integrated fluorescence (anti-CD41) (Bi) and thrombus area (Bii) were calculated, and mean values ± SEM are shown. (Bi) *P = .04; (Bii) *P = .02.
Analysis of the initial rate of increase in median integrated thrombus fluorescence over the first 40 seconds of thrombus development indicates faster kinetics (approximately double the rate) of initial thrombus growth in the PECAM-1–deficient mice. Similar increased initial kinetics were observed for both integrated fluorescence and thrombus area (Figure 1A).
To assess thrombus stability, the duration for which each thrombus remained above 50% of its maximal area or fluorescence intensity was assessed. Mean duration values are shown in Figure 1B and demonstrate significantly greater thrombus stability in PECAM-1–deficient mice. The formation of long thrombi extending along the endothelium downstream from the site of vessel damage appeared more prevalent in PECAM-1–deficient mice. Particularly contrasting examples of this are shown in Figure 2, although it should be noted that as with other aspects of the analysis of thrombi, substantial variability was observed. Videos showing thrombus formation and corresponding to thrombi shown in Figure 2 are available as supplemental Videos S1 and S2 (see the Supplemental Videos link at the top of the online article, at the Blood website).
Figure 2.
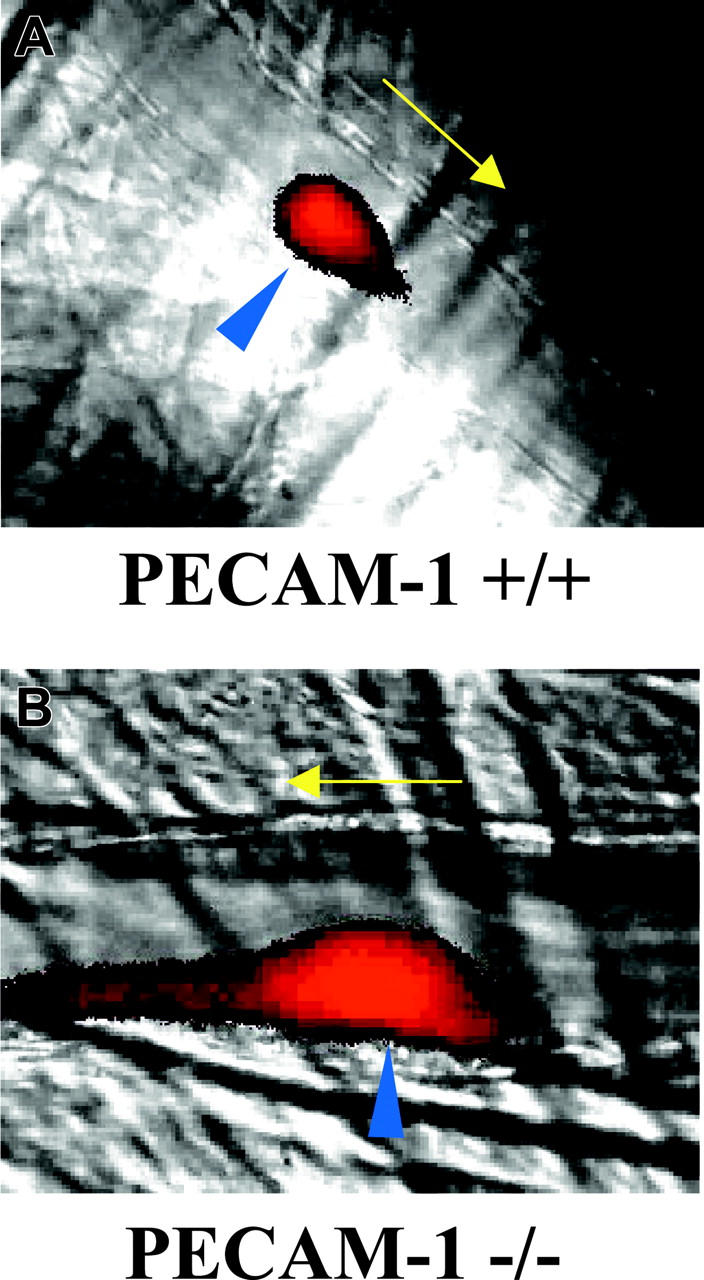
Imaging of thrombus formation in control and PECAM-1–deficient platelets. (A) Control and (B) PECAM-1–deficient mice were infused with Alexa-660–labeled anti-CD41 antibody Fab fragments before thrombus induction in cremaster muscle arterioles using a nitrogen dye laser. Thrombus formation was studied over a period of 3 minutes using a combination of widefield fluorescence and brightfield microscopy. Arrows indicate the direction of blood flow and blue arrowheads indicate the position of laser-induced endothelial damage. Videos representing the formation of thrombi shown in this figure are available as supplemental Videos S1 and S2.
The contributions of platelet and endothelial cell PECAM-1 in the regulation of thrombus formation
In PECAM-1–deficient mice, PECAM-1 is absent from platelets and endothelial cells. It was therefore unclear whether the enhanced thrombus formation observed in these mice was due to the absence of PECAM-1 on platelets, endothelial cells, or both. Experiments were therefore performed using chimeric mice that had been irradiated and that received transplants of either normal or PECAM-1–deficient bone marrow. These included wild-type (WT) mice that received transplants of WT bone marrow, PECAM-1–deficient mice that received transplants of WT bone marrow, and WT mice that received transplants of PECAM-1–deficient bone marrow. Successful bone marrow engraftment was verified by flow cytometric analysis of lymphocytes for expression of PECAM-1 (Figure 3A). Following laser-induced stimulation of thrombus formation, the recruitment of platelets into thrombi was measured by intravital microscopy. Blinded analyses of the area occupied by thrombi and integrated thrombus fluorescence over time were performed as described for full PECAM-1 knockout mice. Median thrombus area and integrated thrombus fluorescence are displayed (Figure 3B) over a period of 120 seconds following thrombus initiation. Thrombus area and integrated anti-CD41 (platelets) fluorescence levels were found to be larger in WT mice that received transplants of PECAM-1–deficient platelets in comparison to WT mice that received transplants of WT bone marrow. Faster kinetics of thrombi formation were observed from the outset of measurements, and larger thrombi were observed for the duration of data collection. These results implicate platelet PECAM-1 in the more rapid formation of larger platelet thrombi in full PECAM-1 knockout mice relative to wild-type mice (Figure 1). By contrast, enlarged thrombi and initial faster kinetics were not observed in PECAM-1–deficient mice that received transplants of WT bone marrow. In these mice similar profiles to those observed in WT mice that received transplants of WT bone marrow were observed until around 90 seconds following thrombus initiation, after which thrombi appeared moderately and transiently larger.
Figure 3.
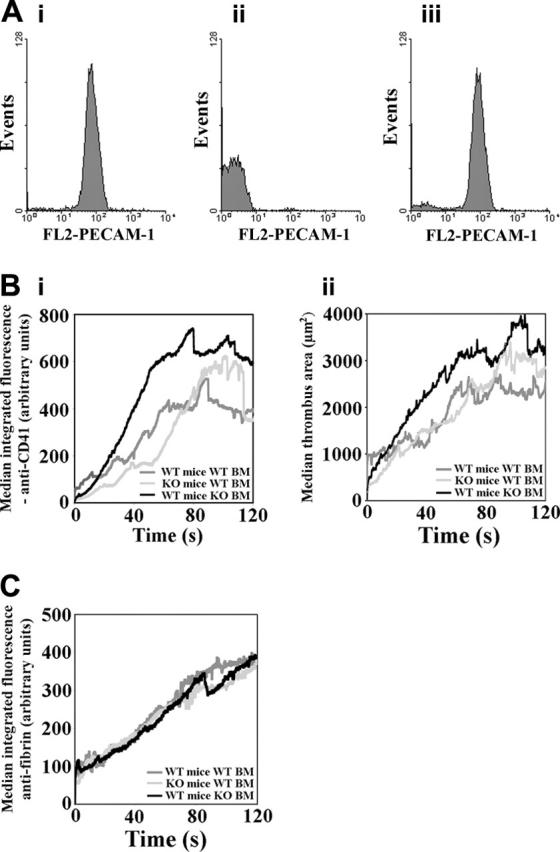
The contributions of platelet and endothelial cell PECAM-1 to the regulation of thrombus formation. (A) Chimeric mice were generated by bone marrow transplantation and, following recovery, successful engraftment was verified by flow cytometric analysis to detect the presence or absence of PECAM-1 on lymphocytes from WT mice that received transplants of WT bone marrow (Ai), WT mice that received transplants of PECAM-1–deficient bone marrow (Aii), and PECAM-1–deficient mice that received transplants of WT bone marrow (Aiii). (B) Median anti-CD41 integrated fluorescence (Bi) and thrombus area (μm2) (Bii) were calculated for thrombi formed in chimeric mice generated by bone marrow transplantation and are plotted against time for a duration of 120 seconds following thrombus initiation (Bi: WT mice with WT BM: n = 30, PECAM-1–deficient mice with WT bone marrow: n = 33, WT mice with PECAM-1–deficient bone marrow: n = 14; ii: WT mice with WT BM: n = 26, PECAM-1–deficient mice with WT bone marrow: n = 33, WT mice with PECAM-1–deficient bone marrow: n = 14). KO indicates knock-out. (C) Fibrin deposition in thrombi was measured simultaneously with platelet accumulation for each type of mouse chimera that received transplants and is plotted against time for the duration of 120 seconds following thrombus initiation (WT mice with WT BM: n = 30; PECAM-1–deficient mice with WT bone marrow: n = 32; WT mice with PECAM-1–deficient bone marrow: n = 14).
To begin to explore the mechanisms that underlie the effects observed in PECAM-1–deficient mice, the accumulation of fibrin in thrombi was measured simultaneously with platelet accumulation in mice that received transplants. Median thrombus fibrin formation (integrated antifibrin fluorescence) was similar in each of the mouse types that received transplants (Figure 3C). Fibrin accumulation was approximately linear over the 120 seconds of thrombus formation shown.
Time to blood vessel occlusion is decreased in PECAM-1–deficient platelets
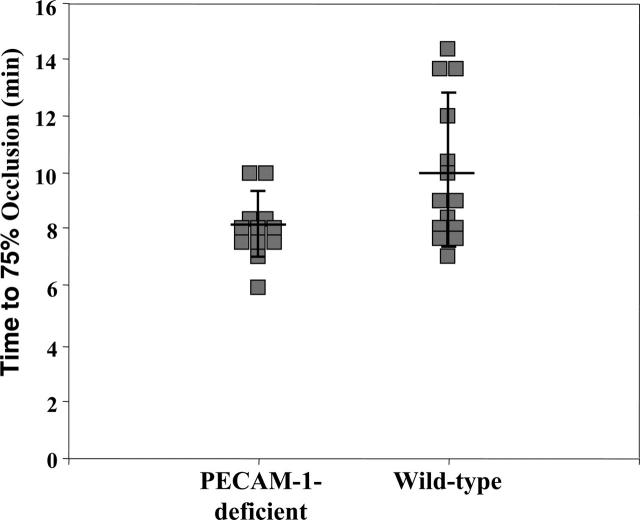
A second model of thrombosis was used in control and PECAM-1–deficient mice. The additional use of the ferric chloride model of thrombosis enabled thrombus formation to be studied in larger vessels and at high flow rates. These assays were also performed blinded for mouse genotype. Thrombus formation was measured in the carotid artery using an ultrasound Doppler flow probe, following exposure to ferric chloride solution. The time to 75% vessel occlusion was measured (ie, until blood flow was reduced to 25% of baseline value) and results are shown in Figure 4. The mean time to 75% occlusion (± standard deviation) for PECAM-1–deficient mice was 8.1 ± 1.1 minutes (n = 12), which was significantly shorter (P < .03) than that observed in wild-type mice (10.0 ± 2.7 minutes, n = 14). The overall magnitude of differences was modest, with results using this model of thrombosis in several wild-type mice appearing comparable to PECAM-1–deficient mice.
Figure 4.
Quantitative analysis of ferric chloride (FeCl3)–induced thrombus formation in the carotid arteries of PECAM-1–deficient and wild-type mice. Filter papers saturated with 10% FeCl3 were applied to exposed carotid arteries for 3 minutes to induce acute injury to the endothelium, after which the vessels were rinsed and saturated with saline solution. Blood flow was monitored with a miniature Ultrasound Doppler flow probe placed under the exposed artery and recorded using a Transonic Model T106 flow meter. The time for blood flow to drop to 25% of baseline values (75% occlusion) was determined. Each data point reflects the time for the left carotid artery to become 75% occluded in PECAM-1–deficient (left) or wild-type (right) animals. The solid line through each data set represents the mean time to 75% occlusion ± standard deviations. The mean time to 75% occlusion (± standard deviation) for PECAM-1–deficient mice was 8.1 ± 1.1 minutes (n = 12), which was significantly shorter (P < .03) than that observed in wild-type mice (10.0 ± 2.7 minutes, n = 14).
Discussion
Immunelike cell signaling is central to the activation of platelets through the collagen receptor GPVI that signals via an immunoreceptor tyrosine-based activatory motif (ITAM) present on an associated protein, the FcR γ-chain.46-48 ITAM signaling has also been implicated in activation of platelets through the immunoglobulin G receptor FcγRIIA49 and the GPIb-V-IX complex.50,51 Studies in vitro have indicated that the relationship between ITAM and ITIM signaling may be important for the regulation of platelets. Stimulation of PECAM-1 has been shown to inhibit platelet signaling and function initiated by a range of receptors, including the GPVI, GPIb-V-IX complex, and FcγRIIA.24,25,52,53 PECAM-1 has also been found to inhibit platelet activation stimulated by G-protein–coupled receptor agonists such as thrombin, albeit to a lesser degree.22
In the present study we have employed PECAM-1–deficient mice to investigate the role of PECAM-1 in thrombus formation in vivo. The use of a highly sensitive intravital microscopic technique to study thrombus formation in real time, and a ferric chloride model of thrombosis, has enabled us to establish a role for this molecule in the regulation of thrombus formation in vivo.
Thrombi formed in cremaster muscle arterioles upon laser injury in PECAM-1–deficient mice were significantly larger than those formed in control mice. Faster kinetics of thrombus formation were observed in the PECAM-1–deficient mice, and the difference in thrombus size appeared constant throughout the time that thrombus development was measured. In addition, thrombi formed in the PECAM-1 knockout mice appeared to have greater stability.
Enhanced thrombus formation in PECAM-1 knockout mice may be due to a lack of PECAM-1 on platelets (and leukocytes), or on endothelial cells, or its absence on both. To address this, laser-induced thrombus formation was examined in chimeric mice generated through bone marrow transplantation. WT mice received transplants of PECAM-1–deficient bone marrow to examine the potential role for platelet PECAM-1 in the regulation of thrombus formation. In comparison to control mice (WT mice that received transplants of WT bone marrow), thrombi formed more rapidly and were larger (Figure 3B). The level of enhanced thrombus development was similar to that observed in full PECAM-1 knockout mice in comparison with controls (Figure 1A). It should be noted that experiments in Figures 1 and 3 were performed separately using different reagents. Direct comparisons of absolute levels of thrombus fluorescence or area should not therefore be made. PECAM-1–deficient mice received transplants of WT bone marrow to examine the effect of PECAM-1 deficiency on endothelial cells with normal levels of PECAM-1 on platelets. The rate of thrombus formation and size in these mice were similar to control mice (Figure 3B). These data implicate platelet PECAM-1 in the elevated platelet responses observed in full knockout mice (Figure 1). Endothelial cell PECAM-1 did not appear to be involved in the initial elevated phase of thrombus formation observed in PECAM-1 knockout mice, although transiently enhanced thrombus formation at later time points in PECAM-1–deficient mice that received transplants of WT bone marrow may indicate a more complex relationship between platelet and endothelial PECAM-1 at different phases of thrombus formation. PECAM-1–deficient mice have been reported to have extended tail-bleeding times that were not rescued by transplantation of WT hematopoietic precursors, indicating an endothelial defect in these mice that results in excessive bleeding.54 This may indicate positive and negative roles for PECAM-1 in thrombus formation. In contrast, however, Vollmar et al39 measured normal bleeding times in PECAM-1–deficient mice. The reason for the discrepancy between these studies is unclear but is likely to reflect differences in experimental procedures.
Upon damage to the endothelium, coagulation is triggered through the exposure of tissue factor in the blood vessel wall and/or recruitment on circulating microparticles,55-57 resulting in thrombin generation and fibrin formation within the thrombus. Fibrin deposition in thrombi was measured simultaneously with platelet accumulation in mice that received bone marrow transplants. The levels and rate of fibrin formation in thrombi were found to be very similar between the 3 mouse types (Figure 3C). This indicates that larger thrombi in PECAM-1–deficient mice are due to hyperreactive platelets rather than enhanced activation of the endothelium or coagulation system.
The laser-induced injury model used has been characterized and found to result in insufficient endothelial damage to cause the exposure of subendothelial collagens.41 This is consistent with direct laser-induced injury of vessels of the mouse ear described by Rosen et al.58 In our experiments, therefore, it is unlikely that the trigger for platelet thrombus formation was platelet-collagen interaction, either through von Willebrand factor–GPIb-V-IX binding or direct platelet interactions through integrin α2β1 or GPVI. More work is required to understand how platelets become activated and recruited to a thrombus in this model, although the reported inhibitory effect of a phosphodiesterase 3A inhibitor on initial platelet accumulation59 indicates an important role of platelet signaling for recruitment and thrombus formation.
A ferric chloride model of thrombosis was used as an alternative approach to examine thrombus formation in a larger artery in PECAM-1–deficient mice. A modest but significant decrease in the time to 75% blood vessel occlusion was recorded in PECAM-1–deficient mice. Ferric chloride models of thrombosis have been shown to cause transmural cell necrosis and substantial damage to the endothelium and, consequently, the exposure of subendothelial collagens (reviewed recently by Day et al42). Indeed, thrombi that form in a ferric chloride model of thrombosis have been reported to involve platelet GPVI, consistent with this model causing the exposure of subendothelial collagens.41
Since laser-induced thrombosis does not involve collagen exposure, it is possible that PECAM-1 is involved in the negative regulation in vivo of thrombin-stimulated platelet activation and thrombus development. Alternative explanations for this phenomenon include PECAM-1–mediated inhibition of signaling stimulated by immobilized fibrinogen through integrin αIIbβ3 or inhibition of responses to secondary mediators released by activated platelets. The involvement of GPVI in thrombus formation in the ferric chloride model is compatible with the role of PECAM-1 in the inhibition of collagen-mediated platelet activation observed in vitro, although the inhibition in vivo of additional mechanisms of platelet activation cannot be excluded.
Our results implicate PECAM-1 in the negative regulation of platelet function in small cremasteric arterioles and in carotid arteries. This is consistent with the observation of Rosenblum et al37,38 that intravenous injection of anti–PECAM-1 antibodies delayed platelet adhesion and aggregation in mice, using a photochemical model of thrombosis shown not to denude the endothelium. Interference by anti–PECAM-1 antibodies in platelet adhesion/aggregation at sites of injury was proposed to underlie this effect, although we would suggest this may have been due to the stimulation of PECAM-1 signaling rather than the inhibition of adhesive interactions.
In most models of thrombosis the mechanism of thrombus formation is not well characterized. This has repercussions in the comparison of data produced using different thrombosis models and potential inconsistencies between studies. For example, our data differ from those of Vollmar et al39 who reported a lack of function of PECAM-1 in photochemically induced vascular thrombosis in cremasteric vessels. Their inability to detect any differences in the PECAM-1–deficient mice probably reflects the different experimental systems employed, methods of detection and analysis, and lower sensitivity. It is also possible that different in vivo models of thrombosis may produce different results depending on the combination of prothrombotic factors and/or negative regulators that are exposed, released, or generated. Given this, it is possible that the role of PECAM-1 in vivo may also be dependent on the nature of an injury and the activatory factors present, between healthy or diseased vessels and the position of platelets within a thrombus.
We have speculated previously that the ligation and thereby oligomerization of PECAM-1 between platelets and endothelial cells at the periphery of a developing thrombus may prevent platelet activation and therefore limit the spread and size of the developing thrombus.24 It has also been reported that activation of PECAM-1 on endothelial cells results in the stimulation of secretion of prostacyclin, which would also inhibit platelet activation.60 While our results indicate platelet PECAM-1 to be involved in the regulation of thrombus growth, a role for endothelial PECAM-1 in the early phase of thrombus development is not supported by the results of this study. The more rapid kinetics of thrombus formation observed in PECAM-1–deficient mice suggests that PECAM-1 in circulating platelets in normal mice is primed for its inhibitory role. Whether PECAM-1 ligation is required for the inhibitory function of this molecule in vivo and where this may occur remains to be addressed. It is presently unclear whether leukocyte PECAM-1 may also contribute to thrombus formation, since the ablation strategy used in these experiments did not target platelet PECAM-1 selectively. While leukocyte rolling and recruitment to laser-induced thrombi generally require longer duration of thrombus formation than measured in this study, the potential role of PECAM-1 in this deserves future scrutiny.
Our data suggest that the balance between ITIM and activatory signaling in platelets may regulate the balance between platelet inhibition and activation. This may be important to determine the stimulation threshold level for thrombus formation and to ensure that the thrombus is restricted to the initial site of injury.
Supplementary Material
Acknowledgments
We are grateful to Dr Tak Mak (Amgen Institute, Toronto, ON, Canada) for generating the PECAM-1–deficient mice and to Drs Boris Tchernychev, Monica Schenone, Erik Vandendries, and Derek Sim for technical advice, the use of labeled antibodies, and helpful discussions during the course of this study. The authors also wish to thank Dr Julie Lovegrove (University of Reading, United Kingdom) for advice on statistical analysis.
Dr Gross's current address is Department of Medicine, St Michael's Hospital, University of Toronto, ON, Canada.
Prepublished online as Blood First Edition Paper, September 15, 2005; DOI 10.1182/blood-2005-04-1512.
Supported by funding from the US National Institutes of Health (HL51926 and HL69435, B.C.F. and B.F.; HL-40926 and HL-44612, P.J.N. and D.K.N.), the Biotechnology and Biological Sciences Research Council (J.M.G.), the Medical Research Council (United Kingdom) (J.M.G.), the British Heart Foundation (J.M.G.), and the Wellcome Trust (J.M.G.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Ohto H, Maeda H, Shibata Y, et al. A novel leukocyte differentiation antigen: two monoclonal antibodiesTM2 and TM3 define 120-kDa molecule present on neutrophils, monocytes, platelets, and activated lymphoblasts. Blood. 1985;66: 873-881. [PubMed] [Google Scholar]
- 2.Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intracellular junctions. J Exp Med. 1989;178: 449-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114: 1059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman PJ, Berndt MJ, Gorski J, et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247: 1219-1222. [DOI] [PubMed] [Google Scholar]
- 5.Vaporciyan AA, DeLisser HM, Yan H-C, et al. Involvement of platelet-endothelial cell adhesion molecule-1 in neutriphil recruitment in vivo. Science. 1993;262: 1580-1582. [DOI] [PubMed] [Google Scholar]
- 6.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178: 449-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan GS, Andrew DP, Takimoto H, et al. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162: 3022-3030. [PubMed] [Google Scholar]
- 8.Tanaka Y, Albelda SM, Horgan KJ, et al. CD31 expressed on distinctive T cell subsets is a preferential amplifier of b1 integrin-mediated adhesion. J Exp Med. 1992;176: 245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leavesley DI, Oliver JM, Swart BW, Berndt MC, Haylock DN, Simmons PJ. Signals from platelet endothelial-cell adhesion molecule enhance the adhesive activity of the very late antigen-4 integrin of human Cd34(+) hematopoietic progenitor cells. J Immunol. 1994;153: 4673-4683. [PubMed] [Google Scholar]
- 10.Piali L, Albelda SM, Baldwin HS, Hammel P, Gisler RH, Imhof BA. Murine platelet endothelial cell adhesion molecule (PECAM-1/CD31) modulates β2 integrins on lymphokine-activated killer cells. Eur J Immunol. 1993;23: 2464-2471. [DOI] [PubMed] [Google Scholar]
- 11.Berman ME, Muller WA. Ligation of platelet endothelial-cell adhesion molecule-1 (Pecam-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte Cr3 (CD11b/CD18). J Immunol. 1995;154: 299-307. [PubMed] [Google Scholar]
- 12.Berman ME, Xie Y, Muller WA. Roles of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in natural killer cell transendothelial migration and beta(2) integrin activation. J Immunol. 1996;156: 1515-1524. [PubMed] [Google Scholar]
- 13.Varon D, Jackson DE, Shenkman B, et al. Platelet/endothelial cell adhesion molecule-1 serves as a co-stimulatory agonist receptor that modulates integrin dependent adhesion and aggregation of human platelets. Blood. 1998;91: 500-507. [PubMed] [Google Scholar]
- 14.Chiba R, Nakagawa N, Kuroasawa K, Tanaka Y, Saito Y, Iwamoto I. Ligation of CD31(PECAM-1) on endothelial cells increases adhesive function of αvβ3 integrin and enhances β1 integrin mediated adhesion of eosinophilis to endothelial cells. Blood. 1999;94: 1319-1329. [PubMed] [Google Scholar]
- 15.Reedquist KA, Ross E, Koop EA, et al. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol. 2000;148: 1151-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao TM, Newman PJ. Integrin activation by regulated dimerization and oligomerization of platelet endothelial cell adhesion molecule (PECAM)-1 from within the cell. J Cell Biol. 2001;152: 65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton-Nash DK, Newman PJ. A new role for platelet-endothelial cell adhesion molecule-1 (CD31): inhibition of TCR-mediated signal transduction. J Immunol. 1999;163: 682-688. [PubMed] [Google Scholar]
- 18.Newman DK, Hamilton C, Newman PJ. Inhibition of antigen-receptor signaling by platelet endothelial cell adhesion molecule-1 (CD31) requires functional ITIMs, SHP-2, and p56(lck). Blood. 2001;97: 2351-2357. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson R, Lyons AB, Roberts D, Wong MX, Bartley PA, Jackson DE. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) acts as a regulator of B-cell development, B-cell antigen receptor (BCR)-mediated activation, and autoimmune disease. Blood. 2002;100: 184-193. [DOI] [PubMed] [Google Scholar]
- 20.Brier G, Brevario F, Caveda L, et al. Molecular cloning and expression of murine vascular endothelial cdherin in early stage development of the cardiovascular system. Blood. 1996;87: 630-641. [PubMed] [Google Scholar]
- 21.Gao CJ, Sun WY, Christofidou-Solomidou M, et al. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood. 2003;102: 169-179. [DOI] [PubMed] [Google Scholar]
- 22.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418: 200-203. [DOI] [PubMed] [Google Scholar]
- 23.Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288: H159-H164. [DOI] [PubMed] [Google Scholar]
- 24.Cicmil M, Thomas JM, Leduc M, Bon C, Gibbins JM. PECAM-1 signalling inhibits the activation of human platelets. Blood. 2002;99: 137-144. [DOI] [PubMed] [Google Scholar]
- 25.Jones KL, Hughan SC, Dopheide SM, Farndale RW, Jackson SP, Jackson DE. Platelet endothelial cell adhesion molecule-1 is a negative regulator of platelet-collagen interactions. Blood. 2001;98: 1456-1463. [DOI] [PubMed] [Google Scholar]
- 26.Patil S, Newman DK, Newman PJ. Platelet endothelial cell adhesion molecule-1 serves as an inhibitory receptor that modulates platelet responses to collagen. Blood. 2001;97: 1727-1732. [DOI] [PubMed] [Google Scholar]
- 27.Sun QH, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually distinct Ig homology domains in PECAM-1 regulate hemophilic binding and modulate receptor affinity. J Biol Chem. 1996;271: 11090-11098. [DOI] [PubMed] [Google Scholar]
- 28.Sun J, Williams J, Yan H-C, Amin KM, Albelda SM, DeLisser HM. Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilin adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996;271: 18561-18570. [DOI] [PubMed] [Google Scholar]
- 29.Newton JP, Buckley CD, Jones EY, Simmons DL. Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites of PECAM-1/CD31. J Biol Chem. 1997;272: 20555-20563. [DOI] [PubMed] [Google Scholar]
- 30.Buckley CD, Doyonnas R, Newton JP, et al. Identification of αvβ3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci. 1996;109: 437-445. [DOI] [PubMed] [Google Scholar]
- 31.Deaglio S, Morra M, Mallone R, et al. Human CD38 (ADP-Ribosyl Cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol. 1998;160: 395-402. [PubMed] [Google Scholar]
- 32.Burshtyn DN, Yang WT, Yi TL, Long EO. A novel phosphotyrosine motif with a critical amino acid at position-2 for the SH2 domain-mediated activation of the tyrosine phosphatase SHP-1. J Biol Chem. 1997;272: 13066-13072. [DOI] [PubMed] [Google Scholar]
- 33.Cicmil M, Thomas JM, Sage T, et al. Collagen, convulxin, and thrombin stimulate aggregation-independent tyrosine phosphorylation of CD31 in platelets: evidence for the involvement of Src family kinases. J Biol Chem. 2000;275: 27339-27347. [DOI] [PubMed] [Google Scholar]
- 34.Jackson DE, Kupcho KR, Newman PJ. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of platelet/endothelial cell adhesion molecule-1 (PECAM-1) that are required for the cellular association and activation of the protein-tyrosine phosphatase, SHP-2. J Biol Chem. 1997;272: 24868-24875. [DOI] [PubMed] [Google Scholar]
- 35.Hua CT, Gamble JR, Vadas MA, Jackson DE. Recruitment and activation of SHP-1 proteintyrosine phosphatase by human platelet endothelial cell adhesion molecule-1 (PECAM-1): identification of immunoreceptor tyrosine-based inhibitory motif-like binding motifs and substrates. J Biol Chem. 1998;273: 28332-28340. [DOI] [PubMed] [Google Scholar]
- 36.Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. J Biol Chem. 1997;272: 6986-6993. [DOI] [PubMed] [Google Scholar]
- 37.Rosenblum WI, Murata S, Nelson GH, Werner PK, Ranken R, Harmon RC. Anti-Cd31 delays platelet adhesion/aggregation at sites of endothelial injury in mouse cerebral arterioles. Am J Pathol. 1994;145: 33-36. [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenblum WI, Nelson GH, Wormley B, Werner P, Wang J, Shih CC-Y. Role of platelet-endothelial cell adhesion molecule (PECAM) in platelet adhesion/aggregation over injured but not denuded endothelium in vivo and ex vivo. Stroke. 1996;27: 709-711. [DOI] [PubMed] [Google Scholar]
- 39.Vollmar B, Schmits R, Kunz D, Menger MD. Lack of in vivo function of CD31 in vascular thrombosis. Thromb Haemost. 2001;85: 160-164. [PubMed] [Google Scholar]
- 40.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8: 1175-1180. [DOI] [PubMed] [Google Scholar]
- 41.Dubois C, Panicot-Dubois L, Furie B, Furie BC. Importance of GPVI in platelet activation and thrombus formation in vivo [abstract]. Blood. 2004;104: 240a. Abstract 842. [Google Scholar]
- 42.Day SM, Reeve JL, Myers DD, Fay WP. Murine thrombosis models. Thromb Haemost. 2004;92: 486-494. [PubMed] [Google Scholar]
- 43.Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric-chloride. Thromb Res. 1990;60: 269-280. [DOI] [PubMed] [Google Scholar]
- 44.Farrehi PM, Ozaki CK, Carmeliet P, Fay WP. Regulation of arterial thrombolysis by plasminogen activator inhibitor-1 in mice. Circulation. 1998;97: 1002-1008. [DOI] [PubMed] [Google Scholar]
- 45.Lyle EM, Lewis SD, Lehman ED, Gardell SJ, Motzel SL, Lynch JJ. Assessment of thrombin inhibitor efficacy in a novel rabbit model of simultaneous arterial and venous thrombosis. Thromb Haemost. 1998;79: 656-662. [PubMed] [Google Scholar]
- 46.Gibbins J, Asselin J, Farndale R, Barnes M, Law CL, Watson SP. Tyrosine phosphorylation of the Fc receptor gamma-chain in collagen-stimulated platelets. J Biol Chem. 1996;271: 18095-18099. [DOI] [PubMed] [Google Scholar]
- 47.Gibbins JM, Okuma M, Farndale R, Barnes M, Watson SP. Glycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor γ-chain. FEBS Lett. 1997;413: 255-259. [DOI] [PubMed] [Google Scholar]
- 48.Poole A, Gibbins JM, Turner M, et al. The Fc receptor γ-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16: 2333-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanaga F, Poole A, Asselin J, et al. Syk interacts with tyrosine-phosphorylated proteins in human platelets activated by collagen and cross-linking of the Fcγ-IIA receptor. Biochem J. 1995;311: 471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falati S, Edmead CE, Poole AW. Glycoprotein Ib-V-IX, a receptor for von Willebrand factor, couples physically and functionally to the Fc receptor gamma-chain, Fyn, and Lyn to activate human platelets. Blood. 1999;94: 1648-1656. [PubMed] [Google Scholar]
- 51.Marshall SJ, Asazuma N, Best D, et al. Glycoprotein IIb-IIIa-dependent aggregation by glycoprotein Ib alpha is reinforced by a Src family kinase inhibitor (PP1)-sensitive signalling pathway. Biochem J. 2002;361: 297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rathore V, Stapleton MA, Hillery CA, et al. PECAM-1 negatively regulates GPIb/V/IX signaling in murine platelets. Blood. 2003;102: 3658-3664. [DOI] [PubMed] [Google Scholar]
- 53.Thai LM, Ashman LK, Harbour SN, Hogarth PM, Jackson DE. Physical proximity and functional interplay of PECAM-1 with the Fc receptor Fc gamma RIIa on the platelet plasma membrane. Blood. 2003;102: 3637-3645. [DOI] [PubMed] [Google Scholar]
- 54.Mahooti S, Graesser D, Patil S, et al. PECAM-1 (CD31) expression modulates bleeding time in vivo. Am J Pathol. 2000;157: 75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falati S, Liu QD, Gross P, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197: 1585-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004;104: 3190-3197. [DOI] [PubMed] [Google Scholar]
- 57.Day SM, Reeve JL, Pedersen B, et al. Macrovscular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105: 192-198. [DOI] [PubMed] [Google Scholar]
- 58.Rosen ED, Raymond S, Zollman A, et al. Laser-induced noninvasive vascular injury models in mice generate platelet- and coagulation-dependent thrombi. Am J Pathol. 2001;158: 1613-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sim DS, Merrill-Skoloff G, Furie BC, Furie B, Flaumenhaft R. Initial accumulation of platelets during arterial thrombus formation in vivo is inhibited by elevation of basal cAMP levels. Blood. 2004;103: 2127-2134. [DOI] [PubMed] [Google Scholar]
- 60.Gurubhagavatula I, Amrani Y, Practico D, Ruberg FL, Albelda SM, Panettieri RA. Engagement of human PECAM-1 (CD31) on human endothelial cells increases intracellular calcium ion concentration and stimulates prostacyclin release. J Clin Invest. 1998;101: 212-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.