Abstract
This 2-part, double-blind, placebo-controlled study was conducted to determine the safety and efficacy of etoricoxib, a COX-2 selective inhibitor, for the treatment of hemophilic arthropathy. In part 1 (6 weeks), 102 patients (≥ 12 years old) with hemophilic arthropathy were randomized to receive 90 mg etoricoxib once daily or placebo (1:1 ratio). In part 2 (6 months), 51 patients taking placebo in part 1 were randomized to receive 90 mg etoricoxib or 25 mg rofecoxib once daily; patients taking etoricoxib in part 1 continued the same treatment. Efficacy end points included Patient Assessment of Arthropathy Pain, Patient Global Assessment of Arthropathy Disease Status, and Investigator Global Assessment of Arthropathy Disease Status. Safety was evaluated at each study visit. Etoricoxib provided significant improvement in all end points versus placebo (P < .001). Fewer patients taking etoricoxib discontinued due to a lack of efficacy versus placebo (P = .048). During part 2, efficacy was maintained; etoricoxib and rofecoxib demonstrated similar results. The most common adverse experiences were upper respiratory infection and headache. The incidence of joint bleeding during part 1 was similar between etoricoxib (66.7%) and placebo (72.6%) and during part 2 between etoricoxib (77.0%) and rofecoxib (78.9%). We conclude that etoricoxib provided superior efficacy versus placebo for the treatment of hemophilic arthropathy and was generally safe and well tolerated.
Introduction
Hemophilia is a bleeding disorder caused by inherited or spontaneous mutations in genes that code for clotting factor. The most common forms of hemophilia are the result of deficiencies in the coagulation factors VIII (hemophilia A) and IX (hemophilia B); both are X-linked recessive disorders making the prevalence of the disease in women rare. The most common form of hemophilia is factor VIII deficiency, which occurs in 1 of every 5000 male live births.1
The severity of hemophilia is directly linked to the magnitude of coagulation factor deficiency and is generally classified as mild, moderate, or severe.2 Severe hemophilia is defined as less than 1% of normal factor activity and accounts for about 40% of the cases.
In patients with hemophilia, prophylactic treatment consists of clotting factor replacement given at regularly scheduled intervals, often 2 to 3 times per week, to maintain plasma factor concentrations at levels sufficient to minimize recurrent bleeding into joints and muscles. Both prophylactic and “on demand” clotting factor replacement therapies have made major impacts for the treatment of hemophilia.3 Despite this success, intra-articular bleeding is still a major clinical manifestation of the disease. Bleeding occurs in the ankles, knees, elbows, hips, and shoulders and is often evident from early childhood. The inflammatory response resulting from intra-articular bleeding severely affects patients' quality of life by causing acute and chronic debilitating pain as well as a loss of function; this complication from hemophilia is known as hemophilic arthropathy.
In the early stages of hemophilic arthropathy, intra-articular hemorrhaging produces synovial inflammation (hemophilic synovitis) and results in synovial hypertrophy and new blood vessel growth.4 The new blood vessels are susceptible to further hemorrhage and may cause further inflammation. Although synovitis may be involved in the progression of the disease by inducing hyperplasia of vascular tissue of the synovium, inflammation in hemophilic arthropathy is mild in comparison to a highly inflammatory disorder such as rheumatoid arthritis. There is also evidence that direct exposure of cartilage to blood induces adverse effects such as inhibition of cartilage matrix synthesis and loss of matrix over time.5 Later stages of hemophilic arthropathy are characterized by advanced cartilage degeneration and joint destruction.2 These effects on cartilage are degenerative in nature, similar to what is seen in osteoarthritis.
Management of chronic hemophilic arthropathy is difficult. Narcotics can be used to alleviate the pain, but long-term use of this mode of analgesic therapy also may lead to tachyphylaxis, dependence, and the potential for abuse.6 Nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) have been used with caution in patients with bleeding disorders due to their inhibition of platelet function.7,8
Cyclooxygenase-2 (COX-2) selective inhibitors, which preferentially inhibit the COX-2 enzyme over the COX-1 enzyme, do not affect platelet thromboxane production9 and do not impair platelet function. Additionally, these agents present a lower risk for developing upper and lower gastrointestinal (GI) bleeding events compared with traditional NSAIDs.10,11 Therefore, potent COX-2 selective inhibitors such as etoricoxib may be well suited for use in patients with hemophilia. The present study was conducted to evaluate the efficacy and safety profiles of 90 mg etoricoxib, a COX-2 selective inhibitor, for the treatment of hemophilic arthropathy.
Patients, materials, and methods
Study population
For the 6-week base study, inclusion criteria were the following: a diagnosis of hemophilia A or B (factor VIII or factor IX deficiency with or without inhibitor), a history of joint bleeding, and chronic symptomatic pain in one or more joints on 20 of the 30 days prior to enrollment, and a diagnosis of hemophilic arthropathy at least 6 months prior to study start, with the primary source of pain or disability in the hip, knee, ankle, or elbow.
Patients also had to be at least 12 years of age, with a minimum weight of 40 kg, and judged to be in relatively good health with the exception of hemophilic arthropathy. Excess alcohol consumption (> 2 drinks a day) and unaccustomed physical activity were not permitted. Additionally, patients were required to have taken analgesic medication for at least 20 of the 30 days prior to enrollment and to have had a history of positive therapeutic benefit from these agents. Use of opioids/narcotics for more than 4 consecutive days during the month prior to enrollment was not permitted.
The study design included a flare period where prior analgesics were withdrawn to establish a baseline level of pain. Patients previously using acetaminophen were required to have demonstrated a minimum of 40 mm (arthropathy pain on a 0- to 100-mm visual analog scale [VAS]) at both screening (visit 1) and baseline (visit 2). Patients who used a nonacetylated salicylate or COX-2 inhibitor as prior therapy were required to have demonstrated hemophilic arthropathy pain defined as at least 40 mm (arthropathy pain on a 0- to 100-mm VAS) at screening and an increase of 15 mm following discontinuation of prior therapy.
For inclusion in the 6-month extension, patients had to either complete the 6-week base study or complete at least 2 weeks of therapy before discontinuing due to lack of efficacy; patients originally randomized to placebo were randomized to either etoricoxib 90 mg daily or rofecoxib 25 mg daily. Those who had experienced any serious or clinically important adverse experiences (AEs) during the first 6 weeks of treatment were ineligible for continuation. All patients who were screened and randomized into the study gave written informed consent. The study protocol was reviewed and approved by the local investigational review boards at each site.
Study design
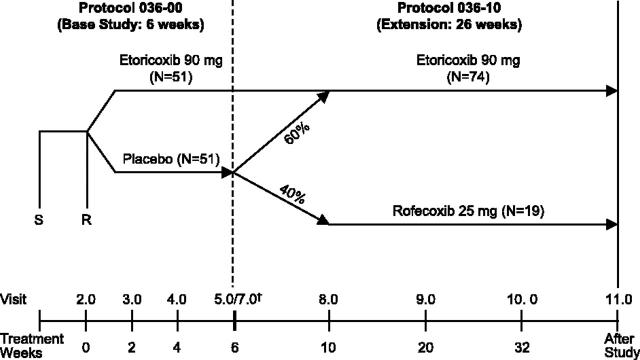
This 6-week, double-blind, placebo-controlled trial was conducted in 8 domestic (United States) and 7 international sites to evaluate the efficacy and safety profiles of 90 mg etoricoxib once daily versus placebo in the treatment of hemophilic arthropathy. This was followed by a 6-month double-blind, active comparator-controlled extension study with etoricoxib 90 mg and rofecoxib 25 mg (Figure 1).
Figure 1.
Study design and patient accounting. The dagger indicates that visit 7 was the first in the extension period. Visits 5.0 (last visit of the base study) and 7 (first visit of the extension study) occurred at the same time. Patients who did not continue into the extension completed the base study with a poststudy visit 6. S indicates screening visit; R, randomization visit.
Patients who met entry criteria were randomized 1:1 to placebo or 90 mg etoricoxib daily. Randomization to therapy was based on a computer-generated schedule and was stratified for prior analgesic use. Acetaminophen (paracetamol) was provided as “rescue therapy” for breakthrough arthropathy pain, and its use was recorded. Patients were instructed to not use acetaminophen within at least 12 hours before a study visit. Additional supplemental analgesic therapy (non-cyclooxygenase inhibitors), including short-acting opioids, was permitted for severe pain. Use of supplemental analgesia was not to exceed 3 days in duration and these therapies were to be withheld 48 hours prior to an efficacy evaluation visit. Patients were evaluated for safety and efficacy at weeks 2, 4, and 6.
Patients who completed this study, or who participated at least 2 weeks in part 1 and discontinued due to lack of efficacy, were eligible for the 6-month extension study. In contrast to the base study, concomitant analgesic therapy was allowed except for NSAIDs, COX-2 selective inhibitors, and nonacetylated salicylates. Patients who received placebo in the base study were randomized to etoricoxib 90 mg (60%) or rofecoxib 25 mg (40%). Patients who received etoricoxib 90 mg in the base study continued on the same therapy in the extension. Patients and investigators remained blinded with respect to study drug allocation. During the extension, patients were evaluated for safety and efficacy at weeks 10, 20, and 32 (Figure 1).
Efficacy measurements
The primary clinical end point of the study was the Patient Assessment of Arthropathy Pain (Visual Analog Scale; VAS). Secondary end points included the Patient Global Assessment of Arthropathy Disease Status (0-100-mm VAS) and Investigator Global Assessment of Arthropathy Disease Status (5-point Likert Scale). Other secondary end points were only measured during the base study: Patient's Global Assessment of Response to Therapy (0-4-point Likert Scale), Investigator's Assessment of Response to Therapy, Patient Discontinuation Due to Lack of Efficacy, and Average Rescue Acetaminophen Usage per Day.
Safety and tolerability assessments
AEs reported by the patient and investigator were documented at each study visit. All potential cardiovascular thrombotic events and GI events that included perforations, ulcers, or bleeding (PUBs) were submitted to external, blinded adjudication committees for review using prespecified case definitions.
Statistical analysis
The primary efficacy assessment for the base study was based on a comparison of change from baseline between etoricoxib 90 mg and placebo computed from a time-weighted average of responses across weeks 2, 4, and 6, and any discontinuation visit using a modified intention-to-treat approach. All patients who had a baseline value and at least one posttreatment measurement in the base study were included in this analysis population. Responses were weighted by the time since the previous visit. Efficacy end points were analyzed by an analysis of covariance (ANCOVA) model, including terms for treatment and baseline measurements of appropriate efficacy variables as covariates. For end points with no baseline values, measurements from similar end points that did have baseline values were used as the covariate. The study sample size had 90% power (α = 0.05, 2-tailed) to detect a between-treatment difference of 14.5 mm in patient assessment of arthropathy pain, based on variability in pain measurements (100-mm VAS) seen in an etoricoxib phase 2B study by Gottesdiener et al24 in osteoarthritis patients.
In both the base study and the 6-month extension study, safety and tolerability were addressed by summarizing counts and percentages of patients with AEs and comparing between treatment groups. Maintenance of clinical efficacy during the 6-month extension was addressed by computing the least-squares (LS) mean changes from baseline (randomization visit in the base study) plus or minus SE over time.
Results
Patient accounting
The primary study joints were the ankle, elbow, hip, or knee; the percent of patients with each primary study joint was similar between treatment groups. Of the 120 patients screened for the 6-week base study, 102 patients were randomized to treatment with 51 patients in each treatment group. Baseline characteristics were generally similar between the placebo and 90-mg etoricoxib treatment groups (Table 1). Of the 102 patients who entered the study, 76 completed the 6-week base study. Twenty-one patients discontinued due to lack of efficacy (29.4% of patients in the placebo group and 11.8% of patients in the etoricoxib group). One patient in each group discontinued due to clinical AEs. Three patients were discontinued due to noncompliance.
Table 1.
Baseline patient characteristics
|
6-wk base study
|
6-mo extension study
|
|||
|---|---|---|---|---|
| Characteristic | Placebo | Etoricoxib 90 mg | Rofecoxib 25 mg | Etoricoxib 90 mg |
| No. of patients | 51 | 51 | 19 | 74 |
| Age, y | ||||
| Mean (SD) | 35.3 (13.2) | 33.9 (13.5) | 31.3 (10.79) | 35.3 (13.6) |
| Range* | 12-60 | 14-71 | 12-47 | 12-71 |
| Race, no. (%) | ||||
| Hispanic American | 11 (21.6) | 8 (15.7) | 4 (21.1) | 14 (18.9) |
| Multiracial | 15 (29.4) | 20 (39.2) | 6 (31.6) | 28 (37.8) |
| White | 24 (47.1) | 22 (43.1) | 8 (42.1) | 31 (41.9) |
| Other | 1 (2.0) | 1 (2.0) | 1 (5.3) | 1 (1.4) |
| Body weight, kg | ||||
| Mean (SD) | 68.76 (14.59) | 68.97 (15.94) | 68.1 (15.8) | 68.5 (14.5) |
| Range | 39.30-95.00 | 42.10-101.90 | 40.8-92.3 | 42.1-101.9 |
| Degree of hemophilia†, no. (%) | ||||
| Mild | 5 (9.8) | 0 (0.0) | 0 (0.0) | 5 (6.8) |
| Moderate | 13 (25.5) | 16 (31.4) | 8 (42.1) | 17 (23.0) |
| Severe | 33 (64.7) | 35 (68.6) | 11 (57.9) | 52 (70.3) |
| History of ulcer or upper GI bleeding‡, no. (%) | 11 (21.6) | 9 (17.6) | 2 (15.8) | 12 (16.2) |
| Factor VIII deficiency, no. (%) | 44 (86.3) | 46 (90.2) | 17 (89.5) | 66 (89.2) |
| Factor IX deficiency, no. (%) | 7 (13.7) | 5 (9.8) | 2 (10.5) | 8 (10.8) |
| HIV as secondary diagnosis§, no. (%) | 13 (25.5) | 11 (21.6) | 5 (26.3) | 18 (24.3) |
| Hepatitis C as secondary diagnosis∥, no. (%) | 18 (35.3) | 22 (43.1) | 7 (36.8) | 30 (40.5) |
Six and 5 patients in the placebo group and etoricoxib group, respectively, were younger than 18 years of age
Mild is factor level > 5%; moderate, factor level 1% to 5%; severe, factor level < 1%
All patients with a history of an ulcer also had a history of upper GI bleeding
HIV serology was not evaluated as part of the study. The data are based on reported history
Patients had active disease at the time of randomization
Seventy-five patients who completed the base study entered the extension study. An additional 18 patients who discontinued due to lack of efficacy in the base study entered the extension. Patients taking etoricoxib in the base study continued on the same therapy during the extension. Patients on placebo in the base study were either given etoricoxib or rofecoxib. There were 74 patients in the 90-mg etoricoxib treatment group and 19 patients in the 25-mg rofecoxib treatment group. Of the 93 patients who entered the extension, 81 (87.1%) completed the study. No discontinuations occurred in the rofecoxib group. Of the patients who discontinued in the etoricoxib group, 9 (12.2%) discontinued due to clinical AEs, 1 (1.4%) due to lack of efficacy, and 2 (2.7%) withdrew consent.
Efficacy
During the base study, etoricoxib demonstrated significantly superior efficacy relative to placebo. There was a significantly larger decrease in pain for patients taking etoricoxib compared with those on placebo according to the primary end point, Patient Assessment of Arthropathy Pain (P < .001). This was observed at the first study visit (week 2) after therapy began and was sustained throughout the 6-week period (Figure 2). The LS mean difference from placebo was -19.44 mm (95% CI, -28.87, -10.01).
Figure 2.
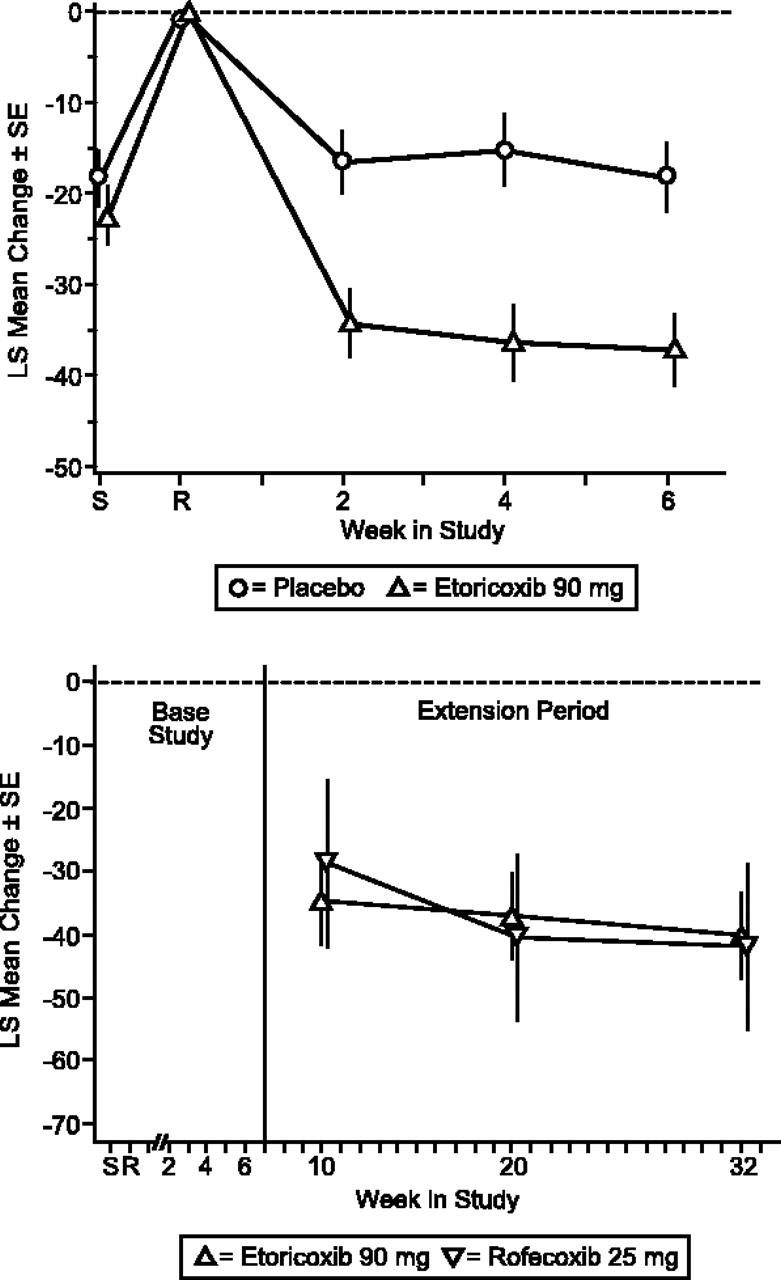
Patient Global Assessment of Arthropathy Pain (VAS). LS mean change from baseline ± SE placebo-controlled (top) and extension period (bottom).
Etoricoxib treatment resulted in significantly greater efficacy versus placebo for Patient and Investigator Global Assessments of Disease Status (P < .001; Figures 3, 4). The LS mean difference between etoricoxib and placebo was -14.64 (95% CI, -23.01, -6.28) for Patient's Global Assessment of Arthropathy Disease Status and -0.70 (95% CI, -1.05, -0.36) for Investigator's Global Assessment of Arthropathy Disease Status. Additionally, the difference in LS mean between etoricoxib and placebo was significant (P < .001) for Patient's Global and Investigator's Assessments of Response to Therapy. The number of patients discontinuing due to lack of efficacy was significantly less in the etoricoxib group relative to the placebo group as well (P = .048). Patients in the etoricoxib group used less acetaminophen (0.49 tablets/d) than those in the placebo group (0.75 tablets/d), although this difference was not statistically significant. There were also 5 (9.8%) patients in the placebo group and 1 (2.0%) patient in the etoricoxib group who took acetaminophen plus codeine phosphate; 3 (5.9%) patients receiving etoricoxib took meperidine hydrochloride as well.
Figure 3.
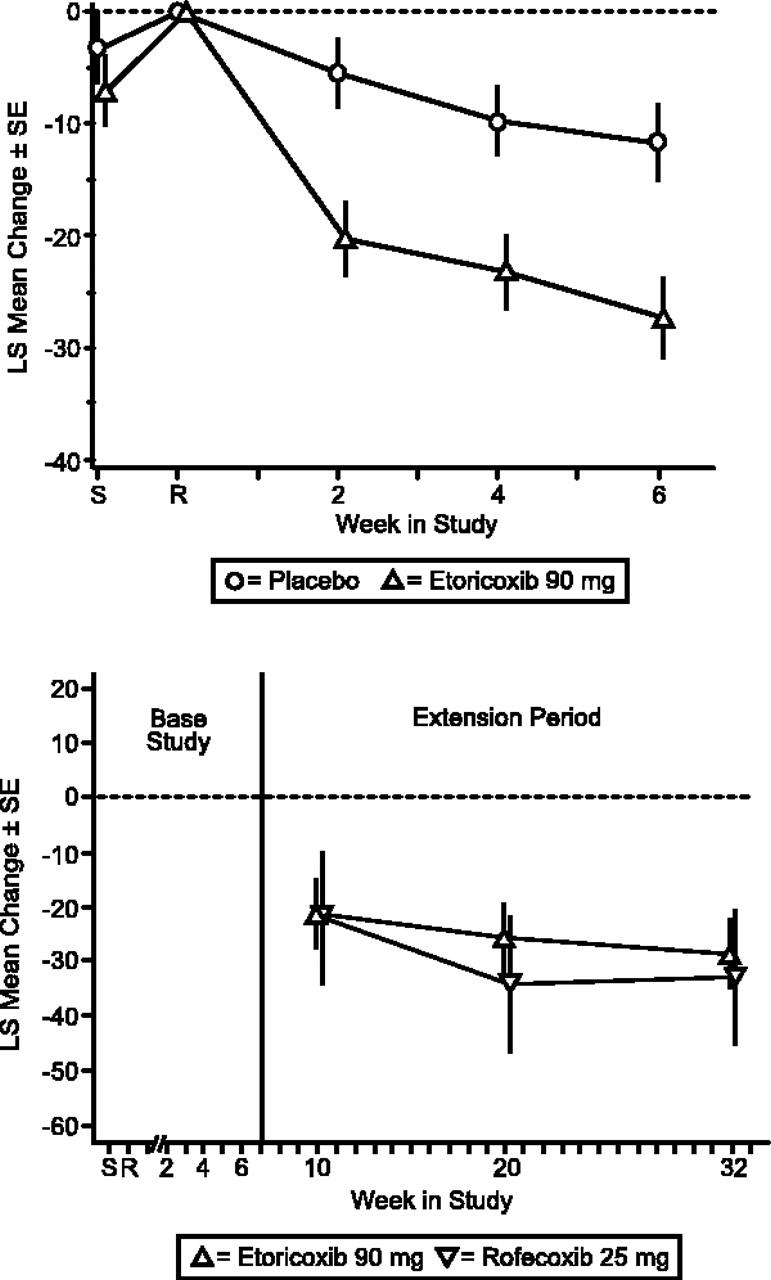
Patient Global Assessment of Arthropathy Disease Status (VAS). LS mean change from baseline ± SE extension period.
Figure 4.
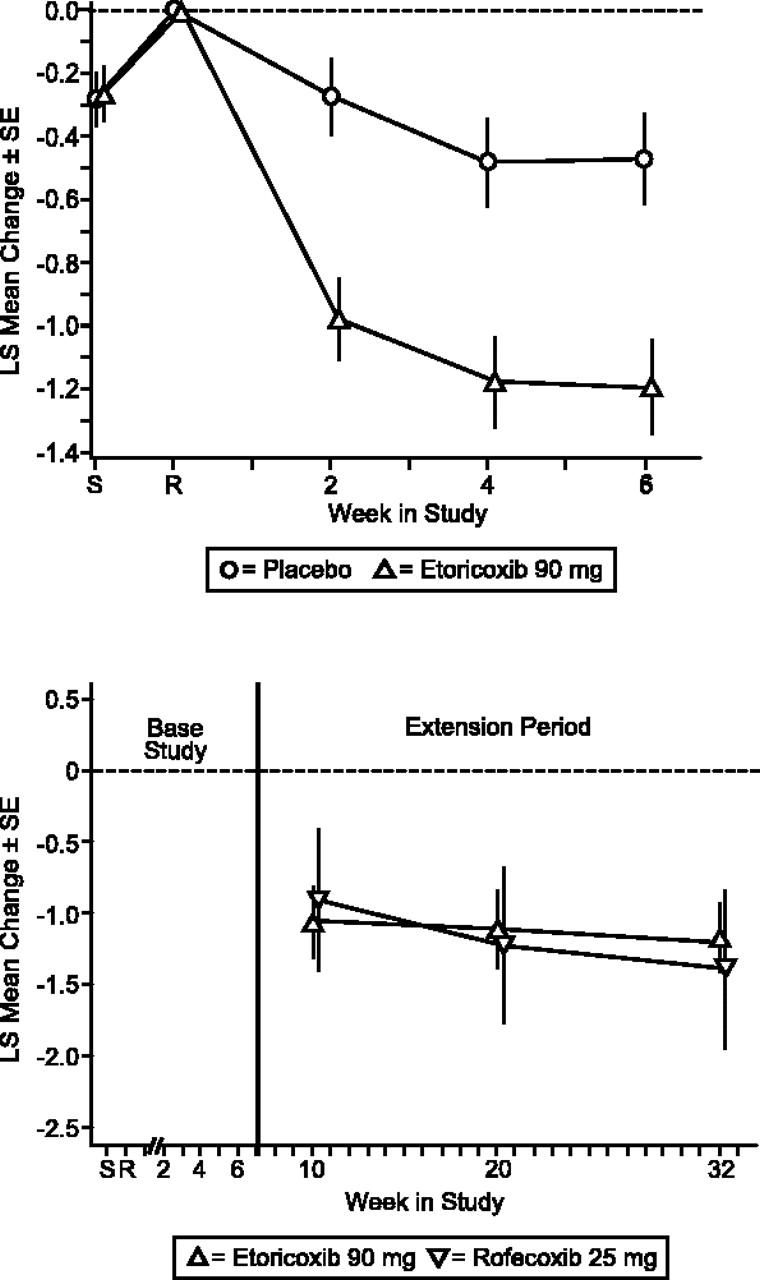
Investigator Global Assessment of Arthropathy Disease Status (Likert). LS mean change from baseline ± SE extension period.
The clinical improvements demonstrated by the etoricoxib patient group during the base period were maintained throughout the 6-month extension period. Treatment with etoricoxib and rofecoxib produced a similar magnitude and maintenance of efficacy over baseline in the extension period for the primary end point, Patient Global Assessment of Arthropathy Pain, and the 2 secondary end points, Patient Global Assessment of Arthropathy Disease Status, and Investigator Global Assessment of Arthropathy Disease Status (Figures 2, 3, 4).
Safety
Six-week placebo-controlled period. During the 6-week base study, 51% of patients on etoricoxib had a clinical AE compared with 29% of patients on placebo; this difference was statistically significant (P = .043). The most common AEs were upper respiratory infection and headache (Table 2). In the digestive body system, there were more AEs with etoricoxib than with placebo. There were 2 patients (3.9%), both in the etoricoxib group, who had serious AEs; one patient had traumatic arthropathy and hemarthrosis and another patient had a bleeding duodenal ulcer. The patient with the bleeding duodenal ulcer discontinued the study. The only other discontinuation due to a clinical AE, asthma, was a patient in the placebo group.
Table 2.
Summary of AEs
|
6-wk base study
|
6-mo extension study
|
|||
|---|---|---|---|---|
| Placebo | Etoricoxib 90 mg | Rofecoxib 25 mg | Etoricoxib 90 mg | |
| No. of patients | 51 | 51 | 19 | 74 |
| Any AE (%) | 15 (29.4) | 26 (51.0) | 10 (52.6) | 47 (63.5) |
| Any serious AE* (%) | 0 (0.0) | 2 (3.9) | 1 (5.3) | 5 (6.8) |
| Most common AEs†, no. of patients (%) | ||||
| Upper respiratory infection | 1 (2.0) | 3 (5.9) | 2 (10.5) | 2 (2.7) |
| Headache | 5 (9.8) | 1 (2.0) | 1 (5.3) | 4 (5.4) |
| Nausea | 0 (0.0) | 1 (2.0) | 1 (5.3) | 3 (4.1) |
| Vomiting | 0 (0.0) | 1 (2.0) | 1 (5.3) | 3 (4.1) |
| Cough | 0 (0.0) | 0 (0.0) | 1 (5.3) | 3 (4.1) |
| Renovascular AEs, no. of patients (%) | ||||
| Lower extremity edema (LEE) | 0 (0.0) | 1 (2.0) | 1 (5.3) | 0 (0.0) |
| Congestive heart failure | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypertension (HTN)‡ | 0 (0.0) | 1 (2.0) | 1 (5.3) | 2 (2.7) |
No discontinuations due to LEE or HTN occurred.
Serious AEs included hemorrhoids, hemorrhagic duodenal/gastric ulcer, erosive gastritis, amputation, subdural hematoma, hemorrhage and hypotension
Incidence ≥ 5% in any treatment group
In addition to the hypertension AEs reported in the table, an additional patient receiving etoricoxib 90 mg experienced an AE of borderline hypertension in the 6-mo extension period
The incidence of joint bleeding during the 6-week treatment period was generally similar between the placebo and etoricoxib groups: 72.6% for the placebo group and 66.7% for the 90-mg etoricoxib group. The number of patients with factor replacement usage for joint bleeding episodes was also similar between placebo and etoricoxib: 68.6% for the placebo group and 64.7% for the etoricoxib group (Table 3).
Table 3.
Joint bleeding episodes and factor replacement usage
|
6-wk base study
|
6-mo extension study
|
|||
|---|---|---|---|---|
| Placebo | Etoricoxib 90 mg | Rofecoxib 25 mg | Etoricoxib 90 mg | |
| Joint bleeding episodes, no. of patients (%) | 37 (72.6) | 34 (66.7) | 15 (78.9) | 57 (77.0) |
| Factor use for joint bleeding episodes, no. of patients (%) | 35 (68.6) | 33 (64.7) | 15 (78.9) | 56 (75.7) |
Six-month active comparator controlled extension study. In the 6-month extension, 63.5% of patients in the etoricoxib group had clinical AEs compared with 52.6% of patients in the rofecoxib group. The proportion of patients with serious AEs was similar between the 2 treatment groups (Table 2). The most common AEs were upper respiratory infection, headache, nausea, vomiting, and cough (Table 2). In the etoricoxib group, 9 of 74 patients (12.2%) discontinued due to clinical AEs, whereas none of the 19 patients discontinued in the rofecoxib group. Events that led to discontinuations included GI AEs as well as those typical of hemophilic arthropathy patients: hemorrhagic duodenal ulcer, erosive gastritis, edema, subdural hematoma, dark stool, abdominal pain, heartburn, and nausea. All AEs that led to discontinuation were moderate in intensity with the exception of the hemorrhagic duodenal ulcer, which was severe. The incidence of serious AEs was similar: 6.8% with etoricoxib and 5.3% with rofecoxib. Serious AEs included hemorrhoids, hemorrhagic duodenal/gastric ulcer, erosive gastritis, amputation, and subdural hematoma in the etoricoxib group. In the rofecoxib group, the serious AEs of hemorrhage and hypotension occurred.
The incidence of joint bleeding during the 6-month extension period was similar between the rofecoxib and etoricoxib groups: 78.9% for the rofecoxib group and 77.0% for the 90-mg etoricoxib group. The number of patients with factor replacement usage for joint bleeding episodes was also similar between rofecoxib and etoricoxib: 78.9% for the rofecoxib group and 75.7% for the etoricoxib group (Table 3).
During both the 6-week base study and the 6-month extension, renovascular AEs were uncommon (Table 2) and there were no serious thrombotic cardiovascular events. In general, etoricoxib, rofecoxib, and placebo were well-tolerated.
Discussion
Hemophilic arthropathy is a painful and disabling condition that affects most patients with hemophilia.2 Treatment is difficult and discovering new modes of therapy that provide relief for the symptoms of hemophilic arthropathy without undue risk of serious bleeding events is important. Although physicians and patients have used nonselective NSAIDs for years in the treatment of this disorder, large, well-designed placebo-controlled trials of NSAIDs (or COX-2 selective inhibitors) have not been reported. Etoricoxib is a COX-2 selective inhibitor that effectively treats pain and inflammation in arthritic disorders12 without affecting platelet function.9 In the 6-week placebo-controlled portion of this study, etoricoxib demonstrated efficacy superior to placebo in the treatment of hemophilic arthropathy; in the extension study, efficacy was maintained over an additional 6 months of treatment. Etoricoxib was generally well tolerated in both the 6-week and 6-month extension studies, although there was a greater proportion of patients who had clinical AEs while receiving etoricoxib versus placebo.
Intra-articular bleeding is a common occurrence in patients with hemophilic arthropathy. However, medications with antiplatelet effects (ie, aspirin and nonselective NSAIDs) can potentially affect the number or severity of bleeding episodes and, consequently, the amount of factor replacement used. The possibility of nonselective NSAIDs increasing this risk of bleeding is a major limiting factor in providing adequate pain relief because these episodes can exacerbate the disease process and cause increased joint pain and damage. In this study, the incidence of joint bleeding in the etoricoxib group during the 6-week period was similar to that of placebo. During the 6-month extension, the incidence of these bleeding episodes was similar to that of the rofecoxib group. These data suggest that treatment with etoricoxib is not associated with an increase in joint bleeding when used in patients with hemophilic arthropathy.
Patients with hemophilic arthropathy are at elevated risk of exacerbating or developing GI lesions. Most patients have severe hemophilia and thus are theoretically at high risk for bleeding events; prior to entering this study, 20% of patients had experienced a previous upper GI bleeding event. Previous analyses7,13 have also suggested a prevalence of approximately 20% for upper GI bleeding in patients with hemophilia. The risk of lower GI bleeding is unknown in this patient population, although 32% of hospitalizations due to GI events in patients with osteoarthritis was due to a lower GI event in an analysis of the Arthritis, Rheumatism, and Aging Medical Information System data bank of arthritis patients.14 The absolute risk for developing a new upper or lower GI lesion in the hemophilic arthropathy patient population is unknown. During this study, the incidence of GI bleeding was relatively low.
In previous analyses, etoricoxib was associated with an approximate 50% risk reduction in serious upper GI side effects (ie, PUBs) than in patients treated with nonselective NSAIDs.15 Although the relative risk of serious GI events in patients with hemophilic arthropathy is likely to be lower in patients treated with etoricoxib compared with a nonselective NSAID, the magnitude of potential decreased relative risk is not known.
Prospectively designed outcome studies will be required to quantify the relative risk of GI events following use of COX-2 selective inhibitors in this patient population. In this study, none of the GI bleeding events occurred while patients were taking concomitant H2 blockers or proton pump inhibitors. Because the GI bleeding events occurred in the absence of concomitant gastroprotective agents, it may be wise for patients initiating therapy for hemophilic arthritis to also consider the option of concomitant treatment with proton pump inhibitors or other GI protective agents as an added risk-reducing measure.
Treatment with both NSAIDs and COX-2 selective inhibitors can affect renal physiology.16 In this study, renovascular AEs rarely occurred and only one patient in the etoricoxib group discontinued as a result of a renovascular-related experience (edema) during the 6-month extension. Furthermore, no thrombotic cardiovascular events occurred in either the placebo-controlled or extension treatment periods.
Discontinuations because of clinical AEs were similar to placebo and occurred in only one patient in each treatment group during the 6-week placebo-controlled base study. In the 6-month extension, discontinuations because of clinical AEs occurred in a higher proportion of patients receiving etoricoxib. All patients who discontinued due to a clinical AE in this study recovered after discontinuation. In general, discontinuations due to clinical AEs occur in higher proportions of patients during trials evaluating long-term therapy with analgesic medications.17,18 The proportion of patients that discontinued due to an AE in this study is similar to proportions seen in other trials of long duration that evaluate analgesic treatments. Furthermore, the discontinuation rate may be also reflective of the comorbidity of patients with hemophilic arthropathy, since a large percentage of these patients have serious secondary diagnoses such as HIV or hepatitis C infections as well as the primary disease of hemophilia.
As an alternative to nonselective NSAIDs, acetaminophen is often used for the management of pain in patients with hemophilic arthropathy. Although acetaminophen is effective for mild to moderate pain, such as for the osteoarthritis and acute pain from dental procedures,19,20 it does not possess the anti-inflammatory activity observed with NSAIDs.21,22 In the case of osteoarthritis, some studies performed with NSAIDs suggest greater efficacy in comparison to acetaminophen.23 The anti-inflammatory effects of COX-2 inhibition may explain this difference in efficacy between NSAIDs and acetaminophen. This study was limited, in that it was not designed to compare the effects of etoricoxib with those of acetaminophen. Nonetheless, during the placebo-controlled period of the present study, a greater amount of acetaminophen was used by patients who received placebo compared with those taking etoricoxib. Despite the greater use of acetaminophen in the placebo group, etoricoxib demonstrated superior efficacy versus placebo.
In summary, treatment with etoricoxib provided clinically significant efficacy for the treatment of hemophilic arthropathy symptoms. The onset of efficacy was rapid, with patients showing clinical improvement within 2 weeks, and efficacy was maintained for an additional 6 months beyond the initial 6-week study. Etoricoxib was also generally safe and well tolerated in this study. These results suggest that etoricoxib may be a valuable treatment option for this patient population. Additional studies to further evaluate the efficacy and safety of COX-2 selective inhibitors for hemophilic arthropathy should be considered.
Note added in proof. On September 30, 2004, Merck announced the voluntary, worldwide withdrawal of rofecoxib from the market. This study was conducted prior to the withdrawal of rofecoxib.
Acknowledgments
Primary investigators involved in this study were the following: Thomas Abshire, Diana Beardsley, Cindy Leissinger, Brad Lewis, David Green, Horacio Caviglia, Saúl Mendoza, Henry Ekert, Norma Bosch, Linamara Battistella, Sandra Quintana, and Robert Janco. The authors would also like to thank Anish Mehta for his writing and editorial contribution to the manuscript as well as Paul Cavanaugh and Jan Markind for their critical review.
Prepublished online as Blood First Edition Paper, November 15, 2005; DOI 10.1182/blood-2004-09-3501.
Supported by a grant from Merck.
S.S., S.M., K.M.G., S.P.C., A.S.R., and A.M. are employed by Merck & Co., Inc., whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Mannucci PM, Tuddenham EG. The hemophilias—-from royal genes to gene therapy. N Engl J Med. 2001;344: 1773-1779. [DOI] [PubMed] [Google Scholar]
- 2.Upchurch KS, Brettler DB. Hemophilic Arthropathy. In: Buddy S, Harris ED, Sledge CB, eds. Kelley's Textbook of Rheumatology. Philadelphia, PA: WB Saunders; 2004: 1567-1574.
- 3.Berntorp E, Astermark J, Bjorkman S, et al. Consensus perspectives on prophylactic therapy for haemophilia: summary statement. Haemophilia. 2003;9(suppl 1): 1-4. [DOI] [PubMed] [Google Scholar]
- 4.Luck JV Jr, Silva M, Rodriguez-Merchan EC, et al. Hemophilic arthropathy. J Am Acad Orthop Surg. 2004;12: 234-245. [DOI] [PubMed] [Google Scholar]
- 5.Roosendaal G, Lafeber FP. Blood-induced joint damage in hemophilia. Semin Thromb Hemost. 2003;29: 37-42. [DOI] [PubMed] [Google Scholar]
- 6.Cherny NL. Opioid analgesics—comparative features and prescribing guidelines. Drugs. 1996;51: 713-737. [DOI] [PubMed] [Google Scholar]
- 7.Cagnoni PJ, Aledort L. Gastrointestinal bleeding in hemophilia as a complication of the use of over the counter non-steroidal anti-inflammatory drugs. Am J Hematol. 1994;47: 336-337. [DOI] [PubMed] [Google Scholar]
- 8.Hilgartner MW. Current treatment of hemophilic arthropathy. Curr Opin Pediatr. 2002;14: 46-49. [DOI] [PubMed] [Google Scholar]
- 9.Dallob A, Hawkey CJ, Greenberg H, et al. Characterization of etoricoxib, a novel, selective COX-2 inhibitor. J Clin Pharmacol. 2003;43: 573-585. [PubMed] [Google Scholar]
- 10.Ramey D, Watson DJ, Yu C, et al. The incidence of upper gastrointestinal adverse events in clinical trials of etoricoxib vs. non-selective NSAIDs: an updated combined analysis. Curr Med Res Opin. 2005;21: 715-722. [DOI] [PubMed] [Google Scholar]
- 11.Laine L, Connors LG, Reicin A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003;124: 288-292. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto AK, Cavanaugh PF Jr. Etoricoxib. Drugs Today (Barc). 2004;40: 395-414. [PubMed] [Google Scholar]
- 13.Forbes CD, Barr RD, Prentice CR, Douglas AS. Gastrointestinal bleeding in haemophilia. Q J Med. 1973;42: 503-511. [PubMed] [Google Scholar]
- 14.Singh G, Rosen RD. NSAID induced gastrointestinal complications: the ARAMIS perspective—1997. Arthritis, Rheumatism, and Aging Medical Information System. J Rheumatol Suppl. 1998;51: 8-16. [PubMed] [Google Scholar]
- 15.Hunt RH, Harper S, Callegari P, et al. Complementary studies of the gastrointestinal safety of the cyclo-oxygenase-2-selective inhibitor etoricoxib. Aliment Pharmacol Ther. 2003;17: 201-210. [DOI] [PubMed] [Google Scholar]
- 16.Brater DC, Harris C, Redfern JS, Gertz BJ. Renal effects of cox-2-selective inhibitors. Am J Nephrol. 2001;21: 1-15. [DOI] [PubMed] [Google Scholar]
- 17.Birbara CA, Puopolo AD, Munoz DR, et al. Treatment of chronic low back pain with etoricoxib, a new cyclo-oxygenase-2 selective inhibitor: improvement in pain and disability-a randomized, placebo-controlled, 3-month trial. J Pain. 2003;4: 307-315. [DOI] [PubMed] [Google Scholar]
- 18.Ruperto N, Nikishina I, Pachanov ED, et al. A randomized, double-blind clinical trial of two doses of meloxicam compared with naproxen in children with juvenile idiopathic arthritis: short- and long-term efficacy and safety results. Arthritis Rheum. 2005;52: 563-572. [DOI] [PubMed] [Google Scholar]
- 19.Skjelbred P, Lokken P. Paracetamol versus placebo: effects on post-operative course. Eur J Clin Pharmacol. 1979;15: 27-33. [DOI] [PubMed] [Google Scholar]
- 20.Bertin P, Keddad K, Jolivet-Landreau I. Acetaminophen as symptomatic treatment of pain from osteoarthritis. Joint Bone Spine. 2004;71: 266-274. [DOI] [PubMed] [Google Scholar]
- 21.Boardman PL, Hart FD. Clinical measurement of the anti-inflammatory effects of salicylates in rheumatoid arthritis. Br Med J. 1967;4: 264-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ring EF, Collins AJ, Bacon PA, Cosh JA. Quantitation of thermography in arthritis using multi-isothermal analysis, II: effect of nonsteroidal anti-inflammatory therapy on the thermographic index. Ann Rheum Dis. 1974;33: 353-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pincus T, Koch GG, Sokka T, et al. A randomized, double-blind, crossover clinical trial of diclofenac plus misoprostol versus acetaminophen in patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2001;44: 1587-1598. [DOI] [PubMed] [Google Scholar]
- 24.Gottesdiener K, Schnitzer T, Fisher C, et al. Results of a randomized, dose-ranging trial of etoricoxib in patients with osteoarthritis. Rheumatol. 2002:41: 1052-1061. [DOI] [PubMed] [Google Scholar]