Abstract
Transfection with synthetic mRNA is a safe and efficient method of delivering antigens to dendritic cells for immunotherapy. Targeting antigens to the lysosome can sometimes enhance the CD4+ T-cell response. We transfected antigen-presenting cells (APCs) with mRNA encoding Gag-p24 and cytoplasmic, lysosomal, and secreted forms of Nef. Antigen-specific cytotoxic T cells were able to lyse the majority of transfected targets, indicating that transfection was efficient. Transfection of APCs with a Nef construct bearing lysosomal targeting signals produced rapid and prolonged antigen presentation to CD4+ and CD8+ T cells. Polyclonal CD4+ and CD8+ T-cell lines recognizing multiple distinct epitopes were expanded by coculture of transfected dendritic cells with peripheral blood mononuclear cells from viremic and aviremic HIV-infected subjects. Importantly, lysosome-targeted antigen drove a significantly greater expansion of Nef-specific CD4+ T cells than cytoplasmic antigen. The frequency of recognition of CD8 but not CD4 epitopes by mRNA-expanded T cells was inversely proportional to sequence entropy and was similar to ex vivo responses from a large chronic cohort. Thus human dendritic cells transfected with mRNA encoding lysosome-targeted HIV antigen can expand a broad, polyclonal repertoire of antiviral T cells, offering a promising approach to HIV immunotherapy.
Introduction
There is extensive evidence that cellular immunity plays a key role in control of HIV infection. In particular, certain HLA class I alleles are associated with prolonged survival and nonprogression to AIDS, strongly implicating CD8+ T cells in viral control.1-6 Although functional correlates of immune protection have been very difficult to characterize, recent reports have highlighted the potential importance of persistent CD8+ T-cell proliferative capacity in protection from AIDS.7 Lichterfeld et al8 demonstrated that in vitro this proliferative capacity was dependent on CD4+ T-cell help and could be restored by addition of autologous CD4+ T cells and that in subjects with chronic HIV infection proliferative capacity was restored in vivo by experimental vaccination that boosted the frequency of antiviral CD4+ T cells. Taken together, these findings support the idea that therapeutic immunization targeting both the CD4+ and CD8+ T-cell compartments is a promising method for boosting protective immunity in subjects with chronic HIV infection.
Dendritic cells (DCs) are the most potent antigen-presenting cells in the body and are capable of inducing CD4+ and CD8+ T-cell responses in vitro and in vivo. Because of this there is great interest in developing methods to exploit DCs to boost immune responses in cases where the immune response is qualitatively or quantitatively insufficient, such as cancer and HIV infection. Promising preliminary results have been reported for both these applications.9-12
Numerous methods have been used to load DCs with antigen for use in potential therapeutic vaccines. These include DNA transfection, transduction with live viral or retroviral vectors, and loading with chemically inactivated virus, recombinant protein, or synthetic peptides. In addition, it has been demonstrated that DCs can be loaded by transfection with synthetic mRNA encoding antigen from a variety of sources,13 including HIV.14 This method has several practical advantages, including high efficiency of transfection, lack of safety concerns related to DNA integration into the chromosome or adverse reaction to viral vectors, absence of any irrelevant vector antigens, and relative ease of production of new mRNA transcripts encoding novel antigens, such as autologous clinical isolates. DCs loaded with antigen by this method have already been used in several human trials for cancer immunotherapy, with promising results regarding immunogenicity and preliminary evidence of clinical benefits.11,15,16 Thus mRNA-transfected DCs may be a very useful tool for therapeutic immunization with HIV antigens.
We have adapted a reliable method for transfection of monocyte-derived dendritic cells (MDDCs), derived from subjects with chronic HIV infection, using synthetic mRNA encoding HIV antigens. Previous reports have shown that targeting antigenic constructs to the lysosomal/endosomal pathway in antigen-presenting cells can sometimes enhance presentation of HLA class II–restricted epitopes derived from tumor and viral antigens in both mice and humans.17-23 In this report we show that by expressing Nef protein as a lysosome-targeted chimeric antigen, we are able to stimulate in vitro robust polyclonal T-cell lines from the peripheral blood mononuclear cells (PBMCs) of chronic viremic and aviremic HIV-infected subjects. These polyclonal lines recognize multiple distinct epitopes and have a high proliferative capacity. Because both CD4+ and CD8+ T cells with diverse specificities are expanded under these conditions, targeted lysosomal expression of HIV antigens by mRNA transfection of MDDCs may provide the basis for a promising new form of immunotherapy for subjects with chronic HIV infection on highly active antiretroviral therapy (HAART.)
Patients, materials, and methods
Human subjects
For experiments using MDDCs, 25 subjects with chronic HIV infection were recruited from the Shattuck Hospital and from the Infectious Disease Clinic at the Massachusetts General Hospital (MGH). For ex vivo enzyme-linked immunospot (ELISpot) analysis of PBMC responses, a total of 347 HIV+ subjects were recruited from 4 hospitals in the Boston area. Individuals at all clinical stages with detectable HIV-specific cellular responses were included regardless of CD4 count, viral load, or treatment status. Approval was obtained from the MGH and Shattuck Hospital institutional review boards for these studies. Informed consent was provided according to the Declaration of Helsinki. All blood samples were obtained by venous puncture with anticoagulant citrate dextrose, and PBMCs were separated from fresh blood by Ficoll gradient centrifugation.
Cell culture
All cells were grown in HEPES-buffered RPMI medium supplemented with penicillin, streptomycin, L-glutamine, and plasma or serum. For lymphoblastoid B-cell lines (BLCLs), 10% fetal bovine serum (Sigma, St Louis, MO) was used. For generation of MDDCs, monocytes were adhered to plastic in 5% pooled human AB serum (PHS; Sigma) and propagated in 1% heparinized normal human plasma. T-cell lines were grown and assayed in 5% PHS.
Construction of viral antigen templates and in vitro transcription
Synthetic mRNA used in Figures 1 and 2 was transcribed directly from gag or nef polymerase chain reaction (PCR) products bearing a T7 polymerase promoter. All other synthetic transcripts were transcribed from linearized plasmid templates. HIV antigen genes used to construct templates were cloned from the NL4.3 molecular clone of HIV-1 or from a synthetic nef gene (DNA2.0, Menlo Park, CA) encoding the noncodon-optimized 2001 clade B consensus nucleotide sequence (submitted to GenBank: accession no. AY973547), which was derived from the Los Alamos National Laboratory database.24
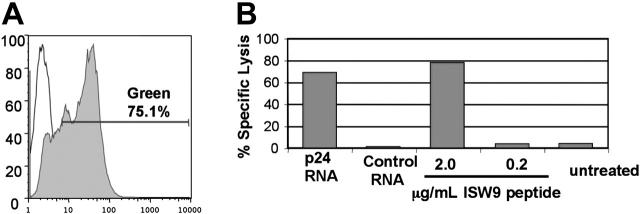
Figure 1.
mRNA transfection is an efficient method for antigen delivery to APCs. (A) Greater than 75% transfection efficiency of DCs electroporated with GFP mRNA. MDDCs were transfected with 10 μg GFP mRNA (filled histogram) or mock transfected (open histogram). The degree of GFP expression was measured by FACS 24 hours after transfection. (B) mRNA transfection primes APCs for lysis by specific CTLs. Lysis of HLA-B57+ BLCLs by a CTL clone specific for the B57-ISW9 epitope (ISPRTLNAW) of HIV p24 was determined with a 51Cr-release assay at an effector-target ratio of 3:1. BLCLs were transfected with 10 μg of mRNA encoding p24 or irrelevant control mRNA. The transfected cells were loaded with ISW9 peptide as positive control or left untreated as a negative control. Results shown are the average of duplicate wells. By comparing the percent specific lysis of p24-transfected targets (68%) to percent specific lysis of targets loaded with 2 μg/mL peptide (78%), we estimate that the transfection efficiency of BLCLs electroporated with p24 mRNA approaches 90%. Dose titration showed that 10 μg mRNA was optimal for this assay (not shown).
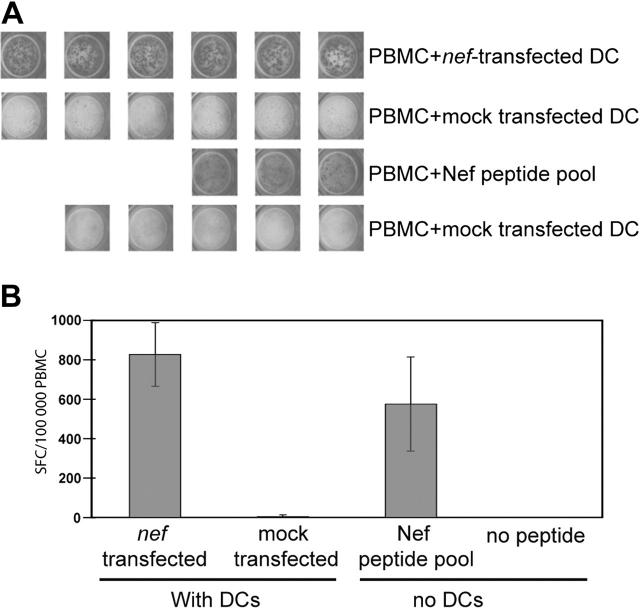
Figure 2.
Transfected MDDCs stimulate PBMC T cells ex vivo. PBMCs from an HIV+ subject were incubated in an overnight IFNγ ELISpot assay with or without transfected autologous DCs. DCs were transfected with 10 μg/106 DCs of consensus nef mRNA or were mock transfected. PBMCs without DCs were incubated with or without a Nef peptide pool matched to the RNA sequence. Each well contained 100 000 PBMCs. The PBMC/DC ratio was 10:1. (A) Digital images of replicate ELISpot wells. (B) Mean response of wells shown in panel A counted with an automated plate reader. Error bars represent 1 standard deviation; SFC, spot-forming cells.
Construction of a template for chimeric lysosome-targeted antigens was generally as described previously.19 We used a vector (pLyso) containing the following sequential elements: a T7 polymerase promoter, a Kozak ribosome binding motif including the start codon of the open reading frame (ORF), the endoplasmic reticulum (ER)–targeting translocation signal of gp96, an antigen insertion locus, the lysosomal targeting motif of LAMP-1, a stop codon, and a polyA tail sequence. Synthetic mRNA was prepared by transcription from linear a DNA template with the Message Machine kit (Ambion, Austin, TX). In Figures 1 and 2, transcripts made from PCR product templates lacking PolyA tails were polyadenylated with the PolyA Tail kit (Ambion). mRNA encoding cytoplasmic transcripts was transcribed from an identical vector lacking the ER-translocation and lysosomal-targeting motifs.
MDDC preparation and transfection
MDDCs were prepared as previously described.25 Briefly, PBMCs were washed several times in RPMI medium to remove platelets and were plated into Corning (Corning, NY) 6-well plates in 5% PHS medium and incubated for 60 minutes at 37°C to adhere monocytes. Nonadherent PBMCs were washed off the plate and frozen in 10% DMSO for future use. Adherent monocytes were grown in 1% normal human plasma for 5 days in the presence of IL-4 and GM-CSF. On day 5, MDDCs were harvested, transfected or mock transfected, and then matured for 24 hours with a cytokine cocktail containing IL-1β, TNFα, IL-6, and PGE2 as previously described.26
Transfection of APCs
For transfection, antigen-presenting cells (APCs) were washed 3 times and resuspended to a concentration of 3.3 × 106/mL in Optimem medium (Invitrogen, Carlsbad, CA). APCs (106) were incubated with 10 μg mRNA (or an equal volume of H2O for mock transfections) on ice for 10 minutes and then electroporated in a 0.2-cm cuvette with a GenePulser Xcell (BioRad, Hercules, CA) at 400 V/0.75 ms, square wave protocol.
T-cell–MDDC coculture assays
Frozen PBMCs were thawed, stained with CFSE, and plated out at 150 000 cells/well in a 96-well plate with 15 000 mature autologous MDDCs per well. Cultures were supplemented with IL-2 (50 U/mL) starting on day 3.
Intracellular cytokine staining and ELISpot
Overlapping peptides (OLPs) were designed and synthesized as previously described.27 To initiate the ICS assay using synthetic peptide, T cells were incubated with 5 μg/mL synthetic peptide for 1 hour, after which brefeldin A (BFA) was added to each sample to a final concentration of 10 μg/mL. T cells were incubated for an additional 5 hours before being stained, fixed, and permeabilized using the Fix/Perm kit (Becton Dickinson, San Jose, CA). Fluorescence-activated cell sorter (FACS) analysis was performed with FACSCalibur or LSR2 flow cytometers (Becton Dickinson).
ELISpot assays were performed as previously described.27 To determine specificity of T-cell lines expanded with Nef mRNA, T-cell lines were tested separately with each of the 27 Nef OLPs. Ex vivo responses were determined as previously described. Briefly, PBMCs were tested by ELISpot with a consensus peptide matrix spanning the HIV genome, and positive responses were confirmed with individual peptides. In some cases prior to ELISpot, T-cell lines were depleted of CD8+ or CD4+ T cells using anti-CD8 or -CD4 magnetic beads (Dynal Biotech, Lake Success, NY) according to manufacturer's instructions.
Statistical analysis
T-cell responses expanded by lysosomal and cytoplasmic constructs (Figure 4B) were compared using the Wilcoxon matched-pairs test. For each subject, the magnitude of the net CD4 response expanded by lysosomal antigen was paired with the magnitude of the net CD4 response expanded by cytoplasmic antigen, and a 2-tailed P value was calculated. The analysis was repeated for CD8 responses.
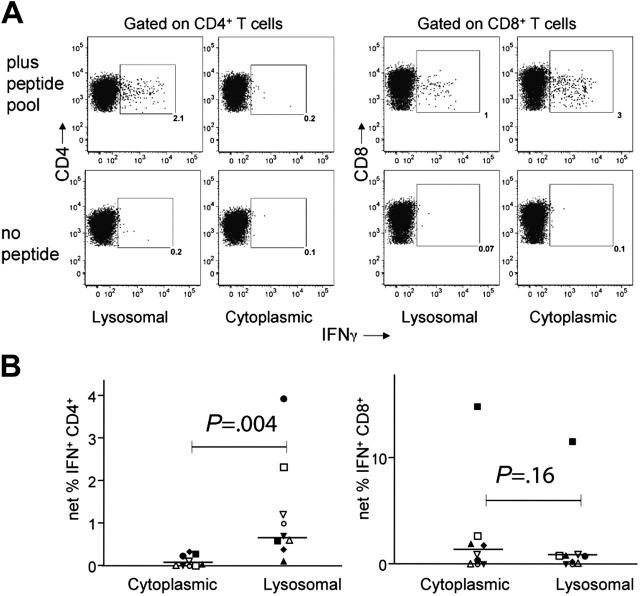
Figure 4.
Lysosomal targeting enhances the expansion of Nef-specific CD4+ T cells from PBMCs. MDDCs were derived from blood of 9 chronic HIV+ subjects, transfected with consensus LysoNef or CytoNef constructs, matured, and cocultured with autologous PBMCs for 12 to 14 days. To determine percent specificity of expanded T cells, resulting T-cell lines were tested in an ICS assay with the consensus pool of overlapping Nef peptides or with no added antigen. Results from a representative subject are shown in panel A; values within the gate represent the percentage IFN+ of CD4+ or CD8+ T cells. To determine the net response, the background response was subtracted from the response to peptide pool; thus for LysoNef and CytoNef, respectively, the net responses in panel A are 1.9% and 0.1% of CD4+ T cells and 0.9% and 2.9% of CD8+ T cells. (B) CD4 and CD8 responses are shown for all 9 subjects; each subject is represented by one symbol. The LysoNef construct expanded a greater CD4 response than the CytoNef construct for 9 of 9 subjects. The magnitudes of responses to LysoNef and CytoNef were compared using the Wilcoxon matched-pairs test (P values as shown). Lysosomal targeting produced a significant increase in CD4 responses compared with cytoplasmic targeting. Conversely, there was no significant difference in the magnitudes of the CD8 responses.
The breadth of responses shown in Table 1 was compared with the corresponding clinical parameters by determining the Spearman rank coefficient and significance level for CD4 count versus breadth of CD4 response, CD4 count versus breadth of CD8 response, viral load versus breadth of CD4 response, and viral load versus breadth of CD8 response; P was greater than .2 in all cases (no significant correlation).
Table 1.
Breadth of response for 13 expanded T-cell lines
| Subject | CD4 count | Viral load, copies/mL | No. of CD4 epitopes, min/max | No. of CD8 epitopes, min/max |
|---|---|---|---|---|
| 1 | 336 | 96 | 3/4 | 1/2 |
| 2 | 276 | < 75 | 2 | 1 |
| 3 | 473 | 8 940 | 0 | 2 |
| 4 | 980 | < 75 | 2/4 | 1 |
| 5 | 215 | < 75 | 3/5 | 2/3 |
| 6 | 308 | < 75 | 1/2 | 2/3 |
| 7 | 337 | < 75 | 2/3 | 4/6 |
| 8 | 116 | 6 299 | 1 | 1-2 |
| 9 | 217 | < 75 | 0 | 2 |
| 10 | 381 | 33 600 | 1 | 1-2 |
| 11 | 255 | < 75 | 1/2 | 3/4 |
| 12 | 505 | < 75 | 0 | 1/2 |
| 13 | 234 | 434 | 0 | 1 |
| Total | NA | NA | 16/24 | 22/31 |
For responses to adjacent OLPs, a minimal value (assuming that any 2 adjacent responses represent a single epitope) and a maximal value (assuming that adjacent responses represent discrete epitopes) were calculated. Limiting dilution analysis (not shown) identified discrete epitopes within adjacent OLPs recognized by some T-cell lines. There were no significant correlations between clinical parameters and breadth of expanded CD4+ or CD8+ T-cell responses (see “Patients, materials, and methods”).
NA indicates not applicable.
The frequencies of recognition of individual peptides recognized by ex vivo PBMCs and expanded T-cell lines (Figure 6B) were compared by determining the Spearman rank coefficient. Spearman r and associated 2-tailed P values are reported. Spearman r and associated 2-tailed P values are also reported for individual peptides and peptide entropy (Figure 6C).
Figure 6.
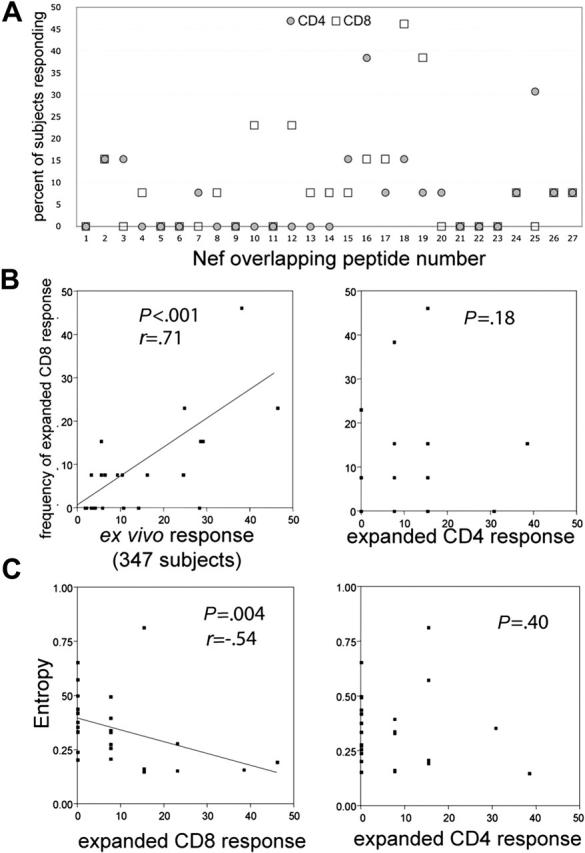
Distribution of epitopes recognized by expanded CD4+ and CD8+ T cells. T-cell lines were expanded from the blood of 13 chronic HIV+ subjects by coculture with LysoNef-transfected DCs. Individual OLPs recognized by each line were identified by ELISpot; each epitope was categorized as being recognized by CD4+ or CD8+ T cells by ICS or by ELISpot following specific depletion with anti-CD4 or -CD8 beads. The relative frequency of recognition was calculated for each of the 27 Nef OLPs. For comparison, the distribution of responses in expanded T cells was compared with the ex vivo responses detected in the PBMCs of 347 chronic HIV+ subjects. (A) The distribution of CD8 and CD4 responses in the DC-expanded T-cell lines. (B) Expanded CD8 responses from the 13 lines were compared with ex vivo responses in PBMCs of 347 chronic subjects and expanded CD4 responses. (C) The frequency of CD8 but not CD4 responses is inversely correlated to OLP entropy. For each OLP, the frequency of recognition was plotted against the average Shannon entropy score for amino acids within the OLP. Entropy values were calculated as previously described.27
Calculations were performed with GraphPad Prism 3.0 for Macintosh (GraphPad Software, San Diego, CA).
Results
Transfection with synthetic mRNA delivers HIV antigen to APCs with high efficiency
In order to boost immune responses in vivo and measure T-cell activity in vitro, it is desirable to deliver HIV antigens reproducibly to a very high percentage of APCs. Previous reports have shown that transfection with synthetic mRNA can be an efficient way to deliver antigens,11 including HIV antigens,14 to APCs. We used 2 methods to estimate the efficiency of antigen delivery to transfected APCs. First, we transfected MDDCs with mRNA encoding GFP. As shown in Figure 1A, greater than 75% of MDDCs expressed GFP after transfection with mRNA. As a functional measure of efficiency of antigen delivery, we determined the percentage of APCs that could be specifically killed by an antigen-specific cytotoxic T lymphocyte (CTL) clone. HLA B57+ BLCLs were transfected with synthetic mRNA encoding HIV p24 antigen and used as targets in a Cr-release assay with a CD8+ T-cell clone specific for an HLA B57–restricted epitope (ISW9) within p24. Figure 1B shows that specific lysis of transfected BLCLs was 87% of the maximal specific lysis of targets loaded with excess peptide. Thus mRNA transfection efficiently delivered antigen to the majority of APCs. These data confirmed that electroporation of APCs with mRNA encoding HIV antigens is practical and leads to the efficient processing and presentation of antigen to CD8+ T cells. The electroporation conditions and RNA dose used for the experiment in Figure 1 were determined to be optimal for transfection of BLCLs (not shown).
Synthetic mRNA encoding the 2001 clade B consensus nucleotide sequence is highly immunogenic
In order to directly compare the immunogenicity of MDDCs transfected with synthetic mRNA to that of synthetic peptides, we created a DNA template from a synthetic gene matched to a noncodon-optimized 2001 clade B HIV-1 consensus sequence. DNA encoding the complete ORF was synthesized de novo, and the synthetic consensus gene was amplified and subcloned for use in subsequent experiments. In all experiments, a Gly-to-Ala point mutation was introduced by PCR at residue position 2 of the Nef consensus sequence; this mutation has been shown to obviate Nef-mediated major histocompatibility complex (MHC) class I down-regulation.28 Except for residue G2, the predicted 207-residue translation product of the consensus Nef sequence is 100% identical to that of an array of synthetic overlapping peptides (OLPs) covering the 2001 B consensus Nef sequence, allowing a direct comparison of immune responses to mRNA and synthetic peptide.
To determine whether the consensus Nef sequence was immunogenic, we generated synthetic mRNA from a template DNA sequence encoding the immunodominant central region of consensus Nef (residues 67-148). MDDCs were derived from the PBMCs of a chronic HIV+ subject, transfected with mRNA, and matured overnight with a cytokine cocktail. The responses of autologous Nef-specific T cells to transfected MDDCs and a sequence-matched peptide pool were compared in an overnight IFNγ ELISpot assay. Figure 2 shows that the response of PBMCs to nef-transfected MDDCs was at least as intense as the response to a sequence-matched peptide pool. Subsequent experiments (Figure 3) confirm that the synthetic consensus Nef sequence is also highly immunogenic when expressed as a full-length protein.
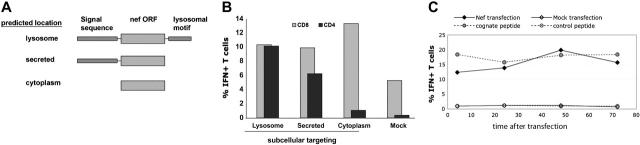
Figure 3.
Targeting consensus Nef protein to the secretory/endosomal pathway enhances presentation of class II–restricted epitopes to CD4+ T cells. (A) Diagram of genetic constructs to target Nef expression to the lysosome, the secretory pathway, or the cytoplasm. Constructs consist of an N-terminal ER-translocation signal sequence, the consensus nef ORF, and a C-terminal LAMP-1 lysosomal targeting motif, as shown. (B) To measure the effect of secretory/endosomal targeting on the presentation of a class II–restricted Nef epitope, BLCLs were transfected with mRNA encoding each of the constructs shown in panel A or were mock transfected. The response of a Nef-specific CD4+ T-cell line (recognizing the consensus epitope KFDSRLAFHHMARELH) was measured by ICS. BLCL and CD4 line were derived from the same subject. As a positive control for antigen expression, the same BLCLs, which are HLA B57+, were used to stimulate a nonautologous B57-restricted CD8+ clone specific for the HW9 epitope HTQGYFPDW. Results are representative of 3 experiments. (C) Rapid and prolonged presentation of a class II–restricted epitope by APCs transfected with lysosome-targeted Nef antigen. BLCLs were transfected with lysosome-targeted Nef chimera, mock transfected, or loaded with 10 μg/mL of cognate peptide or irrelevant peptide and washed 3 times. APCs were then cultured for the indicated time period before being used to stimulate a Nef-specific CD4+ T-cell line in an ICS assay. Value on the x-axis indicates the length of time between transfection of the target and addition of BFA to the ICS assay. Results are representative of 2 experiments.
Targeting Nef into the endosomal/secretory pathway promotes presentation to CD4+ T cells
The antigens shown in Figures 1 and 2 are expressed as cytoplasmic proteins, which are expected to be degraded by the proteasome and enter the MHC class I processing pathway. Given increasing evidence for the importance of functional HIV-specific CD4+ T cells in control of HIV infection,8 we concentrated on developing a system of antigen delivery that would efficiently stimulate both CD4+ and CD8+ T cells. Several previous reports have examined the effects of targeting of antigens into the endosomal/lysosomal pathway to promote access to MHC class II–loading compartments, with some reports finding enhancement of antigen presentation to CD4+ T cells22,23,29 and some finding no effect.20 In order to test the effects on CD4+ and CD8+ T-cell responses of targeting Nef to the lysosome, we expressed the consensus Nef ORF as a chimeric protein, with the nef sequence preceded by an ER-targeting signal sequence and followed by the lysosomal-targeting motif of LAMP-1.29 Thus the translation product of this genetic construct is predicted to be translocated into the ER and directed from the secretory pathway into a lysosomal compartment.
We transfected BLCLs with mRNA encoding consensus Nef expressed as a cytoplasmic protein (“CytoNef”) or as a lysosome-targeted chimera (“LysoNef”) and used transfected BLCLs to stimulate a Nef-specific CD4+ T-cell line and a Nef-specific CD8+ T-cell clone. Figure 3B shows that lysosome-targeted Nef efficiently activates the CD4+ T-cell line, whereas cytoplasm-targeted Nef does not activate a significant CD4+ response. Conversely, both cytoplasm- and lysosome-targeted Nef efficiently stimulated a CD8+ T-cell clone, although the cytoplasmic antigen is somewhat more antigenic for CD8+ T cells. In order to determine whether lysosomal targeting was strictly required for HLA class II presentation, we introduced an in-frame stop codon into the LysoNef sequence between the Nef ORF and the lysosomal targeting motif. The resulting construct (“SecNef”) is predicted to be secreted and not retained in the lysosome. Presentation of SecNef to the CD4+ T-cell line is more efficient than presentation of CytoNef and reduced from that of LysoNef. Thus targeting of Nef protein to the endosomal/secretory pathway permits transfected BLCLs to stimulate both CD4+ and CD8+ T cells.
In order to examine the kinetics of class II–restricted antigen presentation to a Nef-specific CD4+ T-cell line, we transfected BLCLs with LysoNef over a series of time points. As a positive control, BLCLs were loaded with cognate peptide and washed 3 times; as negative controls, BLCLs were mock transfected or loaded with irrelevant peptide. After antigen loading, APCs were returned to culture for increasing periods of time and then assayed for antigen presentation to a CD4+ T-cell line by ICS, as shown in Figure 3C. The ICS assay requires addition of BFA to block IFNγ secretion; this drug would also be expected to block export of nascent LysoNef protein into the endosome. Figure 3C shows that sufficient antigen to activate T cells at near-maximal levels had been exported from the ER when BFA was added as early as 4 hours after transfection. In a separate experiment, when BFA was added only 2 hours after transfection, activation of the T-cell clone was already about 30% of maximal (data not shown). Thus expression and export of chimeric antigen in mRNA-transfected cells is rapid and prolonged.
In order to determine whether targeting Nef antigen to the lysosome would also enhance expansion of specific CD4+ T cells by transfected DCs, we derived MDDCs from the blood of 9 chronic HIV+ subjects and transfected them with either cytoplasmic- or lysosomal-targeted consensus Nef constructs. Matured DCs were cocultured with autologous PBMCs for 12 to 14 days, after which the specificity of the resulting polyclonal T-cell lines was determined by ICS using the pool of overlapping Nef peptides. The LysoNef- and CytoNef-expanded responses of a representative donor are shown in Figure 4A. There was a prominent Nef-specific CD4 response (2.1% of CD4+ T cells) in the T-cell line expanded with the lysosomal construct, whereas the CD4+ response in the line expanded with CytoNef was negligible. Conversely, the CytoNef-expanded CD8 response was greater than the LysoNef-expanded response (3.0% vs 1.0%, respectively).
The overall CD4 and CD8 responses were compared for all T-cell lines from the 9 donors expanded by the 2 constructs (Figure 4B). Expansion with LysoNef produced a significantly greater frequency of Nef-specific CD4+ T cells than expansion with CytoNef (P = .0039). Conversely, there was a slight decrease in frequency of Nef-specific CD8+ T cells expanded in response to LysoNef compared with CytoNef, but this effect was not statistically significant (P = .16). Thus lysosomal targeting of the consensus Nef protein in mRNA-transfected MDDCs produced a significant increase in the expansion of Nef-specific CD4+ T cells without compromising the ability to expand CD8+ T cells.
Transfected DCs expand CD4+ and CD8+ T cells recognizing a broad array of epitopes
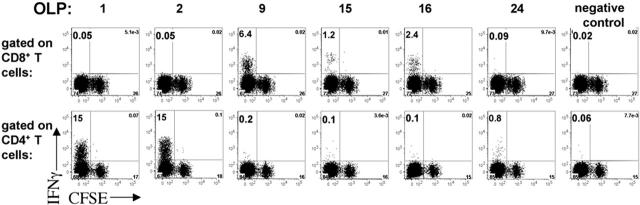
To determine the breadth as well as the frequency of expanded T-cell responses, MDDCs from a subject with chronic HIV infection were transfected with LysoNef mRNA or were mock transfected. Autologous PBMCs were stained with CFSE and cocultured with LysoNef-transfected MDDCs. On day 14, the specificities of the T-cell lines were determined by ELISpot with an array of 27 Nef consensus OLPs. T cells expanded with nef RNA responded to 6 of the OLPs. No significant response was expanded by mock-transfected MDDCs (not shown). In order to determine the phenotype of antigen-specific T cells expanded by transfected DCs, the mRNA-expanded T-cell line was tested in an ICS assay with each of the 6 peptides to which it had a strong ELISpot response. Figure 5 shows that 3 of the 6 peptides tested were recognized by CD4+ T cells, whereas the other 3 peptides were recognized by CD8+ T cells, confirming that transfection is able to induce a broad and diverse T-cell response. Furthermore we note that all of the antigen-specific T cells had undergone many cycles of replication as indicated by CFSE dilution.
Figure 5.
CD4+ and CD8+ T-cell specificity of a DC-expanded T-cell line. MDDCs derived from an HIV+ subject were mock transfected or transfected with LysoNef mRNA and matured with cytokine cocktail. CFSE-stained autologous PBMCs were cocultured with transfected MDDCs for 14 days. The resulting T-cell lines were tested by ELISpot with an array of 27 OLPs spanning the consensus Nef sequence (not shown): T cells expanded with nef RNA responded to 6 of the OLPs, whereas no significant response was expanded by mock-transfected MDDCs. For each of the 6 peptides that elicited a strong response by ELISpot, the T-cell line was tested in an ICS assay to determine the percent specificity and degree of proliferation of CD4+ and CD8+ T cells. A peptide that elicited no response by ELISpot was used as the irrelevant control.
In order to analyze the breadth and specificity of T-cell lines expanded by transfected DCs, MDDCs were derived from the blood of 13 chronic HIV+ subjects and transfected with LysoNef mRNA. For 6 of these subjects MDDCs were also mock transfected. After 12 to 14 days, T-cell lines were tested by ELISpot with the Nef peptide array. Each positive response was determined to be CD8+ or CD4+ (either by ICS or by ELISpot after predepletion with anti-CD4 or -CD8 beads). The 13 T-cell lines each recognized between 1 and 6 CD8 epitopes (mean = 2.3) and between 0 and 5 CD4 epitopes (mean = 1.9). Responses are shown in Table 1 and Figure 6A. T-cell lines expanded with mock-transfected DCs had no Nef-specific responses (data not shown).
Previous studies have investigated the distribution of ex vivo responses against consensus peptides in clade B HIV+ chronic subjects. Frahm et al27 found that PBMC responses were clustered into regions of high and low epitope density within the consensus Nef sequence and that the vast majority of responses detected in that study were CD8+. We compared the distribution of CD4+ and CD8+ specificities in the 13 expanded T-cell lines against the ex vivo Nef-specific responses of 347 chronic HIV+ subjects. Ex vivo responses were determined by testing subject PBMCs with a matrix of OLPs spanning the entire HIV genome as previously described.27 Figure 6B shows that the distribution of Nef CD8 epitopes recognized by the RNA-expanded T-cell lines is very similar to the overall ex vivo responses in the chronic cohort. The correlation between the responses was highly significant (P < .001, r = 0.71). Conversely, there was no significant correlation between the distribution of CD8 epitopes and CD4 epitopes recognized by the expanded T-cell lines (Figure 6B; P = .18) or between ex vivo responses and expanded CD4 responses (not shown), consistent with the observation that CD8+ T cells constitute the majority of ex vivo responses in chronic subjects. These results demonstrate that mRNA-transfected DCs are able to expand CD8+ T cells representing the full repertoire of consensus Nef epitopes recognized by circulating CTLs in vivo. Furthermore, LysoNef-transfected DCs are also able to expand CD4+ T cells, recognizing a distinct array of Nef peptides, from rare precursors in the PBMCs of chronic HIV+ subjects.
Previous studies of CD8+ responses in chronic HIV subjects have noted an inverse correlation between the frequency of recognition of a consensus peptide and the degree of amino acid sequence variability (as indicated by a Shannon entropy score) within the region covered by the peptide.27,30 These studies did not examine CD4 epitopes. For each Nef peptide, we compared the mean entropy of residues within the OLP to the frequency of recognition by PBMCs of 347 chronic subjects or by expanded CD8 and CD4 T cells from the 13 subjects. As found previously,27 the frequency of consensus epitope recognition by ex vivo PBMCs was inversely proportional to sequence entropy (P < .001, r = -0.84; not shown). Likewise, in polyclonal lines expanded by transfected MDDCs, the frequency of recognition by CD8 T cells was also inversely proportional to entropy (Figure 6C; P = .0037, r = -0.54). However, there was no correlation between frequency of recognition and entropy for CD4 epitopes (Figure 6C; P = .40).
In summary, our data show that lysosomal targeting of HIV antigens in APCs leads to the reproducible expansion of polyclonal CD8+ T cells that reflect the specificity of responses occurring in vivo. Furthermore, this strategy empowers APCs with the ability to simultaneously expand HIV-specific CD4+ T cells.
Discussion
In spite of the benefits of antiretroviral chemotherapy in developed countries, there is a significant risk of treatment failure due to viral drug resistance, intolerable side effects, or lack of access to an affordable drug supply. Thus there is great interest in the development of therapeutic vaccines that may boost the immune response to HIV and protect against the worst effects of drug failure. The ability to specifically expand both CD4+ and CD8+ T cells specific for HIV antigens may be a critical factor in the development of an effective immunotherapy. In this report we examined the ability of MDDCs transfected with synthetic mRNA-encoding model HIV antigens to expand HIV-specific CD4+ and CD8+ T cells from the blood of subjects with chronic infection.
As an in vitro approximation of therapeutic vaccination, we cocultured PBMCs from HIV+ subjects with transfected autologous MDDCs and analyzed the resulting T-cell lines for breadth of specificity and magnitude of CD4+ and CD8+ T-cell responses. We determined that expressing Nef as a lysosomal construct increased the expansion of Nef-specific CD4+ T cells without significantly affecting the expansion of CD8+ T cells. Targeting antigen to the lysosome has been shown to improve presentation to CD4+ T cells for several other proteins,22,23,29 although recent reports indicate that class II–restricted epitopes can be generated by dendritic cells from cytoplasmic protein via autophagy31,32 or TAP-dependent transport of proteasomal products.33 The variable ability of different cytoplasmic proteins to access MHC class II antigen-loading compartments is a matter of ongoing investigation.31-34 Indeed, for many of the subjects studied, DCs transfected with cytoplasmic antigen expanded a small but measurable Nef-specific CD4+ response, indicating that cytoplasmic antigen had access to MHC class II–loading compartments in these DCs, although the capacity of cytoplasmic antigen to expand CD4+ T cells was greatly reduced compared with lysosomal antigen.
We also examined the breadth and specificity of T-cell responses expanded by LysoNef-transfected MDDCs. We found that the specificity of expanded CD8+ T cells for individual consensus peptides was highly correlated to the specificity of ex vivo responses found in a very large cohort of chronic subjects. Thus in the context of therapeutic vaccination, LysoNef-transfected MDDCs would be expected to stimulate and expand the full array of circulating Nef-specific CTLs present in chronic HIV+ vaccinees. Unlike the CD8 response, the specificity of LysoNef-expanded CD4+ T cells in the same polyclonal lines had no significant correlation with ex vivo PBMC responses. Thus therapeutic vaccination with this construct would be expected to expand rare circulating CD4+ T cells with a different spectrum of specificities from the CD8+ T cells.
Consistent with previous reports, we find preferential targeting of certain OLPs within the consensus Nef sequence both by ex vivo PBMCs and by expanded CD8+ T cells. Furthermore the frequency of recognition by CD8+ T cells was strongly and inversely correlated with peptide sequence variability (entropy). Thus there was no increase in the frequency of recognition of high-entropy peptides even when T-cell lines were expanded with highly immunogenic MDDCs (Figure 6C). Why high-entropy consensus peptides are not recognized by CTLs in chronic subjects is not fully understood; it may be related to an absence of predicted proteasome cleavage sites35 or to an increased rate of escape in these regions30 or to increased variability between autologous viral sequences and consensus antigen.
We also found that in expanded T-cell lines from chronic subjects, there is no correlation between peptide sequence entropy and frequency of CD4+ T-cell recognition. A similar lack of correlation was found for ex vivo CD4+ T-cell responses in chronic subjects (D.E.K. and E.S.R., unpublished results, 2004). This finding is consistent with the hypothesis that high-entropy peptides correspond to regions of Nef that lack proteasomal cleavage sites but are efficiently processed for class II presentation within the lysosome. Alternatively, class II–restricted responses may simply be more tolerant of sequence variability outside of the anchor residues.
In conclusion, we have demonstrated that MDDCs transfected with mRNA encoding a lysosome-targeted Nef construct can drive the expansion of antigen-specific CD4+ and CD8+ T cells recognizing a broad array of epitopes and provide the basis for a promising new mechanism of antigen delivery for immunotherapy of HIV+ subjects.
Prepublished online as Blood First Edition Paper, October 25, 2005; DOI 10.1182/blood-2005-04-1513.
Supported by grants from the Elizabeth Glazer Pediatric AIDS Foundation (N.B.), the Doris Duke Medical Research Institute (B.D.W.), and National Institutes of Health (N01-A1-15422; C.B.) and fellowships from the Schweizerische Stiftung für medizinisch-biologische Stipendien (D.E.K.) and the National Institutes of Health (1 F32 AI058457-01; D.G.K.).
D.G.K. and D.E.K. contributed equally to this report.
D.G.K. was responsible for overall experimental design, performed experiments, and wrote the paper; D.E.K., N.F., and S.L.G. designed and performed experiments; N.B. and B.D.W. provided supervision and training for D.G.K.; E.S.R. provided supervision and training for D.E.K.; C.B. provided supervision and training for N.F.; S.S. and B.S.W. performed experiments; D.B. and E.G. provided new vectors and technical assistance for mRNA transfection procedures; and M.T.Z., D.R.S., and M.N.J. recruited study subjects and collected blood samples.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Altfeld M, Addo MM, Rosenberg ES, et al. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS. 2003;17: 2581-2591. [DOI] [PubMed] [Google Scholar]
- 2.Trachtenberg E, Korber B, Sollars C, et al. Advantage of rare HLA supertype in HIV disease progression. Nat Med. 2003;9: 928-935. [DOI] [PubMed] [Google Scholar]
- 3.Kaslow RA, Carrington M, Apple R, et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2: 405-411. [DOI] [PubMed] [Google Scholar]
- 4.Hendel H, Caillat-Zucman S, Lebuanec H, et al. New class I and II HLA alleles strongly associated with opposite patterns of progression to AIDS. J Immunol. 1999;162: 6942-6946. [PubMed] [Google Scholar]
- 5.Balla-Jhagjhoorsingh SS, Koopman G, Mooij P, et al. Conserved CTL epitopes shared between HIV-infected human long-term survivors and chimpanzees. J Immunol. 1999;162: 2308-2314. [PubMed] [Google Scholar]
- 6.Frahm N, Adams S, Kiepiela P, et al. HLA-B63 presents HLA-B57/B58-restricted cytotoxic T-lymphocyte epitopes and is associated with low human immunodeficiency virus load. J Virol. 2005; 79: 10218-10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migueles SA, Laborico AC, Shupert WL, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in non-progressors. Nat Immunol. 2002;3: 1061-1068. [DOI] [PubMed] [Google Scholar]
- 8.Lichterfeld M, Kaufmann DE, Yu XG, et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med. 2004;200: 701-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10: 1359-1365. [DOI] [PubMed] [Google Scholar]
- 10.Lu W, Wu X, Lu Y, Guo W, Andrieu JM. Therapeutic dendritic-cell vaccine for simian AIDS. Nat Med. 2003;9: 27-32. [DOI] [PubMed] [Google Scholar]
- 11.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199: 251-263. [DOI] [PubMed] [Google Scholar]
- 12.Walsh SR, Bhardwaj N, Gandhil RT. Dendritic cells and the promise of therapeutic vaccines for human immunodeficiency virus (HIV)-1. Curr HIV Res. 2003;1: 205-216. [DOI] [PubMed] [Google Scholar]
- 13.Sullenger BA, Gilboa E. Emerging clinical applications of RNA. Nature. 2002;418: 252-258. [DOI] [PubMed] [Google Scholar]
- 14.Weissman D, Ni H, Scales D, et al. HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response. J Immunol. 2000;165: 4710-4717. [DOI] [PubMed] [Google Scholar]
- 15.Heiser A, Coleman D, Dannull J, et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002; 109: 409-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su Z, Dannull J, Heiser A, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63: 2127-2133. [PubMed] [Google Scholar]
- 17.Wu TC, Guarnieri FG, Staveley-O'Carroll KF, et al. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci U S A. 1995;92: 11671-11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowell JF, Ruff AL, Guarnieri FG, et al. Lysosome-associated membrane protein-1-mediated targeting of the HIV-1 envelope protein to an endosomal/lysosomal compartment enhances its presentation to MHC class II-restricted T cells. J Immunol. 1995;155: 1818-1828. [PubMed] [Google Scholar]
- 19.Nair SK, Boczkowski D, Morse M, et al. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16: 364-369. [DOI] [PubMed] [Google Scholar]
- 20.Vidalin O, Tanaka E, Spengler U, Trepo C, Inchauspe G. Targeting of hepatitis C virus core protein for MHC I or MHC II presentation does not enhance induction of immune responses to DNA vaccination. DNA Cell Biol. 1999;18: 611-621. [DOI] [PubMed] [Google Scholar]
- 21.Bonini C, Lee SP, Riddell SR, Greenberg PD. Targeting antigen in mature dendritic cells for simultaneous stimulation of CD4+ and CD8+ T cells. J Immunol. 2001;166: 5250-5257. [DOI] [PubMed] [Google Scholar]
- 22.Marques ET Jr, Chikhlikar P, de Arruda LB, et al. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses. J Biol Chem. 2003;278: 37926-37936. [DOI] [PubMed] [Google Scholar]
- 23.de Arruda LB, Chikhlikar PR, August JT, Marques ET. DNA vaccine encoding human immunodeficiency virus-1 Gag, targeted to the major histocompatibility complex II compartment by lysosomal-associated membrane protein, elicits enhanced long-term memory response. Immunology. 2004;112: 126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korber B. Los Alamos National Laboratory HIV Sequence Database; 2003. http://hiv-web.lanl.gov/content/hiv-db/CONSENSUS/M_GROUP/consensus.html. Accessed June 1, 2003.
- 25.Lee AW, Truong T, Bickham K, et al. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20(suppl 4): A8-A22. [DOI] [PubMed] [Google Scholar]
- 26.O'Neill D, Bhardwaj N. Generation of autologous peptide- and protein-pulsed dendritic cells for patient-specific immunotherapy. Methods Mol Med. 2005;109: 97-112. [PubMed] [Google Scholar]
- 27.Frahm N, Korber BT, Adams CM, et al. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J Virol. 2004;78: 2187-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sol-Foulon N, Esnault C, Percherancier Y, et al. The effects of HIV-1 Nef on CD4 surface expression and viral infectivity in lymphoid cells are independent of rafts. J Biol Chem. 2004;279: 31398-31408. [DOI] [PubMed] [Google Scholar]
- 29.Su Z, Vieweg J, Weizer AZ, et al. Enhanced induction of telomerase-specific CD4(+) T cells using dendritic cells transfected with RNA encoding a chimeric gene product. Cancer Res. 2002; 62: 5041-5048. [PubMed] [Google Scholar]
- 30.Bansal A, Gough E, Sabbaj S, et al. CD8 T-cell responses in early HIV-1 infection are skewed towards high entropy peptides. AIDS. 2005;19: 241-250. [PubMed] [Google Scholar]
- 31.Nimmerjahn F, Milosevic S, Behrends U, et al. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol. 2003;33: 1250-1259. [DOI] [PubMed] [Google Scholar]
- 32.Dorfel D, Appel S, Grunebach F, et al. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood. 2005;105: 3199-3205. [DOI] [PubMed] [Google Scholar]
- 33.Tewari MK, Sinnathamby G, Rajagopal D, Eisenlohr LC. A cytosolic pathway for MHC class II-restricted antigen processing that is proteasome and TAP dependent. Nat Immunol. 2005;6: 287-294. [DOI] [PubMed] [Google Scholar]
- 34.Michalek MT, Benacerraf B, Rock KL. The class II MHC-restricted presentation of endogenously synthesized ovalbumin displays clonal variation, requires endosomal/lysosomal processing, and is up-regulated by heat shock. J Immunol. 1992; 148: 1016-1024. [PubMed] [Google Scholar]
- 35.Yusim K, Kesmir C, Gaschen B, et al. Clustering patterns of cytotoxic T-lymphocyte epitopes in human immunodeficiency virus type 1 (HIV-1) proteins reveal imprints of immune evasion on HIV-1 global variation. J Virol. 2002;76: 8757-8768. [DOI] [PMC free article] [PubMed] [Google Scholar]