Abstract
Erythroid Kruppel-like factor (EKLF, KLF1) plays an important role in definitive erythropoiesis and β-globin gene regulation but failure to rectify lethal fetal anemia upon correction of globin chain imbalance suggested additional critical EKLF target genes. We employed expression profiling of EKLF-null fetal liver and EKLF-null erythroid cell lines containing an inducible EKLF-estrogen receptor (EKLF-ER) fusion construct to search for such targets. An overlapping list of EKLF-regulated genes from the 2 systems included α-hemoglobin stabilizing protein (AHSP), cytoskeletal proteins, hemesynthesis enzymes, transcription factors, and blood group antigens. One EKLF target gene, dematin, which encodes an erythrocyte cytoskeletal protein (band 4.9), contains several phylogenetically conserved consensus CACC motifs predicted to bind EKLF. Chromatin immunoprecipitation demonstrated in vivo EKLF occupancy at these sites and promoter reporter assays showed that EKLF activates gene transcription through these DNA elements. Furthermore, investigation of EKLF target genes in the yolk sac led to the discovery of unexpected additional defects in the embryonic red cell membrane and cytoskeleton. In short, EKLF regulates global erythroid gene expression that is critical for the development of primitive and definitive red cells.
Introduction
CACC motifs are common proximal promoter elements in many erythroid genes. For example, the proximal promoters of all of human and mouse β-like and α-like globin genes contain CACC motifs that are important for transcriptional activation in transient reporter assays and transgenic mice.1-5 The critical nature of CACC-binding proteins is best highlighted by natural mutations in the 2 CACC motifs within the human β-globin gene promoter, which lead to β+-thalassemia from promoter dysfunction.6 A large number of studies demonstrated CACC-box dependence of many other erythroid gene promoters in vitro.7-12
There are many CACC-box binding activities within erythroid cells including SP1 and other ubiquitously expressed nuclear factors.13 In 1993, erythroid Kruppel-like factor (EKLF, KLF1),14 the founding member of the KLF family15,16 of transcription factors, was discovered and shown to bind CACC-box or GC-rich motifs through 3 C-terminal SP1-like C2H2 zinc fingers.14 Unlike SP1, mouse and human EKLF are uniquely expressed in erythroid cells of yolk sac, fetal liver, spleen, and bone marrow.14,17 EKLF functions as a transcriptional activator of many erythroid gene promoters in transient reporter assays,9 including the mouse and human β-globin promoters.15,18 In some contexts EKLF may function as a transcriptional repressor, perhaps through recruitment of corepressors such as Sin3A,18 but the importance of this for in vivo function is not clear.
EKLF knockout mice die of anemia by embryonic day 16 (E16),19,20 demonstrating nonredundant in vivo erythroid functions. EKLF-null embryos have a profound defect in β-globin gene expression, but α-globin is relatively spared. There is also iron overload in the fetal liver reticulo-endothelial system and erythroid cells, consistent with ineffective erythropoiesis.20 In the absence of EKLF there is no human β-globin gene expression in transgenic mice that contain the human β-globin locus, and there is persistence of γ-globin gene expression in the fetal liver.21,22 This result has raised interest in pharmacologic suppression of EKLF as a strategy to raise fetal hemoglobin in patients with β-hemoglobinopa-thies such as sickle cell disease and β-thalassemia. However, this approach is potentially dangerous without a more thorough knowledge of potential EKLF target genes.
Although EKLF knockout mice share many characteristics with human β-thalassemia major, the EKLF-/- phenotype is more severe than that generated by deletion of both the β-minor and β-major genes themselves,23 suggesting EKLF-/- erythroid cells have additional nonglobin defects. Furthermore, correction of the α-like-to-β-like globin chain ratio in EKLF-null embryos via transgenic approaches fails to restore red cell morphology or survival.24 This result also argues strongly for additional nonglobin defects in EKLF-null red cells. Knockdown of EKLF to 20% of endogenous levels in the erythroid cell line J2E resulted in poor hemoglobinization in response to erythropoietin and reduced expression of many heme-synthesis enzymes including δ-amino levulinic acid synthase (ALAS-E or ALAS2), ferrochelatase (Fech), 5-aminolevulinic acid dehydratase (Alad), and porphobilinogen deaminase (PBGD).25 Also, immortalized erythroblast cell lines derived from EKLF-null fetal livers demonstrated robust hemoglobinization and morphologic maturation upon restoration of EKLF function.26 Together, these experiments argue that EKLF plays a broad role in gene expression that accompanies terminal erythroid differentiation.
To undertake a nonbiased, genome-wide search for EKLF target genes, we performed expression profiling on EKLF-null E14.5 fetal liver cells and also on an EKLF-null cell line, B1.6, that undergoes EKLF-dependent erythroid maturation.26 This combined approach allowed us to circumvent technical problems associated with either approach and narrow the list of potential EKLF-regulated genes to approximately 100. The list includes genes encoding erythroid differentiation-related factor/α-hemoglobin stabilizing protein (EDRF/AHSP),27 dematin (band 4.9), heme-synthesis enzymes, blood group antigens, transcription factors, and other proteins. We verified a number of target genes and demonstrated direct EKLF gene activation of dematin. In addition, we found that EKLF is influential during primitive erythroid cell differentiation. In short, our findings demonstrate that EKLF plays a much broader role in both primitive and definitive erythropoiesis than previously suspected. It is essential for integrity of the red cell membrane, cytoskeleton, heme synthesis, and globin gene expression in both definitive and primitive red cells.
Materials and methods
Genotyping
Genotyping of the EKLF-null mice was performed by polymerase chain reaction (PCR) with primer pairs that recognize neoR in the targeted locus or a region within the zinc fingers deleted by the targeting event. Primer sequences and PCR conditions are available upon request.
Expression profiling
Total RNA was prepared from fetal livers and cell lines using Trizol or RNA-Easy kits (Invitrogen, Carlsbad, CA). EKLF was induced in EKLF-estrogen receptor (EKLF-ER) cell lines, B1.626 by addition of 200 mM hydroxyl-tamoxifen (Sigma, St Louis, MO), or 0.01% ethanol as a vehicle control in 10% FCS/high-glucose DMEM for 6 hours. RNA quantity and quality was determined using an Agilent Technologies 2100 Bioanalyser (Palo Alto, CA). RNA was linearly amplified using the messageAMP aRNA kit (Ambion, Austin, TX). Labeled samples were hybridized to 22K Compugen mouse long-oligonucleotide microarrays (Compugen, San Jose, CA) and analyzed using previously described methods.28,29 All data and rankings used to define statistically robust differential expression were exported into GeneSpring 7.2 (Agilent Technologies, PaloAlto, CA) for visualization. Full documentation of experimental parameters and raw data are available for download in accordance with MIAME guidelines30 via a Signet interface (Agilent Technologies).
Real time RT-PCR
cDNA was made from total RNA using superscript III (Invitrogen) according to the manufacturer's recommendations. For dematin, reverse transcriptase-PCR (RT-PCR) primers were designed to dematin exons to amplify alternative 5′ transcripts. For validation of target gene expression, primer pairs were designed to cross intron-exon boundaries (Table 1). Quantitative real-time RT-PCR reactions were performed using an ABI-Prism 7500 (Applied Biosystems, Foster City, CA) and SYBR green incorporation. The PCR cycles for all primer sets were as follows: denaturation at 95°C for 10 minutes, followed by 45 cycles of 94°C for 15 seconds and 60°C for 1 minute. Each sample was run in triplicate with biologic replicates for each condition.
Table 1.
Real-time RT-PCR primers
| cDNA | Primer sequences | Product, bp |
|---|---|---|
| HPRT | Forward: 5′-GCA GTA CAG CCC CAA AAT GG | 85 |
| Reverse: 5′-AAC AAA GTC TGG CCT GTA TCC AA | ||
| Pgk1 | Forward: 5′-GGG TCG AGC TAA GCA GAT TG | 102 |
| Reverse: 5′-GCT TTC ACC ACC TCA TCC AT | ||
| Epb4.9 | Forward: 5′-ACC GCA TGA GGC TTG AGA GG | 114 |
| Reverse: 5′-TCT TCT TAA GTT CGT TCC GCT TCC | ||
| Dfy | Forward: 5′-CAG GCT GTA GTC ACT CGA GAG TTC AT | 115 |
| Reverse: 5′-GGA ACT GTC TGT ATC CGG TGG A | ||
| Icam4 | Forward: 5′-CCT GTT ATG TTG ACA GTC CTC GCT TT | 107 |
| Reverse: 5′-ACG TAG ACA GCC CCC ACA GC | ||
| Mgst3 | Forward: 5′-CGC ATA TGG CTA CTA CAC AGG AGA CC | 118 |
| Reverse: 5′-CCA GCC GAG ATG CTG GAA AG | ||
| Lgals | Forward: 5′-CGG GAG CAT CAC AGA GGT GT | 115 |
| Reverse: 5′-GTA GTT GAT GGC CTC CAT GTT GA | ||
| Snca | Forward: 5′-GAC CAG ATG GGC AAG GGT GA | 128 |
| Reverse: 5′-GCT TCA GGC TCA TAG TCT TGG TAG C | ||
| Rhced | Forward: 5′-GGT GAC TGG ACT CCT CAC AGG TT | 109 |
| Reverse: 5′-AAC CGC CAA GTG TGG GAA CT | ||
| Tfrc | Forward: 5′-GGA AAT CAA TGA TCG TAT TAT GAA AGT GG | 91 |
| Reverse: 5′-CCA GAA GAT ATG TCG GAA AGG AGA CT | ||
| LMO2 | Forward: 5′-TGC AGG AGA GAC TAT CTC AGG CTT T | 100 |
| Reverse: 5′-TGT CTT TCA CCC GCA TCG TC | ||
| Bzrp | Forward: 5′-GCA GAT GGG CTG GGC CTT | 135 |
| Reverse: 5′-AGG CCA GGT AAG GGT ACA GCA A | ||
| Acp3 | Forward: 5′-GGA CAT GAC CAC AAC CTG CAG TAT | 103 |
| Reverse: 5′-TTC GTT GAT GTC GCA CAG AGG | ||
| Ermap | Forward: 5′-GGG ATG TGC CGT GTC AGG | 110 |
| Reverse: 5′-AGA AAC TCC GGA GTG AAC TGA AGT | ||
| AHSP | Forward: 5′-CTC AGC ACC ATT AGA CTT GAA | 96 |
| Reverse: 5′-TGC TGA TCC AGC AGA ACA TTA AAC TC |
Western blotting
Protein was extracted from cells using RIPA buffer, and Western blotting was performed by standard techniques with proteins detected by enhanced chemiluminescence (ECL; Amersham Biosciences, Piscataway, NJ). Blots were blocked in 5% milk powder in PBS-0.1% tween20 (PBT) and probed with primary antibodies (1:500) to dematin (BD Biosciences, Franklin Lakes, NJ), AHSP,27 and γ-actin (sc-1616; Santa-Cruz Biotechnology, Santa Cruz, CA) and relevant secondary-HRP antibodies at 1:2000 dilution in PBT (Amersham Biosciences).
FACS
Fetal liver or primitive erythroid cells were passed through a 23-gauge needle, filtered through a 70-μM cell strainer, washed, and resuspended in fluorescent-activated cell sorting (FACS) buffer (PBS, 1% FCS, 10 mM EDTA) at a concentration of 5 × 106 cells/mL. Antibodies TER119-PE, CD117-FITC, and CD71-FTIC (BD Biosciences) were used at 1:200 concentration. Dead cells were detected with propidium iodide. Analysis was performed on a FACSCalibur (BD Biosciences).
Scanning electron microscopy
E11.5 blood cells were centrifuged at 500g onto coverslips fixed in 100% ethanol, critical-point dried, and platinum coated for imaging in a 6300F JEM JEOL scanning electron microscope (JEOL PTY, Tokyo, Japan) according to standard protocols. Images were acquired through ImageSlave 2.14 software (OED, Hornsby, Australia) and processed using Adobe Photoshop 7.01 (Adobe Systems, San Jose, CA).
Bioinformatics
The evolutionary conserved region (ECR) Browser interface31 was used to navigate through regions of human-rat-mouse alignments. Multiple sequence alignment of regions of choice was performed using a threaded blockset aligner (tba).32 Sequences (12.5 kb) from the upstream gene FGF17 to a region in intron 2 of dematin were aligned via the link to Mulan.32 ECRs of more than 100 bp of greater than 70% identity were identified. EKLF binding sites (CCNCNCCC)33 and GATA-1 binding sites (WGATAR)34 were found using the user-defined motif search tool of rVISTA35 within the Mulan interface.32 All human sites as well as phylogenetically conserved sites were identified and graphically represented using Mulan.
ChIP
Chromatin immunoprecipitation (ChIP) assays were performed essentially as previously reported.36,37 Briefly, after induction of B1.6 cells with tamoxifen for 12 hours, ChIP was performed with an ERα antibody (Ab-10; Neomarkers, Fremont, CA) or an irrelevant mouse IgG1 isotype control. Alternatively, ChIP was performed with a rabbit polyclonal antibody raised against the N-terminus of EKLF or preimmune rabbit sera. After formaldehyde fixation (0.4%), chromatin was fragmented to approximately 500 bp by sonication, DNA was precleared with protein G-sepharose, antibody (3 mg/mL) was added (ERα, mIgG1, EKLF, or rabbit IgG) and incubated overnight at 4°C. Following elution and extraction, immunoprecipitated DNA was analyzed by real-time PCR. Primers were designed to amplify 100- to 200-bp amplicons centered on CACC sequences within the ECRs (Table 2). To determine EKLF occupancy, Δ cycle threshold (ΔCT) relative to input DNAs was calculated for each of the 4 conditions and expressed as a percentage of input.
Table 2.
Primers for real-time PCR analysis of ChIP material
| Target region | Central CACC site sequence | Product size, bp, or reference | Primer sequences |
|---|---|---|---|
| β-Major globin | CCACACCCT | Letting et al37 | Forward: 5′-GAC AAA CAT TAT TCA GAG GGA GTA CCC |
| Reverse: 5′-AGG TGC ACC ATG ATG TAT GTT TAT GG | |||
| Nectin | N/A | Letting et al37 | Forward: 5′-TTT ACA TAA GCC TAG TGG TAC CCT |
| Reverse: 5′-ATC GCT GTC CTG CAT CTC ACA GTC G | |||
| ECR a | TGGGTGCGGC | 139 | Forward: 5′-AAT GAG AGG GTG GGT GGT TT |
| Reverse: 5′-CGG AAG ACC CCC TTC AAC | |||
| ECR 1 | CCCCACCCT | 223 | Forward: 5′-CTT GGT GGC CTG AGA AAA GG |
| Reverse: 5′-ACA GGC TCC AGC TAG GTC AC | |||
| ECR 2 | AGGGAGAGT | 205 | Forward: 5′-CAT GCC AGG GAG CTG TGA |
| Reverse: 5′-AGC TCT GCC CAA CAC ATG G | |||
| ECR 3 (1&2)* | 1-TCCCACCCC | 140 | Forward: 5′-AGG GAG ATC AGA CCG CTG AG |
| 2-GCCCACCCA | Reverse: 5′-GAG GCA GCT TCT CCA GGA AC | ||
| ECR 3 (3)* | AGGGTGGGG | 185 | Forward: 5′-ACT CGG ACT CCC CAG CTC |
| Reverse: 5′-GAA GGA GGG GAG CTG GTC | |||
| ECR 3 (4)* | GCCCGCCCT | 103 | Forward: 5′-CGC CAC TAT CAG TTG TTG G |
| Reverse: 5′-CAG TCC CCT GCT GCT CTG | |||
| ECR 6 | CCCCTCCCC | 179 | Forward: 5′-CCC TTC CTG TCT CCT CCA GT |
| Reverse: 5′-CTA TTG GGG CAA AGC TAG GA | |||
| ECR 7 | AGGGTGGGG | 169 | Forward: 5′-CTT GGT CTT CCA GAG GGA TG |
| Reverse: 5′-AGC CTT GGA GGA GGA GCT A | |||
| ECR 8 (1)* | CCACGCCCT | 169 | Forward: 5′-GGA GAG GAG ATG CGC TGA |
| Reverse: 5′-CTA TGA GGC AGG GGA AGA GA | |||
| ECR 8 (2)* | CCCCTCCCT | 182 | Forward: 5′-GCA CTC CTG TAG CCT ATC CTG |
| Reverse: 5′-TGC CAA GAC AAC CTC CTG | |||
| ECR 10 | AGGGTGGGG | 196 | Forward: 5′-GTC CAG GCT CAA CCT TTC C |
| Reverse: 5′-TGG GCT TCG TTC TCC ATA AA | |||
| ECR 12 (1)* | CCCCTCCCTC | 144 | Forward: 5′-GCT GGA GAC AGC TTC AAA GG |
| Reverse: 5′-GCA AGA AAG GAA ACA GTG AGG | |||
| ECR 12 (2&3)* | 2-CCCCTCCCT | 162 | Forward: 5′-CCT CCG ATT ATT GCA GGA |
| 3-GCCCACCCC | Reverse: 5′-CCG AGA AAG GCC TGA GAA G | ||
| ECR 12 (4)* | CGGGAGGGT | 141 | Forward: 5′-CCA TCA CAA AGA GCC TTA ATC C |
| Reverse: 5′-GGG TGT TGC CCC AGT TTT A |
Parenthetic values indicate CACC sites within the ECR
Promoter-reporter assays
Genomic DNA incorporating 1 kb upstream of the published dematin transcription start site38 (P3; -1to -1000) or 1 kb upstream of a discovered erythroid promoter (P2; -8267 to -9269) were cloned into the Asp718 and Bgl2 sites of pGL3-Basic (Promega, Madison, WI) and checked by sequencing. SL2 insect cells were cotransfected with the promoter constructs and an insect cell expression vector for EKLF, pPAC-EKLF, as previously reported.5 Normalizations were performed by cotransfection of a Renilla expression vector, pRL-TK (Promega). The Dual-Luciferase Reporter Assay system (Promega) and a POLARstar OPTIMA machine (BMG Labtech, Offenberg, Germany) were used to measure luciferase and renilla activities sequentially in the same sample.
Results
EKLF-dependent genes in the fetal liver
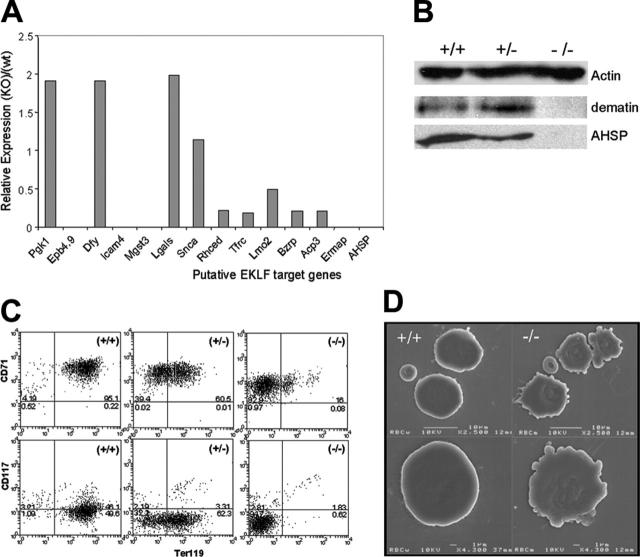
To undertake a genome-wide search for EKLF target genes we used expression-profiling analysis to compare fetal liver mRNAs from E14.5-day-old EKLF-null embryos to wild-type littermate controls. At this time, the fetal liver is almost exclusively composed of erythroid cells. EKLF-null fetal livers are less cellular (35%) than littermate controls, with the major loss being in the late erythroblasts and enucleated red cells.20 A scatter plot of the average expression profiles is shown in Figure 1A. Many more genes are downregulated (600 > 2-fold; Figure 1A green dots) in EKLF-null fetal liver than upregulated (290 > 2-fold; Figure 1A red dots), suggesting EKLF functions primarily as a transcriptional activator in vivo. β-Globin, a known in vivo EKLF target gene,19,20 was one of the most highly EKLF-dependent genes on the array (Figure 1A; Table 3), validating our experimental system. Also, EKLF itself, which is genetically defective in EKLF-null fetal liver cells, was highly downregulated as expected (Figure 1A). Many erythroid-specific genes were significantly downregulated in EKLF-null fetal liver cells, including AHSP (a small protein that binds free α-globin chains),27 dematin (a key red cell cytoskeletal protein),38 heme-synthesis enzymes, and pyruvate kinase, and many important transmembrane proteins including the transferrin receptor 1 (TfR1) and blood group antigens (Rh-cde, Duffy, ICAM4/LW, and Ermap/Scianna).
Figure 1.

EKLF plays a global role as a transcriptional activator in vivo. (A) Scatter plot of gene-expression profiling from EKLF+/+ and EKLF-/- E14.5 fetal livers. Green dots indicate genes that have been downregulated when EKLF is absent; red dots, genes that have been upregulated when EKLF is absent; and yellow dots, genes that are not significantly altered. (B) Relative expression levels of putative EKLF target genes in E14.5 EKLF-/- fetal livers compared with EKLF+/+. Levels were measured by real-time PCR and normalized to hypoxanthine phosphori-bosyltransferase (HPRT). Data are presented as mean ± SEM, n = 3. (C) Scatter plot of gene expression from the control EKLF-ER cell line B1.6 and tamoxifen-treated cells. (D) Relative expression levels of putative EKLF target genes in tamoxifen-treated B1.6 cells compared with control. (E) Venn diagram of the overlap (yellow) between EKLF-activated genes in tamoxifen-treated B1.6 cells (green) and EKLF-activated genes in fetal liver (red). (F) Representative Western blot of dematin and AHSP expression in whole-cell extracts from E14.5 fetal livers. Actin is presented as a loading control.
Table 3.
EKLF-dependent genes in the fetal liver
|
Intensity
|
|||||
|---|---|---|---|---|---|
| Gene description | Common abbreviation | Accession no. | Ratio, —/— to +/+ | EKLF—/— | EKLF+/+ |
| Downregulated genes | |||||
| Cytoskeleton | |||||
| Erythrocyte protein band 4.9/dematin | Epb4.9* | NM_013514 | 0.0507 | 372 | 7 233 |
| Hect domain and RCC1 (CHC1)-like domain (RLD) 2 | rjs | AF061529 | 0.079 | 95 | 741 |
| Talin 1 | Tln1 | AK006088 | 0.0864 | 1 021 | 11 849 |
| Cables | Cables,ik3-1 | AF133208 | 0.137 | 571 | 4 207 |
| P120-catenin | Catns | NM_007615 | 0.224 | 142 | 587 |
| Parvin, gamma | Parvg | AF312712 | 0.265 | 181 | 671 |
| Alpha 1 spectrin | Spna-1 | NM_011465 | 0.749 | 5 607 | 7 401 |
| Transmembrane/blood group antigens/receptors | |||||
| Solute carrier family 2, glucose transporter | Slc2a4; Glut4 | NM_009204 | 0.0249 | 21 | 696 |
| Solute carrier family 22; mitochondrial anion exchange | Slc22a4 OCTN1 | NM_019687 | 0.0346 | 33 | 785 |
| Lysophosphatidic acid-operated K+ channel; TRAAK | Kcnk4 | NM_008431 | 0.0624 | 108 | 1 831 |
| Rhodopsin, olfactory receptor | Rhod | AL133159 | 0.0862 | 281 | 3 661 |
| Mus musculus olfactory receptor 65 | Olfr65 | NM_013617 | 0.0893 | 115 | 639 |
| Calcium channel, voltage-dep, L type, alpha 1C subunit | Cacna1c | NM_009781 | 0.097 | 110 | 668 |
| Neurexophilin 4 | Nxph4 | AK004130 | 0.099 | 189 | 1 912 |
| Duffy blood group | Dfy* | NM_010045 | 0.108 | 1 432 | 13 104 |
| ICAM 4, LW blood group | ICAM-4, Cd242* | AF296282 | 0.219 | 3 518 | 16 029 |
| Roundabout homolog 3 (Drosophila) | Robo3 | NM_011248 | 0.28 | 338.3 | 1 111 |
| Erythroblast membrane-associated protein | Ermap* | NM_013848 | 0.309 | 12 719 | 40 085 |
| Transferrin receptor | Tfrc; CD71* | AK011596 | 0.31 | 7 367 | 23 487 |
| Activin A receptor, type II-like 1 | Acvrl1 | NM_009612 | 0.326 | 1 301 | 3 480 |
| Lectin, galactose binding, soluble 1 | Lgals1; d-Gal1* | NM_008495 | 0.335 | 11 570 | 33 382 |
| Rhesus blood group CDE | Rhced* | NM_011270 | 0.40 | 9 880 | 24 273 |
| Glycophorin A | Gypa† | NM_010369 | 0.572 | 14 932 | 25 518 |
| CD59a antigen | Cd59a† | NM_007652 | 0.626 | 4 744 | 7 127 |
| CD47 antigen, Rh-related antigen | Cd47† | NM_010581 | 0.647 | 9 620 | 14 890 |
| Transcription factors and other nuclear proteins | |||||
| Orphan nuclear receptor subfamily 0, group B, mem 1 | Nr0b1; Dax | NM_007430 | 0.0784 | 200 | 1 717 |
| Retinoic acid receptor, gamma | Rarg, RARγ | M32071 | 0.123 | 160 | 1 240 |
| Fanconi anemia, compl group D2 | 2410150O07Ri | AK019136 | 0.215 | 467 | 2 099 |
| SMC2 structural maintenance of chromosomes 2-like 1 | Smc2l1; CAP | AK017747 | 0.27 | 411 | 1 500 |
| Homeo box B3 | Hox-2.7 | X66177 | 0.278 | 586 | 2 156 |
| Zinc finger protein 297; Bing 1 | Zfp297, Bing1 | NM_020625 | 0.284 | 1 000 | 3 516 |
| CBFA2T1, ETO, MTG8 | Cbfa2t1h, Eto | NM_009822 | 0.319 | 126 | 381 |
| Zinc finger protein 1, Y-linked | Zfy1 | NM_009570 | 0.329 | 180 | 451 |
| Retinoic acid receptor, alpha | Rara, RARα | X56570 | 0.33 | 434 | 1 059 |
| Zinc finger protein 162 | Zfp162 | NM_011750 | 0.335 | 159 | 446 |
| Zinc finger and BTB domain containing 7 | Zbtb7; Pokemon | AK018596 | 0.349 | 1 662 | 4 817 |
| Kruppel-like factor 3 | Klf3 | NM_008453 | 0.38 | 389 | 910 |
| Btebp1; Kruppel-like factor 9 | Bteb1; Klf9 | NM_010638 | 0.474 | 250 | 540 |
| LIM domain only 2 | Lmo2* | NM_008505 | 0.483 | 4 807 | 9 776 |
| GLI-Kruppel family member GLI3 | Gli3 | NM_008130 | 0.51 | 362 | 690 |
| Hemogen | Hemgn | AF269248 | 0.539 | 7 947 | 15 065 |
| TBP-associated factor 140 | TAF140 | AJ292189 | 0.539 | 818.5 | 1 359 |
| Others | |||||
| RIKEN cDNA 1190001M18 - EST | 1190001M18Ri | AK004435 | 0.0594 | 128 | 1 522 |
| RIKEN cDNA 4930518C09 gene | 4930518C09Rik | AK015822 | 0.0762 | 119 | 836 |
| Keratin complex 1, acidic, gene 15 | Krt1-15 | NM_008469 | 0.0457 | 149 | 1 858 |
| Reelin | Reln | U72343 | 0.0458 | 100 | 3 690 |
| Sparc/osteonectin, cwcv and kazal-like domains proteoglycan 1 | Spock1 | NM_009262 | 0.0503 | 56 | 632 |
| Chaperonin subunit 6b (zeta) | Cct6b | NM_009839 | 0.0578 | 66 | 505 |
| IL-3; interleukin-3 | IL3 | S59969 | 0.081 | 268 | 3 479 |
| RIKEN cDNA 1700011M02 gene | 1700011M02Ri | AK005877 | 0.0831 | 300 | 2 917 |
| Male wolffian duct clone: 6720484C15 | 6720484C15Rik | AK020173 | 0.0845 | 419 | 4 694 |
| RIKEN cDNA 4933401L05 gene | 4933401L05Rik | AK016606 | 0.0918 | 125 | 1 587 |
| RIKEN cDNA 4933421O10 gene | 4933421O10Ri | AK016866 | 0.0934 | 224 | 2 456 |
| RIKEN cDNA 3110006O06 gene | 3110006O06Ri | AK014014 | 0.0969 | 207 | 759 |
| Tripartite motif-containing 59 | Trim59 | NM_025863 | 0.358 | 289 | 795 |
| Synuclein, alpha | Snca* | NM_009221 | 0.395 | 9 863 | 24 559 |
| Nonheme enzymes | |||||
| Fructosamine 3 kinase | FN3K | AJ404616 | 0.0746 | 346 | 4 573 |
| ATPase, AAA domain containing 3A | Atad3a | AF343079 | 0.085 | 347 | 4 253 |
| Mitochondrial gst 3 | Mgst3* | NM_025569 | 0.12 | 1 279 | 10 724 |
| Pyruvate kinase | Pklr | NM_013631 | 0.267 | 308.9 | 1 128 |
| Protein tyrosine phosphatase 4a3 | Ptp4a3, Acp5* | NM_008975 | 0.268 | 3 153 | 11 465 |
| Diacylglycerol O-acyltransferase 1 | Dgat1 | NM_010046 | 0.388 | 1 339 | 3 414 |
| Pyruvate carboxylase | Pcx | NM_008797 | 0.436 | 1 086 | 2 471 |
| Ubiquitin-conjugating enzyme E2C | Ube2c | AK003722 | 0.461 | 12 446 | 26 672 |
| Cathepsin B | Ctsb | NM_007798 | 0.536 | 752.1 | 1 419 |
| Globin and heme synthesis | |||||
| Alpha hemoglobin-stabilizing protein | AHSP, EDRF* | AF060220 | 0.0426 | 3 666 | 78 235 |
| β-Globin | β-major* | AF071431 | 0.199 | 13 941 | 72 270 |
| Benzodiazepine receptor, peripheral | Bzrp* | NM_009775 | 0.487 | 3 691 | 7 488 |
| Interferon-stimulated protein | Isg20 | NM_020583 | 0.501 | 1 432 | 2 890 |
| Biliverdin reductase B | Blvrb | BC006617 | 0.524 | 15 936 | 29 989 |
| Protoporphyrinogen oxidase | Ppox | NM_008911 | 0.555 | 1 644 | 2 999 |
| Hydroxymethylbilane synthase | Hmbs, PBGD | BC003861 | 0.693 | 30 672 | 42 426 |
| Aminolevulinic acid synthase 2, erythroid | ALAS-E | M63244 | 0.711 | 38 138 | 53 271 |
| Similar to Cpo protein | Cpox† | NM_007757 | 0.752 | 684 | |
| Uroporphyrinogen decarboxylase | Urod† | NM_009478 | 0.768 | 22 428 | 28 536 |
| Ferrochelatase | Fech† | NM_007998 | 1.195 | 20 752 | 17 629 |
| Aminolevulinic acid synthase 1 | ALAS-H† | M63245 | 1.394 | 846.2 | 625 |
| Uroporphyrinogen III synthase | Uros† | NM_009479 | 1.392 | 17 625 | 12 695 |
| Upregulated genes | |||||
| Carbonic anhydrase 1 | Car1 | NM_009799 | 12.39 | 24 734 | 2 155 |
| BCL2/adenovirus E1B 19-kDa interacting protein 1, NIP3 | Bnip3 | NM_009760 | 5.834 | 9 525 | 1 678 |
| Neoplastic progression 3 | Npn3; Srx; TX01 | Z31362 | 4.732 | 3 036 | 686 |
| RIKEN cDNA 9630039A02 gene | Cntnap2 | NM_025771 | 4.651 | 1 571 | 388 |
| Tnf receptor-associated factor 5 | Traf5 | NM_011633 | 4.464 | 778 | 367 |
| Trafficking protein particle complex 2 | Trappc2 | NM_025432 | 4.039 | 880 | 344 |
| RIKEN cDNA 3110040M04 gene | 3110040M04Ri | AK014162 | 3.973 | 2 038 | 577 |
| Glutathione transferase zeta 1 | Tmed8; Gm1184 | AK018286 | 3.909 | 2 717 | 690 |
| Ubiquitin-conjugating enzyme E2E 1, UBC4/5 homolog | Ube2e1 | NM_009455 | 3.842 | 4 706 | 2 774 |
| Formin homology 2 domain containing 3 | Fhod3 | AK020839 | 3.558 | 1 508 | 498 |
| RIKEN cDNA 1500010M24 gene | 1500010M24Ri | AK005193 | 3.532 | 1 505 | 468.2 |
| Interferon, alpha-inducible protein | G1p2 | NM_015783 | 3.444 | 2 849 | 968.3 |
| SH3 domain and nuclear localization signals, 1 | Samsn1; Hacs1 | AF222928 | 3.44 | 2 597 | 757 |
| Phosphoglycerate kinase 1 | Pgk1 | NM_008828 | 3.367 | 14 878 | 5 269 |
| ST3 beta-galactoside alpha-2,3-sialyltransferase 6 | St3gal6 | NM_018784 | 3.365 | 4 880 | 1 578 |
| G kinase anchoring protein 1 | Gkap1 | NM_019832 | 3.29 | 803 | 460 |
| Tuftelin 1 | Tuft1 | NM_011656 | 3.145 | 3 052 | 966 |
| Kruppel-like factor 6 | Klf6 | AF072403 | 2.843 | 736 | 302 |
| Retinoblastoma-like 2 | Rbl2 | NM_011250 | 2.793 | 812 | 346 |
| Musashi homolog 2 (Drosophila) | msi2 | AB056103 | 2.775 | 1 205 | 449 |
| Annexin A6 | Anxa6 | NM_013472 | 2.622 | 575.2 | 317.8 |
| Annexin A5 | Anxa5 | NM_009673 | 2.62 | 6 065 | 2 436 |
| Phosphoglycerate mutase 1 | Pgam1 | AF283667 | 2.46 | 23 283 | 9 548 |
Gene names are in full description and common abbreviations with a GenBank accession number. The ratio is the normalized mean of the relative expression in EKLF–/– versus EKLF+/+ fetal liver RNA (n = 6). The mean absolute intensities of each gene are given.
Genes verified by real-time RT-PCR
Genes that do not have expression fold differences that are enough for listing as possible EKLF targets but are included for comparison and discussion
We performed real-time RT-PCR for 13 putatively downregulated genes from different functional groups to validate the microarray data (Figure 1B). All 13 showed downregulation in the EKLF-null fetal liver, in most cases more dramatically than anticipated from the microarray fold-change calculation (Table 3). This suggests that the criteria we used to select putative EKLF-regulated genes were strict; that is, most of the 600 putative regulated genes are likely to be true positives.
There were fewer (290) transcripts upregulated greater than 2-fold in EKLF-null fetal liver compared with wild-type fetal liver (Figure 1A; Table 3) and most of these were only just above the 2-fold arbitrary cutoff. Some of these are implicated in apoptosis. However, we found no evidence of apoptosis by FACS staining of fetal liver cells with annexin V (data not shown) nor have others found increased apoptosis when EKLF is reduced.25 Hypoxia could indirectly influence gene expression in EKLF-null fetal liver samples. Indeed, 2 of the most highly upregulated genes, carbonic anhydrase 1 (CA1) and NIP3, are known to be inducible by hypoxia.39 Human γ-globin is known to be upregulated in the fetal livers of EKLF-null mice21,22 but was not present on these arrays, being human specific. Lastly, some of these transcripts could be directly repressed by EKLF. The full list of EKLF-dependent genes (and raw data) is available at Signet.40 A partial list organized by subcellular localization and/or function is shown in Table 3.
EKLF-induced transcripts in EKLF-ER cell lines overlap with EKLF-dependent genes in the fetal liver
We could not exclude the possibility that some of the gene expression differences could have resulted from a relative excess of certain nonerythroid cell types in EKLF-null fetal livers, hypoxia, or a failure of maturation of erythroid precursors. Therefore, we repeated expression profiling in B1.6 cells, an erythroid line derived from E14.5 EKLF-null fetal livers (the same gestational age as the primary fetal liver samples), which undergo EKLF-dependent terminal erythroid maturation. Upon activation of a tamoxifen-inducible form of EKLF (EKLF-ER) the cells differentiate in vivo.26 We undertook expression profiling 6 hours after addition of tamoxifen versus control (vehicle), expecting this time point to be sufficient for upregulation of direct EKLF target genes but insufficient for indirect upregulation via secondary transcription factor hierarchies or as a normal accompaniment of terminal differentiation. A scatter plot of the data is presented in Figure 1C. A total of 286 genes were upregulated greater than 1.3-fold (with B stats > 0 and absolute expression levels > 150) in response to tamoxifen and 154 were downregulated to this degree. Only 6 transcripts were downregulated greater than 2-fold and most of these are expressed sequence tags (ESTs; Table 4). Again, this strongly suggests that EKLF acts primarily as a transcriptional activator in this cell line system, although a few transcripts could be directly repressed by EKLF. The same genes used for the fetal liver were verified by real-time PCR in the cell line (Figure 1D). Interestingly, only a few of these genes were downregulated in the EKLF-null line, suggesting that the B1.6 cells do not exactly mimic the in vivo environment (see “EKLF is primarily a transcriptional activator in vivo”). Nevertheless, there was significant overlap with the gene lists from the fetal liver experiments (100 genes including β-globin, dematin, and AHSP; Figure 1E), which enabled us to focus upon likely direct targets of EKLF.
Table 4.
Tamoxifen-inducible changes in gene expression
|
Intensity
|
|||||
|---|---|---|---|---|---|
| Gene description | Common abbreviation | Accession no. | Control-tamoxifen ratio | Tamoxifen | Control |
| Upregulated genes | |||||
| Reelin | Rein | U72343 | 0.0623 | 5 574 | 279 |
| Erythroid-associated factor | Eraf; AHSP;EDRF | AF060220 | 0.088 | 61 187 | 7 499 |
| Mitochondrial glutathione S-transferase | Mgst3* | NM_025569 | 0.128 | 21 710 | 2 879 |
| Endothelin 2 | Edn2 | NM_007902 | 0.148 | 3 069 | 486 |
| Sparc/osteonectin | Spock1 | NM_009262 | 0.155 | 1 769 | 329 |
| Lipocalin 5 | Lcn5 | NM_007947 | 0.171 | 3 445 | 602 |
| GLI pathogenesis-related 2 | Glipr2; GAPR-1 | AK017557 | 0.172 | 1 607 | 273 |
| Erythrocyte protein band 4.9 | Epb4.9* | NM_013514 | 0.202 | 3 279 | 723 |
| Ubiquilin 1 | Ubqln1; DA41 | AK007651 | 0.206 | 4 783 | 974 |
| Annexin A8 | Anxa8 | NM_013473 | 0.21 | 1 628 | 354 |
| Ephrin A2 | Efna2 | NM_007909 | 0.148 | 3 069 | 486 |
| Solute carrier family 22, member 16 | Slc22a16 | AK014809 | 0.172 | 1 607 | 273 |
| Embryonic ζ-globin | zeta-globin | NM_010405 | 0.172 | 1 607 | 273 |
| Adult β-globin | beta-maj globin | AF071431 | 0.202 | 3 279 | 723 |
| Erythroblast membrane-associated protein | Ermap* | NM_013848 | 0.235 | 23 792 | 5 788 |
| G protein, gamma 2 subunit | Gng2 | NM_010315 | 0.24 | 3 272 | 762 |
| TGFB-inducible early growth response 1 | Tieg1 | NM_013692 | 0.374 | 2 823 | 1 138 |
| Duffy blood group | Dfy* | NM_010045 | 0.432 | 1 525 | 673 |
| Diacylglycerol O-acyltransferase 2 | Dgat2; DGAT-2 | AF384160 | 0.447 | 1 957 | 883 |
| Benzodiazepine receptor, peripheral | Bzrp* | NM_009775 | 0.451 | 26 957 | 12 685 |
| Decay accelerating factor 2 | Daf2 | NM_007827 | 0.452 | 510 | 233 |
| Aminolevulinic acid synthase 2, erythroid | ALAS-E; ALAS2 | M63244 | 0.47 | 36 257 | 17 574 |
| Transferrin receptor | Tfrc; CD71 | AK011596 | 0.483 | 31 429 | 15 360 |
| Interferon-stimulated protein | Isg20 | NM_020583 | 0.484 | 6 661 | 3 293 |
| Nuclear factor, erythroid derived 2 | Nfe2 | L09600 | 0.486 | 5 562 | 2 760 |
| Glycophorin C | Gypc | AK002769 | 0.5 | 11 440 | 5 777 |
| Rhesus blood group CE and D | Rhced* | NM_011270 | 0.579 | 20 406 | 12 047 |
| Zinc finger and BTB domain containing 7 | Zbtb7; Pokemon | AK018596 | 0.58 | 3 943 | 2 313 |
| Zinc finger protein 297 | Zfp297 | NM_020625 | 0.652 | 1 772 | 1 220 |
| Embryonic ε-y globin | ε-y globin | NM_008221 | 0.627 | 61 768 | 39 553 |
| Embryonic βh1 globin | Hbb-βh1 | NM_008219 | 0.593 | 58 027 | 37 087 |
| Porphobilinogen deaminase | PBDG | BC003861 | 0.706 | 42 501 | 28 006 |
| Downregulated genes | |||||
| RIKEN cDNA 1200008O12 gene | 1200008O12Rik | AK004655 | 7.189 | 106 | 438 |
| RIKEN cDNA 1110032E23 gene | 11100032E23Rik | AF285091 | 2.729 | 1 759 | 4 777 |
| RIKEN cDNA 1700029M23 gene | 1700029M23Rik | AK006511 | 2.364 | 4 188 | 9 591 |
| RIKEN cDNA 9530027K23 gene | 9530027K23Rik | AK020578 | 2.347 | 156 | 302 |
| RIKEN cDNA 2600013N14 gene | 2600013N14Rik | AK011203 | 2.223 | 364 | 775 |
| Tumor protein p53-inducible protein 5 | Tp53i5 | AK017334 | 2.138 | 7 662 | 16 485 |
Gene names are in full description and common abbreviations with a GenBank accession number. The ratio is the normalized mean of the relative expression in control versus tamoxifen-treated samples (n = 4). The mean absolute intensities of each gene are given for both taxoxifen- and control-treated samples (n = 4).
Genes verified by real-time PCR
Dematin (band 4.9), the red cell cytoskeleton, and EKLF
Dematin is an important red cell cytoskeleton protein.28,38,41 The array data suggest that dematin is highly dependent on EKLF in the fetal liver (∼20-fold) and also in the EKLF-null erythroid cell line (∼5-fold), which was confirmed in both systems by real-time RT-PCR (Figure 1B,D). By Western blotting, dematin protein expression is also dramatically reduced in EKLF-null fetal liver compared with wild-type and heterozygous littermates (Figure 1F). With regard to other red cell cytoskeleton proteins, α-spectrin (Spna-1) was also mildly EKLF dependent (∼1.5-fold) in the fetal liver (Table 3) but not in the cell line. Gallagher et al29 suggest that β-spectrin, band 3 (AE1), and band 4.2 are also direct EKLF target genes, but we did not find these or other red cell cytoskeletal proteins to be significantly EKLF dependent (Table 3).
AHSP and EKLF
One of the most dramatic putative EKLF-regulated genes is AHSP (Figure 1; Tables 3, 4), also known as EDRF, a recently described GATA-1 target gene that binds and stabilizes free α-globin and Hbα.27 AHSP expression was essentially absent in EKLF-null fetal liver by real-time RT-PCR and it was also dramatically upregulated by tamoxifen in B1.6 cells (Figure 1B,D). AHSP was also absent in EKLF-null fetal liver cells by Western blotting (Figure 1F). AHSP is important for stabilizing free Hbα in vivo27,42 and its loss in EKLF-null mice likely exacerbates the pathology of a chain excess caused by β-globin deficiency.
Transmembrane proteins and EKLF
TfR1 appeared to be an EKLF-dependent gene in fetal liver (Tfrc; Figure 1B). Loss of TfR1 might contribute to poor hemoglobinization in EKLF-null erythroblasts by impairing the uptake of circulating transferrin-bound iron. TfR1 was significantly downregulated in EKLF-null fetal liver (∼10-fold) and induced by tamoxifen (∼2-fold) in B1.6 cells (Figure 1B,D). To investigate this downregulation further we measured the cell surface expression of CD71 by FACS. Although TfR1 transcripts were reduced, CD71 expression at the cell surface was normal (Figure 2); greater than 95% of EKLF-null fetal liver cells were CD71+ and the intensity of expression was identical to wild-type littermates. It is possible that posttranscriptional regulation of TfR1 may play some compensatory role to generate normal CD71 surface expression in the context of reduced mRNA levels.
Figure 2.
TER119 is absent in EKLF-null fetal liver. Representative dot plots of FACS-sorted E14.5 fetal liver cells from EKLF+/+ and EKLF-/- mice stained with TER119-PE and either CD71-FITC (transferrin receptor) or CD117-FITC (c-kit). The top and right quadrants represent positive stained cells. The percentage of cells in each quadrant is indicated.
Interestingly, there was a very dramatic reduction in TER119 expression in EKLF-null fetal liver cells (Figure 2). In the normal fetal liver, greater than 80% of cells are TER119 intermediate or bright as previously published,43,44 whereas most if not all EKLF-null cells fail to express TER119. The antigen recognized by TER119 is an unknown protein of approximately 52 kDa.44 It could be one of the transmembrane proteins upregulated by EKLF (Table 3). Expression of the stem/progenitor cell marker CD117 (c-kit) was normal in EKLF-null fetal liver (Figure 2), which is consistent with the normal progenitor cell numbers as determined by colony assays in SCF and Epo.20
Primitive EKLF-null red cells show defects in gene expression, the red cell membrane, and surface expression of TER119
We and others previously reported that EKLF-null primitive erythrocytes are morphologically normal and express normal amounts of embryonic globins: ζ-, βh1-, and ε-y.19,20 This is in spite of the fact that EKLF is expressed in embryonic red cells45 and is required for expression of a μLCR-β-globin transgene and LacZ reporter gene in primitive cells.46,47 To determine if our target genes identified at E14.5 played any role at earlier time points we examined expression of the novel EKLF target genes in E11.5 embryonic blood. Real-time RT-PCR demonstrated a dramatic reduction in expression of AHSP, dematin, Mgst3, and Ermap/Scianna in EKLF-null embryonic red cells (Figure 3A), and Western blotting showed absence of both dematin and AHSP (Figure 3B). Acp3, the peripheral benzodiazepine receptor (Bzrp), Rh-cde, and ICAM4/LW were also significantly downregulated. Interestingly, not all of the 13 genes tested in the fetal liver were downregulated in embryonic red cells, suggesting that other CACC-box binding proteins may compensate for the loss of EKLF in this environment at some erythroid gene promoters (see “Primitive EKLF-null red cells show defects in morphology and gene expression”).
Figure 3.
EKLF-null primitive erythroid cells show defects in gene expression and structure. (A) Relative expression levels of putative EKLF target genes in E11.5 EKLF-/- blood cells compared with EKLF+/+. Levels were measured by real-time PCR and normalized to HPRT. Data are presented as mean, n = 2. (B) Representative Western blot of dematin and AHSP expression in whole-cell extracts from E11.5 blood cells. Actin is presented as a loading control. (C) Representative dot plots of FACS-sorted E11.5 blood cells from EKLF+/+, EKLF+/-, and EKLF-/- mice stained with TER119-PE and either CD71-FITC (transferrin receptor) or CD117-FITC (c-kit). The top and right quadrants represent positive stained cells. The percentage of cells in each quadrant is indicated. (D) Scanning electron microscopy of cytospin preparations of primitive erythroid cells from E11.5 EKLF+/+ and EKLF-/- embryos. The small enucleated discs represent maternal red cells and the large nucleated cells are yolk sac-derived primitive erythrocytes. Scale bars and magnification are shown.
EKLF-null embryonic red cells, like their fetal liver counterparts, fail to express TER119 but express normal levels of CD71 and CD117/c-kit (Figure 3C). Interestingly, EKLF+/- embryonic red cells express intermediate levels of TER119 at the surface (Figure 3C), suggesting transcript levels in this case precisely define the output of the antigen recognized by TER119. Haploinsufficiency of EKLF at the human β-globin gene promoter also results in reduced production of β-globin.22 To determine if loss of TER119 influences cell membrane morphology we performed scanning electron microscopy. EKLF-null embryonic red cells appear to have an abnormal morphology following gentle cytocentrifugation (Figure 3D). They displayed aberrant folding of the cell membrane that was a reliable surrogate marker of genotype. Despite this abnormal phenotype, the embryonic red cell numbers in EKLF-null embryos were not significantly different from littermates (data not shown), suggesting a relatively normal lifespan for the mutant primitive erythrocytes.
Phylogenetically conserved CACC and GATA sites within dematin gene regulatory regions
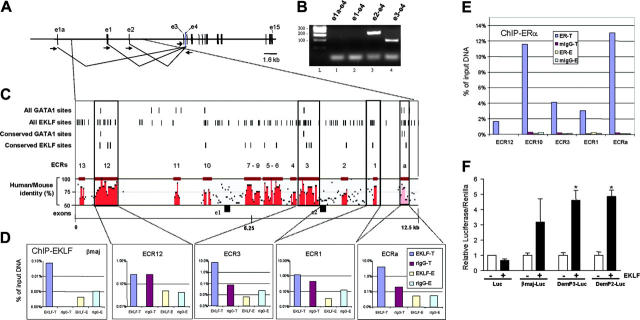
Expression-profiling experiments cannot distinguish whether the putative EKLF-regulated genes are direct targets, activated indirectly via a transcriptional cascade, or activated indirectly because of EKLF-induced differentiation. Thus, we searched for phylogenetically conserved EKLF binding sites (CCNCNCCC)33 within our putative target gene list. In this paper we focused in detail upon regulation of the dematin gene as proof of principal of direct EKLF target validation. This gene was previously reported to contain 14 exons,38 with the first exon containing the transcriptional initiation codon (exon 3 in Figure 4A). However, a search for spliced ESTs using the UCSC genome browser48 demonstrated the presence of additional spliced transcripts from 3 upstream noncoding exons labeled e1a, e1, and e2 in Figure 4A. We confirmed expression of transcripts originating from exon 2 and exon 3, but not exon 1 or 1a, in fetal liver by RT-PCR (Figure 4B).
Figure 4.
EKLF occupies conserved CACC elements in dematin upstream gene regulatory regions and directly activates 2 dematin promoters. (A) Schematic of organization of the dematin gene. The original cDNA starts at exon 3.38 The other putative upstream exons correspond to alternatively spliced transcripts. (B) An RT-PCR analysis of alternative transcript used in E14.5 fetal liver. The arrows represent RT-PCR primers. (C) Schematic of the ECR regions of the dematin genomic sequence. ECR regions are defined as areas of greater than 70% sequence identity of at least 100 bp in length and are numbered in reverse order from intron 2. All putative EKLF and GATA-1 sites within this region are marked, as are the conserved sites between the human, mouse, and rat genomes. A plot of percentage identity between human and mouse sequences is shown, with red regions corresponding to ECRs. The positions of exons 1 and 2 are indicated for reference. (D) ChIP using EKLF antisera or nonimmune sera. Plots show real-time PCR analysis of the precipitated DNA from the corresponding upstream regions. For each region, ChIPed material is expressed relative to input DNA. ChIP was performed on B1.6 cells treated in 4 ways: using EKLF-specific or preimmune sera and after tamoxifen-induced (T) or control (E) induction of EKLF-ER. (E) ChIP using ERα monoclonal antibody or isotype control. Again, ChIP was performed on cells treated in 4 ways: using ERα antibody or mIgG1 and after tamoxifen-induced (T) or control (E) induction of EKLF-ER. (F) Mean relative luciferase levels (as normalized to renilla) ± SEM, n = 3. pGL3-basic (Luc) constructs were transfected into SL2 cells with or without an EKLF expression vector. In each case transfection of the Luc construct alone did not alter luciferase levels (data not shown). EKLF response data are presented normalized to 1 for each construct. The βmaj-Luc contains the β-major globin promoter and is a positive control for EKLF action. DemP3 corresponds to -1 to -1000, and DemP2 to -8267 to -9269 from the transcription start site of the dematin gene.38
We used the ECR Browser31 to find phylogenetically conserved regions of at least 100 bp of greater than 70% identity between the human (hg17), mouse (mm5), and rat (rn3) genomes. We found 14 ECRs within a 12.5-kb region of dematin that extended from the FGF17 gene upstream to intron 2 (Figure 4C). These are numbered in reverse to indicate increasing distance from the coding region. ECR3 abuts exon 2 and so it is likely to be a proximal promoter element (P2) for this alternative transcript. We then used the Mulan interface32 to perform a directed search for conserved sites (between the 3 genomes) corresponding to the EKLF consensus site CCNCNCCC.33 We simultaneously looked for conserved GATA-1 binding sites (WGATAR) as previously defined by PCR site selection,34 since EKLF and GATA-1 are known to work cooperatively in certain contexts.8,49 Locations of conserved as well as nonconserved EKLF and GATA-1 binding sites are indicated in Figure 4C. These reside mostly within the ECR regions. In the case of ECR3, ECR12, and ECRa, 1 or 2 conserved GATA-1 binding sites occur in close proximity to the conserved EKLF sites (Figure 4C).
In vivo occupancy of dematin ECRs by EKLF
We performed ChIP assays using ERα or an irrelevant mouse monoclonal antibody in B1.6 cells treated with either tamoxifen or vehicle control. We also performed ChIP assays with an EKLF antibody or preimmune rabbit sera. In each case, an aliquot of cross-linked input DNA (prior to ChIP) was prepared and analyzed as a reference for ChIP efficiency. In the case of an irrelevant negative control gene, nectin,37 there was no enrichment of DNA in material ChIPed by the ERα or EKLF antibodies, confirming specificity and the lack of DNA occupancy by EKLF at this irrelevant gene (data not shown). In contrast, ChIP with EKLF antisera demonstrated tamoxifen-dependent occupancy of the β-globin promoter by EKLF-ER (Figure 4D, left panel). Dematin ECRa and ECR3 demonstrated significant EKLF occupancy (each ∼100-fold), which was both specific for the EKLF antibody and also tamoxifen dependent. On the other hand, ECR1 and ECR12 demonstrated much less, if any, EKLF occupancy (Figure 4D). We also performed ChIP assays with an ERα monoclonal antibody or irrelevant mIgG1 isotype control. Again, there was strong tamoxifen-dependent EKLF-ER occupancy at ECRa and also ECR10 (∼1000-fold) and reasonable occupancy at ECR1 and ECR3 but weak occupancy at ECR12. Together, these results strongly suggest that many but not all of the phylogenetically conserved regions containing EKLF binding sites within a 12.5-kb region of the dematin gene are bound by EKLF in vivo. Thus, dematin is very likely to be a direct EKLF target.
EKLF directly activates 2 dematin promoters
We cloned a 1-kb region of ECR3 (corresponding to promoter P2), which is bound by EKLF in vivo, and a 1-kb region upstream of the published murine dematin cDNA (promoter P3),38 which also contains 3 (although not conserved) CACC motifs into pGL3-Basic, and performed reporter assays in SL2 cells, which have no endogenous SP1 activity. Cells were cotransfected with an expression vector for EKLF, pPAC-EKLF.5 The β-globin promoter was used as a positive control for EKLF responsiveness.5 EKLF did not activate pGL3-Basic but activated the β-globin promoter approximately 4-fold and both the P2 and P3 promoters approximately 5-fold (Figure 5B). This is further evidence that EKLF directly activates the dematin gene via CACC sites within the 2 putative promoters.
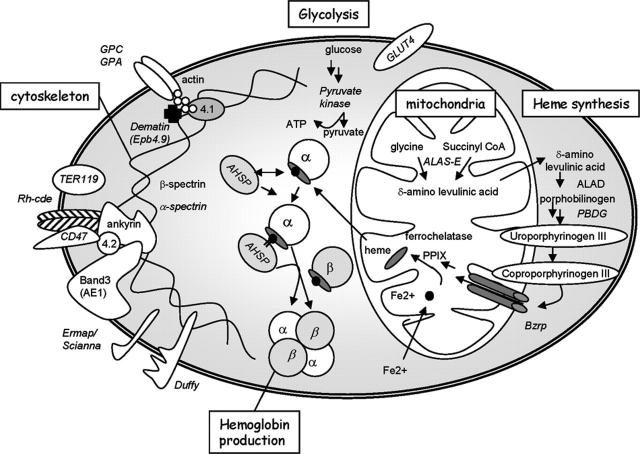
Figure 5.
Schematic of metabolic pathways and structural red cell proteins regulated by EKLF. EKLF coordinates expression of genes that encode proteins that link the membrane and cytoskeleton, heme synthesis, energy production through glycolysis (pyruvate kinase), β-globin, and AHSP. Genes in italics are probable EKLF target genes. Genes in normal font are provided for context. See text for abbreviations.
Discussion
EKLF is primarily a transcriptional activator in vivo
Most analyses have reported that EKLF acts primarily as a transcriptional activator in vitro,50,51 although one proposes that EKLF can bind the transcriptional repressor Sin3A and directly repress γ-globin gene expression.27 Our expression-profiling data suggest EKLF acts primarily as a transcriptional activator in vivo, although a few transcripts may be directly repressed. In contrast, GATA-1 functions more often as a transcriptional repressor in a similar cell line system.52 We conducted expression-profiling analyses using both EKLF-null mice and an EKLF-null cell line to ensure we were targeting genes that were specific in their response to EKLF. This approach yielded an overlap of approximately 100 genes, allowing us to focus our studies on this subset (Figure 1). There were some genes apparently EKLF dependent in the fetal liver but not induced by tamoxifen in B1.6 cells. We think this is most likely due to the immortalization process for B1.6 cells. There were no genes that were both upregulated by EKLF loss in the fetal liver and downregulated by tamoxifen in the cell line, so we have no solid leads for EKLF-repressed genes. Our results are similar and complementary to others recently published.53 These authors used a different inducible EKLF system in primary fetal liver-derived cells and a different array platform. Despite these differences, we found a remarkably similar set of EKLF target genes. Together, our 2 sets for experiments argue very strongly for the validity of the gene lists.
We used real-time PCR to validate many of the putative EKLF target genes (Figure 1B,D), as well as confirming that transcriptional downregulation translated to a decrease in protein expression (Figure 1F). Importantly there did appear to be differences in the expression levels of some genes, such as Lgals and Snca, between the 2 systems (Figure 1B,D), suggesting that we have the ability to focus our studies even further. It is not certain from these expression-profiling experiments whether all or most of the differentially expressed genes are direct EKLF transcriptional targets or not, but an argument for many being so, based on the published literature and on our findings presented here, will follow.
Primitive EKLF-null red cells show defects in morphology and gene expression
To determine if EKLF is active during primitive erythropoiesis we examined expression of some of our target genes at E11.5. Our data show that many of these genes were downregulated at this developmental time point (Figure 3A) and that, at least for dematin, AHSP, and TER119, this translated into a substantial downregulation in protein expression (Figure 3B). Loss of dematin and TER119 might partly explain the abnormally ruffled red cell membrane (Figure 3D), but loss of other transmembrane proteins (Table 3) are likely to contribute to this defect. Our data confirm that EKLF is transcriptionally active in embryonic red cells as previously described for β-globin transgenes46,47 and identify key novel EKLF target genes in embryonic as well as definitive red cells. Given that EKLF-null primitive erythrocytes appear to have a relatively normal lifespan and the embryos survive until late gestation, it is likely that other CACC-binding proteins, such as LKLF (KLF2), may compensate for the absence of EKLF at certain promoters such as the ζ-, ε-y, βh1-globin, and Duffy gene promoters in embryonic red cells.54
The red cell membrane in the absence of EKLF
Dematin, or band 4.9, is a key component of the red cell cytoskeleton that binds F-actin28 and other components of the cytoskeleton (Figure 5) and it is highly downregulated in the absence of EKLF (Figures 1 and 3). A genetic deletion of the headpiece domain of dematin leads to increased red cell fragility and compensated hemolysis,41 suggesting that it is an essential structural component of the red cell cytoskeleton. Thus, the observed membrane defect in primitive erythroid cells at E11.5 (Figure 3D) may also be partially due to dematin loss. To demonstrate that our EKLF target genes may be directly regulated by the transcription factor, we showed that EKLF binds a number of phylogenetically conserved regulatory elements (with CACC cores) in the dematin gene (Figure 4D-E) and activates transcription from 2 independent erythroid promoters (Figure 4F). Both of these promoter elements contain EKLF consensus CACC sites, further suggesting that, like β-globin, dematin is a key in vivo EKLF target gene (Figure 5).
Hemoglobin synthesis pathway gene regulation by EKLF
Heme is synthesized from glycine and succinyl CoA via an enzymatic cascade acting within and without mitochondria (Figure 5). The first enzyme in the biosynthetic pathway is ALAS. There are 2 ALAS genes, ALAS1 (or ALAS-H) and ALAS2 (or ALAS-E). ALAS1 is ubiquitously expressed at low levels and was found to have normal expression levels in EKLF-null fetal liver (Table 3). ALAS2, which is mutated in X-linked sideroblastic anemia,55 is expressed at much higher levels in red cells (compare intensities in Table 3) and is moderately downregulated following EKLF loss (Table 3). Interestingly, a gene knockout of ALAS2 leads to severe anemia with accumulation of siderotic iron and an increase in nucleated definitive red cells,56 and a zebrafish mutant, sauternes, has a similar severe erythroid phenotype.57 The ALAS2 promoter has a conserved CACC element that can bind EKLF, and EKLF can activate ALAS2 in reporter assays.12,58 There is also a well-characterized EKLF-dependent enhancer in intron 8.59
The third enzyme in the heme-synthesis pathway known alternatively as PBGD or hydroxymethylbilane synthase (Hmbs) was induced 2-fold by EKLF in B1.6 cells (Table 4) and downregulated the EKLF-null fetal liver (Table 3). Interestingly, mouse and human PBGD gene promoters also have conserved CACC-box elements60,61 that can bind EKLF in vitro. Although we previously reported PBGD is not EKLF dependent in EKLF-null red cells,20 it could be an EKLF target gene from these expression-profiling results. In addition, the peripheral Bzrp may transport coproporphyrin III into the mitochondria for completion of heme synthesis.62 Bzrp levels are markedly reduced in EKLF-null cells (Table 3; Figure 1B,D). Expression of other heme-synthesis enzymes are not significantly EKLF dependent (Tables 3, 4). These results suggest that the combined lack of ALAS2, PDBG, and Bzrp is likely to be responsible in concert for the defect in heme synthesis and siderotic (mitochondrial) iron accumulation found in EKLF-null red cells25,26 (Figure 5).
AHSP is nearly undetectable when EKLF is absent (Figures 1 and 3). AHSP is a small red cell-specific protein that binds and stabilizes free α-globin chains and alpha hemoglobin.27 AHSP knockout mice have a moderate compensated hemolytic anemia that is worsened significantly by concomitant β-globin deficiency, suggesting that one function of AHSP is to protect cells against the toxic effects of excessive free α-globin or αHb.27 Thus, the loss of AHSP in EKLF-null mice is likely to aggravate the coexisting β-globin deficiency and, therefore, contribute to embryonic demise.
Blood group antigens and EKLF
Our expression-profiling experiments suggest that many blood group antigens are regulated by EKLF. These include Rh-ced (which encodes an allelic Rh antigen complex), Ermap (which encodes the Scianna blood group antigen63,64), Duffy, and ICAM4 (also known as the LW antigen65; Table 3). These genes were validated as EKLF target genes in the fetal liver by real-time PCR (Figures 1 and 3), although only Duffy and Ermap were significantly upregulated by tamoxifen in B1.6 cells (Figure 1D). Also, Duffy was not affected at E11.5 (Figure 3A), suggesting that downregulation in the fetal liver may be an indirect effect of EKLF loss in this case. In addition to these validated genes, other blood group antigens and related proteins are likely EKLF target genes (Table 3). These include glycophorin A (GPA), CD47 (an Rh antigen-associated protein66), and CD59 (a GPI-anchored protein lost in paroxysmal nocturnal hemoglobinuria [PNH]). Interestingly, most of these proteins coexist in a mega-complex that links the membrane to the underlying cytoskeleton.67 Importantly, mouse Rh-cde, GPA, CD59, CD47, and Ermap/Scianna genes all contain CACC sites in their proximal promoters that are likely to bind EKLF.7,28,68 Together, our results strongly suggest that EKLF coordinates expression of many key blood group antigens and associated proteins that link the membrane to the cytoskeleton (Figure 5).
Transcription factor targets
Many of the EKLF-regulated genes encode transcription factors such as p45-NF-E2, CHOP, LMO2, ETO, BKLF/KLF3, RARγ, RARα, pokemon (Zbtb7), Bteb1/KLF9, Gli-3, and hemogen.69 A detailed discussion of the physiologic importance of these is beyond the scope of this paper, but many have proven roles in erythropoiesis.70 Of particular note, BKLF/KLF3 is markedly reduced in EKLF-null fetal liver71 and EKLF-null cell lines.26 BKLF is one of the most abundant CACC-box binding proteins in erythroid cells and its loss in EKLF-null embryos may play a key role in erythropoiesis and/or γ-globin gene silencing, so it is worthy of further investigation.
Conclusion
In summary, we have used expression profiling to discover that EKLF plays a global role in both primitive and definitive erythropoiesis. It participates in the control of expression of many genes including AHSP and β-globin and heme synthesis and transport enzymes, which function together to produce the hemoglobin molecule (Figure 5). EKLF also coordinates expression of many genes that work together to build a flexible red cell that can transverse the microcirculation (Figure 5). Importantly, we show that EKLF can directly regulate a least one (and probably many) potential target gene, suggesting that its action as a transcription factor is direct. Finally, our findings suggest that drugs targeting EKLF may not be an ideal treatment strategy for patients with β-hemoglobinopathies.
Acknowledgments
We thank Chris Vakoc, Gerd Blobel, and Emery Bresnick for advice about ChIP assays. We thank Robert Rea for advice about FACS.
Prepublished online as Blood First Edition Paper, December 27, 2005; DOI 10.1182/blood-2005-07-2888.
Supported by Australian National Health and Medical Research Council (NH & MRC) grant 143701 (A.P.) and National Institutes of Health (NIH) grant R01 DK061692 (M.W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Myers RM, Tilly K, Maniatis T. Fine structure genetic analysis of a beta-globin promoter. Science. 1986;232: 613-618. [DOI] [PubMed] [Google Scholar]
- 2.Myers RM, Cowie A, Stuve L, Hartzog G, Gaensler K. Genetic and biochemical analysis of the mouse beta-major globin promoter. Prog Clin Biol Res. 1989;316A: 117-127. [PubMed] [Google Scholar]
- 3.Charnay P, Mellon P, Maniatis T. Linker scanning mutagenesis of the 5′-flanking region of the mouse beta-major-globin gene: sequence requirements for transcription in erythroid and nonerythroid cells. Mol Cell Biol. 1985;5: 1498-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowie A, Myers RM. DNA sequences involved in transcriptional regulation of the mouse beta-globin promoter in murine erythroleukemia cells. Mol Cell Biol. 1988;8: 3122-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon CT, Fox VJ, Najdovska S, Perkins AC. C/EBPdelta and C/EBPgamma bind the CCAAT-box in the human beta-globin promoter and modulate the activity of the CACC-box binding protein, EKLF. Biochim Biophys Acta. 2005;1729: 74-80. [DOI] [PubMed] [Google Scholar]
- 6.Orkin SH, Antonarakis SE, Kazazian HH Jr. Base substitution at position -88 in a beta-thalassemic globin gene: further evidence for the role of distal promoter element ACACCC. J Biol Chem. 1984;259: 8679-8681. [PubMed] [Google Scholar]
- 7.Rahuel C, Vignal A, London J, et al. Structure of the 5′ flanking region of the gene encoding human glycophorin A and analysis of its multiple transcripts. Gene. 1989;85: 471-477. [DOI] [PubMed] [Google Scholar]
- 8.Gregory RC, Taxman DJ, Seshasayee D, Kensinger MH, Bieker JJ, Wojchowski DM. Functional interaction of GATA1 with erythroid Kruppel-like factor and Sp1 at defined erythroid promoters. Blood. 1996;87: 1793-1801. [PubMed] [Google Scholar]
- 9.Tsai SF, Strauss E, Orkin SH. Functional analysis and in vivo footprinting implicate the erythroid transcription factor GATA-1 as a positive regulator of its own promoter. Genes Dev. 1991;5: 919-931. [DOI] [PubMed] [Google Scholar]
- 10.Raich N, Romeo PH. Erythroid regulatory elements. Stem Cells (Dayt). 1993;11: 95-104. [DOI] [PubMed] [Google Scholar]
- 11.Youssoufian H, Zon LI, Orkin SH, D'Andrea AD, Lodish HF. Structure and transcription of the mouse erythropoietin receptor gene. Mol Cell Biol. 1990;10: 3675-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surinya KH, Cox TC, May BK. Transcriptional regulation of the human erythroid 5-aminolevulinate synthase gene: identification of promoter elements and role of regulatory proteins. J Biol Chem. 1997;272: 26585-26594. [DOI] [PubMed] [Google Scholar]
- 13.Hartzog GA, Myers RM. Discrimination among potential activators of the beta-globin CACCC element by correlation of binding and transcriptional properties. Mol Cell Biol. 1993;13: 44-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller IJ, Bieker JJ. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Kruppel family of nuclear proteins. Mol Cell Biol. 1993;13: 2776-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philipsen S, Suske G. A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucleic Acids Res. 1999;27: 2991-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner J, Crossley M. Mammalian Kruppel-like transcription factors: more than just a pretty finger. Trends Biochem Sci. 1999;24: 236-240. [DOI] [PubMed] [Google Scholar]
- 17.van Ree JH, Roskrow MA, Becher AM, et al. The human erythroid-specific transcription factor EKLF localizes to chromosome 19p13.12-p13.13. Genomics. 1997;39: 393-395. [DOI] [PubMed] [Google Scholar]
- 18.Benzeno S, Narla G, Allina J, et al. Cyclin-dependent kinase inhibition by the KLF6 tumor suppressor protein through interaction with cyclin D1. Cancer Res. 2004;64: 3885-3891. [DOI] [PubMed] [Google Scholar]
- 19.Nuez B, Michalovich D, Bygrave A, Ploemacher R, Grosveld F. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature. 1995;375: 316-318. [DOI] [PubMed] [Google Scholar]
- 20.Perkins AC, Sharpe AH, Orkin SH. Lethal beta-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature. 1995;375: 318-322. [DOI] [PubMed] [Google Scholar]
- 21.Perkins AC, Gaensler KM, Orkin SH. Silencing of human fetal globin expression is impaired in the absence of the adult beta-globin gene activator protein EKLF. Proc Natl Acad Sci U S A. 1996;93: 12267-12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wijgerde M, Gribnau J, Trimborn T, et al. The role of EKLF in human beta-globin gene competition. Genes Dev. 1996;10: 2894-2902. [DOI] [PubMed] [Google Scholar]
- 23.Yang B, Kirby S, Lewis J, Detloff PJ, Maeda N, Smithies O. A mouse model for beta 0-thalassemia. Proc Natl Acad Sci U S A. 1995;92: 11608-11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkins AC, Peterson KR, Stamatoyannopoulos G, Witkowska HE, Orkin SH. Fetal expression of a human Agamma globin transgene rescues globin chain imbalance but not hemolysis in EKLF null mouse embryos. Blood. 2000;95: 1827-1833. [PubMed] [Google Scholar]
- 25.Spadaccini A, Tilbrook PA, Sarna MK, Crossley M, Bieker JJ, Klinken SP. Transcription factor erythroid Kruppel-like factor (EKLF) is essential for the erythropoietin-induced hemoglobin production but not for proliferation, viability, or morphological maturation. J Biol Chem. 1998;273: 23793-23798. [DOI] [PubMed] [Google Scholar]
- 26.Coghill E, Eccleston S, Fox V, et al. Erythroid Kruppel-like factor (EKLF) coordinates erythroid cell proliferation and hemoglobinization in cell lines derived from EKLF null mice. Blood. 2001;97: 1861-1868. [DOI] [PubMed] [Google Scholar]
- 27.Kihm AJ, Kong Y, Hong W, et al. An abundant erythroid protein that stabilizes free alpha-haemoglobin. Nature. 2002;417: 758-763. [DOI] [PubMed] [Google Scholar]
- 28.Frank BS, Vardar D, Chishti AH, McKnight CJ. The NMR structure of dematin headpiece reveals a dynamic loop that is conformationally altered upon phosphorylation at a distal site. J Biol Chem. 2004;279: 7909-7916. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher PG, Pilon AM, Nilson DG, Wong E, Bodine DM. Multiple defects in erythroid gene expression in erythroid Kruppel-like factor (EKLF) target genes in EKLF-deficient mice [abstract]. Blood Cells Mol Dis. 2005;34: 71-134. [Google Scholar]
- 30.European Bioinformatics Institute. MIAME: minimum information about a microarray experiment. http://www.mged.org/Workgroups/MIAME/miame.html. Accessed January 2005.
- 31.Ovcharenko I, Nobrega MA, Loots GG, Stubbs L. ECR Browser: a tool for visualizing and accessing data from comparisons of multiple vertebrate genomes. Nucleic Acids Res. 2004;32: W280-W286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ovcharenko I, Loots GG, Giardine BM, et al. Mulan: multiple-sequence local alignment and visualization for studying function and evolution. Genome Res. 2005;15: 184-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng WC, Southwood CM, Bieker JJ. Analyses of beta-thalassemia mutant DNA interactions with erythroid Kruppel-like factor (EKLF), an erythroid cell-specific transcription factor. J Biol Chem. 1994;269: 1493-1500. [PubMed] [Google Scholar]
- 34.Merika M, Orkin SH. DNA-binding specificity of GATA family transcription factors. Mol Cell Biol. 1993;13: 3999-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loots GG, Ovcharenko I. rVISTA 2.0: evolutionary analysis of transcription factor binding sites. Nucleic Acids Res. 2004;32: W217-W221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Im H, Grass JA, Christensen HM, Perkins A, Bresnick EH. Histone deacetylase-dependent establishment and maintenance of broad low-level histone acetylation within a tissue-specific chromatin domain. Biochemistry. 2002;41: 15152-15160. [DOI] [PubMed] [Google Scholar]
- 37.Letting DL, Rakowski C, Weiss MJ, Blobel GA. Formation of a tissue-specific histone acetylation pattern by the hematopoietic transcription factor GATA-1. Mol Cell Biol. 2003;23: 1334-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azim AC, Kim AC, Lutchman M, Andrabi S, Peters LL, Chishti AH. cDNA sequence, genomic structure, and expression of the mouse dematin gene (Epb4.9). Mamm Genome. 1999;10: 1026-1029. [DOI] [PubMed] [Google Scholar]
- 39.Manalo DJ, Rowan A, Lavoie T, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105: 659-669. [DOI] [PubMed] [Google Scholar]
- 40.Signet. http://spring.imb.uq.edu.au. Accessed 2005.
- 41.Khanna R, Chang SH, Andrabi S, et al. Headpiece domain of dematin is required for the stability of the erythrocyte membrane. Proc Natl Acad Sci U S A. 2002;99: 6637-6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong Y, Zhou S, Kihm AJ, et al. Loss of alpha-hemoglobin-stabilizing protein impairs erythropoiesis and exacerbates beta-thalassemia. J Clin Invest. 2004;114: 1457-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papathanasiou P, Perkins AC, Cobb BS, et al. Widespread failure of hematolymphoid differentiation caused by a recessive niche-filling allele of the Ikaros transcription factor. Immunity. 2003;19: 131-144. [DOI] [PubMed] [Google Scholar]
- 44.Dorsky RI, Raible DW, Moon RT. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev. 2000;14: 158-162. [PMC free article] [PubMed] [Google Scholar]
- 45.Southwood CM, Downs KM, Bieker JJ. Erythroid Kruppel-like factor exhibits an early and sequentially localized pattern of expression during mammalian erythroid ontogeny. Dev Dyn. 1996;206: 248-259. [DOI] [PubMed] [Google Scholar]
- 46.Guy LG, Mei Q, Perkins AC, Orkin SH, Wall L. Erythroid Kruppel-like factor is essential for beta-globin gene expression even in absence of gene competition, but is not sufficient to induce the switch from gamma-globin to beta-globin gene expression. Blood. 1998;91: 2259-2263. [PubMed] [Google Scholar]
- 47.Tewari R, Gillemans N, Wijgerde M, et al. Erythroid Kruppel-like factor (EKLF) is active in primitive and definitive erythroid cells and is required for the function of 5′HS3 of the beta-globin locus control region. EMBO J. 1998;17: 2334-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.University of California at Santa Cruz. UCSC genome browser. http://genome.ucsc.edu. Accessed March 2005.
- 49.Merika M, Orkin SH. Functional synergy and physical interactions of the erythroid transcription factor GATA-1 with the Kruppel family proteins Sp1 and EKLF. Mol Cell Biol. 1995;15: 2437-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donze D, Townes TM, Bieker JJ. Role of erythroid Kruppel-like factor in human gamma- to beta-globin gene switching. J Biol Chem. 1995;270: 1955-1959. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Bieker JJ. Erythroid Kruppel-like factor (EKLF) contains a multifunctional transcriptional activation domain important for inter- and intramolecular interactions. EMBO J. 1996;15: 5888-5896. [PMC free article] [PubMed] [Google Scholar]
- 52.Welch JJ, Watts JA, Vakoc CR, et al. Global regulation of erythroid gene expression by transcription factor GATA-1. Blood. 2004;104: 3136-3147. [DOI] [PubMed] [Google Scholar]
- 53.Drissen R, von Lindern M, Kolbus A, et al. The erythroid phenotype of EKLF-null mice: defects in hemoglobin metabolism and membrane stability. Mol Cell Biol. 2005;25: 5205-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basu P, Morris PE, Haar JL, et al. KLF2 is essential for primitive erythropoiesis and regulates the human and murine embryonic {beta}-like globin genes in vivo. Blood. 2005;106: 2566-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edgar AJ, Vidyatilake HM, Wickramasinghe SN. X-linked sideroblastic anaemia due to a mutation in the erythroid 5-aminolaevulinate synthase gene leading to an arginine170 to leucine substitution. Eur J Haematol. 1998;61: 55-58. [DOI] [PubMed] [Google Scholar]
- 56.Nakajima O, Takahashi S, Harigae H, et al. Heme deficiency in erythroid lineage causes differentiation arrest and cytoplasmic iron overload. EMBO J. 1999;18: 6282-6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brownlie A, Donovan A, Pratt SJ, et al. Positional cloning of the zebrafish sauternes gene: a model for congenital sideroblastic anaemia [see comments]. Nat Genet. 1998;20: 244-250. [DOI] [PubMed] [Google Scholar]
- 58.Kramer MF, Gunaratne P, Ferreira GC. Transcriptional regulation of the murine erythroid-specific 5-aminolevulinate synthase gene. Gene. 2000;247: 153-166. [DOI] [PubMed] [Google Scholar]
- 59.Surinya KH, Cox TC, May BK. Identification and characterization of a conserved erythroid-specific enhancer located in intron 8 of the human 5-aminolevulinate synthase 2 gene. J Biol Chem. 1998;273: 16798-16809. [DOI] [PubMed] [Google Scholar]
- 60.Mignotte V, Eleouet JF, Raich N, Romeo PH. Cis-and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc Natl Acad Sci U S A. 1989;86: 6548-6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Porcher C, Pitiot G, Plumb M, Lowe S, de Verneuil H, Grandchamp B. Characterization of hypersensitive sites, protein-binding motifs, and regulatory elements in both promoters of the mouse porphobilinogen deaminase gene. J Biol Chem. 1991;266: 10562-10569. [PubMed] [Google Scholar]
- 62.Rebeiz N, Arkins S, Kelley KW, Rebeiz CA. Enhancement of coproporphyrinogen III transport into isolated transformed leukocyte mitochondria by ATP. Arch Biochem Biophys. 1996;333: 475-481. [DOI] [PubMed] [Google Scholar]
- 63.Ye TZ, Gordon CT, Lai YH, et al. Ermap, a gene coding for a novel erythroid specific adhesion/receptor membrane protein. Gene. 2000;242: 337-345. [DOI] [PubMed] [Google Scholar]
- 64.Wagner FF, Poole J, Flegel WA. Scianna antigens including Rd are expressed by ERMAP. Blood. 2003;101: 752-757. [DOI] [PubMed] [Google Scholar]
- 65.Bailly P, Tontti E, Hermand P, Cartron JP, Gahmberg CG. The red cell LW blood group protein is an intercellular adhesion molecule which binds to CD11/CD18 leukocyte integrins. Eur J Immunol. 1995;25: 3316-3320. [DOI] [PubMed] [Google Scholar]
- 66.Furusawa T, Yanai N, Hara T, Miyajima A, Obinata M. Integrin-associated protein (IAP, also termed CD47) is involved in stroma-supported erythropoiesis. J Biochem (Tokyo). 1998;123: 101-106. [DOI] [PubMed] [Google Scholar]
- 67.Van Kim CL, Colin Y, Cartron JP. Rh proteins: key structural and functional components of the red cell membrane. Blood Rev. 2005. Epub ahead of print. [DOI] [PubMed]
- 68.Petranka JG, Fleenor DE, Sykes K, Kaufman RE, Rosse WF. Structure of the CD59-encoding gene: further evidence of a relationship to murine lymphocyte antigen Ly-6 protein. Proc Natl Acad Sci U S A. 1992;89: 7876-7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang LV, Nicholson RH, Kaplan J, Galy A, Li L. Hemogen is a novel nuclear factor specifically expressed in mouse hematopoietic development and its human homologue EDAG maps to chromosome 9q22, a region containing breakpoints of hematological neoplasms. Mech Dev. 2001;104: 105-111. [DOI] [PubMed] [Google Scholar]
- 70.Shivdasani RA, Orkin SH. Erythropoiesis and globin gene expression in mice lacking the transcription factor NF-E2. Proc Natl Acad Sci U S A. 1995;92: 8690-8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crossley M, Whitelaw E, Perkins A, Williams G, Fujiwara Y, Orkin SH. Isolation and characterization of the cDNA encoding BKLF/TEF-2, a major CACCC-box-binding protein in erythroid cells and selected other cells. Mol Cell Biol. 1996;16: 1695-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]